Abstract
While neural stem/progenitor cells (NSCs) in the dentate gyrus of the hippocampus have been extensively characterized, the behavior of NSCs in the CA1 and CA3 subfields of the hippocampus is mostly unclear. Therefore, we compared the in vitro behavior of NSCs expanded from the micro-dissected CA1 and CA3 subfields of postnatal day (PND) 4 and 12 Fischer 344 rats. A small fraction (~1%) of dissociated cells from CA1 and CA3 subfields of both PND 4 and 12 hippocampi formed neurospheres in the presence of EGF and FGF-2. A vast majority of neurosphere cells expressed NSC markers such as nestin, Sox-2 and Musashi-1. Differentiation assays revealed the ability of these NSCs to give rise to neurons, astrocytes, and oligodendrocytes. Interestingly, the overall neuronal differentiation of NSCs from both subfields decreased with age (23-28% at PND4 to 5-10% at PND12) but the extent of oligodendrocyte differentiation from NSCs increased with age (24-32% at PND 4 to 45-55% at PND 12). Differentiation of NSCs into astrocytes was however unchanged (40-48%). Furthermore, NSCs from both subfields gave rise to GABA-ergic neurons including subclasses expressing markers such as calbindin, calretinin, neuropeptide Y and parvalbumin. However, the fraction of neurons that expressed GABA decreased between PND4 (59-67%) and PND 12 (25-38%). Additional analyses revealed the presence of proliferating NSC-like cells (i.e. cells expressing Ki-67 and Sox-2) in different strata of hippocampal CA1 and CA3 subfields of both PND4 and PND 12 animals. Thus, multipotent NSCs persist in both CA1 and CA3 subfields of the hippocampus in the postnatal period. Such NSCs also retain their ability to give rise to both GABA-ergic and non-GABA-ergic neurons. However, their overall neurogenic potential declines considerably in the early postnatal period.
Keywords: cell transplantation, GABA-ergic neurons, hippocampal neurogenesis, hippocampal pyramidal neurons, neural stem cells, neural stem cell proliferation, neural stem cell differentiation, posterior subventricular zone
Introduction
Multipotent neural stem/progenitor cells (NSCs) from diverse regions of the developing brain can be easily expanded in vitro for extended periods using mitogenic factors such as the fibroblast growth factor-2 (FGF-2) and the epidermal growth factor (EGF) (Reynolds et al., 1992; Shetty and Turner, 1998; Svendsen and Caldwell, 2000; Shetty, 2004; Moses et al., 2006; Louis et al., 2013). In contrast, in the postnatal and adult brain, such NSCs are abundantly seen in mainly the neurogenic regions such as the subventricular zone (SVZ) of the forebrain and the subgranular zone (SGZ) of the dentate gyrus (DG) (Reynolds and Weiss, 1992; Gage et al., 1995; Palmer et al., 1999; Ming and Song, 2011; Sakaguchi and Okano, 2012). Although studies point out that limited numbers of multipotent NSCs persist in numerous non-neurogenic regions of the postnatal and adult brain (Palmer et al., 1999), their properties such as ability for producing different neuronal cell types, and possible alterations in their neurogenic and gliogenic potential with age are not fully understood.
In the hippocampus, it is well known that NSCs (radial astrocytes) persist in the SGZ of the DG where they proliferate and add new neurons to the hippocampal circuitry throughout life (Eriksson et al., 1998; Rao et al., 2005; Shetty et al., 2012). These SGZ-NSCs proliferate extensively when cultured on a substrate in the presence of FGF-2 (Gage et al., 1995; Suhonen et al., 1996) but show limited proliferation in suspension cultures (neurosphere cultures) even in the presence of EGF and FGF2 (Seaberg and van der Kooy, 2002; Becq et al., 2005). On the other hand, NSCs in non-neurogenic regions of the hippocampus such as those in the CA1 and CA3 subfields of the postnatal and adult brain have not been fully characterized though the presence of NSCs in the CA1 region has been noted in several studies (Rietze et al., 2000; Becq et al., 2005). It is of interest to analyze the proliferative and differentiation behavior of putative NSCs generated from CA1 and CA3 subfields of the postnatal hippocampus because these regions are generally considered to be non-neurogenic after birth, in contrast to the conspicuously neurogenic DG region that generates new neurons throughout life (Gage et al., 1995; Eriksson et al., 1998; Ming and Song, 2011). The perception of postnatal hippocampal CA subfields as non-neurogenic regions mainly comes from the fact that all principal neurons in the rat hippocampal CA regions are generated in the prenatal period (mainly between embryonic days 17-19) from the hippocampal primordium (Bayer, 1980a,b). Furthermore, virtually all hippocampal CA region GABA-ergic interneurons are derived from the ganglionic eminences in the embryonic forebrain, which tangentially migrate along the cortex into the hippocampus (Li et al., 2012). Likely because of these reasons, NSCs in the CA1 and CA3 regions are believed to be part of the posterior SVZ lining the hippocampus (Becq et al., 2005). Indeed, a previous study has shown that NSCs in the periventricular zone of the CA1 subfield generate pyramidal neurons following ischemia-induced CA1 neuron loss (Nakatomi et al., 2002). Thus, understanding the properties of these NSCs may help in developing strategies that stimulate them to produce new neurons and glia to repair the damaged areas of the hippocampus and to improve function after insults such as status epilepticus (Rao et al., 2006), traumatic brain injury (Pitkanen et al., 2009), ischemia or stroke (Bellenchi et al., 2012). Additionally, these NSCs, if expandable in culture, may also be useful as donor cells for intrahippocampal grafting in neurological disease conditions associated with hippocampal lesions and dysfunction such as in temporal lobe epilepsy (Shetty et al., 2005; Waldau et al., 2010; Shetty, 2011), stroke (Lindvall and Kokaia, 2011), neonatal brain injury (Titomanlio et al., 2011) and Alzheimer’s disease (Borlongan, 2012).
In this study, we examined the properties of multipotent NSCs responsive to FGF-2 and EGF in CA subfields of the hippocampus in the postnatal period. Particularly, we addressed the following issues pertaining to NSCs isolated from the hippocampal CA1 and CA3 subfields of postnatal day (PND) 4 and PND 12 rats. (1) Do significant numbers of NSCs responsive to FGF-2 and EGF persist in both CA1 and CA3 subfields of the postnatal hippocampus? If yes, are there any disparities in the differentiation potential between CA1 and CA3 region NSCs? (2) Are the progeny of NSCs in both CA1 and CA3 subfields capable of differentiating into both GABA-ergic and non-GABA-ergic neurons? (3) Are there any changes in the number and differentiation potential of NSCs with advancing age in the early postnatal period?
Materials and Methods
Dissection of CA1 and CA3 subfield tissues
Postnatal day 4 (PND4) and PND12 rats (n=16-20/age group obtained from 6 litters) were deeply anesthetized with isoflurane, heads were gathered through decapitation and stored in a 10 ml tube containing calcium and magnesium free Hank’s balanced salt solution (HBSS; Sigma, St. Louis, MO) with 0.6% glucose, 10mM HEPES, and 1% penicillin-streptomycin. The brains were next dissected out in a petri plate containing the same medium. For separation of CA1 and CA3 tissues from the dentate gyrus, every cerebral hemisphere was severed from the brain stem and cut coronally into 4-6 slices. Slices containing the hippocampus were distinguished under a Nikon dissection microscope. From each of these slices, CA1 and CA3 subfield tissues were carefully severed through sharp cuts using a scalpel blade and collected in fresh HBSS. The SVZ tissue was carefully avoided while dissecting the CA1 subfield tissue. Similarly, the DG tissue and most of fimbria were excluded while dissecting the CA3 subfield tissue. A schematic in Figure 1 systematically shows the dissection procedure employed in this study.
Figure 1.
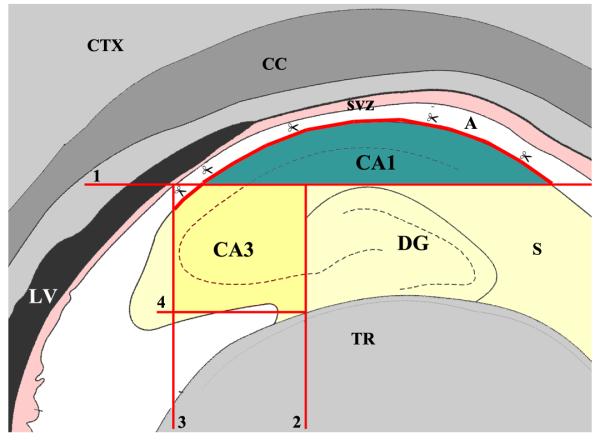
A schematic showing the procedure employed for micro-dissection of hippocampal CA1 and CA3 subfield tissues from the postnatal hippocampus for neural stem/progenitor cell (NSC) culture preparation. Micro-dissection was performed on coronal slices containing the hippocampus using a scalpel blade and microscissors. The horizontal line indicated by number 1 denotes the first sharp cut that was made to separate the CA1 tissue from the rest of hippocampus. Scissors depict areas of the alveus (A) where CA1 tissue was separated from the posterior subventricular zone (SVZ) using microscissors. The vertical line indicated by number 2 shows the second sharp cut that was made to separate the CA3 region tissue from the dentate gyrus. The vertical lines denoted by numbers 3 and 4 show the location of sharp cuts that were made to separate the CA3 tissue from the major parts of the fimbria (F). CC, corpus callosum; CTX, cerebral cortex; LV, lateral ventricle; S, subiculum; TR, thalamic region.
Neurosphere cultures and quantification of the yield of neurospheres
Using a fire-polished Pasteur pipette, CA1 and CA3 tissue pieces (pooled from multiple animals for each age group) were separately triturated ~40 times in 2 ml of HBSS. The ensuing cell suspension was diluted with 10 ml of fresh HBSS and filtered through a steel mesh (pore size, 175μm), centrifuged at 800 rpm for 10 minutes, and the pellet was resuspended in HBSS. Cells were washed thrice through resuspension in HBSS and centrifugation. The final pellet was resuspended in 50 μl of HBSS and the viability was calculated using trypan blue test. The density of viable cells was then attuned to 0.2 × 105 viable cells/10 ml of NSC proliferation medium containing Dulbecco’s modified Eagle’s medium (DMEM)/F12 (3:1 mixture; Life Technologies, Gaithersburg, MD), B-27 supplement (1 ml/50 ml of medium; Life Technologies), EGF (20 ng/ml; Sigma) or FGF-2 (20 ng/ml; Peprotech), heparin (5 μg/ml), penicillin (100 U/ml; Sigma), and streptomycin (100 μg/ml; Sigma). The cell suspension was plated onto 50-ml (25-cm2) Falcon tissue culture flasks with canted necks (10-ml/flask: Becton Dickinson). For measuring the yield of neurospheres, the cell suspension was plated onto individual wells of 24-well culture plates (0.5 ml [1,000 live cells] per well). Cultures were maintained at 37°C in a humidified atmosphere of 5% CO2 and 19.8% O2. Proliferation of NSCs and neurosphere formation was examined daily using an inverted phase-contrast microscope. Most cells died within the first few days after plating and were found floating close to the surface of the medium. These are likely differentiated cells, which failed to thrive in this suspension culture because of a lack of substrate. Moreover, no aggregates were found at this time-point. However, some cells got attached to the surface of the plastic in every well and appeared to undergo divisions. Such clusters of dividing cells (putative NSCs) got detached from the plates and formed clear smaller floating spheres when examined 4 days after plating. These smaller spheres grew in size over the next 5 days and became clear larger neurospheres. We counted only such larger neurospheres with smooth margins 9 days after plating. The numbers of neurospheres per well were scored using an inverted phase-contrast Nikon microscope. Counting was made from eight independent cultures for each of the two tissues (CA1 and CA3) collected at PND4 and PND12 (n=8/tissue type/time-point; total, 32 cultures). Statistical comparison used two-way ANOVA with Bonferroni post-hoc tests, and postnatal age and hippocampal region as two variables.
Differentiation of NSC derived neurospheres
Primary neurospheres from CA1 and CA3 tissues grown in multiple tissue culture flasks (n=6-8/tissue type/time point) were separately collected via centrifugation, washed thrice in HBSS, and resuspended in differentiation medium comprising DMEM (95.5 ml/100 ml), B-27 (2 ml/100 ml), L - glutamine (1.25 ml/100 ml), BDNF (20 ng/ml), penicillin (100 U/ml) and streptomycin (100 μg/ml) (Shetty, 2004). Neurosphere containing differentiation medium was next transferred onto sterile and coated (poly-L – lysine, 0.05 mg/ml) 35-mm Falcon dishes, and cultured (1.5 ml of medium/dish). One-third of the culture medium (500 μl) was replaced every 3 days with a fresh differentiation medium. We chose primary neurospheres for differentiation assays because of the likelihood that primary neurosphere cells exhibit a differentiation pattern that is closer to the differentiation potential of progeny of NSCs in CA regions, and that NSCs that have been exposed longer to factors and passaged several times in culture may not show properties of original NSCs from which they are derived, as various factors and passaging can modify the behavior of NSCs and their progeny.
Cultures were observed daily for growth and differentiation until 13 days, and their differentiation examined at different time points by immunostaining using markers of NSCs (nestin, Sox-2, and Musashi-1), neurons (ß-III tubulin [TuJ1]), astrocytes (glial fibrillary acidic protein [GFAP]) and oligodendrocytes (rip). Also, the presence of different subtypes of hippocampal neurons (GABA-ergic neurons, and neurons expressing calbindin, calretinin, neuropeptide Y, and parvalbumin) was investigated. Percentages of TuJ1+/GABA+ neurons, GFAP+ astrocytes, and Rip+ oligodendrocytes were also measured using DAPI counterstaining. Counting was made from multiple regions of 4-6 independent cultures for each marker, as described in our earlier reports (Shetty and Turner, 1998; Shetty, 2004). Following plating, neurospheres exhibited migration of cells away from the core and hence, individual cells in the peripheral and intermediate regions (representing >75% of a neurosphere field) were counted. Individual cells in the residual core region were difficult to discriminate from other cells and hence were excluded from quantification. In each culture, cells derived from neurospheres were counted from five different regions. Cell numbers counted per region varied from 78-202. Statistical comparison used two-way ANOVA with Bonferroni post-hoc tests, and postnatal age and hippocampal region as two variables.
5′-bromodeoxyuridine (BrdU) labeling experiments
In order to determine whether the TuJ1+/GABA+ neurons obtained from neurosphere cells in differentiation cultures were indeed generated in the culture during the expansion of neurospheres, neurosphere cultures were pulsed with 0.5-μM 5′-bromodeoxyuridine (BrdU; Sigma) for 5-7 days. Addition of BrdU to cultures was done after the appearance of smaller neurospheres (i.e. 2 days after the initial plating of cells). Following 5-7 days of incubation in BrdU containing proliferation medium, neurospheres were washed thrice in HBSS and plated onto poly-L-lysine coated dishes containing differentiation medium. After different time points of incubation in the differentiation medium, cultures were processed for BrdU-TuJ1 and BrdU-GABA dual immunofluorescence to examine the presence of different types of BrdU+ neurons in the culture.
Antibodies and immunocytochemistry
Immunocytochemical characterization of cultures and hippocampal tissues utilized primary antibodies from multiple sources. This comprised mouse monoclonal antibodies to nestin (Rat 401; 1:10, Hybridoma Bank, Department of Biological Sciences, University of Iowa, Iowa City, IA), rip (1:100; Hybridoma Bank), BrdU (1:250, Becton Dickenson), calbindin-D (1:1,000; Sigma), calretinin (1:1000; Sigma) and parvalbumin (1:1000; Sigma), and rabbit antisera to Sox-2 (1:1000; Millipore Billerica, MA) and Ki-67 (1:1000; Santa Cruz), Musashi-1 (1:1000; Millipore), GABA (1:5000; Sigma), GFAP (1:1000; Dako, Carpinteria, CA) and NPY (1:10000; Bachem; Torrance, CA). Affinity-purified and biotinylated secondary antibodies (horse anti-mouse IgG and goat anti-rabbit IgG; 1:200), and avidin-biotin reagents (Elite ABC kit) were obtained from Vector Labs (Burlingame, CA). The other secondary antibodies (goat anti-mouse FITC, goat anti-rabbit FITC, Alexa-fluor 488/594 conjugated goat anti-mouse IgG, and goat anti-rabbit IgG [1:200]) and streptavidin Texas Red were from Invitrogen (Eugene, OR). Immunocytochemical staining was done using procedures described in our earlier report (Shetty and Turner, 1998, 1999, Shetty, 2004; Hattiangady and Shetty, 2011, 2012).
Immunohistochemical analyses of PND4 and PND 12 hippocampal tissues
On postnatal days 4 and 12, rat pups (n=6-8/age group) were deeply anesthetized with isoflurane and fixed with 4% paraformaldehyde via intracardiac perfusions. Brains were removed, post-fixed in 4% paraformaldehyde for 18 hours, cryoprotected in sucrose solution and cut coronally through the hippocampus at 40 μm using a cryostat. Representative sections through the hippocampus were processed for Ki-67 and Sox-2 dual immunofluorescence (Hattiangady and Shetty, 2008) to identify putative NSCs that are undergoing cell division in the hippocampal CA1 and CA3 subfield tissues. Individual dual labeled cells were identified via Z-section analyses in a confocal microscope (Hattiangady and Shetty, 2008).
Results
Yield of neurospheres from hippocampal CA1 and CA3 subfield tissues
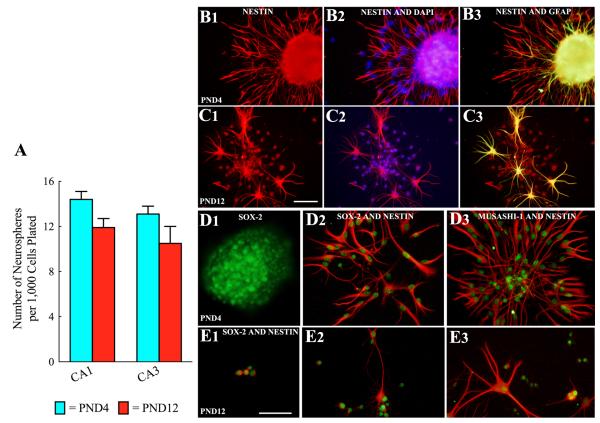
Two-way ANOVA analyses revealed that the overall yield of neurospheres from CA1 and CA3 subfield cells were comparable between tissues obtained from PND4 and PND12 hippocampi (Fig. 2 [A]). In PND4 cultures, the yield of neurospheres per 1,000 cells plated was 14.4 ± 0.7 (Mean ± SEM) for CA1 cells and 13.1 ± 0.7 for CA3 cells (n=8/group). In PND12 cultures, the yield was 11.9 ± 0.8 for CA1 cells and 10.5 ± 1.5 for CA3 cells (Fig. 2 [A]; n=8/group). Thus, 1-1.4% of plated cells formed neurospheres from both CA1 and CA3 subfield tissues obtained from PND4 and PND12 hippocampi. This suggests that the number of neurosphere forming NSCs remains stable between PND4 and PND12 in both CA1 and CA3 subfields.
Figure 2.
Yield of neurospheres and cell types within neurospheres generated from CA1 and CA3 subfield cells of postnatal day 4 (PND 4) and PND12 hippocampi. The bar chart illustrates the yield of neurospheres per 1,000 cells after 9 days of incubation in neural stem cell (NSC) proliferation medium (A). Note that the yield of neurospheres from CA1 and CA3 subfield cells were comparable between tissues obtained from PND4 and PND12 hippocampi. Figures B1-E3 illustrate cell types in neurospheres after 24 hours of incubation in the differentiation medium. Virtually all cells derived from neurospheres expressed NSC markers such as the intermediate neurofilament protein nestin (B1-B3, C1-C3), Sox-2 (D1-D2, E1-E2) and Musashi-1 (D3, E3) at this stage. However, GFAP (a marker of NSCs and astrocytes) is also expressed in some cells (D3, C3). Scale bars = 50μm.
Expression of NSC markers in neurospheres obtained from postnatal CA1 and CA3 subfields
We investigated the presence of several NSC markers in neurospheres obtained from both CA1 and CA3 subfield tissues of PND4 and PND12 hippocampi. Examination of cells migrated away from larger neurospheres (Fig. 2 [B1-B3]), cells fully dispersed from smaller neurospheres (Fig. 2 [C1-C3, D2-D3] and dissociated neurosphere cells (Fig. 2 [E1-E3] 24 hours after plating on a substrate containing differentiation medium revealed robust expression of several NSC markers. These include the intermediate neurofilament protein nestin (Fig. 2 [B1-B3, C1-C3, E1-E3]), Sox-2 (Fig. 2 [D1, D2, E1-E3] and Musashi-1 (Fig. 2 [D3]). Only a small fraction among these cells expressed GFAF (a marker of NSCs and astrocytes, Fig. 2 [B3, C1-C2]). However, many cells within the core of larger neurospheres appeared to express GFAP (Fig. 2 [B3]). Furthermore, no TuJ-1+ cells were observed within neurospheres or in cells that migrated away from the neurospheres at this time-point. Thus, neurospheres obtained from PND4 and PND12 hippocampal CA1 and CA3 subfield tissues comprise putative NSCs.
Differentiation of neurosphere-derived cells into neurons
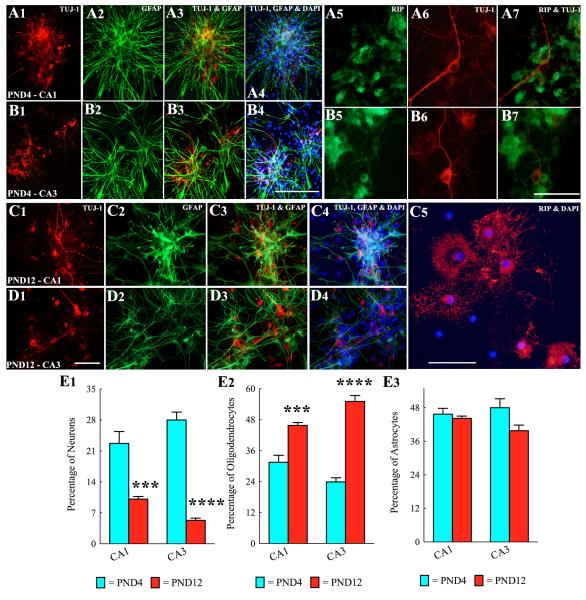
Neurospheres derived from both CA1 and CA3 subfield tissues of PND4 or PND12 hippocampus gave rise to TuJ1+ neurons, GFAP+ astrocytes and rip+ oligodendrocytes when plated onto poly-L-lysine coated dishes and incubated in the differentiation medium for 7 days (Fig. 3 [A1-D4]). Neurons immunopositive for TuJ1 were mostly concentrated in the central and intermediate zones of neurospheres (Fig. 3 [A1, B1, C1, D1]). Quantification revealed that the extent of neuronal differentiation from both CA1 and CA3 region NSCs decreases with increasing age during the postnatal period (Fig. 3 [E1]). Two-way ANOVA analyses confirmed that neuronal differentiation from CA1 and CA3 subfield neurospheres is affected by the postnatal age (p<0.0001) but not by the hippocampal region where NSCs reside (p>0.05). Bonferroni post-hoc tests showed that, between postnatal days 4 and 12, the overall percentage of neurons derived from both CA1 and CA3 subfield neurospheres significantly decreased (CA1 neurospheres, 22.7-10.1%, p<0.001; CA3 neurospheres, 28.0-5.3%, p<0.0001; Fig. 3 [E1], n=4-6/group).
Figure 3.
Neurospheres derived from CA1 and CA3 subfield cells of postnatal day 4 (PND4) and PND12 hippocampi generate all three cell types of the central nervous system when examined after 7 days of incubation in the differentiation medium. A1-B7: Tuj1+ neurons (A1, A3-A4, B1, B3-B4), GFAP+ astrocytes (A2-A4, B2-B4) and rip+ oligodendrocytes (A5, A7, B5, B7) from neurospheres generated from CA1 subfield (A1-A7) and CA3 subfield (B1-B7) tissues of PND4 hippocampi. Scale bar, A1-A4, B1-B4 = 200μm. A5-A7, B5-B7 = 100μm. C1-C5: Tuj1+ neurons (C1, C3-C4, D1, D3-D4), GFAP+ astrocytes (C2-C4, D2-D4) and rip+ oligodendrocytes (C5) from neurospheres generated from CA1 subfield (C1-C5) and CA3 subfield (D1-D4) tissues of PND4 hippocampi. Scale bar, C1-C4, D1-D4 = 200μm. C5 = 100μm. Bar charts in E1-E3 illustrate percentages of neurons, oligodendrocytes and astrocytes derived from neurospheres of CA1 and CA3 subfield cells of PND4 and PND12 hippocampi. Note that, between PND4 and PND12, the extent of differentiation of neurosphere cells into neurons decreases (E1). However, differentiation of neurosphere cells into oligodendrocytes increases (E2) and into astrocytes remains stable (E3) during this period. ***, p<0.001; ****, p<0.0001.
Differentiation of neurosphere-derived cells into oligodendrocytes
Oligodendrocytes immunopositive for rip were found mostly in the intermediate and peripheral zones of neurospheres (Fig. 3 [A5, C5]). Oligodendrocytes that were located farther away from the core of neurospheres appeared to have more mature branching processes (Fig. 3 [C5]). In contrast to neuronal differentiation, the extent of oligodendrocyte differentiation from both CA1 and CA3 region NSCs increased with advanced age during the postnatal period (Fig. 3 [E2]). Two-way ANOVA analyses confirmed that oligodendrocyte differentiation from CA1 and CA3 subfield neurospheres is affected by the postnatal age (p<0.0001) but not by the hippocampal region where NSCs reside (p>0.05). Bonferroni post-hoc tests showed that, between postnatal days 4 and 12, the overall percentage of oligodendrocytes derived from both CA1 and CA3 subfield neurospheres significantly increased (CA1 neurospheres, 31.5-45.8%, p<0.001; CA3 neurospheres, 23.9-55.1%, p<0.0001; Fig. 3 [E2], n=4-6/group).
Differentiation of neurosphere-derived cells into astrocytes
Astrocytes immunopositive for GFAP and exhibiting long processes were found in all three zones (central, intermediate and peripheral) of neurospheres (Fig. 3 [A2, B2, C2, D2]). In contrast with both neuronal and oligodendrocyte differentiation, astrocyte differentiation from both CA1 and CA3 region NSCs remained comparable between postnatal days 4 and 12 (CA1 region neurospheres, 44.2-45.7%; CA3 region neurospheres, 39.8-48%; Fig. 3 [E3]). Two-way ANOVA analyses further confirmed that astrocyte differentiation from CA1 and CA3 subfield neurospheres is affected by neither the postnatal age (p>0.05) nor the hippocampal region where NSCs reside (p>0.05; Fig. 3 [E3], n=4-6/group).
Differentiation of neurosphere-derived cells into GABA-ergic interneurons and non-GABA-ergic neurons
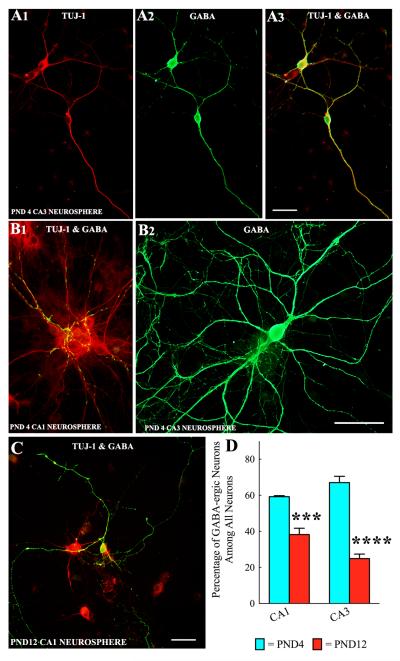
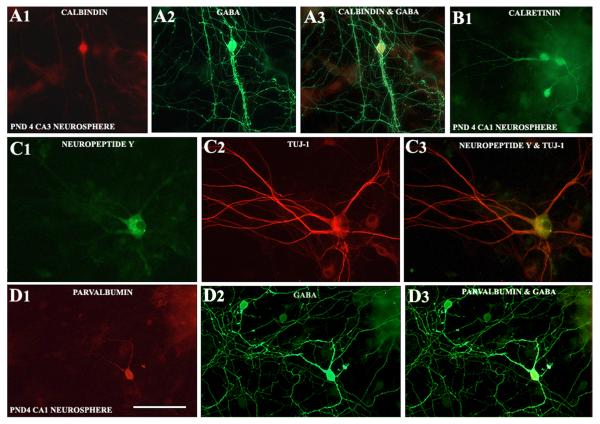
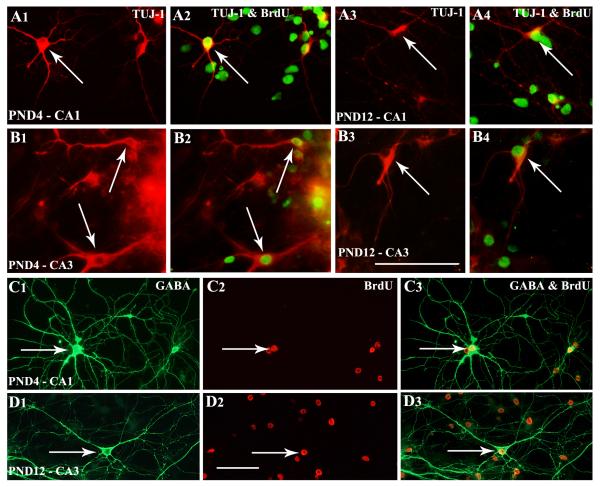
Neurospheres obtained from both CA1 and CA3 subfield tissues of PND4 and PND12 hippocampus generated well-differentiated GABA-ergic interneurons when maintained in the differentiation medium for 13 days (Fig. 4 [A1-A3, B2, C]). In many instances, GABA-ergic axon terminals were found on soma and dendrites of non-GABA-ergic hippocampal pyramidal-like neurons in the core of neurospheres (Fig. 4 [B1]). However, quantification revealed that the extent of differentiation of both CA1 and CA3 subfield NSCs into GABA-ergic interneurons decreases with increasing age during the postnatal period (Fig. 4 [D]). Two-way ANOVA analyses confirmed that GABA-ergic interneuron differentiation from CA1 and CA3 subfield neurospheres is affected by the postnatal age (p<0.0001) but not by the hippocampal region where NSCs reside (p>0.05). Bonferroni post-hoc tests showed that, between postnatal days 4 and 12, the overall percentage of GABA-ergic neurons among all neurons derived from both CA1 and CA3 subfield neurospheres significantly decreased (CA1 subfield neurospheres, 59.2-38.2%, p<0.001; CA3 subfield neurospheres, 67.0-24.9%, p<0.0001; Fig. 4 [D], n=4-6/group). As a consequence, percentages of non-GABA-ergic neurons (among all neurons) increased from 41-68% for CA1 subfield neurospheres and 33-75% for CA3 subfield neurospheres during the same period. Many of these non-GABA-ergic neurons exhibited pyramidal shaped soma and dendrites having GABA-ergic axon terminals (Fig. 4 [B1] Neurosphere cultures maintained for 13 days also revealed the presence of different subclasses of GABA-ergic interneurons (Fig. 5). This comprised neurons positive for calbindin (Fig. 5 [A1-A3]), calretinin (Fig. 5 [B1]), neuropeptide Y (Fig. 5 [C1-C3]) and parvalbumin (Fig. 5 [D1-D3]).
Figure 4.
Neurospheres derived from CA1 and CA3 subfield cells of postnatal day 4 (PND4) and PND12 hippocampi generate GABA-ergic interneurons. Figures A1-A3 show examples and morphology of TuJ-1 positive neurons generated from PND4 CA3 neurosphere cells that expressed GABA. Figure B1 illustrates GABA-ergic axon terminals on soma and dendrites of non-GABA-ergic hippocampal pyramidal-like neurons derived from PND4 CA1 neurosphere cells. B2 is an example of a well-developed GABA-ergic neuron generated from a PND4 CA3 neurosphere cell. Figure C shows an example of a well-developed GABA-ergic neuron (orange) generated from a PND12 CA1 neurosphere cell. Scale bar = 50μm. The bar chart in D shows percentages of GABA-ergic interneurons (among TuJ1+ neurons) derived from neurospheres of CA1 and CA3 subfield cells of PND4 and PND12 hippocampi. Note decreased percentages of GABA-ergic neurons in neurospheres of PND 12 subfields (25-38%), in comparison to neurospheres of PND 4 subfields (59-67%). ***, p<0.001; ****, p<0.0001.
Figure 5.
Various subclasses of interneurons emerge from long-term differentiation cultures of CA1 and CA3 subfield neurospheres. Figures A1-A3 illustrate a GABA-ergic interneuron expressing calbindin derived from a PND4 CA3 neurosphere cell. B1 shows calretinin expressing interneurons derived from PND4 CA1 neurosphere cells. Figures C1-C3 illustrate a neuropeptide Y expressing interneuron from a PND12 CA3 neurosphere cell. Figures D1-D3 show a parvalbumin expressing interneuron derived from a PND4 CA1 neurosphere cell. Scale bar = 50μm.
Neurons from CA1 and CA3 subfield neurospheres are indeed generated from NSCs expanded in culture
Administration of BrdU to NSC proliferation cultures commencing two days after the initial plating of cells demonstrated that TuJ-1+ and GABA+ neurons generated from neurospheres of postnatal CA1 and CA3 subfield tissues were indeed derived from NSCs that proliferated in culture. This was clearly evidenced by the presence of BrdU in neurosphere-derived cells that differentiated into TuJ1-immunopositive neurons (Fig. 6 [A1-B4]) or GABA-ergic interneurons exhibiting extensive branching of dendrites (Fig. 6 [C1-D3]). Maintenance in BrdU containing proliferation medium for 5 days during the expansion of neurospheres resulted in labeling of 62-64% of neurosphere-derived cells (Mean ± S.E.M: CA1 neurospheres, 62 ± 4.3%; CA3 neurospheres, 64 ± 3.3%; n=4/group) whereas maintenance for 7 days in BrdU containing proliferation medium resulted in labeling of 77-81% of neurospheres-derived cells (CA1 neurospheres, 77 ± 2.7%; CA3 neurospheres, 85 ± 1.7%; n=4/group).
Figure 6.
TuJ1+ and GABA+ neurons derived from postnatal CA1 and CA3 subfield neurospheres are indeed derived from cells that proliferated in neurosphere cultures, which was evidenced through incorporation of BrdU by these cells when grown in a proliferation medium containing 0.5 μM BrdU. Figures A1-A4 illustrate BrdU labeling in Tuj1+ neurons derived from CA1 subfield neurosphere cells of PND4 (A1, A2) and PND12 (A3, A4) hippocampi. Figures B1-B4 show BrdU labeling in Tuj1+ neurons derived from CA3 subfield neurosphere cells of PND4 (B1, B2) and PND12 (B3, B4) hippocampi. Figures C1-D3 illustrate BrdU labeling in GABA-ergic interneurons derived from CA1 subfield neurosphere cells of PND4 hippocampus (C1-C3) and CA3 subfield neurosphere cells of PND12 hippocampus (D1-D3). Scale bars, A1-B4 = 200μm; C1-D3 = 50μm.
Proliferating NSC-like cells are seen in different layers of CA1 and CA3 subfields in the postnatal period
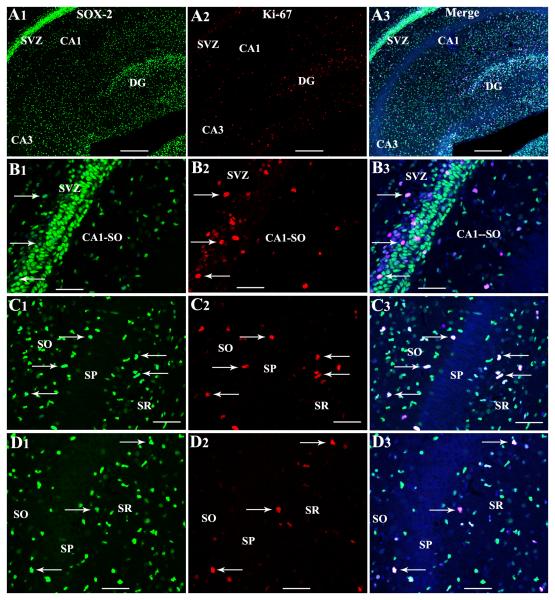
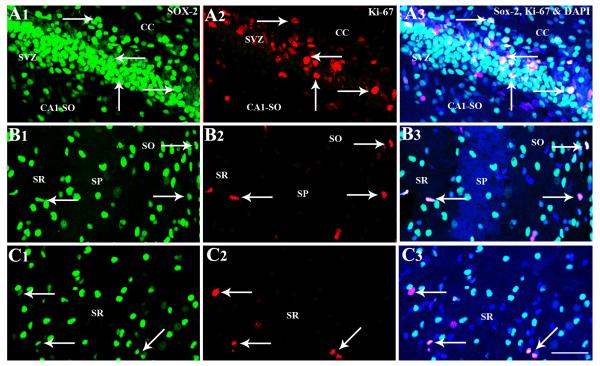
Immunohistochemical and confocal microscopic analyses of cells positive for Sox-2 (a putative marker of neural stem/progenitor cells) and Ki-67 (an endogenous marker of dividing cells) revealed the presence of proliferating NSC-like cells throughout the hippocampus and the surrounding periventricular regions of PND4 and PND12 rat pups (Figs. 7 and 8). Although such cells were more conspicuous in the posterior SVZ overlying the hippocampal CA1 region (Figs. 7 [A1-B3] and 8 [A1-A3]) and the DG (Fig. 7 [A3]), individual cells positive for Sox-2 and Ki-67 are clearly discernible in CA1 and CA3 subfield parenchymal tissues (Figs. 7 [C1-D3] and 8 [B1-C3]). In the CA1 subfield, proliferating NSC-like cells were seen mostly in strata oriens and radiatum adjoining the stratum pyramidale (Figs. 7 [C1-C3] and 8 [B1-B3]). In the CA3 subfield, such cells were mainly seen in the stratum radiatum (Figs. 7 [D1-D3] and 8 [C1-C3]). However, some NSC-like cells could also be seen in the stratum pyramidale (Fig. 7 [D1-D3]) and the fimbria underlying the CA3b sub region (not illustrated).
Figure 7.
Proliferating NSC-like cells in the postnatal day 4 hippocampus visualized through Sox-2 and Ki-67 dual immunofluorescence and Z-sectioning analyses in a confocal microscope. Figures A1-A3 illustrate Sox-2+ cells and Ki-67+ cells in hippocampal regions as well as the posterior subventricular zone (SVZ) overlying the CA1 region. Figures B1-D3 show cells expressing both Sox-2 and Ki-67 in the posterior SVZ (arrows in B1-B3), strata oriens (SO) radiatum (SR) of CA1 subfield (arrows in C1-C3), and the SR and stratum pyramidale (SP) of the CA3 subfield (arrows in D1-D3). Scale bar, A1-A3 = 100μm. B1-D3 = 50μm.
Figure 8.
Proliferating NSC-like cells in the postnatal day 12 posterior subventricular zone (SVZ) and hippocampal regions visualized through Sox-2 and Ki-67 dual immunofluorescence and Z-sectioning in a confocal microscope. Figures A1-C3 show cells expressing both Sox-2 and Ki-67 in the posterior SVZ (arrows in A1-A3), strata oriens (SO) and radiatum (SR) of the CA1 subfield (arrows in B1-B3), and the SR of the CA3 subfield (arrows in D1-D3). Scale bar, A1-C3 = 50μm.
Discussion
This study provides novel evidence that NSCs capable of generating GABA-ergic interneurons and non-GABA-ergic neurons persist in both CA1 and CA3 subfields of the rat hippocampus during the postnatal period. The results also showed that the potential of CA1 and CA3 subfield NSCs to generate neurospheres in vitro remains stable between postnatal days 4 and 12. Additional analyses revealed that both CA1 and CA3 subfield NSCs maintain multipotent property but display reduced predilection for generating neurons and increased propensity for generating oligodendrocytes with age during the early postnatal period. However, their ability for differentiation into astrocytes remains stable during this period. Thus, the extent of differentiation of cells derived from CA1 and CA3 subfield NSCs into neurons and oligodendrocytes in vitro depends on the postnatal age of NSCs at the time of harvesting.
Neural stem/progenitor cells in the CA1 subfield
Previous studies have documented the presence of NSCs in the CA1 subfield of adult mice (Becq et al., 2005) and young rats (Nakatomi et al., 2002). By analyzing neurospheres from the CA1 subfield tissues of adult CD1 mice, Becq et al (2005) showed that ~0.07-0.2% of cells from the adult CA1 subfield are capable of forming multipotent neurospheres in culture in the presence of EGF or EGF+FGF-2. In the current study, 1.2-1.4% of cells from the CA1 subfield of PND4/PND12 rats formed multipotent neurospheres in the presence of EGF+FGF-2. The discrepancy in the yield of neurospheres between the two studies likely reflects the age-related reduction in the number of NSCs in this subfield.
The current study did not specifically analyze NSCs located in different layers of the CA1 subfield, as cultures comprised cells from all layers of CA1 subfield tissue. However, we carefully micro-dissected CA1 tissues from the overlying posterior SVZ using coronal slices through the hippocampus and the cerebral cortex. Therefore, there is no likelihood for inclusion of a major part of the SVZ tissue in CA1 tissues used for cultures in this study though one cannot rule out the possible inclusion of streaks of SVZ NSC-like cells that invade ventrally into the CA1 stratum oriens. Furthermore, our immunohistochemical analyses of hippocampal tissues from PND4 and PND12 rat pups indeed reveal the presence of proliferating NSC-like cells (i.e. cells expressing Sox-2 and Ki-67) in strata oriens and radiatum of the CA1 subfield. Likewise, a previous study using postnatal hippocampal slice cultures has shown that mitogen treatment leads to an increased proliferation of NSC-like cells in the CA1 stratum oriens along with some proliferation of cells in the CA1 strata pyramidale and radiatum (Becq et al., 2005). Collectively, these results imply that NSC-like cells are scattered all over the CA1 subfield. However, a previous study suggested that neurospheres generated from the hippocampus are mostly derived from the posterior SVZ lining the hippocampus (Seaberg and van der Kooy, 2002). Considering these, it may be that NSC-like cells found in the CA1 strata represent invading progenitor cells derived from NSCs in the adjacent posterior SVZ.
While the ability of CA1 subfield NSCs to give rise to neurons, astrocytes and oligodendrocytes in vitro has been noted earlier (Becq et al., 2005), the current study extends these findings by demonstrating that these NSCs are capable of generating different subclasses of GABA-ergic interneurons (expressing calbindin, calretinin, neuropeptide Y, and parvalbumin) as well as non-GABA-ergic neurons (presumably CA1 pyramidal-like neurons). Ability of the CA1 subfield NSCs to generate CA1 pyramidal-like neurons has also been noted in a rat model of ischemia where intraventricular infusion of EGF+FGF-2 recruited endogenous NSCs resulting in a massive regeneration of CA1 pyramidal neurons after ischemia (Nakatomi et al., 2002). The latter study also suggested that NSCs in the CA1 subfield remain dormant in normal conditions but get activated after injury which allows them to be mobilized further with exogenous application of NSC mitogenic factors (Nakatomi et al., 2002). However, another study in a rat model suggested that global ischemia induced degeneration of CA1 neurons can lead to spontaneous regeneration of CA1 neurons through activation of NSCs residing in the posterior SVZ (Bendel et al., 2005).
Nevertheless, it remains to be examined whether such massive response of CA1 subfield NSCs is limited to ischemic injury or any type of injury. While proliferation of putative nestin/Mash-1 immunopositive NSCs have been observed in the CA1 stratum oriens and the SVZ lining the CA1 subfield after status epilepticus, neuronal differentiation of newly born cells seemed to be limited (Becq et al., 2005). However, a few of the newly born cells in the stratum oriens seemed to express NeuN but none expressed markers of GABA-ergic interneurons (Becq et al., 2005). It will be interesting to examine in future studies whether mobilization of CA1 subfield NSCs to repopulate the lost neurons (both GABA-ergic interneuron and CA1 pyramidal neurons) can be accomplished with specific growth factors in conditions such as after status epilepticus, traumatic brain injury, temporal lobe epilepsy or early stages of Alzheimer’s disease.
Neural stem/progenitor cells in the CA3 subfield
This is the first study to report the presence of multipotent NSCs capable of forming both GABA-ergic interneurons and non-GABA-ergic neurons in the rat CA3 subfield in the postnatal period. A previous study has noted the presence of such NSCs in the CA3 subfield of embryonic day 19 rat hippocampus (Shetty, 2004). Our immunohistochemical analyses of hippocampal tissues demonstrated proliferating NSC-like cells scattered mostly over the stratum radiatum of CA3 subfield at both postnatal days 4 and 12. However, such cells were also seen in the fimbria underlying the CA3b sub region. Furthermore, earlier studies in rat models of CA3 subfield injury also suggested the presence of NSC-like cells (radial glia-like cells expressing GFAP and nestin) along the fimbria lining the CA3a,b subregions, which represents an extension of the posterior SVZ (Clark et al., 1994, Abdel-Rahman et al., 2004). Since our micro-dissection of CA3 tissues included parts of fimbria underlying CA3a,b sub regions, it is unclear whether NSCs expanded in culture are derived from cells in CA3 parenchyma or fimbria. Additional NSC culture studies from small punch samples of only CA3 parenchymal tissues are needed in the future to resolve this issue. Interestingly, in vitro properties of both CA1 and CA3 subfield NSCs in terms of their proliferative ability, differentiation into neurons, oligodendrocytes and astrocytes, GABA-ergic interneurons and non GABA-ergic neurons were comparable at both PND4 and PND12 time-points in this study. Therefore, it is plausible that CA1 and CA3 region NSCs are not regionally specified in the postnatal period examined in this study. It may be possible that NSC-like cells found in both subfields during the postnatal period emerge from the posterior SVZ lining the respective subfields and hence have similar properties.
Properties of CA1 and CA3 subfield NSCs and Postnatal Age
Our results suggest that CA1 and CA3 subfield NSCs maintain multipotent property at both PND4 and PND12 time-points. However, they displayed reduced predilection for generating neurons (including GABA-ergic interneurons) and increased propensity for generating oligodendrocytes with age in the postnatal period examined in this study. Interestingly, their ability for differentiation into astrocytes remained stable during this period. This could be due to the plasticity of NSCs and/or their progeny to match the developmental stage of the hippocampus. Increased propensity of NSC derived cells for differentiation into oligodendrocytes at the expense of neurons at PND12 may be due to the fact that: (i) both CA1 and CA3 subfields are already filled with the requisite numbers of principal pyramidal neurons and GABA-ergic interneurons at this time-point; and (ii) myelination is one of the major processes ongoing at this stage of hippocampal development. Indeed, a previous study has revealed that mature myelinated fibers in the rodent hippocampus first appear in the second postnatal week, which eventually increase in density and reach adult levels by PND25 (Meier et al., 2004). Thus, the extent of differentiation of cells derived from CA1 and CA3 subfield NSCs into neurons and oligodendrocytes in vitro depends on the postnatal age of NSCs at the time of harvesting. However, certain injury-induced signals in vivo likely enhance neurogenesis from these NSCs as observed in ischemia-induced CA1 injury models (Nakatomi et al, 2002; Bendel et al., 2005).
Conclusions
Our results demonstrate that considerable numbers of multipotent NSCs persist in both CA1 and CA3 subfields of the hippocampus in the first two weeks of the postnatal period. These NSCs also retain their ability to give rise to both GABA-ergic interneurons and non-GABA-ergic neurons. Additional results underscore that the postnatal age governs the amount of genesis of neurons and oligodendrocytes by these NSCs though their potential for generating astrocytes remains stable during this postnatal period. Further studies are needed in the future to examine whether intrahippocampal grafting of these NSCs is a useful approach for improving function in neurological disease conditions associated with hippocampal lesions and dysfunction.
Multipotent neural stem cells (NSCs) persist in CA1 and CA3 subfields of the postnatal hippocampus
Neuronal differentiation potential of NSCs in CA1 and CA3 subfields decreases with postnatal age
Oligodendrocyte differentiation of NSCs in CA1 and CA3 subfields increases with postnatal age
NSCs from postnatal CA1 and CA3 subfields can generate GABA-ergic neurons
NSCs from postnatal CA1 and CA3 subfields can generate different subclasses of GABA-ergic neurons
Acknowledgments
This research was supported by Emerging Technology Funds from the State of Texas through TAMHSC and grants from the National Institutes Health (NINDS RO1 NS043507 to A.K.S.) and Department of Veterans Affairs (Merit Review Awards to A.K.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdel-Rahman A, Rao MS, Shetty AK. Nestin expression in hippocampal astrocytes after injury depends on the age of the hippocampus. Glia. 2004;47:299–313. doi: 10.1002/glia.20047. [DOI] [PubMed] [Google Scholar]
- Bayer SA. Development of the hippocampal region in the rat. I. Neurogenesis examined with “3Hthymidine autoradiography. J Comp Neurol. 1980a;190:87–114. doi: 10.1002/cne.901900107. [DOI] [PubMed] [Google Scholar]
- Bayer SA. Development of the hippocampal region in the rat. II. Morphogenesis during embryonic and early postnatal period. J Comp Neurol. 1980b;190:115–134. doi: 10.1002/cne.901900108. [DOI] [PubMed] [Google Scholar]
- Becq H, Jorquera I, Ben-Ari Y, Weiss S, Represa A. Differential properties of dentate gyrus and CA1 neural precursors. J Neurobiol. 2005;62:243–61. doi: 10.1002/neu.20089. [DOI] [PubMed] [Google Scholar]
- Bellenchi GC, Volpicelli F, Piscopo V, Perrone-Capano C, Porzio UD. Adult neural stem cells: an endogenous tool to repair brain injury? J Neurochem. 2012 Nov 7; doi: 10.1111/jnc.12084. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Bendel O, Bueters T, von Euler M, Ogren SO, Sandin J, von Euler G. Reappearance of hippocampal CA1 neurons after ischemia is associated with recovery of learning and memory. J Cereb Blood Flow Metab. 2005;25:1586–1595. doi: 10.1038/sj.jcbfm.9600153. [DOI] [PubMed] [Google Scholar]
- Borlongan CV. Recent preclinical evidence advancing cell therapy for Alzheimer’s disease. Exp Neurol. 2012;237:142–146. doi: 10.1016/j.expneurol.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccolini F, Svendsen CN. Fibroblast growth factor 2 (FGF-2) promotes acquisition of epidermal growth factor (EGF) responsiveness in mouse striatal precursor cells: identification of neural precursors responding to both EGF and FGF-2. J Neurosci. 1998;18:7869–7880. doi: 10.1523/JNEUROSCI.18-19-07869.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke SR, Shetty AK, Bradley JL, Turner DA. Reactive astrocytes express the embryonic intermediate neurofilament nestin. Neuroreport. 1994;5:1885–1888. doi: 10.1097/00001756-199410000-00011. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1337. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Gage FH, Ray J, Fisher LJ. Isolation, characterization, and use of stem cells from the CNS. Annu Rev Neurosci. 1995;18:159–192. doi: 10.1146/annurev.ne.18.030195.001111. [DOI] [PubMed] [Google Scholar]
- Hattiangady B, Shetty AK. Aging does not alter the number or phenotype of putative stem/progenitor cells in the neurogenic region of the hippocampus. 2008;29:129–147. doi: 10.1016/j.neurobiolaging.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattiangady B, Shetty AK. Neural stem cell grafting in an animal model of chronic temporal lobe epilepsy. Curr Protoc Stem Cell Biol. 2011 doi: 10.1002/9780470151808.sc02d07s18. Chapter 2:Unit2D.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattiangady B, Shetty AK. Neural Stem Cell Grafting Counteracts Hippocampal Injury-Mediated Impairments in Mood, Memory, and Neurogenesis. Stem Cells Transl Med. 2012;1:696–708. doi: 10.5966/sctm.2012-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Chou S-J, Hamasaki T, Perez-Garcia CG, O’Leary DDM. Neuregulin repellent signaling via ErbB4 restricts GABAergic interneurons to migratory paths from ganglionic eminence to cortical destinations. Neural Development. 2012;7:10. doi: 10.1186/1749-8104-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindvall O, Kokaia Z. Stem cell research in stroke: how far from the clinic? Stroke. 2011;42:2369–2375. doi: 10.1161/STROKEAHA.110.599654. [DOI] [PubMed] [Google Scholar]
- Louis SA, Mak CK, Reynolds BA. Methods to culture, differentiate, and characterize neural stem cells from the adult and embryonic mouse central nervous system. Methods Mol Biol. 2013;946:479–506. doi: 10.1007/978-1-62703-128-8_30. [DOI] [PubMed] [Google Scholar]
- Meier S, Bräuer AU, Heimrich B, Nitsch R, Savaskan NE. Myelination in the hippocampus during development and following lesion. Cell Mol Life Sci. 2004;61:1082–1094. doi: 10.1007/s00018-004-3469-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses D, Teper Y, Gantois I, Finkelstein DI, Horne MK, Drago J. Murine embryonic EGF-responsive ventral mesencephalic neurospheres display distinct regional specification and promote survival of dopaminergic neurons. Exp Neurol. 2006;199:209–221. doi: 10.1016/j.expneurol.2006.02.120. [DOI] [PubMed] [Google Scholar]
- Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, Kawahara N, Tamura A, Kirino T, Nakafuku M. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002;110:429–441. doi: 10.1016/s0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Markakis EA, Willhoite AR, Safar F, Gage FH. Fibroblast growth factor-2 activates a latent neurogenic program in neural stem cells from diverse regions of the adult CNS. J Neurosci. 1999;19:8487–8497. doi: 10.1523/JNEUROSCI.19-19-08487.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkänen A, Immonen RJ, Gröhn OH, Kharatishvili I. From traumatic brain injury to posttraumatic epilepsy: what animal models tell us about the process and treatment options. Epilepsia. 2009;50(Suppl 2):21–29. doi: 10.1111/j.1528-1167.2008.02007.x. [DOI] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, Abdel-Rahman A, Stanley DP, Shetty AK. Newly born cells in the ageing dentate gyrus display normal migration, survival and neuronal fate choice but endure retarded early maturation. Eur J Neurosci. 2005;21:464–476. doi: 10.1111/j.1460-9568.2005.03853.x. [DOI] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, Reddy DS, Shetty AK. Hippocampal neurodegeneration, spontaneous seizures, and mossy fiber sprouting in the F344 rat model of temporal lobe epilepsy. J Neurosci. 2006;83:1088–1005. doi: 10.1002/jnr.20802. [DOI] [PubMed] [Google Scholar]
- Rietze R, Poulin P, Weiss S. Mitotically active cells that generate neurons and astrocytes are present in multiple regions of the adult mouse hippocampus. J Comp Neurol. 2000;424:397–408. [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Tetzlaff W, Weiss S. A multipotent EGF-responsive striatal embryonic progenitor cell produces neurons and astrocytes. J Neurosci. 1992;12:4565–4574. doi: 10.1523/JNEUROSCI.12-11-04565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi M, Okano H. Neural stem cells, adult neurogenesis, and galectin-1: from bench to bedside. Dev Neurobiol. 2012;72:1059–1067. doi: 10.1002/dneu.22023. [DOI] [PubMed] [Google Scholar]
- Seaberg RM, van der Kooy D. Adult rodent neurogenic regions: the ventricular subependyma contains neural stem cells, but the dentate gyrus contains restricted progenitors. J Neurosci. 2002;22:1784–1793. doi: 10.1523/JNEUROSCI.22-05-01784.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty AK. Progenitor cells from the CA3 region of the embryonic day 19 rat hippocampus generate region-specific neuronal phenotypes in vitro. Hippocampus. 2004;14:595–614. doi: 10.1002/hipo.10206. [DOI] [PubMed] [Google Scholar]
- Shetty AK. Progress in cell grafting therapy for temporal lobe epilepsy. Neurotherapeutics. 2011;8:721–735. doi: 10.1007/s13311-011-0064-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty AK, Zaman V, Hattiangady B. Repair of the injured adult hippocampus through graft-mediated modulation of the plasticity of the dentate gyrus in a rat model of temporal lobe epilepsy. J Neurosci. 2005;25:8391–8401. doi: 10.1523/JNEUROSCI.1538-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty AK, Turner DA. In vitro survival and differentiation of neurons derived from epidermal growth factor-responsive postnatal hippocampal stem cells: inducing effects of brain-derived neurotrophic factor. J Neurobiol. 1998;35:395–425. doi: 10.1002/(sici)1097-4695(19980615)35:4<395::aid-neu7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Turner DA. Neurite outgrowth from progeny of epidermal growth factor-responsive hippocampal stem cells is significantly less robust than from fetal hippocampal cells following grafting onto organotypic hippocampal slice cultures: effect of brain-derived neurotrophic factor. J Neurobiol. 1999;38:391–413. doi: 10.1002/(sici)1097-4695(19990215)38:3<391::aid-neu8>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Hattiangady B, Rao MS, Shuai B. Neurogenesis response of middle-aged hippocampus to acute seizure activity. PLoS One. 2012;7:e43286. doi: 10.1371/journal.pone.0043286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhonen JO, Peterson DA, Ray J, Gage FH. Differentiation of adult hippocampus-derived progenitors into olfactory neurons in vivo. Nature. 1996;383:624–627. doi: 10.1038/383624a0. [DOI] [PubMed] [Google Scholar]
- Svendsen CN, Caldwell MA. Neural stem cells in the developing central nervous system: implications for cell therapy through transplantation. Prog Brain Res. 2000;127:13–34. doi: 10.1016/s0079-6123(00)27003-9. [DOI] [PubMed] [Google Scholar]
- Titomanlio L, Kavelaars A, Dalous J, Mani S, El Ghouzzi V, Heijnen C, Baud O, Gressens P. Stem cell therapy for neonatal brain injury: perspectives and challenges. Ann Neurol. 2011;70:698–712. doi: 10.1002/ana.22518. [DOI] [PubMed] [Google Scholar]
- Waldau B, Hattiangady B, Kuruba R, Shetty AK. Medial ganglionic eminence-derived neural stem cell grafts ease spontaneous seizures and restore GDNF expression in a rat model of chronic temporal lobe epilepsy. Stem Cells. 2010;28:1153–1164. doi: 10.1002/stem.446. [DOI] [PMC free article] [PubMed] [Google Scholar]