Abstract
Recently it was demonstrated that treatment with a nonselective endothelin (ET) receptor antagonist significantly reduces myocardial infarct size, which suggests a major role for ET in tissue repair following myocardial infarction (MI). Tissue repair and remodeling found at the site of MI are mainly attributed to myofibroblasts (myoFbs), which are phenotypically transformed fibroblasts that express α-smooth muscle actin. It is unclear whether myoFbs generate ET peptides and consequentially regulate pathophysiological functions de novo through expression of the ET-1 precursor (prepro-ET-1), ET-converting enzyme-1 (ECE-1), a metalloprotease that is required to convert Big ET-1 to ET-1 and ET receptors. To address these intriguing questions, we used cultured myoFbs isolated from 4-wk-old MI scar tissue. In cultured cells, we found: 1) expression of mRNA for ET precursor gene (ppET1), ECE-1, and ETA and ETB receptors by semiquantitative RT-PCR; 2) phosphoramidon-sensitive ECE-1 activity, which converts Big ET-1 to biologically active peptide ET-1; 3) expression of ETA and ETB receptors; 4) elaboration of Big ET-1 and ET-1 peptides in myoFb culture media; and 5) upregulation of type I collagen gene expression and synthesis by ET, which was blocked by bosentan (a nonselective ETA- and ETB receptor blocker). These studies clearly indicated that myoFbs express and generate ET-1 and receptor-mediated modulation of type I collagen expression by ET-1. Locally generated ET-1 may contribute to tissue repair of the infarcted heart in an autocrine/paracrine manner.
Keywords: converting enzyme, receptors, type I collagen, bosentan, ppET1 gene
Tissue remodeling is a common response to various insults or injuries; it can be the outcome of any perturbation in the cellular function of any tissue and is characterized by excessive expression and accumulation of type I collagen (α, RI). Often following myocardial infarction (MI), the remodeling process (extracellular matrix deposition) stands as an obstacle to normal physiological functioning of the heart. Studies have identified a deleterious role for endothelin (ET) after MI (7, 8, 16, 22). In a clinical study that consisted of 142 cardiac patients, plasma ET levels 3 days post-MI were strongly correlated to survival probability, with high ET levels being associated with an unfavorable prognosis (23). Additional correlations between ET and negative survival probability after MI were observed in rats with left coronary artery ligation, where blockade of ETA receptors with BQ-123 significantly increased the survival rate of animals (29).
In vivo and in vitro studies in several organ systems and cell cultures using ET receptor antagonists strongly indicate a correlation between endogenous ET and fibrous tissue formation. In the liver, studies demonstrated that antagonism of ET after the establishment of fibrosing injury reduced hepatic stellate cell activation and matrix production, which suggests a role for ET in the development of fibrogenesis (26). Addition of ET-1 to cultured cells led to increased collagen synthesis of adult rat cardiac fibroblasts (10) and neonatal bone organ cultures (41).
It has been shown that treatment with bosentan, an ETA and ETB receptor blocker, attenuated deoxycorticosterone acetate-induced cardiac fibrosis in rats (11). Furthermore, treatment with TAK-044, a nonselective ETA and ETB receptor antagonist, significantly reduced rat heart infarct size, which suggests a role for ET in tissue repair following MI (16).
Although several studies support the concept that administration of ET antagonists mitigates ET-mediated adverse remodeling (27, 30), some reports demonstrate that antagonizing ET action may aggravate remodeling after MI (7). However, the time of treatment with these receptor antagonists and the choice of antagonist used have been shown to play important roles in alleviating the ET-induced adverse remodeling (19, 22, 29, 39). In rats, subsequent to MI, ET was elevated in plasma and heart interstitial fluid (3, 8). To determine areas of ET-1 production in the infarcted heart, Oie et al. (21) examined infarcted regions of rat heart with immunohistochemistry and found heavy immunostaining for ET-1 in the granulation tissue at the site of infarction. Fibroblasts and endothelial cells also displayed marked immunoreactivity, and interestingly, the time course of the immunoreactivity correlated with wound healing and was consistent with ET production by the wound-healing cells that produce the granulation tissue (21).
Myofibroblasts (myoFbs) are the predominant wound-healing fibroblast-like cells and have morphological features of both smooth muscle cells and fibroblasts. They express extracellular matrix and contractile proteins and play major roles in matrix remodeling and wound contraction in diverse tissues (9, 15, 37). The contribution of myoFbs to tissue repair in the injured heart is of considerable interest. Recently it was demonstrated that ET along with vascular endothelial growth factor (VEGF) is a major player in angiogenic response and gastric ulcer healing processes (1). Our previous and recent studies on cardiac myoFbs demonstrated that these cells express ANG II (12), transforming growth factor (TGF)-β1 (4), and VEGF (5), which play major roles in tissue regeneration and angiogenesis (4, 5, 12, 15). However, it is unclear whether these cells also produce ET de novo. To study the local production of ET at the site of MI during tissue regeneration and/or remodeling, we isolated, cultured, and examined myoFbs from the site of MI for various components involved in de novo generation of ET. These components are composed of the ET precursor (prepro-ET-1) gene ppET1, Big ET-1, ET-converting enzyme-1 (ECE-1, a metalloprotease that converts Big ET-1 to ET-1), and stereospecific ETA and ETB receptors. Furthermore, we also investigated the influence of ET on type I collagen expression in this study.
Materials and Methods
Materials
Protease inhibitors (leupeptin, aprotinin, pepstatin A, and PMSF) were purchased from Sigma Chemical (St. Louis, MO). Phosphoramidon, bestatin, and actinonin were obtained from Peptide International (Louisville, KY). The 125I-labeled ET-1 was purchased from DuPont-NEN (Boston, MA). Rat ET-1 and Big ET-1 were purchased from Peninsula Laboratories (Belmont, CA). Bosentan was a generous gift from Roche Pharmaceuticals. Enzymes and other reagents required for RT-PCR were obtained from Promega Biotech (Madison, WI). The β2-microglobulin (β2-MG) oligonucleotide primers were purchased from ClonTech (Palo Alto, CA), and other oligonucleotide primers were synthesized in the DNA Core Facility at the University of Missouri Health Sciences Center (Columbia, MO). Rat-specific anti-ETA and anti-ETB receptor antibodies were obtained from Alomone Laboratories (Jerusalem, Israel). All other chemicals used in this study were of reagent grade.
Isolation and Culture of MyoFbs
Left ventricular MI was created in 8-wk-old male Sprague-Dawley rats (250–300 g body wt) via ligation of the left coronary artery. Rats were euthanized 28 days following ligation, hearts were removed, and scar tissue from the infarcted area was isolated for harvesting the myoFbs. Rat MI models were created using a protocol approved by the Institutional Animal Care and Use Committee. Previous in vivo studies confirmed the presence of an abundant population of myoFbs at the site of infarction at 28 days post-MI (37). The border region was removed by microdissection to avoid possible contamination of blood vessels and cardiac myocytes from this region. Recovered MI scar tissue was washed four times with 5 ml of saline. Microdissected scar tissue was sliced into smaller pieces and placed in a six-well plate that contained 20% FCS in DMEM with amphotericin-B (2 μg/ml), gentamycin (20.5 μg/ml), and penicillin-streptomycin (20 U/ml and 20 μg/ml, respectively). After 6 h, additional medium with 10% FCS was added. Cells were washed after 24 h with DMEM that contained 10% FCS, and medium was subsequently replaced every 2–3 days. Cells were grown and maintained under the conditions previously described (4, 12). After 2 wk, cells reached confluence and were routinely split at a 1:3 ratio. Early passages of cells (passages 3 and 4) were frozen in 10% DMSO, stored in liquid nitrogen, and used as needed.
Morphological and phenotypical characterization of cultured myoFbs were studied by immunohistochemical and confocal microscopy as previously reported (4). Briefly, myoFbs were found to be positive to α-smooth muscle actin, vimentin, and desmin and negative to anti-factor VIII. Vimentin staining is specific for fibroblast-like cells and excludes the presence of vascular smooth muscle cells from our preparations. Absence of a positive factor VIII result indicated that these cells were nonendothelial. Therefore, cultured myoFbs were neither endothelial nor vascular smooth muscle cells. We observed little or no contamination of these cells in culture, and these cells were stable up to 12 passages. We used cells from early passages for these studies to avoid possible phenotypic instability.
RT-PCR Analysis
We used previously established semiquantitative RT-PCR assays (5, 12) for prepro-ET-1, ECE-1, and ETA and ETB receptor expression. To monitor cDNA synthesis efficiency, β2-MG was used as an internal control.
RNA extraction and cDNA synthesis
As reported previously (4, 12), total RNA was extracted from myoFbs using the guanidine thiocyanate/phenol method. Briefly, confluent myoFbs were washed with PBS, and total cellular RNA was extracted with RNAzol B solution (Biotecx; Houston, TX), which was followed by chloroform extraction and precipitation with isopropanol. RNA pellets were washed with 75% ethanol, air dried, and dissolved in distilled water. cDNA was synthesized by reverse transcription of cellular RNA as previously described (5).
Oligonucleotide primer design
PCR primers for amplifying rat prepro-ET-1 (31), ECE-1 (43), and ETA (18) and ETB receptor cDNA were selected from the rat gene sequence as previously reported (32). The β2-MG cDNA was PCR amplified using primers obtained from ClonTech. The specificity of these primers was confirmed by purifying the respective PCR products (QiaQuick gel extraction system, Qiagen; Chats-worth, CA) and sequencing them. The sequences of the oligonucleotide primers used and the expected PCR product sizes are summarized in Table 1.
Table 1. Oligonucleotide primers for PCR amplification.
| Gene | GenBank Accession No. | Primer Name | Primer Sequence 5′ → 3′ | Product Size, bp |
|---|---|---|---|---|
| ppET1 | M64711 | ET-1-5′ | GAAGTGTATCTATCAGCAGC | 334 |
| ET-1-3′ | GGAACACCTCAACCTCTCTTGG | |||
| ECE-1 | U29196 | ECE-5′ | CGTAGCGATAGTCTTAGCAC | 529 |
| ECE-3′ | GTGCCACACCAAAACTACAG | |||
| ETA | M60786 | ETA-5′ | CGAGGTCATGAGGCTTTTGG | 787 |
| ETA-3′ | GTGTTTAAGCTGTTGGCGGG | |||
| ETB | AF074963 | ETB-5′ | TGCACACCTTTCCGCAAGCACG | 919 |
| ETB-3′ | AGCTGGTGCCCTTCATACAGAAGGC | |||
| β2-MG | Y00441 | β2-MG-5′ | CTCCCCAAATTCAAGTGTACTCTCG | 249 |
| β2-MG-3′ | GAGTGACGTGTTTAACTCTGCAAGC |
β2-MG, β2-microglobulin (used as internal control); ET, endothelin.
Polymerase chain reaction
With the use of primer-specific sequences of β2-MG, prepro-ET-1, ECE-1, and ETA and ETB receptor genes were PCR amplified as described previously (5, 12). Briefly, 25-μl PCR reaction volumes that contained 20 mmol/l Tris·HCl (pH 8.3), 50 mmol/l KCl, 1.5 mmol/l MgCl2, 0.01% (wt/vol) gelatin, 200 μmol/l of each dNTP, 40 pmol of the appropriate oligonucleotide primers, 1 unit of Taq DNA polymerase, and 5–10 μl of the diluted myoFb cDNAs were amplified in a GeneAmp model 9600 thermocycler (Perkin-Elmer; Norwalk, CT) using the following conditions: after an initial denaturation at 94°C for 1 min, β2-MG sequences were PCR amplified using 25 cycles of denaturation at 94°C for 15 s, annealing at 55°C for 1 min, and primer extension at 72°C for 2 min with the denaturation time increasing an additional 1 s/cycle. A final extension at 72°C for 5 min was performed to ensure that all reactions were completed. Pre-pro-ET-1, ECE-1, and ETA and ETB receptor sequences were PCR amplified using a similar procedure except that the number of cycles was increased to 30 and the annealing temperature was increased to 58°C. PCR products were elec-trophoretically size fractionated on 1.5% agarose gels that contained ethidium bromide and were visualized with UV light.
Separation and Determination of ET-1 in MyoFb Culture Media
Serum-free culture media (each dish contained 5–10 ml media/≈2 million cells) from four separate experiments was utilized in triplicate, and the medium was collected in the presence of protease inhibitor cocktail (final concentrations were 1 μM each of pepstatin, aprotinin, leupeptin, bestatin, actonin, and phosphoramidon, and 0.1 mM PMSF), separated on C-2 columns, and concentrated before performance of ELISA for ET-1 or Big ET-1 (Amersham; Arlington Heights, IL).
Preconditioned solid-phase C-2 extraction columns were equilibrated with Tris·HCl buffer (0.1 mol/l, pH 7.3), and the samples (5–10 ml/sample) were drawn through the cartridge by vacuum. ET peptides were eluted with 0.1% trifluoroacetic acid in acetonitrile, evaporated to dryness in a Speed Vac concentrator equipped with a cold-finger trap, dissolved in ELISA buffer, and then stored at −20°C until further analysis by ELISA. ET peptide extraction efficiency in myoFb culture media ranged from 70 to 83%, which suggests a possible 13–30% underestimation of these peptides.
Protein Analysis
MyoFb lysate protein content was determined using a modified Lowry method (Bio-Rad protein assay kit, Bio-Rad Laboratories; Hercules, CA).
Collagen ELISA
Collagen ELISAs were performed using previously reported methods (24, 25) with minor alterations. Briefly, 96-well Costar EIA/RIA (enzyme immunoassay/radioimmunoassay) plates were coated with 100 μl of 100 mM carbonate-bicarbonate buffer. Collagen standards (type I collagen, Sigma Chemical) and 100-μl media samples were added directly to the buffer in triplicate, and plates were incubated at 4°C overnight. Wells were washed three times with PBS that contained 0.05% Tween 20 (PBS-Tween 20) buffer. Nonspecific binding sites were blocked by 1 h of incubation with 250 μl/well of 1% BSA in Tris-buffered saline (TBS, 100 mM Tris·HCl with 150 mM NaCl). After the wells were washed with PBS three times, 100 μl of primary antibody (1:1,000 dilution, rabbit anti-rat type I collagen polyclonal antibody, Bio Design International; Kennebunk, ME) in TBS was added and wells were incubated for 1 h at room temperature. Plates were washed five times before addition of 100 μl of secondary antibody (1:20,000 dilution, goat anti-rabbit monoclonal antibody with alkaline phosphatase conjugate, Sigma Chemical) in TBS and incubated at room temperature for 1 h. Wells were then washed five times with PBS-Tween 20 and twice with distilled water before 100 μl (1 mg/ml) of the color-development agent p-nitrophenyl phosphate in 1.0 M diethanolamine buffer was added. Optical density readings at 405 nm were taken at half-hour intervals with the Vmax microplate reader (Molecular Devices; Sunnyvale, CA).
Conversion of Exogenous Big ET-1 to ET-1 by MyoFbs
Confluent myoFbs (100-mm dishes in triplicate; ≈2 million cells/dish) were washed three times (3 × 10 ml/dish) with serum-free media and then incubated in serum-free media (5 ml/dish) with and without Big ET-1 (10 nM) in the absence and presence of the ECE-1 inhibitor phosphoramidon (0.1 μM) for 20 h. Media were collected, processed as above, and analyzed for ET-1 contents.
Western Immunoblotting for ET receptors
Confluent myoFbs were washed with PBS, and cell lysates were prepared (12). Aliquots with 50 μg of protein were electrophoretically fractionated on 10% SDS-PAGE minigels and were electroblotted onto a nitrocellulose membrane. The membrane was sequentially incubated in TBS (pH 7.4) that contained 5% dry milk for 30 min and diluted (1:200) rat anti-ETA and anti-ETB receptor primary antibody (Alomone Laboratories) in 5% Blotto (Bio-Rad nonfat dry milk powder in TBS) for 12 h on a shaker at 4°C. The blot was washed with TBS that contained 0.1% Tween 20 and was incubated with a 1:1,000 dilution of horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (Sigma Chemical) in 5% Blotto for 1 h at room temperature, washed with TBS, and incubated with the peroxidase substrate. Immunoreactive bands were visualized within 10 min using an ECL detection kit (Amersham Pharmacia Biotech; Piscataway, NJ).
Statistical Analysis
Results are reported as means ± SE for a minimum of five determinations, each of which was performed either in duplicate or triplicate. Statistical analysis was performed using one-way ANOVA with P ≤ 0.05 considered significant.
Results
Expression of Prepro-ET-1 and ECE-1 by MyoFbs
Expression of the prepro-ET-1 gene (ppET1, the only known precursor gene that encodes Big ET-1) and ECE-1 were examined in myoFbs by semiquantitative RT-PCR technique. RT-PCR studies resulted in PCR products of predicted sizes for ppET1 (334 bp) and ECE-1 (529 bp) (Fig. 1). These results demonstrated that myoFbs express ppET1 and the key enzyme ECE-1 that converts Big ET-1 to the active peptide ET-1. The PCR products for ET-1 and ECE-1 were of predicted sizes, and their identities were confirmed by cycle sequencing.
Fig. 1.
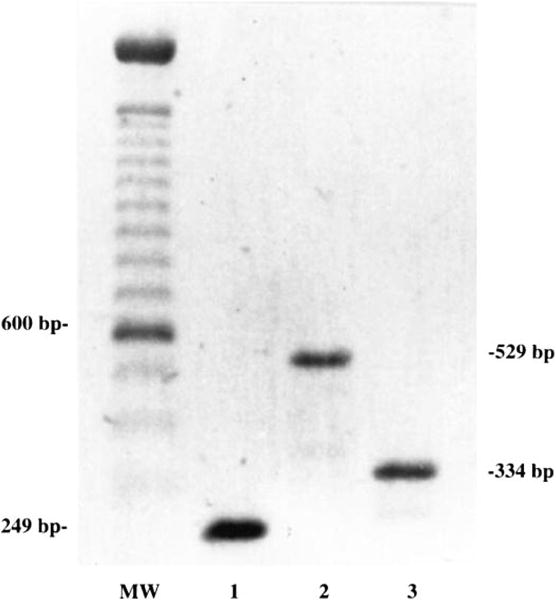
Myofibroblasts (myoFbs) express endothelin (ET) precursor gene ppET1 and ET-converting enzyme-1 (ECE-1); RT-PCR results demonstrate expression of transcripts for ppET1 and ECE-1 genes. Lane 1, internal control β2-microglobulin; lane 2, ECE-1; lane 3, ppET1. MW, mol wt marker.
Determination of Big ET-1 and ET-1 Contents in MyoFb Culture Media
The elaboration of Big ET-1 and the production of ET-1 in the media of serum-deprived myoFbs were studied in a time-dependent manner. The results indicated that myoFbs generate both Big ET-1 and ET-1 (Figs. 2 and 3). The time-dependent pattern of Big ET-1 elaboration (0.5, 2, 4, and 8 h) indicated maximum levels of Big ET-1 in myoFb culture media at 2 and 4 h. In another set of experiments with an 8-h incubation in the presence of phosphoramidon (a specific inhibitor for ECE-1), Big ET-1 levels were increased two- to threefold (Fig. 2, fifth bar), which suggests that the gradual drop seen in Big ET-1 concentration at 4 and 8 h is due to its conversion to ET-1 by ECE-1 (Fig. 2, third and fourth bars). The production of ET-1 was time dependent (6, 12, 24, and 36 h) with the highest levels of ET-1 found at 24 h. ET-1 concentrations after 24 h in the presence of bosentan (10−6 M) were highly elevated (90 pg/ml) compared with 24 h without bosentan (Fig. 3, fifth bar), because a significant portion of ET-1 loss due to its binding to cellular ET receptors was prevented by bosentan.
Fig. 2.
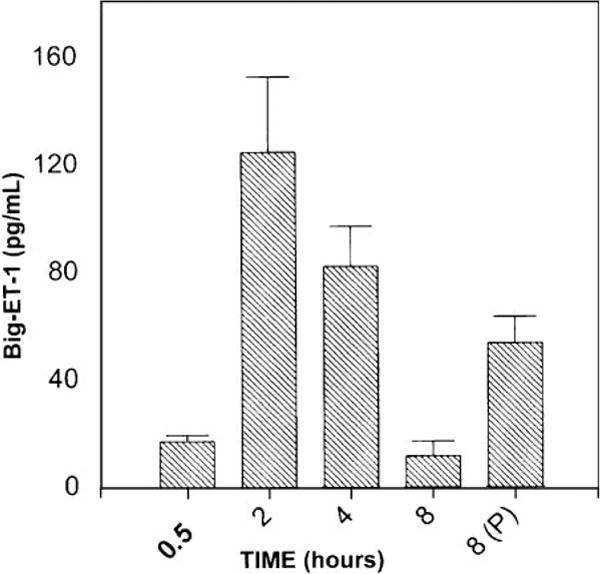
Time-dependent elaboration of Big ET-1 by myoFbs. Big ET-1 levels in serum-free culture media were analyzed by ELISA. Big ET-1 levels were elevated at 8 h in the presence of 0.1 mM phosphoramidon (P), which is an ECE-1-specific inhibitor (fifth bar). Values are means ± SE.
Fig. 3.
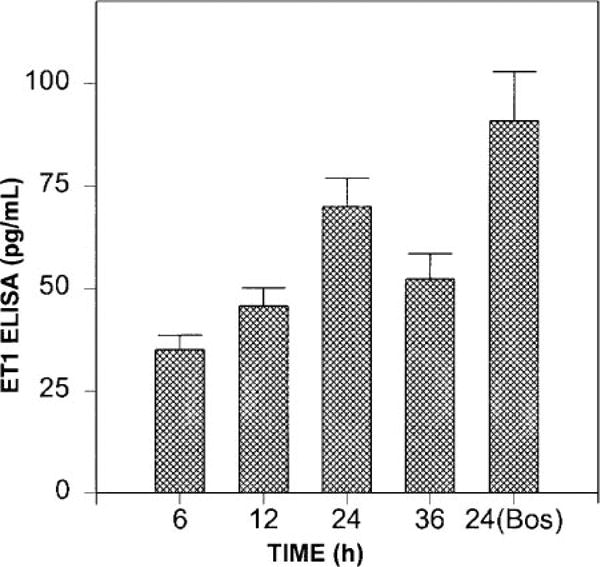
Time-dependent production of ET-1 by myoFbs. ET-1 levels in serum-free culture media determined at different time points by ELISA are shown. Fifth bar represents 24-h incubation values in the presence of 10−6 M bosentan (Bos), a nonselective ETA and ETB receptor agonist. Values are means ± SE.
Protease Involved in Conversion of Big ET-1 to ET-1 and Effects of Exogenous Big ET-1 on MyoFb ET-1 Production
ECE-1 is a membrane-bound, phosphoramidon-sensitive, zinc metalloprotease that converts Big ET-1 to ET-1. The presence of ECE-1 was detected in the myoFb cell preparation, which converted exogenous Big ET-1 to ET-1 and was blocked by 100 μM phosphoramidon (Fig. 4).
Fig. 4.
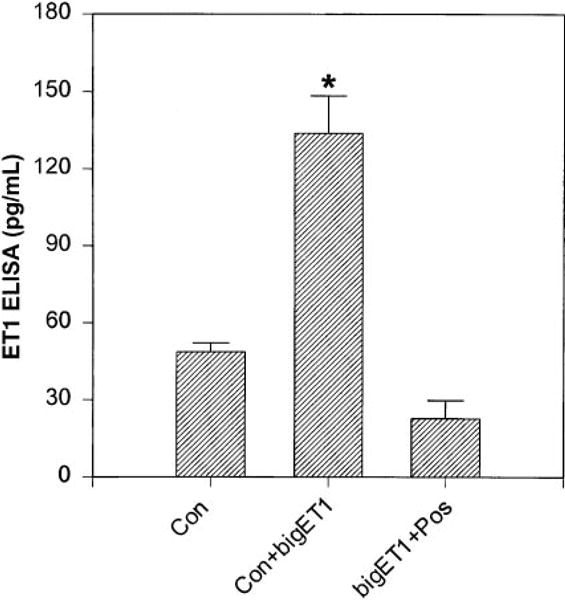
ECE-1 in myoFbs. ET-1 levels in 24-h serum-deprived media were detected after 20-h pretreatment with 10 μM Big ET-1 (middle) and Big ET-1 with 0.1 M phosphoramidon (right). Values are means ± SE. *P < 0.05.
The ability of confluent myoFbs to generate ET-1 was significantly (P ≤ 0.05) increased in the presence of exogenous Big ET-1 (Fig. 4, second bar) and was significantly reduced in the presence of the ECE-1 inhibitor phosphoramidon (Fig. 4, third bar), which suggests possible involvement of an active metalloprotease ECE-1 in generating ET-1 de novo.
Expression of ETA and ETB Receptors by RT-PCR
Using a standard semiquantitative RT-PCR method (5, 12, 14), we examined the expression of both ETA and ETB receptors in myoFbs. The results clearly demonstrated the amplification of a single predicted band of PCR product for ETA (787 bp) and ETB (919 bp) receptors, respectively (Fig. 5), which suggests that myoFbs express both ETA and ETB receptors.
Fig. 5.
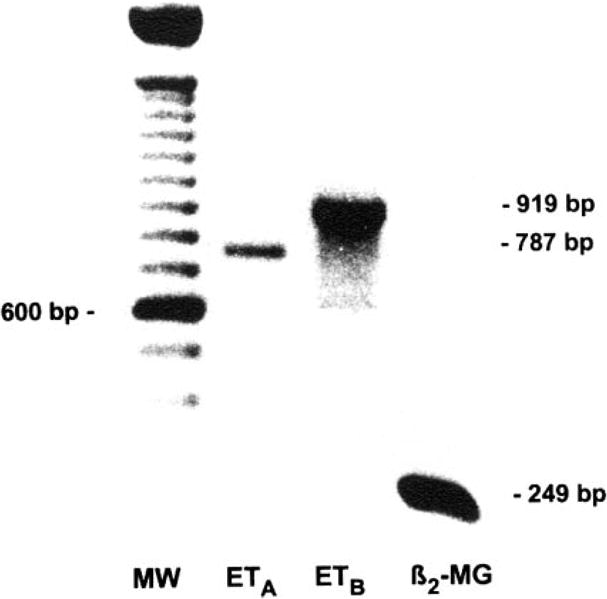
RT-PCR results demonstrate the expression of both ETA and ETB receptor genes in myoFbs. Lane 1, MW (100-bp ladder); lane 2, ETA receptors (787 bp); lane 3, ETB receptors (919 bp); lane 4, internal control, β2-microglobulin (β2-MG, 249 bp).
Western Immunoblotting for ETA and ETB Receptors
Cultured myoFb and coronary endothelial cell (as positive control) pellets were used to detect the presence of ET receptors by Western blot analysis using ETA and ETB receptor-specific antibodies. Results obtained from Western immunoblotting revealed two major bands of ∼60- and 39-kDa protein for the ETA receptor and a single band of ∼40 kDa for the ETB receptor (Fig. 6). These protein bands were within the predicted range as specified by the commercial supplier of these antibodies. These results confirm that cultured myoFbs express both ETA and ETB receptors.
Fig. 6.
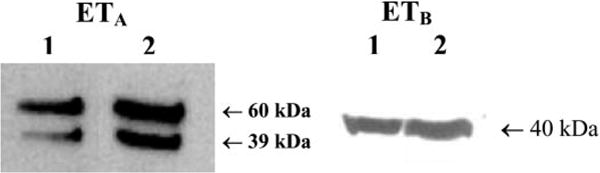
Identification of ETA and ETB receptor protein by Western blot using ETA (left) and ETB (right) receptor-specific antibodies. Lanes 1 and 2 represent myoFbs and coronary endothelial cells, respectively. ETA immunoblot with anti-ETA-specific antibody resulted in two bands of ≈60- and 39-kDa proteins, whereas immunoblot with anti-ETB receptor antibody identified a protein band of ≈40 kDa. Preimmune serum served as a control (not shown).
ET-1-Receptor Binding Studies
The presence of ET receptors was also confirmed by ET-1-receptor binding assays using 125I-labeled ET-1 as the radioligand (13). ET-1 receptor densities as determined at the saturation of binding for fibroblasts and myoFbs were 31.80 ± 1.03 and 35.89 ± 1.93 fmol/mg of protein, respectively, which suggests that these were the active target cells for the autocoid influence of ETs on the induction of type I collagen gene expression.
Type I Collagen mRNA Induction by ET-1
The influence of ET on type I collagen mRNA expression was evaluated by Northern blot analysis, and the results indicated an induction of type I collagen mRNA by ET at the concentrations studied (10−7 to 10−11 M; Fig. 7). As shown in Fig. 7, the upregulation of type I collagen was effectively blocked by the ETA and ETB receptor antagonist bosentan (10−6 M), which suggests that induction of α1-type I collagen mRNA is an ET receptor-mediated mechanism.
Fig. 7.
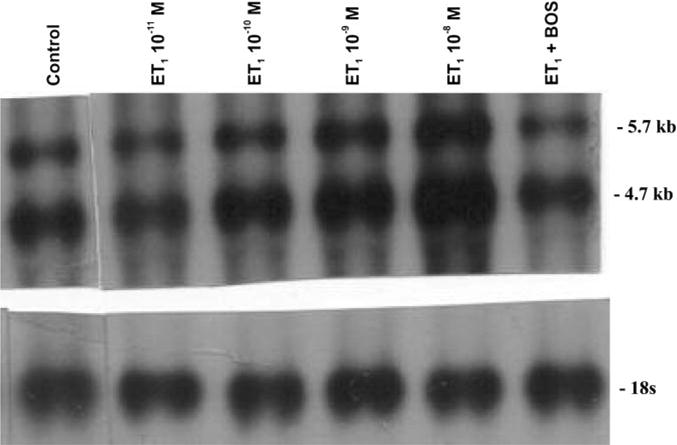
Type I collagen mRNA analysis by Northern blot. ET-1 has a concentration-dependent effect on type I collagen gene expression. ET induction of myoFb type I collagen mRNA was determined by Northern hybridization (band sizes: top, 5.7 kb; bottom, 4.7 kb). Lane 1, untreated control. Lanes 2–5, treated with ET-1 (10−11 to 10−8 M). Lane 6, treated with ET-1 (10−9 M) and bosentan (10−6 M).
Type I Collagen Synthesis by ELISA
The influence of ET-1 on type I collagen protein synthesis was evaluated by ELISA, and the results indicated a dose-dependent increase in type I collagen synthesis induced by ET-1 (Fig. 8). The upregulation of type I collagen synthesis by ET-1 was significantly higher (P ≤ 0.05 at 10−10 to 10−8 M), which was attenuated with bosentan treatment (Fig. 8, seventh bar; 10−9 M ET-1 plus 10−6 M bosentan). These results clearly indicate that induction of type I collagen expression by ET-1 was at both transcriptional (mRNA; see Fig. 7) and translational (protein; see Fig. 8) levels.
Fig. 8.
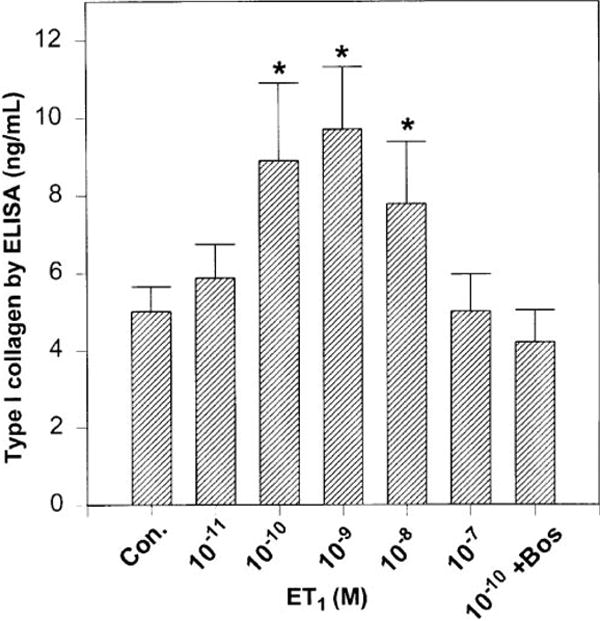
Quantitation of soluble type I collagen by ELISA. A concentration-dependent effect of ET-1 on myoFb type I collagen protein expression was determined by ELISA using rat type I collagen-specific antibody. First bar, untreated control; second through sixth bars, treated with ET-1 (10−11 to 10−7 M); seventh bar, treated with ET-1 (10−9 M) plus bosentan (10−6 M). Values are means ± SE. *P < 0.05.
Discussion
Several in vivo studies using ET-receptor antagonists implicated a role for ET in modulating extracellular matrix turnover, tissue repair, and remodeling (7, 8, 11, 16, 19, 21, 22, 27, 30, 39). ET-1 production in the failing heart as examined immunohistochemically revealed heavy ET-1 immunostaining that was confined to granulation tissue and proliferating fibroblasts (21). In many organs including heart, tissue repair in response to injury involves myoFbs, a phenotypically transformed cell type that is commonly referred to as a wound-healing cell. Studies from our laboratory and others have shown that myoFbs are the predominant wound-healing cells that appear at various sites of injury and express ANG II and are responsible for collagen turnover and tissue repair (6, 9, 12, 15, 34–37). These studies have consistently shown the presence of these cells at the site of injury and regeneration and also at sites of high collagen turnover such as MI (6, 12), skin-pouch tissue regeneration (15), and heart-valve leaflets (as valvular interstitial cells, a normal site of high collagen turnover; Ref. 14), where they have demonstrated generation of ANG II and regulation of collagen turnover. MyoFbs have morphological features of both smooth muscle cells and fibroblasts (4, 9). In our study, we isolated myoFbs from rat MI scar tissue and verified that these cells express ET peptides. RT-PCR experiments demonstrated that myoFbs express mRNA for the ET precursor prepro-ET-1. Furthermore, we demonstrated the presence of both Big ET-1 and ET-1 in the culture media by ELISA. The findings of Oie et al. (21) support our results that myoFbs isolated from the site of infarction generate ET-1 and could serve as a local source of ET at the site of MI.
Our concurrent objective of this investigation was to demonstrate the expression of the key enzyme ECE-1 by myoFbs. The data showed the presence of mRNA for ECE-1 in myoFbs, and addition of exogenous Big ET-1 to myoFb culture media significantly increased ET-1 levels. This suggests the presence of ECE-1 activity, which was significantly reduced in the presence of the ECE-1 inhibitor phosphoramidon. Expression of phosphoramidon-sensitive ECE-1 by myoFbs strengthens the fact that these cells have all the necessary elements to produce biologically active ET-1 from ET precursors.
Earlier reports have shown that ET-1 is mitogenic and that it stimulates fibroblast proliferation (40). Media obtained from bovine aortic endothelial cells induced collagen accumulation and protein formation by human fibroblasts, which suggests that an endothelial cell-derived factor can stimulate collagen production by fibroblasts (42). We have previously demonstrated a positive influence of ET-1 on cardiac fibroblasts (10) and vascular smooth muscle cell (25) collagen expression. To determine whether myoFbs, a cell type that is isolated from infarcted rat heart, is responsive to ET-1, we investigated the presence of specific receptors for ET-1 for an autocrine influence. Through RT-PCR and Western blot analyses, we found the presence of both ETA- and ETB-receptor subtypes and also demonstrated a significant 125I-labeled ET binding to myoFb cell membranes. In our studies, we have demonstrated that myoFbs isolated from the site of infarction not only express ANG II (12) and transforming growth factor (TGF)-β1 (4), but also express ppET1, ECE-1, and ETA and ETB receptors thus implicating de novo expression of ET by myoFbs and suggesting a possible role for locally generated ET-1 in tissue regeneration and remodeling at the site of MI.
As stated earlier, when Oie et al. (21) examined infarcted regions of rat heart with immunohistochemistry, they found heavy immunostaining for ET-1 in the granulation tissue at the site of infarction, which indicated that wound-healing myoFbs found at this site may be responsible for producing ET. Apart from the vascular endothelial cells, the possibility of myocytes, smooth muscle cells, and fibroblasts expressing ET cannot be ruled out, but these are not the classical wound-healing cells that are found at the site of tissue repair or remodeling. However, these cells can be the target cells for the paracrine action of ET as they express ET receptors (13, 25).
The regulation of ET-1 expression in these cells is unclear and it may be similar to the autoregulation that is reported for endothelial cells (28), or it could be influenced by TGF-β1 and/or other cytokines. Understanding the regulatory aspects of ET generation by myoFbs at the site of infarction is important and has become the subject of our ongoing study. A study by Boffa et al. (2) on renal vasculature showed ET as a mediator of fibrogenic action of ANG II. The fibrotic response induced by ANG II was mitigated by bosentan, a nonselective ET-receptor antagonist. Similarly, it was shown that TGF-β1 is known to upregulate ET-1 expression and production in several cell types (17, 20, 33, 40). Studies by Sun et al. demonstrated that ANG II plays a vital role in collagen turnover and remodeling in several tissue-injury models including myocardium (36, 37). Our earlier study (15) on tissue regeneration in response to injury in a rat-pouch tissue model showed the production of ANG II, which regulates TGF-β1. Previous studies from our group conclusively showed that myoFbs produce ANG II, TGF-β1, and VEGF (4, 5, 12), and the present study confirms the de novo production of ET and its receptors. From these studies, it is evident that myoFbs play a unique role in tissue repair and regeneration by expressing ANG II, ET, VEGF, and TGF-β1, which modulate collagen turnover in response to injury in different organs.
Collectively, results from the present study demonstrate the generation of Big ET-1 and ET-1 de novo by cultured myoFbs isolated from infarct scar tissue and the influence of ET on type I collagen expression. Such locally generated ET-1, ANG II, TGF-β1, and VEGF could influence collagen turnover and tissue repair and remodeling at and remote to the site of injury in an autocrine/paracrine manner as well as in cell-cell and cell-matrix contractions, which promote matrix remodeling and scar thinning.
Acknowledgments
The author thanks Rouzanna Burton for excellent technical assistance and Drs. Vishnu Chintalgattu and Sashidhar Nakerakanti for assistance in revising this article.
Disclosures: This work was supported by National Heart, Lung, and Blood Institute Grant HL-60047 and in part by American Heart Association, National Grant-in-Aid 96012350.
Footnotes
Part of this work was performed at the Dalton Cardiovascular Research Center, Univ. of Missouri, Columbia, MO 65211.
References
- 1.Akimoto M, Hashimoto H, Maeda A, Shigemoto M, Yamashita K. Roles of angiogenic factors and endothelin-1 in gastric ulcer healing. Clin Sci (Lond) 2002;103(Suppl 48):450S–454S. doi: 10.1042/CS103S450S. [DOI] [PubMed] [Google Scholar]
- 2.Boffa JJ, Tharaux PL, Placier S, Ardaillou R, Dussaule JC, Chatziantoniou C. Angiotensin II activates collagen type I gene in the renal vasculature of transgenic mice during inhibition of nitric oxide synthesis: evidence for an endothelin-mediated mechanism. Circulation. 1999;100:1901–1908. doi: 10.1161/01.cir.100.18.1901. [DOI] [PubMed] [Google Scholar]
- 3.Brunner F. Cardiac tissue endothelin-1 levels under basal, stimulated, and ischemic conditions. J Cardiovasc Pharm. 1995;26(Suppl 3):S44–S46. [PubMed] [Google Scholar]
- 4.Campbell SE, Katwa LC. Angiotensin II stimulated expression of transforming growth factor-β1 in cardiac fibroblasts and myofibroblasts. J Mol Cell Cardiol. 1997;29:1947–1958. doi: 10.1006/jmcc.1997.0435. [DOI] [PubMed] [Google Scholar]
- 5.Chintalgattu V, Nair DM, Katwa LC. Cardiac myofibroblasts: a novel source of vascular endothelial growth factor (VEGF) and its receptors Flt-1 and KDR. J Mol Cell Cardiol. 2003;35:277–286. doi: 10.1016/s0022-2828(03)00006-3. [DOI] [PubMed] [Google Scholar]
- 6.Cleutjens JPM, Verluyten MJA, Smits JFM, Daemen MJAP. Collagen remodeling after myocardial infarction in the rat heart. Am J Pathol. 1995;147:325–338. [PMC free article] [PubMed] [Google Scholar]
- 7.Fraccarollo D, Galuppo P, Bauersachs J, Ertl G. Collagen accumulation after myocardial infarction: effects of ETA receptor blockade and implications for early remodeling. Cardiovasc Res. 2002;3:559–567. doi: 10.1016/s0008-6363(02)00256-0. [DOI] [PubMed] [Google Scholar]
- 8.Fraccarollo D, Hu K, Galuppo P, Gaudron P, Ertl G. Chronic endothelin receptor blockade attenuates progressive ventricular dilation and improves cardiac function in rats with myocardial infarction. Possible involvement of myocardial endothelin system in ventricular remodeling. Circulation. 1997;96:3963–3973. doi: 10.1161/01.cir.96.11.3963. [DOI] [PubMed] [Google Scholar]
- 9.Gabbiani G, Ryan GB, Majno G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia. 1971;27:549–550. doi: 10.1007/BF02147594. [DOI] [PubMed] [Google Scholar]
- 10.Guarda E, Katwa LC, Myers PR, Tyagi SC, Weber KT. Effects of endothelins on collagen turnover in cardiac fibroblasts. Cardiovasc Res. 1993;27:2130–2134. doi: 10.1093/cvr/27.12.2130. [DOI] [PubMed] [Google Scholar]
- 11.Karam H, Heudes D, Hess P, Gonzales MF, Loffler BM, Clozel M, Clozel JP. Respective role of humoral factors and blood pressure in cardiac remodeling of DOCA hypertensive rats. Cardiovasc Res. 1996;31:287–295. [PubMed] [Google Scholar]
- 12.Katwa LC, Campbell SE, Tyagi SC, Lee SJ, Cicila GT, Weber KT. Cultured myofibroblasts generate angiotensin peptides de novo. J Mol Cell Cardiol. 1997;29:1375–1386. doi: 10.1006/jmcc.1997.0376. [DOI] [PubMed] [Google Scholar]
- 13.Katwa LC, Guarda E, Weber KT. Endothelin receptors in cultured adult rat cardiac fibroblasts. Cardiovasc Res. 1993;27:2125–2129. doi: 10.1093/cvr/27.12.2125. [DOI] [PubMed] [Google Scholar]
- 14.Katwa LC, Tyagi SC, Campbell SE, Lee SJ, Cicila GT, Weber KT. Valvular interstitial cells express angiotensinogen and cathepsin D, and generate angiotensin peptides. Int J Biochem Cell Biol. 1996;28:807–821. doi: 10.1016/1357-2725(96)00012-x. [DOI] [PubMed] [Google Scholar]
- 15.Katwa LC, Sun Y, Campbell SE, Tyagi SC, Dhalla AK, Kandala JC, Weber KT. Pouch tissue and angiotensin peptide generation. J Mol Cell Cardiol. 1998;30:1401–1413. doi: 10.1006/jmcc.1998.0708. [DOI] [PubMed] [Google Scholar]
- 16.Kojima M, Kusumoto K, Fujiwara S, Watanabe T, Fujino M. Role of endothelin in the extension of myocardial infarct size studies with the endothelin receptor antagonist, TAK044. J Cardiovasc Pharmacol. 1995;26:S365–S368. [PubMed] [Google Scholar]
- 17.Kurihara H, Yoshizumi M, Sugiyama T, Takaku F, Yanagi-sawa M, Masaki T, Hamaoki M, Kato H, Yazaki Y. Transforming growth factor-β stimulates the expression of endothelin mRNA by vascular endothelial cells. Biochem Biophys Res Commun. 1989;159:1435–1440. doi: 10.1016/0006-291x(89)92270-5. [DOI] [PubMed] [Google Scholar]
- 18.Lin HY, Kaji EH, Winkel GK, Ives HE, Lodish HF. Cloning and functional expression of a vascular smooth muscle endothelin-1 receptor. Proc Natl Acad Sci USA. 1991;88:3185–3189. doi: 10.1073/pnas.88.8.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mulder P, Boujedaini H, Richard V, Derumeaux G, Henry JP, Renet S, Wessale J, Opgenorth T, Thuillez C. Selective endothelin-A versus combined endothelin-A/endothelin-B receptor blockade in rat chronic heart failure. Circulation. 2000;102:491–493. doi: 10.1161/01.cir.102.5.491. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura T, Ebihara I, Fukui M, Tomino Y, Koide H. Effect of a specific endothelin receptor A antagonist on mRNA levels for extracellular matrix components and growth factors in diabetic glomeruli. Diabetes. 1995;44:895–899. doi: 10.2337/diab.44.8.895. [DOI] [PubMed] [Google Scholar]
- 21.Oie E, Vinge LR, Tonnessen T, Grogaard HK, Kjekshus H, Christensen G, Smiseth OA, Attramadal H. Transient, isopeptide-specific induction of myocardial endothelin-1 mRNA in congestive heart failure in rats. Am J Physiol Heart Circ Physiol. 1997;273:H1727–H1736. doi: 10.1152/ajpheart.1997.273.4.H1727. [DOI] [PubMed] [Google Scholar]
- 22.Oie E, Yndestad A, Robins SP, Bornerheim R, Asberg A, Attramadal H. Early intervention with a potent endothelin-A/endothelin-B receptor antagonist aggravates left ventricular remodeling after myocardial infarction in rats. Basic Res Cardiol. 2002;97:239–247. doi: 10.1007/s003950200017. [DOI] [PubMed] [Google Scholar]
- 23.Ormland T, Lie RT, Aakvaag A, Aarsland T, Dickstein K. Plasma endothelin determination as a prognostic indicator of 1-year mortality after acute myocardial infarction. Circulation. 1994;89:1573–1579. doi: 10.1161/01.cir.89.4.1573. [DOI] [PubMed] [Google Scholar]
- 24.Rennard SI, Berg R, Martin GR, Foidart JM, Robey PG. Enzyme-linked immunoassay (ELISA) for connective tissue components. Anal Biochem. 1980;104:205–214. doi: 10.1016/0003-2697(80)90300-0. [DOI] [PubMed] [Google Scholar]
- 25.Rizvi MAD, Katwa LC, Spadone DP, Myers PR. The effects of endothelin-1 on collagen type I and III synthesis in cultured coronary artery vascular smooth muscle cells. J Mol Cell Cardiol. 1996;28:243–252. doi: 10.1006/jmcc.1996.0023. [DOI] [PubMed] [Google Scholar]
- 26.Rockey DC, Chung JJ. Endothelin antagonism in experimental hepatic fibrosis: implications for endothelin in the pathogenesis of wound healing. J Clin Invest. 1996;98:1381–1388. doi: 10.1172/JCI118925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubin LJ, Roux S. Bosentan: a dual endothelin receptor antagonist. Expert Opin Investig Drugs. 2002;11:991–1002. doi: 10.1517/13543784.11.7.991. [DOI] [PubMed] [Google Scholar]
- 28.Saito S, Hirata Y, Imai T, Marumo F. Autoregulation of endothelin-1 gene in rat endothelial cells. J Cardiovasc Pharmacol. 1995;26:S84–S87. [PubMed] [Google Scholar]
- 29.Sakai S, Miyaucyhi T, Kobayashi M, Yamaguchi I, Goto K, Sugishita Y. Inhibition of myocardial endothelin pathway improves long-term survival in heart failure. Nature. 1996;384:353–355. doi: 10.1038/384353a0. [DOI] [PubMed] [Google Scholar]
- 30.Sakai S, Miyauchi T, Yamaguchi I. Long-term endothelin receptor antagonist administration improves alterations in expression of various cardiac genes in failing myocardium of rats with heart failure. Circulation. 2000;101:2849–2853. doi: 10.1161/01.cir.101.24.2849. [DOI] [PubMed] [Google Scholar]
- 31.Sakurai T, Yanagisawa M, Inoue A, Ryan US, Kimura S, Mitsui Y, Goto K, Masaki T. cDNA cloning, sequence analysis, and tissue distribution of rat preproendothelin-1 mRNA. Biochem Biophys Res Commun. 1991;175:44–47. doi: 10.1016/s0006-291x(05)81197-0. [DOI] [PubMed] [Google Scholar]
- 32.Sakurai T, Yanagisawa M, Takuwa Y, Miyazaki H, Kimura S, Goto K, Masaki T. Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature. 1990;348:732–735. doi: 10.1038/348732a0. [DOI] [PubMed] [Google Scholar]
- 33.Schnermann JB, Zhu XL, Shu XQ, Yang TX, Huang YG, Kretzler M, Briggs JP. Regulation of endothelin production and secretion in cultured collecting duct cells by endogenous transforming growth factor-beta. Endocrinology. 1996;137:5000–5008. doi: 10.1210/endo.137.11.8895374. [DOI] [PubMed] [Google Scholar]
- 34.Sun Y, Kiani MF, Postlethwaite AE, Weber KT. Infarct scar as living tissue. Basic Res Cardiol. 2002;97:343–347. doi: 10.1007/s00395-002-0365-8. [DOI] [PubMed] [Google Scholar]
- 35.Sun Y, Ramires FJA, Zhou G, Ganjam VK, Weber KT. Fibrous tissue and angiotensin II. J Mol Cell Cardiol. 1997;29:2001–2012. doi: 10.1006/jmcc.1997.0451. [DOI] [PubMed] [Google Scholar]
- 36.Sun Y, Weber KT. Angiotensin converting enzyme and myofibroblasts during tissue repair in the heart. J Mol Cell Cardiol. 1996;28:851–858. doi: 10.1006/jmcc.1996.0080. [DOI] [PubMed] [Google Scholar]
- 37.Sun Y, Weber KT. Cells expressing angiotensin II receptors in fibrous tissue of rat heart. Cardiovasc Res. 1996;31:518–525. [PubMed] [Google Scholar]
- 38.Takada K, Matsumura Y, Dohmen S, Mitsutomi N, Takaoka M, Morimoto S. Endothelin-1 secretion from cultured vascular endothelial cells of DOCA-salt hypertensive rats. Life Sci. 1996;59:PL111–PL116. doi: 10.1016/0024-3205(96)00366-9. [DOI] [PubMed] [Google Scholar]
- 39.Takahashi C, Kagaya Y, Namiuchi S, Takeda M, Fukuchi M, Otani H, Ninomiya M, Yamane Y, Kohzuki M, Watanabe J, Shirato K. Nonselective endothelin receptor antagonist initiated soon after the onset of myocardial infarction may deteriorate 24-hour survival. J Cardiovasc Pharmacol. 2001;38:29–38. doi: 10.1097/00005344-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 40.Takuwa N, Takuwa Y, Yanagisawa M, Yamashita K, Masaki T. A novel vasoactive peptide endothelin stimulates mitogenesis through inositol lipid turnover in Swiss 3T3 fibroblasts. J Biol Chem. 1989;264:7856–7861. [PubMed] [Google Scholar]
- 41.Tatrai A, Foster S, Lakatos P, Shankar G, Stern PH. Endothelin-1 actions on resorption, collagen and noncollagen protein synthesis, and phosphatidylinositol turnover in bone organ cultures. Endocrinology. 1992;131:603–607. doi: 10.1210/endo.131.2.1639010. [DOI] [PubMed] [Google Scholar]
- 42.Villanueva AG, Farber HW, Rounds S, Goldstein RH. Stimulation of fibroblast collagen and total protein formation by an endothelial cell-derived factor. Circ Res. 1991;69:134–141. doi: 10.1161/01.res.69.1.134. [DOI] [PubMed] [Google Scholar]
- 43.Wang X, Douglas SA, Louden C, Vickery-Clark LM, Feuer-stein GZ, Ohlstein EO. Expression of endothelin-1, endothelin-3, endothelin-converting enzyme-1, and endothelin-A and endothelin-B receptor mRNA after angioplasty-induced neointimal formation in the rat. Circ Res. 1996;78:322–328. doi: 10.1161/01.res.78.2.322. [DOI] [PubMed] [Google Scholar]


