Abstract
Spinal cord injury (SCI) initiates a cascade of processes that ultimately form a nonpermissive environment for axonal regeneration. Emerging evidence suggests that regenerative failure may be due in part to inhibitory factors expressed by reactive spinal cord glial cells and meningeal fibroblasts, such as the Eph receptor protein-tyrosine kinases and their corresponding ligands (ephrins). Here we sought to assess the role of ephrin B2, an inhibitory axonal guidance molecule, as an inhibitor of the recovery process following SCI. To determine the extent of ephrin B2 involvement in axonal regenerative failure, a SCI model was performed on a conditional ephrin B2 knockout mouse strain (ephrin B2−/−), in which the ephrin B2 gene was deleted specifically in astrocytes. The expression of ephrin B2 was significantly decreased in astrocytes of injured and uninjured ephrin B2−/− mice compared to wild type mice. Notably, in the ephrin B2−/− mice, the deletion of ephrin B2 reduced astrogliosis, and accelerated motor function recovery after SCI. Anterograde axonal tracing on a hemisection model of SCI further showed that ephrin B2−/− mice exhibited increased regeneration of injured corticospinal axons and a reduced glial scar, when compared to littermate controls exposed to similar injury. These results were confirmed by an in vitro neurite outgrowth assay and ephrin B2 functional blockage, which showed that ephrin B2 expressed on astrocytes inhibited axonal growth. Combined these findings suggest that ephrin B2 ligands expressed by reactive astrocytes impede the recovery process following SCI.
Keywords: Spinal cord injury, axonal guidance molecules, ephrin B2, axonal regeneration, astrocytic gliosis
1. Introduction
Traumatic insult to the adult mammalian central nervous system (CNS), such as spinal cord injury (SCI), initiates a cascade of events that ultimately lead to regenerative failure. It is widely recognized that various components of the post-traumatic spinal cord (SC) milieu are inhibitory to axonal re-growth, and that this inhibitory microenvironment is a major contributor to poor functional recovery following SCI. A number of myelin-derived inhibitors including, the reticulon family member RTN4a/Nogo-A (Bandtlow and Schwab, 2000, GrandPre et al., 2000) myelin-associated glycoprotein (McKerracher et al., 1994, DeBellard et al., 1996, Schafer et al., 1996, Filbin, 2003) oligodendrocyte myelin glycoprotein (Wang et al., 2002), and chondroitin sulfate proteoglycans (CSPG) (Bradbury et al., 2002) have been intensively studied as potential mediators of the inhibitory microenvironment. Laboratory experiments and treatment strategies based on these identified inhibitory factors have met with some success.
Many studies have shown that ephrin/Eph signaling plays a role in the regulation of axon guidance through contact repulsion, inducing neuronal growth cone collapse in the developing brain and SC (Flanagan and Vanderhaeghen, 1998, Wilkinson, 2001). Members of this family are up-regulated following CNS injury (Miranda et al., 1999, Willson et al., 2003) and in a previous study, ephrin B3 was shown to inhibit axonal regeneration (Duffy et al., 2012). Another member of the family, ephrin B2 has been suggested to be a possible mediator of astrogliosis and scar formation (Fabes et al., 2006, Goldshmit et al., 2006, Curinga and Smith, 2008), through its receptor EphB2, expressed by meningeal fibroblasts in the injured adult SC (Bundesen et al., 2003). Ephrin B2 has also been shown to bind to EphA4, expressed on the corticospinal tract (CST) axonal stump (Fabes et al., 2006). EphA4 has been implicated in the response to injury, with expression in astrocytes and neurons (Goldshmit et al., 2004, Fabes et al., 2006, Herrmann et al., 2010). Several lines of evidence, including Eph-A4 knockout mice, peptide antagonist, or soluble recombinant blocker, suggest that the lack of EphA4 enhanced axonal regeneration of the corticospinal tract and improved functional recovery following traumatic SCI (Goldshmit et al., 2004, Fabes et al., 2007, Goldshmit et al., 2011).
Although ephrin B2 has been proposed to play an inhibitory role in axonal regeneration after SCI, no studies have so far directly tested the effect of deletion of ephrin B2. In this study, a conditional ephrin B2 knockout mouse strain, ephrin B2−/−, was established with a Cre-LoxP system, in which the ephrin B2 gene was deleted in astrocytes. This astrocyte conditional ephrin B2 knockout mouse line provided a novel tool to test the effect of astrocytic derived ephrin B2 on axonal regeneration. We found that deletion of ephrin B2 in astrocytes enhanced axonal regeneration after SCI. The study also provides additional evidence suggesting that the effect of ephrin B2 deletion is likely a result of suppressing the glial scar formation.
2. Experimental procedures
2.1 Establishment of ephrin B2 conditional knockout mice
We used two transgenic lines to establish the astrocytic conditional ephrin B2 knockout mice (ephrin B2−/−): GFAP-Cre mice [FVB-Tg (GFAP-cre) 25Mes/J] bearing Cre recombinase enzyme gene under control of GFAP promoter in astrocytes, which were obtained from the Jackson Laboratory (Bar Harbor, ME, USA) (Zhuo et al., 2001), and ephrin B2-Lox mice, in which exon 1 of the endogenous ephrin B2 gene was flanked by LoxP sites, which were obtained from the California Institute of Technology (Gerety and Anderson, 2002). All animal housing and procedures were performed in compliance with guidelines established by the University Committee of Animal Resources at the University of Rochester. For genotyping purposes, genomic DNA was isolated from the tails of 4 to 6-week-old mice and used for PCR. PCR genotyping for the conditional ephrin B2 allele (floxed allele) was performed with a 5′primer specific for the 5′loxP site insertion, 5′-AAGTTATAAGCTTCAACGCGTCC-3′ (TF3), and a 3′ primer in the genomic region downstream of exon 1, 5′-GAGCCCCAGGTTCTAGAATAACTTCG-3′ (RF1) (product size of 320bp). The wild-type ephrin B2 locus was detected with a 5′ primer that includes sequence flanking the inserted 5′ loxP site, and a 3′ primer downstream of the first exon, 5′-GCTGCCCGCGGCCGGTCCCAACG-3′ (BrgF1) and 5′-CCGTTAGTGGCAACGTCCTCCGTCCTCG-3′ (HL-I-R2h) (product size of 500bp). The GFAP-Cre transgene was detected by allele specific primers, with the oIMR1900, 5′-ACTCCT TCATAAAGCCCT-3′ and the oIMR1901, 5′-ATCACTCGTTGCATCGACCG-3′ (product size of 190 bp). Ephrin B2−/− mice were identified by the presence of loxP-allele specific and GFAP-Cre specific PCR products. The mice with preservation of ephrin B2 gene (ephrin B2+/+) in the same litter were used as control (wild type mice).
2.2 Spinal cord injury model
For evaluation of CST regeneration, the lateral hemisection SCI model (Goldshmit et al., 2004) was used for the histological study of axonal regeneration, in addition to behavioral testing. All the surgical procedures were approved by the University Committee of Animal Resources at the University of Rochester. The lateral hemisection SCI model was used because it produced a more consistent and reproducible SCI, with respect to the lesion size and severity, which is important for quantification of the glial scar size and the degree of injured axonal regeneration. A shortcoming however is that it is sometimes difficult to ensure all CST axons are severed, especially the CST that is close to the midline. To avoid this issue and maintain injury consistency, one of the authors, who is an experienced neurosurgeon trained in microsurgical techniques, performed all the surgeries under blinded conditions using a surgical microscope. Sectioning began at the midline and proceeded to the lateral side to ensure consistency and complete injury. Following behavioral analysis, histological assessments of each injury were performed to confirm the complete section of the CST. If it was found that the transection failed to fully extend to the midline, the mice were removed from the behavioral data. Briefly, the animals were anesthetized with intraperitoneal injections of a mixture of ketamine (8mg/kg) and xylazine (10mg/kg), a midline incision was made on the back region and a laminectomy was performed at T10–T11 level. Spinal hemisection at T10 was performed on the right side of the cord, using a fine corneal blade (cut twice in the same place to ensure complete section). The overlying layers of muscles and skin were closed with absorbable sutures. After surgery, bladders were manually expressed twice per day or as necessary. Animals received sulfamethoxazole (4mg/100gm) and trimethoprim (0.8mg/100gm) twice per day orally to prevent infection. Animal weight and hydration were carefully monitored. If dehydrated, animals received subcutaneous injections of D5-lactated Ringer’s solution.
2.3 Real-time RT- PCR analysis and immunohistochemistry
Real-time quantitative PCR was used to measure levels of ephrin B2 mRNA of cultured astrocytes. Data are expressed as relative quantification units. Standard immunohistochemical staining, as described previously (Peng et al., 2009), was employed. Primary antibodies of mouse anti-GFAP (1:500, Sigma) and goat anti-ephrin-B2 (1:200, R & D Systems) were used. For double-labeling immunohistochemistry, donkey secondary antibodies conjugated to FITC or Texas Red (Jackson ImmunoResearch) were used.
2.4 Anterograde tracing and glial scar size quantification
Anterograde labeling of the CST was done by injection of biotinylated dextran amine (BDA) (Molecular Probes, Oregon, USA) into the contralateral motor cortex two weeks before sacrificing the animal using standard coordinates (1.0mm lateral, 0.5 mm deep to the cortical surface and +0.5, −0.5 mm with respect to Bregma) (Steward et al., 2008). BDA is injected into a total of 2 sites (0.5μl per site over a 10 minute time period) using a 10μl Hamilton microsyringe tipped with a pulled glass micropipette. In our experiments, BDA was injected to the injury model 6 weeks after injury (n=11 respectively, for wild type and ephrin B2 knockout animals). After an additional 14-day survival period, the animals were perfused with 4% paraformaldehyde. Longitudinal serial sections of SC were cut at 30 μm with a freezing microtome, and sections were mounted on gelatinized slides and examined using fluorescence and confocal microscopy. This technique labeled the CST in the SC, contralateral to the injection site, with no labeling on the ipsilateral side. The glial scar formed after SCI was demonstrated by the GFAP immunostaining described previously (Lee et al., 2010). To obtain scar size, scar tissue area (A) was outlined and measured using the freeform outline tool in Image J. The total scar volume was calculated by means of Calvieri’s estimator of volume equation (V=[Σ(A1+A2+…An) x D] − [Amax x Y], where D = Distance between measurements; Y = thickness of each section) (Paul et al., 2009). In addition, total volume of 2mm semi-SC including scar area along the longitudinal axis was calculated as previously described (Paul et al., 2009). The percentage of total scar volume over total cord volume for each animal within a group was determined using the three dimensional volume values.
2.5 Behavioral analysis
The Basso Mouse Scale (BMS) locomotor rating scale (Basso et al., 2006) was used to assess motor function. It is a 9-point scale, designed to assess hind limb locomotor recovery after SCI to the thoracic SC in mice. This scale categorizes combinations of ankle movements, plantar placement, stepping, coordination, paw position, trunk instability and tail position. Functional assessments began on post-operative day 1 by the staff that was not involved in this project and blind to the assignments of the groups. In our experiments, the mice were evaluated every day for the first 3 days and every 3.5 days for the remaining 8 weeks after injury. BMS scores for ephrin B2−/− (n=11) or ephrin B2+/+ (n=11) mice were compared with ANOVA.
2.6 Astrocyte and neuronal co-cultures and neurite outgrowth assay
Astrocyte culture
The culture was done as described previously (Nedergaard et al., 1991, Nedergaard, 1994, Cotrina et al., 1998a, Cotrina et al., 1998b). Briefly, newborn (1–3 day postnatal) mouse brains were dissected, and the meninges and pia mater were carefully removed. The tissue was trypsinized, mechanically triturated, and seeded in tissue culture flasks. Cultures were maintained in DMEM-F12 (Life Technologies, Gaithersburg, MD) supplemented with 10% fetal bovine serum (Atlanta Biologica), penicillin (100U/ml) and streptomycin (100ug/ml) (Life Technologies), and 5% glucose (Sigma, St. Louis, MO) in a 5% CO2 humidified incubator at 37°C. The medium was changed every 2–3 day, and the cultures were re-plated when confluent. Ephrin B2+/+ or ephrin B2−/− pups were used for the astrocyte culture. The purity of astrocyte culture is demonstrated by more than 95% of cells being positive of GFAP immunostaining.
Neuron culture
Cerebral cortical neuron cultures were obtained from ephrin B2+/+ newborn (1–3 day postnatal) mice. Meninges were carefully removed and cortices were enzymatically and mechanically dissociated as previously reported (Kaech and Banker, 2006, Brewer and Torricelli, 2007). Cells were re-suspended in NeurobasalA/B27 medium with Glutamax and growth factors. The medium contains 25ml NeurobasalA (Invitrogen,10888), 0.5 ml B27 (B27 serum-free supplement, Invitrogen 17504-044), 0.5mM Glutamax (Invitrogen 35050-061), 100IU/ML Penicillin and 100ug/ml streptomycin (Invitrogen 15140-122) and 1.25ul growth factors mouse FGF2 (0.1mg/ml) (Invitrogen, Biosource). Cells were plated onto poly-D-lysine-precoated wells; and maintained at 37°C in a humidified incubator containing 5% CO2 and atmospheric oxygen. The procedure produces highly pure (> 99%) primary neuron cultures confirmed by neurofilament immunostaining.
Neuron and astrocyte co-culture
Purified astrocyte and neuronal cultures were prepared as above. For analysis of neurite length, cortical neurons were plated at 40,000 per well in 24 well plates containing ephrin B2+/+, ephrin B2−/− and ephrin B2-siRNA treated ephrin B2+/+ astrocyte monolayer, which were poly-D-ornithine-laminin coated (Winslow et al., 1995). After 4 days, cells were fixed and immunostained for the neurofilament (1:300, AB1981. Anti-Neurofilament M) to visualize the neurites. Neurite length was measured using NIH Image J software analysis as previously described (Turnley and Bartlett, 1998, Goldshmit et al., 2004). The results represent the average length of the longest neurite of each individual neuron, taken from three separate wells (approximately 25 neurites for each group). Significance of differences in the mean neurite lengths were analyzed using multi-factorial ANOVA.
Small interfering RNA transfection
Small interfering RNA (siRNA) duplex oligonucleotides directed against ephrin B2 were synthesized and obtained from AB (s65352, Applied Biosystems, Foster City, CA). Control cultures were transfected with a negative control siRNA (Applied Biosystems, Foster City, CA). One day before transfection, plate cells were placed in 500μl of growth medium without antibiotics. On the following day, cells were transfected with a siRNA (final concentration of RNA when added to the cells was 33 nM) targeting ephrin B2, using the oligomer-Lipofectamine™ 2000 complexes in serum- and antibiotic-free medium, in accordance with the manufacturer’s instructions. The medium was changed to DMEM with 10% FBS after 4 hours. The response of the cells to treatments was measured 72 hours later. Gene silencing was confirmed by real-time quantitative PCR.
3. Results
3.1 Suppression of astrocytic ephrin B2 expression in ephrin B2 conditional knockout mice
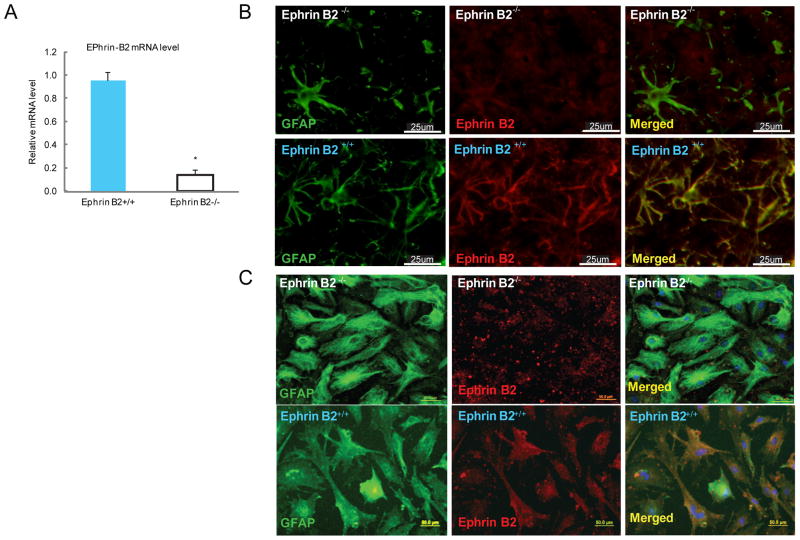
The transgenic, astrocyte specific ephrin B2 genetic knockout mouse line, was developed in this study to assess the role of ephrin B2’s function in the regenerative failure characteristic of SCI. Conditional deletion was necessary because global ephrin B2−/− mice die shortly after birth due to severe cardiac and vascular abnormality (Wang et al., 1998, Adams et al., 1999). In the established conditional ephrin B2−/− mice, the ephrin B2 was specifically knocked out in astrocytes. The histology and biochemistry studies showed no obvious developmental abnormalities in the conditional transgenic mice. The conditional ephrin B2 knockout mouse had a normal life span with respect to the ephrin B2+/+ mice. The immunochemistry performed on SC tissue sections at injury site 2 weeks after SCI (Fig. 1 B) and cultured astrocytes (Fig. 1 C) showed significantly decreased ephrin B2 immunoreactivity on astrocytes of ephrin B2−/− mice compared to ephrin B2+/+ mice. RT-PCR also confirmed the significant decrease of ephrin B2 mRNA in cultured SC astrocytes from the ephrin B2−/− mouse (Fig. 1 A). Use of BDA anterograde tracing technique in non-lesioned ephrin B2+/+ and ephrin B2−/− mice showed equivalent labeling of descending axonal pathways (Fig. 2 C).
Figure 1. Establishment of ephrin B2 conditional knockout mice.
A: Comparison of ephrin B2 mRNA level between ephrin B2+/+ and ephrin B2−/− cultured astrocytes by semi-quantification RT-PCR showed significant decrease of mRNA level in ephrin B2−/− astrocytes. * P<0.001 (unpaired t-test) compared to ephrin B2+/+ group (mean +/− SD); B: Immunolabeling against ephrin B2 (red), GFAP (green) of SC tissue sections at injury site 2 weeks after SCI from ephrin B2+/+ and ephrin B2−/− mice. C: Immunolabeling against ephrin B2 (red), GFAP (green) and DAPI (blue) of cultured astrocytes from ephrin B2+/+ and ephrin B2−/− mice;
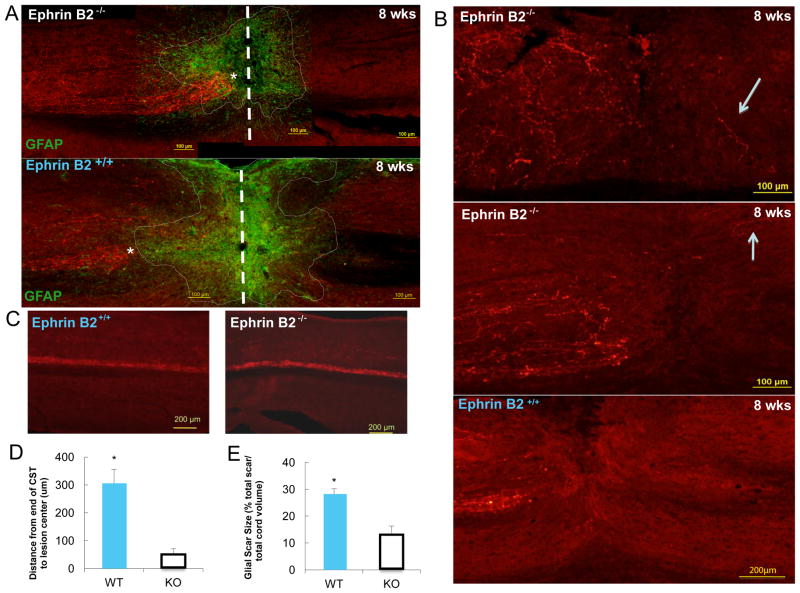
Figure 2. Increased axonal regeneration of injured corticospinal tract and decreased astrocytic gliosis following SCI in ephrin B2−/− mouse.
A: The difference of axonal regeneration of CST between ephrin B2−/− and ephrin B2+/+ mice. The center of the injury is indicated by thick dotted line. * Indicates the end of CST. Injured axons in the CST proximal to the lesion in ephrin B2−/− mice grow much closer to the center of lesion compared to ephrin B2+/+ mice at 8 weeks after SCI; The merge of BDA–labeled CST with GFAP-labeled glial scar shows decreased astrocytic gliosis (indicated by fine dotted line) around injury site in ephrin B2−/− mice compared to ephrin B2+/+ mice and CST grows into the glial scar in ephrin B2−/− mice while stop in the border of the glial scar in ephrin B2+/+ mice; B: High magnification showing some BDA positive fibers just distal to lesion site in ephrin B2−/− mice after SCI, No BDA positive fibers visualized distal to lesion site on ephrin B2+/+ mice after SCI; C: BDA anterograde tracing of CST showed equivalent labeling of descending axonal pathways in ephrin B2+/+ and ephrin B2−/− mice; D: Quantification of the distance of the most caudally reaching CST axons to the lesion center in 2 groups. There is significant difference between ephrin B2+/+ (n=6) and ephrin B2−/− (n=6) groups (P<0.01 unpaired t-test, mean +/− SD); E: Quantification of the glial scar size in 2 groups. There is significant difference between ephrin B2+/+ (n=5) and ephrin B2−/− (n=5) groups (P<0.001 unpaired t-test, mean +/− SD).
3.2 Increased regeneration of injured corticospinal axons and less astrocytic gliosis in ephrin B2−/− mice
The BDA anterograde tracing technique was employed to visualize the CST, to assess the extent of axonal regeneration after SCI. We injected BDA into the motor cortex instead of cervical SC in order to visualize the CST specifically. BDA injection on ephrin B2+/+ and ephrin B2−/− mice showed equivalent labeling of descending axonal pathways, contralateral to the injection site, and no labeling to the ipisilateral injection site (Fig. 2 C). The BDA injection did not provide any evidence for abnormal midline crossing of CST axons on the ephrin B2−/− mice, which has been reported by ephrin B3 and EphA4 knockout studies (Dottori et al., 1998). After SCI, the astroglial scar was formed and mainly surrounded the fibrovascular scar, in the center of the lesion, and diminished peripherally. The injured axons in the CST proximal to the lesion grew closer to the lesion center in the ephrin B2−/− mice compared to the ephrin B2+/+ mice (Fig. 2 A). The distance between the stump of the injured axons and the lesion center was much shorter in the ephrin B2−/− mice, when compared to ephrin B2+/+ mice (Fig. 2 D). CST as a tract dose not cross the lesion site in either group, but just distal to the lesion site scattered BDA positive fibers can be visualized in ephrin B2−/− mice, not in ephrin B2+/+ mice (Fig. 2 B). The immunostaining against GFAP showed a much smaller astroglial scar in ephrin B2−/− mice as well as greater penetration from the regenerated axons (Fig. 2 A, E).
3.3 Ephrin B2 on cultured astrocytes inhibits neurite outgrowth
The cortical neuron and astrocyte co-culture neurite outgrowth assay was used to test the ephrin B2’s effect in vitro. The differences in neurite outgrowth, from cortical neurons that were co-cultured on ephrin B2+/+ and ephrin B2−/− astrocytes, were compared. In addition, siRNA was used to knock down the ephrin B2 gene, as a functional blockade test. The knockdown of ephrin B2 mRNA with different concentrations of siRNA was confirmed by RT-PCR (Fig. 3 C). Cortical neurons seeded on astrocytes from ephrin B2−/− mice or astrocytes treated with siRNA, exhibited increased neurite outgrowth when compared to neurons co-cultured with untreated ephrin B2+/+ astrocytes (Fig. 3 A). A two to three-fold increase in axonal outgrowth was noticed on ephrin B2−/− astrocytes over the ephrin B2+/+ astrocytes (Fig. 3 B). The neurite growth in the astrocytes treated by siRNA also proved to be longer than neurite growth from the ephrin B2+/+ astrocytes (Fig. 3 A and B).
Figure 3. Ephrin B2 expression on astrocytes was inhibitory to cortical neuronal neurite outgrowth.
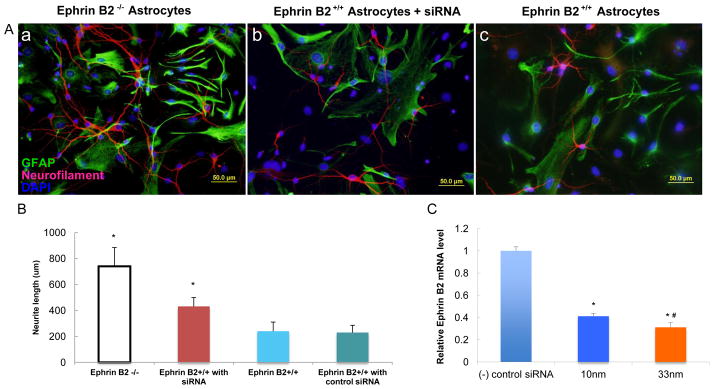
A: Immunolabeling against neurofilament (red), GFAP (green) and DAPI (blue) of the cortical neuron and astrocyte co-culture of three groups: a: ephrin B2+/+ cortical neuron + ephrin B2−/− astrocytes; b: ephrin B2+/+ cortical neuron + ephrin B2+/+ astrocytes treated with blocking siRNA; c: ephrin B2+/+ cortical neuron + ephrin B2+/+ astrocytes; B: Comparison of average longest neurite length of cultured cortical neurons among the above three groups and control group. * P < 0.05 ANOVA compared to the group c, data reported as mean +/− SD; C: Ephrin B2 gene silencing with siRNA: The mRNA level of ephrin B2 was knocked down by different concentration of siRNA. n=5 in all three groups; * P<0.01 to the negative control siRNA treated group, # P<0.05 to 10nm group, ANOVA, data reported as mean +/− SD.
3.4 Improved functional recovery of ephrin B2−/− mice
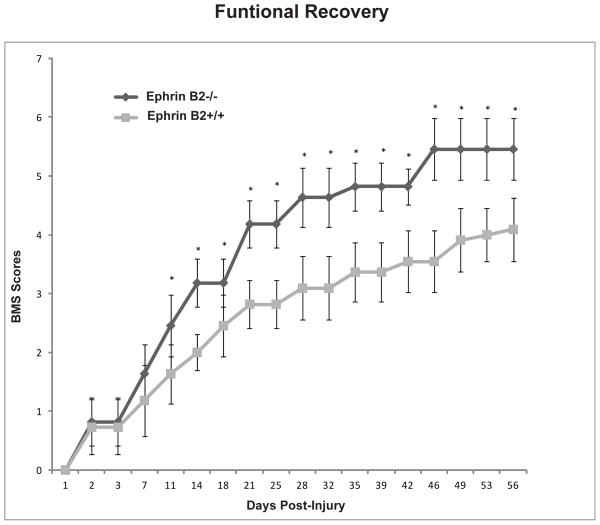
Although the CST as a tract did not cross the injury site and did not reach the distal target, the ephrin B2−/− mice demonstrated a faster recovery than ephrin B2+/+ mice. The functional recovery after SCI, scaled by BMS score, was compared between ephrin B2+/+ and ephrin B2−/− groups. The BMS score showed that the functional recovery in the ephrin B2−/− group was faster and more complete than in the ephrin B2+/+ group (Fig. 4). During the first 3 days, the mice showed complete paralysis of the lower hindlimbs on the side of hemisection, corresponding to a BMS score of 0–1, which then proceeded with different extents of recovery thereafter. Starting from approximately 11 days after injury, the extent of functional recovery began to show the difference between the two groups. This difference remained statistically significant up to 8 weeks after SCI (Fig. 4). At 11 days after SCI, the ephrin B2−/− mice exhibited occasional plantar stepping of the paralyzed hindlimbs, albeit rarely with weight support, while the ephrin B2+/+ mice only displayed ankle movement and occasional plantar placing of the paw without weight support. By 4 weeks after injury, most of the ephrin B2−/− mice exhibited frequent or consistent plantar stepping, some coordination, paws rotated at initial contact. In contrast, the ephrin B2+/+ mice could perform plantar placing of the paw with or without weight support, or occasional, frequent or consistent dorsal stepping but no plantar stepping, By 8 weeks after injury, most of the ephrin B2−/− mice exhibited frequent or consistent plantar stepping, some coordination, paws parallel at initial contact; while the ephrin B2+/+ mice could perform occasional plantar stepping.
Figure 4. Ephrin B2−/− mice have a better functional recovery after spinal cord injury.
Ephrin B2−/− mice exhibit greater functional recovery after lateral thoracic SC hemisection injury. BMS scores for functional recovery evaluation in ephrin B2+/+ and ephrin B2−/− mice. Ephrin B2−/− mice recover to a greater extent from 11 days after injury (n=11), *P < 0.001 ANOVA, data reported as mean +/− SD.
4. Discussion
In the present study, we examined the role of ephrin B2, the membrane bound ligand of several Eph receptor protein tyrosine kinases, including Eph-B2 and Eph-A4, in astrocytic gliosis, glial scar formation, and axonal growth inhibition following SCI. Multiple previous studies have shown that following SCI, ephrin B2 is continually up-regulated in reactive astrocytes and exhibits heightened colocalization with GFAP expression (Bundesen et al., 2003, Fabes et al., 2006, Giger et al., 2010). This temporal and spatial expression pattern suggests that ephrin B2 on astrocytes may play a role in the post-SCI astrogliosis that is believed to create an inhibitory microenvironment for axonal regrowth (Bundesen et al., 2003). To test whether ephrin B2 suppresses axonal regeneration following SCI, we constructed a mouse model of conditional deletion of ephrin B2, specifically in astrocytes.
The astrocytic conditional ephrin B2 knock out mouse line was established by crossing GFAP-Cre mice and ephrin B2-Lox mice. While it is true that the GFAP promoter is capable of driving Cre expression in both neurons and glial cells, it is also acknowledged that the relative expression of GFAP is far larger in astrocytes than in neurons (Su et al., 2004). Moreover, robust upregulation of astrocytic GFAP has been shown numerous times by our lab (Wang et al., 2004, Peng et al., 2009, Chen et al., 2012, Huang et al., 2012), and by others (reviewed by Eng et al., 2000) to be a hallmark of post-SCI astrogliosis. Expression of GFAP in neurons has been reported as only occasional and specific to certain cell types, such as the granule cells of the cerebellum, olfactory bulb, dentate gyrus, and hippocampal pyramidal cells, but importantly for studies of SCI, GFAP expression was show to be distinctly absent in motor cortex neurons, where the CST axons originate (Zhuo et al., 2001). Consequently, Cre-recombinase inserted under the GFAP promoter is thought to be activated to a far larger extent in SC astrocytes than in neurons. Ephrin B2 immunohistochemical analysis of SC tissue after SCI (Fig. 1 B), gene expression studies (Fig. 1 A), and in vitro cell cultures (Fig. 1 C) confirmed that ephrin B2 was significantly decreased in SC astrocytes. To our knowledge, ephrin B2 is mainly expressed in astrocytes and not neurons (Bundesen et al., 2003, Ashton et al., 2012), so the conditional knockout of the Epherin-B2 gene was unlikely to directly affect the neurons of the CST, which indeed appeared similar in organization and morphology to CST neurons from the ephrin B2+/+ mice (Fig. 2 C).
In our study, the suppression of ephrin B2 expression on SC astrocytes significantly decreased the post-SCI astrogliosis at the injury site. This finding supports the hypothesis that the function of ephrin B2 contributes to the formation of the glial scar following SCI (Bundesen et al., 2003). A possible mechanism could include a disruption of the interaction of astrocytic ephrin B2 with EphB2 expressed by meningeal fibroblasts. In fact, previous studies showed that EphB2 on meningeal fibroblasts interact with ephrin B2 on astrocytes, causing significant gliosis (Bundesen et al., 2003) and resulting in a segregation of ephrin B2-expressing astrocytes from EphB2-positive meningeal fibroblasts. Another possible mechanism is through interaction between ephrin B2 and EphA4 in astrocytes that are next to each other at the lesion epicenter. Many studies (Willson et al., 2002, Goldshmit et al., 2004, Herrmann et al., 2010) showed that EphA4 receptor is upregulated in reactive astrocytes after SCI. This mechanism was supported by a previous study that showed EphA4 knockout mice also exhibit decreased astrogliosis at injury site (Goldshmit et al., 2004). However, because a similar but subsequent study did not reproduce those findings, this mechanism needs further confirmation. Even so, many studies have shown that a decrease of glial scarring can improve functional recovery and axonal repair (Goldshmit et al., 2004, Curinga et al., 2007, Fabes et al., 2007). Thus, the increased axonal regeneration after SCI on ephrin B2−/− mice may have been due in part to a decrease in the amount of astrogliosis surrounding the injury site. On the other hand, several groups including Sofroniew and colleagues, among others, have shown that reactive astrocytes can restrict the spreading of the cyst at the lesion epicenter, isolating the injured cells from the healthy ones, and preventing further uncontrolled tissue damage (Faulkner et al., 2004, Myer et al., 2006). In addition, the glial scar may reduce the infiltration of inflammatory cells (Bush et al., 1999). There are two distinct areas of the scar after SCI: a largely GFAP-negative fibrotic scar core/cyst surrounded by a largely GFAP-positive lesion border (astroglial scar). Although this study raises the question of whether the observed increase in axonal regeneration after SCI on ephrin B2−/− mice may be due to decreased fibrotic scar, rather than reduced astrogliosis, this remains to be tested.
Although CST fibers from the ephrin B2−/− mice did not regrow to fully span the lesion, enhanced penetration into the lesion epicenter was observed when compared to the wild type animals exposed to the same injury (Fig. 2 A). Additionally it was possible to visualize scattered BDA positive fibers just distal to lesion site on ephrin B2−/− mice, but not on the wild type littermates. This difference may account for the increased functional recovery seen on ephrin B2−/− mice. This is supported by the findings from studies on receptor protein tyrosine phosphatase sigma (RPTPσ) or ephrin B3 deficient mice with SCI (Fry et al., 2010, Duffy et al., 2012), which have reported improved locomotor performance with regenerated CST fibers but no re-establishment of whole tract. In some of these studies the greater hindlimb function even was noticed in the absence of any CST axon fibers regrowing across the lesion site, as in the case of severe total transection SCI performed on ephrin B3 knockout mice (Duffy et al., 2012). A possible explanation of these findings is that multiple pathways relevant to functional recovery may be sensitive to inhibition by myelin and the astroglial scar. These other functional pathways, such as the rubrospinal and raphespinal tracts, may also benefit from a favorable growing environment in the ephrin B2−/− mice, which may also contribute to the improved functional performance. Studies showed recovery of hindlimb locomotion after a complete SCI in cats, rats, and mice rely on a spinal circuitry, which is below the lesion site and is capable of generating the basic locomotor pattern independently of descending commands. This local circuitry is a specialized network of interneurons termed the central pattern generator (CPG) (reviewed in Goulding, 2009, Rossignol and Frigon, 2011). The CPG is fully functioning when it receives inputs from both descending pathways and sensory afferents (Rossignol, 2006). However, following SCI, the new fibers through regeneration and sprouting (directly or indirectly) reach a CPG that has been modified to function more autonomously (Rossignol and Frigon, 2011). Observed increased regeneration of descending tracts in this study, including the CST on ephrin B2−/− mice, may have increased the likelihood for these descending pathways to directly or indirectly (through sprouting and synapse plasticity) reach the CPG area below the injury site, which in turn might explain the observed functional recovery.
The decreased glial scarring observed in our study likely contributed to the functional recovery and increased axonal regeneration in the ephrin B2−/− mice, since glial scarring has been shown numerous times to be inhibitory to axonal re-growth (Yiu and He, 2006), and additional studies have suggested that the amount of glial scarring correlates with the degree of tissue loss during the secondary injury process, which is thought to impact functional recovery following acute SCI (Bethea et al., 1999, Teng et al., 2002, Ito et al., 2009). However, it remains unclear how ephrin B2 exerted its effect on axonal growth. Following SCI, EphA4 receptor expression has been shown to localize on the axonal stump, at the injury site, and SC astrocytes have likewise been shown to concomitantly up-regulate ephrin B2, in the glial scar surrounding the injury site (Fabes et al., 2006). Our finding that compared to wild type controls, BDA positive CST fibers from ephrin B2−/− mice grew further into the lesion site is consistent with the hypothesis that the interactions between EphA4 on the axonal stump and ephrin B2 on astrocytes may repel axonal re-growth away from the glial scar (Goldshmit et al., 2006). This hypothesis was additionally supported by the longer processes on cultured cortical neurons when co-cultured with ephrin B2−/− mouse astrocytes or astrocytes with the ephrin B2 gene silenced by siRNA (Fig. 3 A,B). This mechanism is further supported by the findings from SCI studies on EphA4 deficient mice (Goldshmit et al., 2004) and EphA4 functional blockades following SCI (Fabes et al., 2007), which found that inhibition of the EphA4 receptor promoted axonal re-growth and functional recovery.
Activated astrocytes at the injury site proliferate to form a glial scar (Barrett et al., 1981, Bush et al., 1999) and enhance the deposition of inhibitory CSPG (McKeon et al., 1999, Bradbury et al., 2002, Jones et al., 2003). Mice deficient in CSPG receptors, such as RPTPσ, showed CST regeneration after SCI (Fry et al., 2010), similar to our study. Transgenic mice deficient in two newly recognized CSPG receptors, NgR1 and NgR3 also exhibited similar regenerative outcomes following SCI (Dickendesher et al., 2012). Due to the close correlation of CSPG deposition and astrogliosis, it is conceivable that when glial scar is decreased, the inhibitory effect between CSPG/RPTPs and NgRs will also be decreased. The CSPG and its receptors likely exert their inhibitory effect together with the ephrin B2/EphA4 pathway discussed above.
5. Conclusion
The present findings demonstrate that ephrin B2 expression on SC astrocytes is critically involved in the establishment of the inhibitory microenvironment for axonal re-growth. Following SCI, ephrin B2 likely exerts its inhibitory effect by facilitating astrogliosis, which is possibly mediated by interactions with EphB2 on meningeal fibroblasts and interaction of ephrin B2 and EphA4 on adjacent astrocytes (or next to each other on the same astrocyte) at the lesion site. Ephrin B2 on astrocytes may also trigger axonal collapse through its interaction with EphA4 on the axonal stump. The CSPG produced by astrocytes may also be involved in the inhibitory effect of ephrin B2 through facilitating astrogliosis (Fig. 5). To clarify and confirm the above mechanisms, further studies are needed, such as a quantification of GFAP expression (as a quantitative indicator of astrogliosis) at the lesion site from ephrin B2 −/− vs. ephrin B2 +/+ mice in a time-dependent manner, which will provide more evidence whether ehrin B2 promotes astrogliosis; the immunostaining for CSPG in lesioned ephrin B2 −/− mice or ephrin B2+/+ mice treated with siRNA vs. wild type mice, which will testify the model of ephrin B2 needed for astrogliosis with a consequence of repulsive environment due to CSPG production; and a separate quantification of the two areas of scar at injury site (i.e. the central fibrotic scar/cyst and the surrounding glial scar), which may clarify whether the beneficial effect from deletion of ephrin B2 is from decreased astrogliosis or rather from a decrease in the fibrotic scar.
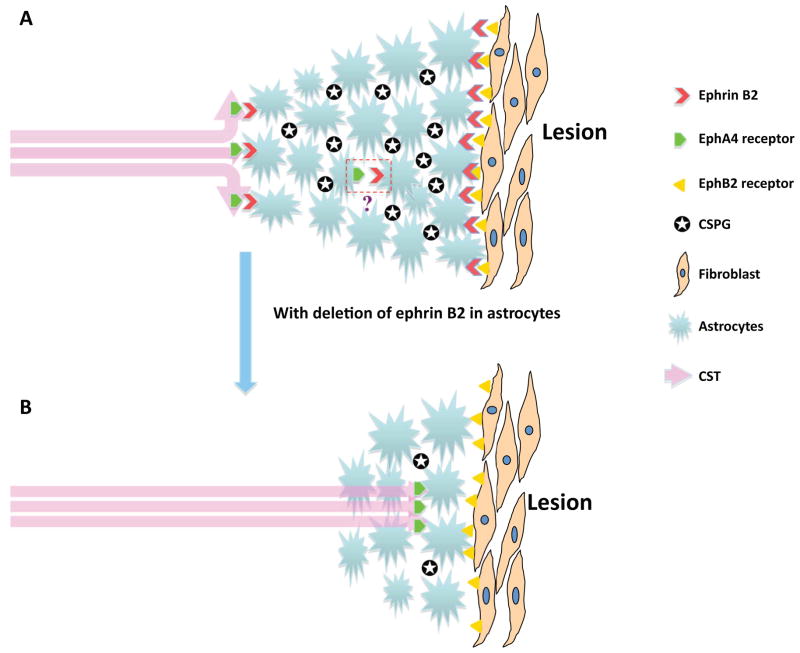
Figure 5. Schematic model for the possible mechanisms of ephrin B2 in the spinal cord injury repair.
A: In wild type mice ephrin B2 on astrocytes interacts with EphB2 on fibroblasts, inducing astrogliosis following SCI. The proliferation of astrocytes causes deposition of CSPG within the glial scar. CSPG interactions with their receptors produce an inhibitory effect to axonal re-growth. Interactions between EphA4 on axonal stump and ephrin B2 on astrocytes may repel axonal re-growth away from the glial scar due to the repulsive nature of ephrin/Eph signaling. The interaction of ephrin B2 and EphA4 on adjacent astrocytes (or next to each other on the same astrocyte) at the lesion site may also play a role in affecting growth inhibition.
B: Deletion of ephrin B2 induces decreased astrogliosis and less CSPG deposition and mitigates the inhibitory effect exerted by astrogliosis and CSPG. It also weakens the axonal collapse induced by ephrin B2 /EphA4 repulsive signaling. This model is suggestive based on our data and others; further studies are needed to firmly establish the pathway.
Highlights.
Astrocyte specific deletion of ephrin B2 resulted in decreased gliosis over controls
Ephrin B2 on astrocytes was inhibitory to neurite outgrowth in an in-vitro assay
Ephrin B2 knockout mice exhibited improved regeneration of injured corticospinal tract axons
Ephrin B2 deletion resulted in improved functional recovery over wild type controls
Acknowledgments
We thank Gabriele Mosconi (California Institute of Technology) for kindly providing the ephrin B2-loxP mice; and the Neurosurgical Research and Education Foundation (NREF) from the American Association of Neurological Surgeons (AANS) for providing funding (Z.R.).
Abbreviations
- ANOVA
analysis of variance
- BDA
biotinylated dextran amine
- BMS
Basso mouse scale
- CNS
central nervous system
- CPG
central pattern generator
- CSPG
chondroitin sulfate proteoglycans
- CST
corticospinal tract
- GFAP
glial-fibrillary-acidic-protein
- RPTPσ
receptor protein tyrosine phosphatase sigma
- siRNA
small interfering RNA
- SC
Spinal cord
- SCI
Spinal cord injury
Footnotes
Author contributions: Z.R., M.N. and Y.Z. designed research; Z.R., X.C, J.Y., and J.T. performed research at Center for Translational Neuromedicine; Z.R., X.C., H.L., Y.Z., T.T and M.N. analyzed data and Z.R., M.N. and B. K, wrote the paper.
References
- Adams RH, Wilkinson GA, Weiss C, Diella F, Gale NW, Deutsch U, Risau W, Klein R. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 1999;13:295–306. doi: 10.1101/gad.13.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton RS, Conway A, Pangarkar C, Bergen J, Lim KI, Shah P, Bissell M, Schaffer DV. Astrocytes regulate adult hippocampal neurogenesis through ephrin-B signaling. Nature neuroscience. 2012;15:1399–1406. doi: 10.1038/nn.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandtlow CE, Schwab ME. NI-35/250/nogo-a: a neurite growth inhibitor restricting structural plasticity and regeneration of nerve fibers in the adult vertebrate CNS. Glia. 2000;29:175–181. doi: 10.1002/(sici)1098-1136(20000115)29:2<175::aid-glia11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Barrett CP, Guth L, Donati EJ, Krikorian JG. Astroglial reaction in the gray matter lumbar segments after midthoracic transection of the adult rat spinal cord. Experimental neurology. 1981;73:365–377. doi: 10.1016/0014-4886(81)90272-7. [DOI] [PubMed] [Google Scholar]
- Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma. 2006;23:635–659. doi: 10.1089/neu.2006.23.635. [DOI] [PubMed] [Google Scholar]
- Bethea JR, Nagashima H, Acosta MC, Briceno C, Gomez F, Marcillo AE, Loor K, Green J, Dietrich WD. Systemically administered interleukin-10 reduces tumor necrosis factor-alpha production and significantly improves functional recovery following traumatic spinal cord injury in rats. J Neurotrauma. 1999;16:851–863. doi: 10.1089/neu.1999.16.851. [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- Brewer GJ, Torricelli JR. Isolation and culture of adult neurons and neurospheres. Nat Protoc. 2007;2:1490–1498. doi: 10.1038/nprot.2007.207. [DOI] [PubMed] [Google Scholar]
- Bundesen LQ, Scheel TA, Bregman BS, Kromer LF. Ephrin-B2 and EphB2 regulation of astrocyte-meningeal fibroblast interactions in response to spinal cord lesions in adult rats. J Neurosci. 2003;23:7789–7800. doi: 10.1523/JNEUROSCI.23-21-07789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush TG, Puvanachandra N, Horner CH, Polito A, Ostenfeld T, Svendsen CN, Mucke L, Johnson MH, Sofroniew MV. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron. 1999;23:297–308. doi: 10.1016/s0896-6273(00)80781-3. [DOI] [PubMed] [Google Scholar]
- Chen MJ, Kress B, Han X, Moll K, Peng W, Ji RR, Nedergaard M. Astrocytic CX43 hemichannels and gap junctions play a crucial role in development of chronic neuropathic pain following spinal cord injury. Glia. 2012;60:1660–1670. doi: 10.1002/glia.22384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotrina ML, Kang J, Lin JH, Bueno E, Hansen TW, He L, Liu Y, Nedergaard M. Astrocytic gap junctions remain open during ischemic conditions. J Neurosci. 1998a;18:2520–2537. doi: 10.1523/JNEUROSCI.18-07-02520.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotrina ML, Lin JH, Nedergaard M. Cytoskeletal assembly and ATP release regulate astrocytic calcium signaling. J Neurosci. 1998b;18:8794–8804. doi: 10.1523/JNEUROSCI.18-21-08794.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curinga G, Smith GM. Molecular/genetic manipulation of extrinsic axon guidance factors for CNS repair and regeneration. Experimental neurology. 2008;209:333–342. doi: 10.1016/j.expneurol.2007.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curinga GM, Snow DM, Mashburn C, Kohler K, Thobaben R, Caggiano AO, Smith GM. Mammalian-produced chondroitinase AC mitigates axon inhibition by chondroitin sulfate proteoglycans. J Neurochem. 2007;102:275–288. doi: 10.1111/j.1471-4159.2007.04530.x. [DOI] [PubMed] [Google Scholar]
- DeBellard ME, Tang S, Mukhopadhyay G, Shen YJ, Filbin MT. Myelin-associated glycoprotein inhibits axonal regeneration from a variety of neurons via interaction with a sialoglycoprotein. Molecular and cellular neurosciences. 1996;7:89–101. doi: 10.1006/mcne.1996.0007. [DOI] [PubMed] [Google Scholar]
- Dickendesher TL, Baldwin KT, Mironova YA, Koriyama Y, Raiker SJ, Askew KL, Wood A, Geoffroy CG, Zheng B, Liepmann CD, Katagiri Y, Benowitz LI, Geller HM, Giger RJ. NgR1 and NgR3 are receptors for chondroitin sulfate proteoglycans. Nature neuroscience. 2012;15:703–712. doi: 10.1038/nn.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dottori M, Hartley L, Galea M, Paxinos G, Polizzotto M, Kilpatrick T, Bartlett PF, Murphy M, Kontgen F, Boyd AW. EphA4 (Sek1) receptor tyrosine kinase is required for the development of the corticospinal tract. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:13248–13253. doi: 10.1073/pnas.95.22.13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy P, Wang X, Siegel CS, Tu N, Henkemeyer M, Cafferty WB, Strittmatter SM. Myelin-derived ephrinB3 restricts axonal regeneration and recovery after adult CNS injury. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:5063–5068. doi: 10.1073/pnas.1113953109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng LF, Ghirnikar RS, Lee YL. Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000) Neurochemical research. 2000;25:1439–1451. doi: 10.1023/a:1007677003387. [DOI] [PubMed] [Google Scholar]
- Fabes J, Anderson P, Brennan C, Bolsover S. Regeneration-enhancing effects of EphA4 blocking peptide following corticospinal tract injury in adult rat spinal cord. Eur J Neurosci. 2007;26:2496–2505. doi: 10.1111/j.1460-9568.2007.05859.x. [DOI] [PubMed] [Google Scholar]
- Fabes J, Anderson P, Yanez-Munoz RJ, Thrasher A, Brennan C, Bolsover S. Accumulation of the inhibitory receptor EphA4 may prevent regeneration of corticospinal tract axons following lesion. Eur J Neurosci. 2006;23:1721–1730. doi: 10.1111/j.1460-9568.2006.04704.x. [DOI] [PubMed] [Google Scholar]
- Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci. 2004;24:2143–2155. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbin MT. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nature reviews Neuroscience. 2003;4:703–713. doi: 10.1038/nrn1195. [DOI] [PubMed] [Google Scholar]
- Flanagan JG, Vanderhaeghen P. The ephrins and Eph receptors in neural development. Annual review of neuroscience. 1998;21:309–345. doi: 10.1146/annurev.neuro.21.1.309. [DOI] [PubMed] [Google Scholar]
- Fry EJ, Chagnon MJ, Lopez-Vales R, Tremblay ML, David S. Corticospinal tract regeneration after spinal cord injury in receptor protein tyrosine phosphatase sigma deficient mice. Glia. 2010;58:423–433. doi: 10.1002/glia.20934. [DOI] [PubMed] [Google Scholar]
- Gerety SS, Anderson DJ. Cardiovascular ephrinB2 function is essential for embryonic angiogenesis. Development. 2002;129:1397–1410. doi: 10.1242/dev.129.6.1397. [DOI] [PubMed] [Google Scholar]
- Giger RJ, Hollis ER, 2nd, Tuszynski MH. Guidance molecules in axon regeneration. Cold Spring Harbor perspectives in biology. 2010;2:a001867. doi: 10.1101/cshperspect.a001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldshmit Y, Galea MP, Wise G, Bartlett PF, Turnley AM. Axonal regeneration and lack of astrocytic gliosis in EphA4-deficient mice. J Neurosci. 2004;24:10064–10073. doi: 10.1523/JNEUROSCI.2981-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldshmit Y, McLenachan S, Turnley A. Roles of Eph receptors and ephrins in the normal and damaged adult CNS. Brain Res Rev. 2006;52:327–345. doi: 10.1016/j.brainresrev.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Goldshmit Y, Spanevello MD, Tajouri S, Li L, Rogers F, Pearse M, Galea M, Bartlett PF, Boyd AW, Turnley AM. EphA4 blockers promote axonal regeneration and functional recovery following spinal cord injury in mice. PLoS One. 2011;6:e24636. doi: 10.1371/journal.pone.0024636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulding M. Circuits controlling vertebrate locomotion: moving in a new direction. Nature reviews Neuroscience. 2009;10:507–518. doi: 10.1038/nrn2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GrandPre T, Nakamura F, Vartanian T, Strittmatter SM. Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature. 2000;403:439–444. doi: 10.1038/35000226. [DOI] [PubMed] [Google Scholar]
- Herrmann JE, Shah RR, Chan AF, Zheng B. EphA4 deficient mice maintain astroglial-fibrotic scar formation after spinal cord injury. Experimental neurology. 2010;223:582–598. doi: 10.1016/j.expneurol.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Han X, Li X, Lam E, Peng W, Lou N, Torres A, Yang M, Garre JM, Tian GF, Bennett MV, Nedergaard M, Takano T. Critical role of connexin 43 in secondary expansion of traumatic spinal cord injury. J Neurosci. 2012;32:3333–3338. doi: 10.1523/JNEUROSCI.1216-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Natsume A, Takeuchi H, Shimato S, Ohno M, Wakabayashi T, Yoshida J. Type I interferon inhibits astrocytic gliosis and promotes functional recovery after spinal cord injury by deactivation of the MEK/ERK pathway. J Neurotrauma. 2009;26:41–53. doi: 10.1089/neu.2008.0646. [DOI] [PubMed] [Google Scholar]
- Jones LL, Sajed D, Tuszynski MH. Axonal regeneration through regions of chondroitin sulfate proteoglycan deposition after spinal cord injury: a balance of permissiveness and inhibition. J Neurosci. 2003;23:9276–9288. doi: 10.1523/JNEUROSCI.23-28-09276.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech S, Banker G. Culturing hippocampal neurons. Nat Protoc. 2006;1:2406–2415. doi: 10.1038/nprot.2006.356. [DOI] [PubMed] [Google Scholar]
- Lee H, McKeon RJ, Bellamkonda RV. Sustained delivery of thermostabilized chABC enhances axonal sprouting and functional recovery after spinal cord injury. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:3340–3345. doi: 10.1073/pnas.0905437106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeon RJ, Jurynec MJ, Buck CR. The chondroitin sulfate proteoglycans neurocan and phosphacan are expressed by reactive astrocytes in the chronic CNS glial scar. J Neurosci. 1999;19:10778–10788. doi: 10.1523/JNEUROSCI.19-24-10778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKerracher L, David S, Jackson DL, Kottis V, Dunn RJ, Braun PE. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron. 1994;13:805–811. doi: 10.1016/0896-6273(94)90247-x. [DOI] [PubMed] [Google Scholar]
- Miranda JD, White LA, Marcillo AE, Willson CA, Jagid J, Whittemore SR. Induction of Eph B3 after spinal cord injury. Experimental neurology. 1999;156:218–222. doi: 10.1006/exnr.1998.7012. [DOI] [PubMed] [Google Scholar]
- Myer DJ, Gurkoff GG, Lee SM, Hovda DA, Sofroniew MV. Essential protective roles of reactive astrocytes in traumatic brain injury. Brain. 2006;129:2761–2772. doi: 10.1093/brain/awl165. [DOI] [PubMed] [Google Scholar]
- Nedergaard M. Direct signaling from astrocytes to neurons in cultures of mammalian brain cells. Science. 1994;263:1768–1771. doi: 10.1126/science.8134839. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Goldman SA, Desai S, Pulsinelli WA. Acid-induced death in neurons and glia. J Neurosci. 1991;11:2489–2497. doi: 10.1523/JNEUROSCI.11-08-02489.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul C, Samdani AF, Betz RR, Fischer I, Neuhuber B. Grafting of human bone marrow stromal cells into spinal cord injury: a comparison of delivery methods. Spine (Phila Pa 1976) 2009;34:328–334. doi: 10.1097/BRS.0b013e31819403ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng W, Cotrina ML, Han X, Yu H, Bekar L, Blum L, Takano T, Tian GF, Goldman SA, Nedergaard M. Systemic administration of an antagonist of the ATP-sensitive receptor P2X7 improves recovery after spinal cord injury. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:12489–12493. doi: 10.1073/pnas.0902531106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol S. Plasticity of connections underlying locomotor recovery after central and/or peripheral lesions in the adult mammals. Philos Trans R Soc Lond B Biol Sci. 2006;361:1647–1671. doi: 10.1098/rstb.2006.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol S, Frigon A. Recovery of locomotion after spinal cord injury: some facts and mechanisms. Annual review of neuroscience. 2011;34:413–440. doi: 10.1146/annurev-neuro-061010-113746. [DOI] [PubMed] [Google Scholar]
- Schafer M, Fruttiger M, Montag D, Schachner M, Martini R. Disruption of the gene for the myelin-associated glycoprotein improves axonal regrowth along myelin in C57BL/Wlds mice. Neuron. 1996;16:1107–1113. doi: 10.1016/s0896-6273(00)80137-3. [DOI] [PubMed] [Google Scholar]
- Steward O, Zheng B, Tessier-Lavigne M, Hofstadter M, Sharp K, Yee KM. Regenerative growth of corticospinal tract axons via the ventral column after spinal cord injury in mice. J Neurosci. 2008;28:6836–6847. doi: 10.1523/JNEUROSCI.5372-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su M, Hu H, Lee Y, d’Azzo A, Messing A, Brenner M. Expression specificity of GFAP transgenes. Neurochemical research. 2004;29:2075–2093. doi: 10.1007/s11064-004-6881-1. [DOI] [PubMed] [Google Scholar]
- Teng YD, Lavik EB, Qu X, Park KI, Ourednik J, Zurakowski D, Langer R, Snyder EY. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:3024–3029. doi: 10.1073/pnas.052678899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnley AM, Bartlett PF. MAG and MOG enhance neurite outgrowth of embryonic mouse spinal cord neurons. Neuroreport. 1998;9:1987–1990. doi: 10.1097/00001756-199806220-00013. [DOI] [PubMed] [Google Scholar]
- Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- Wang KC, Koprivica V, Kim JA, Sivasankaran R, Guo Y, Neve RL, He Z. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature. 2002;417:941–944. doi: 10.1038/nature00867. [DOI] [PubMed] [Google Scholar]
- Wang X, Arcuino G, Takano T, Lin J, Peng WG, Wan P, Li P, Xu Q, Liu QS, Goldman SA, Nedergaard M. P2X7 receptor inhibition improves recovery after spinal cord injury. Nat Med. 2004;10:821–827. doi: 10.1038/nm1082. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG. Multiple roles of EPH receptors and ephrins in neural development. Nature reviews Neuroscience. 2001;2:155–164. doi: 10.1038/35058515. [DOI] [PubMed] [Google Scholar]
- Willson CA, Irizarry-Ramirez M, Gaskins HE, Cruz-Orengo L, Figueroa JD, Whittemore SR, Miranda JD. Upregulation of EphA receptor expression in the injured adult rat spinal cord. Cell transplantation. 2002;11:229–239. [PubMed] [Google Scholar]
- Willson CA, Miranda JD, Foster RD, Onifer SM, Whittemore SR. Transection of the adult rat spinal cord upregulates EphB3 receptor and ligand expression. Cell transplantation. 2003;12:279–290. doi: 10.3727/000000003108746830. [DOI] [PubMed] [Google Scholar]
- Winslow JW, Moran P, Valverde J, Shih A, Yuan JQ, Wong SC, Tsai SP, Goddard A, Henzel WJ, Hefti F, et al. Cloning of AL-1, a ligand for an Eph-related tyrosine kinase receptor involved in axon bundle formation. Neuron. 1995;14:973–981. doi: 10.1016/0896-6273(95)90335-6. [DOI] [PubMed] [Google Scholar]
- Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nature reviews Neuroscience. 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo L, Theis M, Alvarez-Maya I, Brenner M, Willecke K, Messing A. hGFAP-cre transgenic mice for manipulation of glial and neuronal function in vivo. Genesis. 2001;31:85–94. doi: 10.1002/gene.10008. [DOI] [PubMed] [Google Scholar]