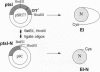
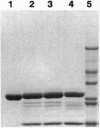
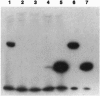
Abstract
The bacterial phosphoenolpyruvate/glycose phosphotransferase system (PTS) comprises a group of proteins that catalyze the transfer of the phosphoryl group from phosphoenolpyruvate (PEP) to sugars concomitant with their translocation. The first two steps of the phosphotransfer sequence are PEP <--> Enzyme I (EI) <--> HPr (the histidine-containing phosphocarrier protein). We have proposed that many functions of the PTS are regulated by EI, which undergoes a monomer/dimer transition. EI monomer (63.5 kDa) comprises two major domains: a flexible C-terminal domain (EI-C) and a protease-resistant, structurally stable N-terminal domain (EI-N) containing the active site His. Trypsin treatment of Salmonella typhimurium EI yielded EI-N, designated EI-N(t). Homogeneous recombinant Escherichia coli EI-N [i.e., EI-N(r)], has now been prepared in quantity, shows the expected thermodynamic unfolding properties and, similarly to EI-N(t), is phosphorylated by phospho-HPr, but not by PEP. In addition, binding of EI-N(r) to HPr was studied by isothermal titration calorimetry: K/a = 1.4 x 10(5) M(-1) and delta H = +8.8 kcal x mol(-1). Both values are comparable to those for HPr binding to intact EI. Fluorescence anisotropy [dansyl-EI-N(r)] and gel filtration of EI-N(r) show that it does not dimerize. These results emphasize the role of EI-C in dimerization and the regulation of intact EI.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chauvin F., Brand L., Roseman S. Sugar transport by the bacterial phosphotransferase system. Characterization of the Escherichia coli enzyme I monomer/dimer equilibrium by fluorescence anisotropy. J Biol Chem. 1994 Aug 12;269(32):20263–20269. [PubMed] [Google Scholar]
- Chauvin F., Brand L., Roseman S. Sugar transport by the bacterial phosphotransferase system. Characterization of the Escherichia coli enzyme I monomer/dimer transition kinetics by fluorescence anisotropy. J Biol Chem. 1994 Aug 12;269(32):20270–20274. [PubMed] [Google Scholar]
- Dannelly H. K., Roseman S. NAD+ and NADH regulate an ATP-dependent kinase that phosphorylates enzyme I of the Escherichia coli phosphotransferase system. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11274–11276. doi: 10.1073/pnas.89.23.11274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox D. K., Meadow N. D., Roseman S. Phosphate transfer between acetate kinase and enzyme I of the bacterial phosphotransferase system. J Biol Chem. 1986 Oct 15;261(29):13498–13503. [PubMed] [Google Scholar]
- Freire E. Statistical thermodynamic analysis of differential scanning calorimetry data: structural deconvolution of heat capacity function of proteins. Methods Enzymol. 1994;240:502–530. doi: 10.1016/s0076-6879(94)40062-8. [DOI] [PubMed] [Google Scholar]
- Lee B. R., Lecchi P., Pannell L., Jaffe H., Peterkofsky A. Identification of the N-terminal domain of enzyme I of the Escherichia coli phosphoenolpyruvate:sugar phosphotransferase system produced by proteolytic digestion. Arch Biochem Biophys. 1994 Jul;312(1):121–124. doi: 10.1006/abbi.1994.1289. [DOI] [PubMed] [Google Scholar]
- LiCalsi C., Crocenzi T. S., Freire E., Roseman S. Sugar transport by the bacterial phosphotransferase system. Structural and thermodynamic domains of enzyme I of Salmonella typhimurium. J Biol Chem. 1991 Oct 15;266(29):19519–19527. [PubMed] [Google Scholar]
- Meadow N. D., Fox D. K., Roseman S. The bacterial phosphoenolpyruvate: glycose phosphotransferase system. Annu Rev Biochem. 1990;59:497–542. doi: 10.1146/annurev.bi.59.070190.002433. [DOI] [PubMed] [Google Scholar]
- Postma P. W., Lengeler J. W., Jacobson G. R. Phosphoenolpyruvate:carbohydrate phosphotransferase systems of bacteria. Microbiol Rev. 1993 Sep;57(3):543–594. doi: 10.1128/mr.57.3.543-594.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privalov G., Kavina V., Freire E., Privalov P. L. Precise scanning calorimeter for studying thermal properties of biological macromolecules in dilute solution. Anal Biochem. 1995 Nov 20;232(1):79–85. doi: 10.1006/abio.1995.9957. [DOI] [PubMed] [Google Scholar]
- Reizer J., Hoischen C., Reizer A., Pham T. N., Saier M. H., Jr Sequence analyses and evolutionary relationships among the energy-coupling proteins Enzyme I and HPr of the bacterial phosphoenolpyruvate: sugar phosphotransferase system. Protein Sci. 1993 Apr;2(4):506–521. doi: 10.1002/pro.5560020403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffen D. W., Presper K. A., Doering T. L., Roseman S. Sugar transport by the bacterial phosphotransferase system. Molecular cloning and structural analysis of the Escherichia coli ptsH, ptsI, and crr genes. J Biol Chem. 1987 Nov 25;262(33):16241–16253. [PubMed] [Google Scholar]
- Saier M. H., Jr, Reizer J. Proposed uniform nomenclature for the proteins and protein domains of the bacterial phosphoenolpyruvate: sugar phosphotransferase system. J Bacteriol. 1992 Mar;174(5):1433–1438. doi: 10.1128/jb.174.5.1433-1438.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel N., Kukuruzinska M. A., Nakazawa A., Waygood E. B., Roseman S. Sugar transport by the bacterial phosphotransferase system. Phosphoryl transfer reactions catalyzed by enzyme I of Salmonella typhimurium. J Biol Chem. 1982 Dec 10;257(23):14477–14491. [PubMed] [Google Scholar]
- Xu Y., McGuire M., Dunaway-Mariano D., Martin B. M. Separate site catalysis by pyruvate phosphate dikinase as revealed by deletion mutants. Biochemistry. 1995 Feb 21;34(7):2195–2202. doi: 10.1021/bi00007a013. [DOI] [PubMed] [Google Scholar]