Abstract
Odors of predators are often co-opted by prey species to serve as warning signals. Perceptual properties of such kairomonal communication are under studied despite their common use in many mammals. We demonstrate that the kairomonal response in mice to rat odors varies monotonically with the volume of rat odor. Moreover, the ability of mice to differentiate between two strengths of rat odors is dependent on the ratio of the two concentrations. These results show that mice can compare kairomonal strength over a large range of values, and that kairomonal communication follows Weber’s law.
Introduction
Foraging animals continually face a conflict between 1) the need to seek opportunities such as food and mating partners; and, 2) the need to avoid exposure to predators. In response to predation pressure, many prey species have co-opted predator odors as kairomones; chemicals emitted by one species, usually for inter-species communication, but intercepted by other species resulting in benefit for the receiver and detriment of the emitter. In this role, predator odors such as urine, fecal material or body odors initiate a rapid avoidance response in prey, thus reducing the probability of successful predation 1, 2. Such avoidance of predator cues needs to be ‘traded-off’ against foraging opportunities. In view of this, it can be speculated that a kairomonal responses may not be an absolute all-or-nothing phenomenon. Rather avoidance is expected to be relative to the intensity of the predator cue. Implicit in this speculation is the idea that animals can quantitatively perceive differences in kairomonal strength.
A wide variety of animals can make quantitative estimates of percepts such as time, foraging opportunities, efforts and rewards 3– 7. These quantitative estimates are often derived using a comparative representational system that is dependent on the ratios between opposing quantities 3– 7. The ability to make quantitative estimates is important because it allows calibration of behavioral responses to incipient environmental opportunities and challenges. In accordance with the comparative nature of such perceptual systems, it can be predicted that greater quantities of kairomones evoke greater response i.e. that the response is dose-dependent. More importantly, sensitivity to changes in the magnitude of a stimulus decreases when stimulus magnitude increases. In other words, the discrimination threshold (i.e. the ‘just-noticeable difference’ between two stimuli of different intensities) is smaller when both stimuli are weak compared to when both stimuli are strong. This formulation is often termed Weber’s law 8, and is a fundamental property of many percepts.
Kairomonal communication has been widely studied in insects 9– 12. Additionally, the neurobiology and physiology of rodent kairomones has attracted significant scientific interest in the recent past 1, 13. Yet, the perceptual properties of kairomonal communication in mammals have so far been under studied, including, the dose-responsivity of kairomonal communication 14 and the relationship of discrimination threshold to stimulus magnitude.
House mice ( Mus musculus) are predated by rats ( Rattus norvegicus) 15– 17 and accordingly, the mice express innate avoidance to rat odors 18, 19. In this report, we investigate the dose-responsivity and discrimination threshold of kairomonal communication in mice.
Materials and methods
Animals
The Nanyang Technological University (IACUC number: ARF SBS/NIE-A-0106AZ) institutional animal care and use committee reviewed and approved all procedures. Fifteen male Balb/c mice (7–8 weeks old, housed five per cage (369 x 156 x 132 mm; 1145T, Tecniplast, UK)) were obtained from the vivarium of the National University of Singapore. Eight male Wistar rats (48 days old, housed two per cage (425 x 266 x 185 mm; 1291H, Tecniplast, UK)) were obtained from the same vivarium and used as a kairomonal source. Standard corn cob cage bedding was changed twice a week. Animals were maintained on a 12 hours light-dark cycle, with temperature and relative humidity ranging between 20–25 degree celsius and 70–80%, respectively. Experiments were conducted during the light phase. Food and water was available ad libitum. The diet consisted of standard laboratory chow (PicoLab Rodent Diet 20, 5053) with 20% protein content.
Kairomone collection
Rat urine was collected using metabolic cages (Harvard Apparatus). Rat urine contains volatile compounds and major urinary proteins (MUPs). The urine was treated with menadione (M5625 Sigma-Aldrich, Singapore) to competitively displace volatile compounds bound to the MUPs, followed by centrifugation (Millipore, 3000 g for 5 minutes) through a size-exclusion column (>3 kDa). Only the high molecular-weight fraction containing MUPs and devoid of volatiles was used, in accordance with the prior demonstration that rat MUPs serve as kairomones to mice 13.
Dose-responsivity to kairomones
The response of mice (n = 10) to increasing doses of MUP fraction of rat urine ((henceforth referred to as rat urine) was studied (trial duration = 600s). Avoidance was quantified by comparing time spent by mice in two opposing bisects of an arena (76 × 9 cm; 15 cm high). Data on time spent in each bisect was collected by automated behavioral tracking software (ANY-maze, version 4.3, Stoelting). Opposing arms contained either rat urine or phosphate-buffered saline. The amount of rat urine was systematically varied from 1X to 16X (3.125, 6.25, 12.5, 25 and 50 µl). X was arbitrarily defined as 3.125 µl of rat urine. The same set of mice was used in successive testing for all doses (starting from lower to higher doses) with 24 hours elapsing between two successive trials).
Discrimination threshold
Both arms of the arena contained rat urine in this condition. The amount of rat urine in one arm was varied in five discrete doses (6.25–25 μl), equidistant on a Log 2 scale. The opposing arm contained volume that was greater by ratio of either 1.2 or 1.3. The percentage of time that mice (n = 15, the same mice that were used in the previous experiment) spent in the arm with the greater volume of urine, was quantified. The same set of mice were used in successive testing for all doses (starting from lower to higher doses), with two successive trials (24 hours apart).
Statistics
All statistical tests were conducted using IBM SPSS software (version 20) One-way analysis of variance followed by ‘Fisher’s least significant difference’ (LSD) post-hoc test was used to analyze increasing doses of kairomones on mouse behaviour. A two-way analysis of variance was carried out to determine main effect and/or interaction of kairomone dose and its corresponding ratios.
Results
Dose responsivity of kairomonal communication
One-way analysis of variance (ANOVA) revealed that the amount of time spent near rat urine decreased with an incremental increase in the amount of rat urine ( Figure 1; n = 10; F (4,45) = 6.9; p = 0.0002). Animals spent significantly more time near the weakest odor, compared to the strongest (LSD, Fisher's least significant difference, p = 0.0002). The stimulus-response curve exhibited a robust fit to sigmoidal curve ( Figure 1; R 2 > 0.99; p < 0.01), showing a monotonic linear response between concentrations 2X to 8X.
Figure 1. Kairomonal communication in mice is dose-dependent (mean±SEM).
Aversion of mice to increasing doses of rat urine was quantified by comparing the time spent in two opposing arms of an arena, with one arm containing incremental doses of rat urine and the other containing buffered saline (trial duration = 600 s, n = 10 mice). The graph depicts average time spent in rat urine arm. The gray line depicts sigmoidal fit. Abscissa depicts dose of rat urine employed (log 2 scale; x arbitrarily set as 3.125 µl of rat urine).
In order to rule out carry-over effects during repeated trials, we tested a separate set of five mice repeatedly at a singular dose (4X) over five days. This set of mice exhibited a comparable aversion to rat urine across all trials, showing a lack of habituation, sensitization or conditioning during repeated testing (one-way ANOVA; F (4,20) = 0.314, p > 0.8).
Proportional discrimination threshold
The discrimination threshold (i.e the ‘just-noticeable difference’) was studied for five equidistant doses (Log 2 scale) encompassing the linear part of the dose-response curve ( Figure 2). One arm of the arena in this case contained the dose depicted in the abscissa. The discrimination around this dose was studied in two successive trials by providing a greater amount of kairomone in the opposing arm, differing by a ratio of either 1.2 or 1.3. A positive discrimination was noted as less time was spent in the arm containing the greater volume of urine.
Figure 2. Detection threshold in mice is proportional to kairomone strength (mean±SEM).
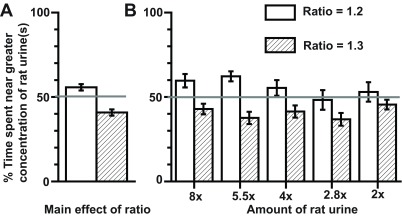
The detection threshold at varying doses of rat urine was further examined by setting up an avoidance-avoidance conflict, where mice chose to spend time in arms containing either lower or higher amounts of rat urine. The higher dose was of either a 20% (un-shaded bars) or 30% (shaded bars) greater magnitude (e.g. 120% or 130% of 2X). Abscissa depicts the lower dose used in each of the comparisons (e.g. 2X). Ordinate depicts time spent in arm with the greater amount of rat urine divided by the sum of the time spent in both arms (gray line = 50% chance). N = 15 mice for all comparisons.Log 2 scale; arbitrarily set as 3.125 µl of rat urine.
A two-way ANOVA for dose and ratio revealed a significant main effect of the ratio ( Figure 2A; F (1,138) = 32.1, p = 0.00000008). The main effect of doses themselves did not reach statistical significance (F (4,138) = 1.347, p = 0.256). Similarly, interaction between doses and ratios was not significant (F (4,138) = 1.214, p = 0.308). Thus, regardless of the dose studied, detection threshold was constantly proportional to the kairomone strength ( Figure 2B) by a ratio ≤ 1.3 but above >1.2. In other words, the discrimination threshold was smaller for weaker stimuli and bigger for stronger stimuli
Discussion
Kairomones are compounds emitted by one species and co-opted by another receiving species, resulting in benefit for the receiver and detriment for the emitter. Kairomones can be used by both predators to locate prey and by prey to secure advanced warning of predator presence. Odors used as pheromones in intra-species communication are often the most vulnerable for co-option as kairomones. This is because use in conspecific communication requires robust expression of odors, making them more liable for eavesdropping by other species (reviewed in Kolluru and Zuk 20). In agreement with this formulation, rats use urine marks to communicate status and sexual attractiveness 21– 23; and, proteins secreted with rat urine are sufficient to initiate innate avoidance in mice 13, a prey species of rats 15– 17. The salient kairomone in this case has been identified to be a major urinary protein, MUP13 13. These involatile rodent kairomones provide a unique opportunity to study kairomonal perception by virtue of their stability in the ambient environment and due to their ease of reliably controlling their dosing. This is in contrast with the volatile nature of many kairomones that require onerous delivery methods using olfactometers.
Several pheromonal responses in insects and mammals show dose responsivity whereby a stronger pheromonal stimulus evokes a greater response (e.g. Coureaud et al., He et al. and Perna et al. 24– 26). In contrast, the dose responsivity of kairomonal communication has not been well-studied. In this report, we demonstrate that mice perceive rat kairomones in a dose-dependent manner. We speculate that the relative nature of kairomonal communication permits prey to calibrate foraging responses according to perceived predatory threats. For example, a weak kairomonal stimulus might signal the passage of a long period of time since the urine mark was laid, and thereby evoke lesser avoidance. In contrast, a stronger odor is likely to be fresh and a better indicator of predator presence. Similar dose-responsivity has been previously described during the perception of cat odors by rats 14.
The ability to calibrate avoidance also suggests that mice are able to differentiate between various amounts of kairomones. Animals can indeed discriminate between different amounts of many percepts, including olfactory sensations 3– 7. In the absence of an absolute numerical system, these discriminations are often dependant on a relative estimation based on comparative perceptions. Weber and Fletcher 8 formalized one of the hallmarks of such relative estimation by showing that the discrimination threshold is a constant fraction of the stimulus intensity in many perceptual systems ( k = ∆I/I; where k is a constant, I is intensity and ∆I is just-noticeable difference). In this report, we demonstrate that kairomonal communication in mice follows Weber’s law with Weber’s fraction valued at greater than 0.2 but smaller than 0.3. Weber’s law has been previously studied in human olfaction for volatile odors, yielding a comparable sensitivity of 0.28 7. Similarly, pheromonal communication in Argentine Ants ( Linepithema humile) has been recently shown to follow Weber’s law 26. To the best of our knowledge, this is the first demonstration of Weber’s law applied to kairomones.
Dose responsiveness file: Dose responsiveness of mice (n=10) to differing volumes (ul) of rat urine (kairomone) or control (PBS). Time was measured in seconds; total time in the enclosure was 600 seconds. Mice were exposed to concentrations in ascending order, with a 24 hour gap between tests. Data corresponds to Fig 1 in the main manuscript.
Single dose responsiveness file: Responsiveness of mice (n=5) to a single volume (ul) of rat urine (kairomone) or control (PBS) over 5 days. Time was measured in seconds; total time in the enclosure was 600 seconds. Mice were exposed to concentrations in ascending order, with a 24 hour gap between tests.
Detection threshold: Discrimination threshold of mice (n=15) when exposed to two different volumes (ul) of rat urine (kairomone): a specific volume vs. either a 20% or a 30% increase in this volume. Time was measured in seconds; total time in the enclosure was 600 seconds. Mice were exposed to concentrations in ascending order, with a 24 hour gap between tests. Data corresponds to Fig 2 in the main manuscript.
Funding Statement
This research was funded by Nanyang Technological University and the Ministry of Education, Singapore (MOE2011-T2-2-111).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
v1; ref status: approved 1
References
- 1.Apfelbach R, Blanchard CD, Blanchard RJ, et al. : The effects of predator odors in mammalian prey species: a review of field and laboratory studies. Neurosci Biobehav Rev. 2005;29(8):1123–1144 10.1016/j.neubiorev.2005.05.005 [DOI] [PubMed] [Google Scholar]
- 2.Wisenden BD: Olfactory assessment of predation risk in the aquatic environment. Philos Trans R Soc Lond B Biol Sci. 2000;355(1401):1205–1208 10.1098/rstb.2000.0668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker JM, Shivik J, Jordan KE: Tracking of food quantity by coyotes (Canis latrans). Behav Processes. 2011;88(2):72–75 10.1167/10.7.238 [DOI] [PubMed] [Google Scholar]
- 4.Meck WH, Church RM: A mode control model of counting and timing processes. J Exp Psychol Anim Behav Process. 1983;9(3):320–334 10.1037/0097-7403.9.3.320 [DOI] [PubMed] [Google Scholar]
- 5.Shafiei N, Gray M, Viau V, et al. : Acute Stress Induces Selective Alterations in Cost/Benefit Decision-Making. Neuropsychopharmacology. 2012;37(10):2194–209 10.1038/npp.2012.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohring W, Libertus ME, Bertin E: Speed discrimination in 6- and 10-month-old infants follows Weber's law. J Exp Child Psychol. 2012;111(3):405–418 10.1016/j.jecp.2011.11.002 [DOI] [PubMed] [Google Scholar]
- 7.Stone H, Bosley JJ: Olfactory Discrimination and Weber's Law. Percept Mot Skills. 1965;20:657–665 10.2466/pms.1965.20.2.657 [DOI] [PubMed] [Google Scholar]
- 8.Glimcher PW: Foundations of neuroeconomic analysis.(Oxford University Press, New York) pp xix,2011;467 10.1093/acprof:oso/9780199744251.001.0001 [DOI] [Google Scholar]
- 9.Svensson GP, Larsson MC, Hedin J: Attraction of the larval predator Elater ferrugineus to the sex pheromone of its prey, Osmoderma eremita, and its implication for conservation biology. J Chem Ecol. 2004;30(2):353–363 10.1023/B:JOEC.0000017982.51642.8c [DOI] [PubMed] [Google Scholar]
- 10.Larsson MC, Svensson GP: Pheromone monitoring of rare and threatened insects: exploiting a pheromone-kairomone system to estimate prey and predator abundance. Conserv Biol. 2009;23(6):1516–1525 10.1111/j.1523-1739.2009.01263.x [DOI] [PubMed] [Google Scholar]
- 11.Rutledge C: A survey of identified kairomones and synomones used by insect parasitoids to locate and accept their hosts. Chemoecology. 1996;7(3):121–131 10.1007/BF01245964 [DOI] [Google Scholar]
- 12.Miller DR, Asaro C, Crowe CM, et al. : Bark beetle pheromones and pine volatiles: attractant kairomone lure blend for longhorn beetles (Cerambycidae) in pine stands of the southeastern United States. J Econ Entomol. 2011;104(4):1245–1257 10.1603/EC11051 [DOI] [PubMed] [Google Scholar]
- 13.Papes F, Logan DW, Stowers L: The vomeronasal organ mediates interspecies defensive behaviors through detection of protein pheromone homologs. Cell. 2010;141(4):692–703 10.1016/j.cell.2010.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takahashi LK, Nakashima BR, Hong H, et al. : The smell of danger: a behavioral and neural analysis of predator odor-induced fear. Neurosci Biobehav Rev. 2005;29(8):1157–1167 10.1016/j.neubiorev.2005.04.008 [DOI] [PubMed] [Google Scholar]
- 15.Galef BG, Jr: Aggression and timidity: responses to novelty in feral Norway rats. J Comp Physiol Psychol. 1970;70(3):370–381 10.1037/h0028719 [DOI] [PubMed] [Google Scholar]
- 16.Hsuchou H, Ho YJ, Shui HA, et al. : Effects of incisor-cutting on muricidal behavior induced by olfactory bulbectomy in rats. Physiol Behav. 2002;76(4–5):669–675 10.1016/S0031-9384(02)00807-7 [DOI] [PubMed] [Google Scholar]
- 17.Karli P: The Norway rat's killing response to the white mouse: an experimental analysis. Behaviour. 1956;10:81–103 10.1163/156853956X00110 [DOI] [Google Scholar]
- 18.Amaral VC, Santos Gomes K, Nunes-de-Souza RL: Increased corticosterone levels in mice subjected to the rat exposure test. Horm Behav. 2010;57(2):128–133 10.1016/j.yhbeh.2009.09.018 [DOI] [PubMed] [Google Scholar]
- 19.Yang M, Augustsson H, Markham CM, et al. : The rat exposure test: a model of mouse defensive behaviors. Physiol Behav. 2004;81(3):465–473 10.1016/j.physbeh.2004.02.010 [DOI] [PubMed] [Google Scholar]
- 20.Kolluru GR, Zuk M: Exploitation of sexual signals by predators and parasitoids. Q Rev Biol. 1998;73(4):415–438 10.1086/420412 [DOI] [Google Scholar]
- 21.Taylor GT, Haller J, Bartko G, et al. : Conspecific urine marking in male-female pairs of laboratory rats. Physiol Behav. 1984;32(4):541–546 10.1016/0031-9384(84)90307-X [DOI] [PubMed] [Google Scholar]
- 22.Taylor GT, Regan D, Haller J: Sexual experience, androgens and female choice of a mate in laboratory rats. J Endocrinol. 1983;96(1):43–52 10.1677/joe.0.0960043 [DOI] [PubMed] [Google Scholar]
- 23.Taylor GT, Haller J, Regan D: Female rats prefer an area vacated by a high testosterone male. Physiol Behav. 1982;28(6):953–958 10.1016/0031-9384(82)90159-7 [DOI] [PubMed] [Google Scholar]
- 24.Coureaud G, Langlois D, Sicard G, et al. : Newborn rabbit responsiveness to the mammary pheromone is concentration-dependent. Chem Senses. 2004;29(4):341–350 10.1093/chemse/bjh037 [DOI] [PubMed] [Google Scholar]
- 25.He J, Ma L, Kim S, et al. : Distinct signals conveyed by pheromone concentrations to the mouse vomeronasal organ. J Neurosci. 2010;30(22):7473–7483 10.1523/JNEUROSCI.0825-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perna A, Granovskiy B, Garnier S, et al. : Individual Rules for Trail Pattern Formation in Argentine Ants (Linepithema humile). PLoS Comput Biol. 2012;8(7):e1002592 10.1371/journal.pcbi.1002592 [DOI] [PMC free article] [PubMed] [Google Scholar]



