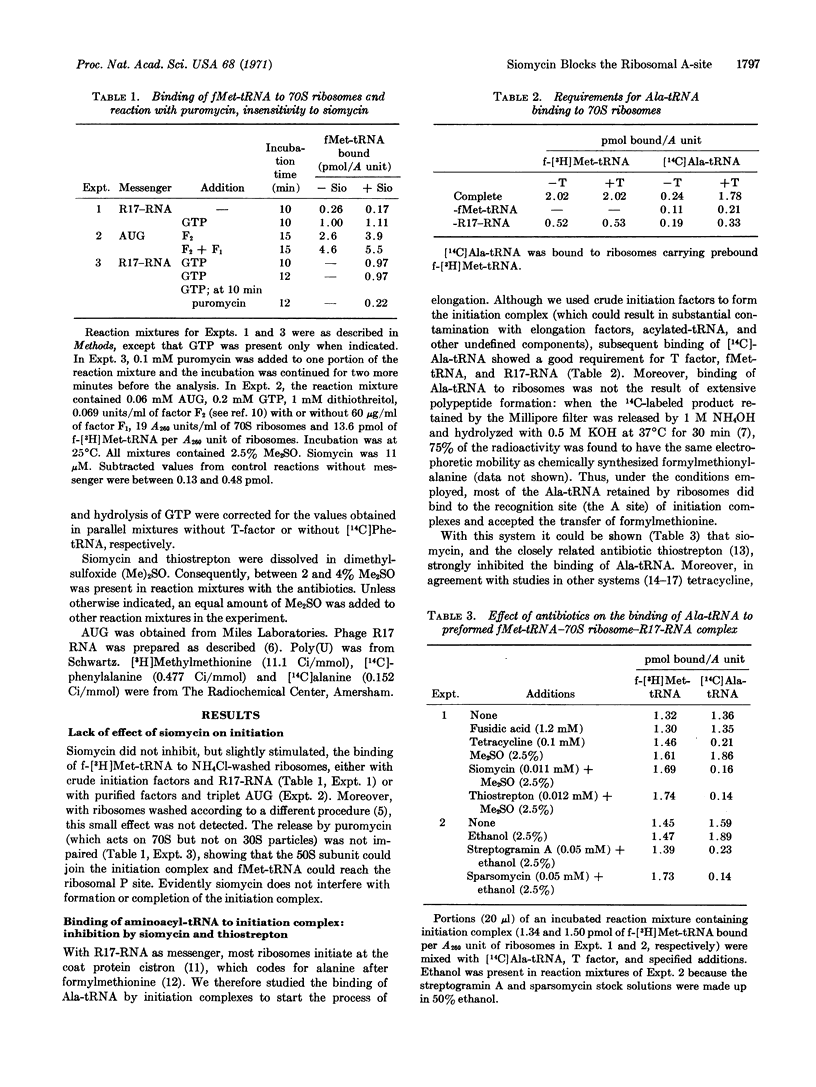
Abstract
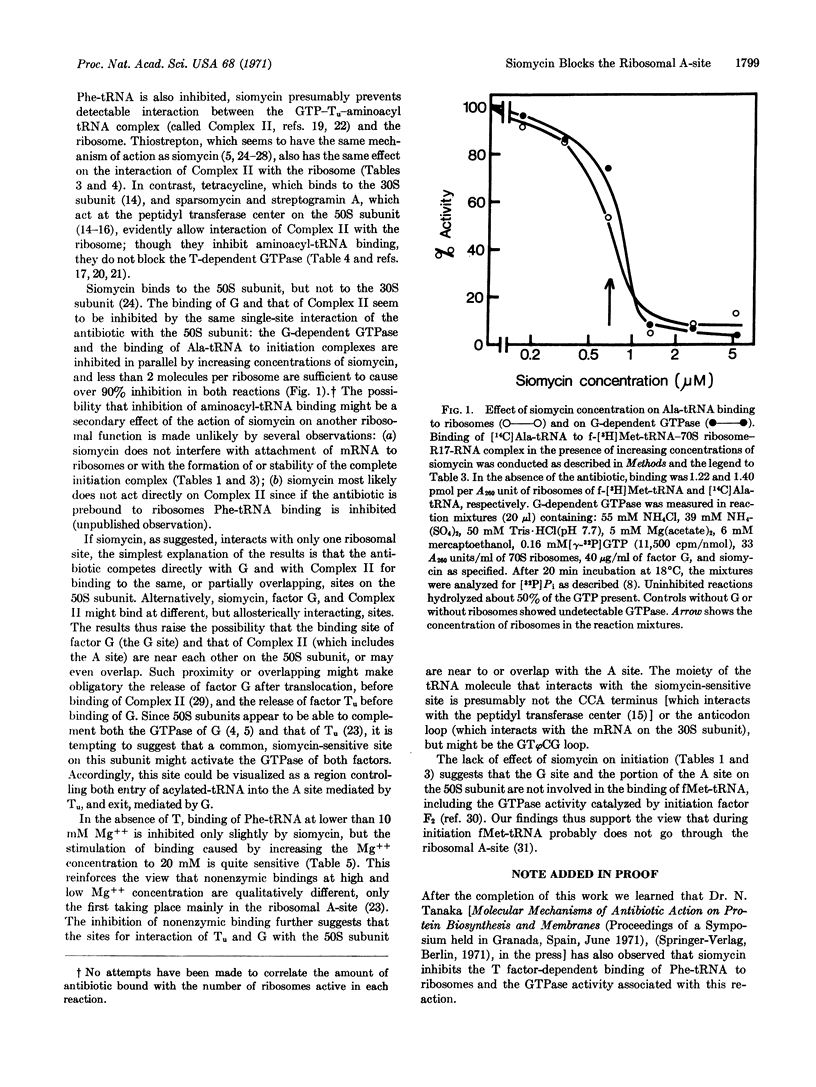
Siomycin, a peptide antibiotic that interacts with the 50S ribosomal subunit and inhibits binding of factor G, is shown also to inhibit binding of aminoacyl-tRNA; however, it does not impair binding of fMet-tRNA and completion of the initiation complex. Moreover, unlike other inhibitors of aminoacyl-tRNA binding (tetracycline, sparsomycin, and streptogramin A), siomycin completely abolishes the GTPase activity associated with the binding of aminoacyl-tRNA catalyzed by factor Tu. A single-site interaction of siomycin appears to be responsible for its effect on both the binding of the aminoacyl-tRNA-Tu-GTP complex and that of factor G.
Keywords: Millipore filter, Tu, tetracycline, streptogramin, puromycin
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodley J. W., Lin L., Highland J. H. Studies on translocation. VI. Thiostrepton prevents the formation of a ribosome-G factor-guanine nucleotide complex. Biochem Biophys Res Commun. 1970 Dec 24;41(6):1406–1411. doi: 10.1016/0006-291x(70)90543-7. [DOI] [PubMed] [Google Scholar]
- Bodley J. W., Lin L. Interaction of E. coli G factor with the 50S ribosomal subunit. Nature. 1970 Jul 4;227(5253):60–61. doi: 10.1038/227060a0. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S., Marcker K. A. Polypeptidyl-sigma-ribonucleic acid and amino-acyl-sigma-ribonucleic acid binding sites on ribosomes. Nature. 1966 Jul 23;211(5047):380–384. doi: 10.1038/211380a0. [DOI] [PubMed] [Google Scholar]
- CONWAY T. W., LIPMANN F. CHARACTERIZATION OF A RIBOSOME-LINKED GUANOSINE TRIPHOSPHATASE IN ESCHERICHIA COLI EXTRACTS. Proc Natl Acad Sci U S A. 1964 Dec;52:1462–1469. doi: 10.1073/pnas.52.6.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celma M. L., Monro R. E., Vazquez D. Substrate and antibiotic binding sites at the peptidyl transferase centre of E. coli ribosomes: Binding of UACCA-Leu to 50 S subunits. FEBS Lett. 1971 Mar 16;13(4):247–251. doi: 10.1016/0014-5793(71)80546-x. [DOI] [PubMed] [Google Scholar]
- Chae Y. B., Mazumder R., Ochoa S. Polypeptide chain initiation in E. coli: isolation of homogeneous initiation factor E2 and its relation to ribosomal proteins. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1181–1188. doi: 10.1073/pnas.62.4.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. Hydrolysis of guanosine 5'-triphosphate associated wh binding of aminoacyl transfer ribonucleic acid to ribosomes. J Biol Chem. 1969 Oct 25;244(20):5680–5686. [PubMed] [Google Scholar]
- Igarashi K., Kaji A. Relationship between sites 1,2 and acceptor, donor sites for the binding of aminoacyl tRNA to ribosomes. Eur J Biochem. 1970 May 1;14(1):41–46. doi: 10.1111/j.1432-1033.1970.tb00258.x. [DOI] [PubMed] [Google Scholar]
- Kolakofsky D., Dewey K. F., Hershey J. W., Thach R. E. Guanosine 5'-triphosphatase activity of initiation factor f2. Proc Natl Acad Sci U S A. 1968 Nov;61(3):1066–1070. doi: 10.1073/pnas.61.3.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipmann F. Polypeptide chain elongation in protein biosynthesis. Science. 1969 May 30;164(3883):1024–1031. doi: 10.1126/science.164.3883.1024. [DOI] [PubMed] [Google Scholar]
- Lodish H. F. Bacteriophage f2 RNA: control of translation and gene order. Nature. 1968 Oct 26;220(5165):345–350. doi: 10.1038/220345a0. [DOI] [PubMed] [Google Scholar]
- Modolell J., Davis B. D. Breakdown by streptomycin of initiation complexes formed on ribosomes of Escherichia coli. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1148–1155. doi: 10.1073/pnas.67.3.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modolell J., Davis B. D. Rapid inhibition of polypeptide chain extension by streptomycin. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1279–1286. doi: 10.1073/pnas.61.4.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modolell J., Vazquez D., Monro R. E. Ribosomes, G-factor and siomycin. Nat New Biol. 1971 Mar 24;230(12):109–112. doi: 10.1038/newbio230109a0. [DOI] [PubMed] [Google Scholar]
- Moldave K., Galasinski W., Rao P., Siler J. Studies on the peptidyl tRNA translocase from rat liver. Cold Spring Harb Symp Quant Biol. 1969;34:347–356. doi: 10.1101/sqb.1969.034.01.041. [DOI] [PubMed] [Google Scholar]
- NIRENBERG M., LEDER P. RNA CODEWORDS AND PROTEIN SYNTHESIS. THE EFFECT OF TRINUCLEOTIDES UPON THE BINDING OF SRNA TO RIBOSOMES. Science. 1964 Sep 25;145(3639):1399–1407. doi: 10.1126/science.145.3639.1399. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y., Lipmann F. The interrelationship between guanosine triphosphatase and amino acid polymerization. Arch Biochem Biophys. 1966 Sep 26;116(1):344–351. doi: 10.1016/0003-9861(66)90040-3. [DOI] [PubMed] [Google Scholar]
- Ono Y., Skoultchi A., Waterson J., Lengyel P. Peptide chain elongation: GTP cleavage catalysed by factors binding aminoacyl-transfer RNA to the ribosome. Nature. 1969 May 17;222(5194):645–648. doi: 10.1038/222645a0. [DOI] [PubMed] [Google Scholar]
- Ono Y., Skoultchi A., Waterson J., Lengyel P. Stoichiometry of aminoacyl-transfer RNA binding and GTP cleavage during chain elongation and translocation. Nature. 1969 Aug 16;223(5207):697–701. doi: 10.1038/223697a0. [DOI] [PubMed] [Google Scholar]
- Pestka S. Studies on the formation of transfer ribonucleic acid-ribosome complexes. XI. Antibiotic effects on phenylalanyl-oligonucleotide binding to ribosomes. Proc Natl Acad Sci U S A. 1969 Oct;64(2):709–714. doi: 10.1073/pnas.64.2.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka S. Thiostrepton: a ribosomal inhibitor of translocation. Biochem Biophys Res Commun. 1970 Aug 11;40(3):667–674. doi: 10.1016/0006-291x(70)90956-3. [DOI] [PubMed] [Google Scholar]
- Ravel J. M., Shorey R. A., Shive W. Relationship between peptidyl transferase activity and interaction of ribosomes with phenylalanyl transfer ribonucleic acid--guanosine 5'-triphosphate--TIu complex. Biochemistry. 1970 Dec 8;9(25):5028–5033. doi: 10.1021/bi00827a030. [DOI] [PubMed] [Google Scholar]
- Shorey R. L., Ravel J. M., Garner C. W., Shive W. Formation and properties of the aminoacyl transfer ribonucleic acid-guanosine triphosphate-protein complex. J Biol Chem. 1969 Sep 10;244(17):4555–4564. [PubMed] [Google Scholar]
- Steitz J. A. Polypeptide chain initiation: nucleotide sequences of the three ribosomal binding sites in bacteriophage R17 RNA. Nature. 1969 Dec 6;224(5223):957–964. doi: 10.1038/224957a0. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Watanabe S., Teraoka H., Tamaki M. Effect of siomycin on protein synthesizing activity of Escherichia coli ribosomes. Biochem Biophys Res Commun. 1970;39(6):1189–1193. doi: 10.1016/0006-291x(70)90686-8. [DOI] [PubMed] [Google Scholar]
- Tanaka N., Kinoshita T., Masukawa H. Mechanism of protein synthesis inhibition by fusidic acid and related antibiotics. Biochem Biophys Res Commun. 1968 Feb 15;30(3):278–283. doi: 10.1016/0006-291x(68)90447-6. [DOI] [PubMed] [Google Scholar]
- Weisblum B., Davies J. Antibiotic inhibitors of the bacterial ribosome. Bacteriol Rev. 1968 Dec;32(4 Pt 2):493–528. [PMC free article] [PubMed] [Google Scholar]
- Weisblum B., Demohn V. Inhibition by thiostrepton of the formation of a ribosome-bound guanine nucleotide complex. FEBS Lett. 1970 Dec;11(3):149–152. doi: 10.1016/0014-5793(70)80515-4. [DOI] [PubMed] [Google Scholar]
- Weisblum B., Demohn V. Thiostrepton, an inhibitor of 5OS ribosome subunit function. J Bacteriol. 1970 Mar;101(3):1073–1075. doi: 10.1128/jb.101.3.1073-1075.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]


