Abstract
Manganese (Mn) intoxication results in neurological conditions similar, but not identical, to idiopathic Parkinson’s disease. While the mechanism(s) by which Mn exposure leads to neurotoxic effects remains unclear, studies by magnetic resonance imaging demonstrate a high Mn accumulation in the hippocampal formation (HPCf) of the brain. Metal quantification using this method is not possible. Using x-ray fluorescence imaging, we measured the distribution of Mn in the HPCf for a rodent model of chronic Mn exposure and quantitatively compared it with distributions of other biologically relevant metals. We found considerable increases in average Mn concentrations in all analyzed areas and we identified the dentate gyrus (DG) and the cornus ammonis 3 (CA3) layer as areas accumulating the highest Mn content (~1.2 µg Mn/g tissue). The DG is significantly enriched with iron (Fe), while the CA3 layer has high zinc (Zn) content. Additionally, significant spatial correlations were found for Mn/Zn concentrations across the identified substructures of the HPCf and for Mn/Fe concentrations in the DG. Combined results support that at least two mechanisms may be responsible for Mn transport and/or storage in the brain, associated with either Fe or Zn. Subcellular resolution images of metal distribution in cells of the CA3 show diffuse Mn distributions consistent with Mn localization in both the cytoplasm and nucleus. Mn was not increased in localized intracellular Fe or copper accumulations. A consistent Mn/Zn correlation both at the tissue (40 µm × 40 µm) and cellular (0.3 µm × 0.3 µm) levels suggests that a Zn transport/storage mechanism in the HPCf is likely associated with Mn accumulation.
Introduction
Trace metals serve an important role in proper development and function of the brain. Under physiological conditions manganese (Mn) serves as a cofactor to various proteins including phosphoenolpyruvate carboxykinase1–2, mitochondrial superoxide dismutase3, glutamine-synthetase4–6, pyruvate carboxylase7–8, and arginase9. The disruption of trace metal homeostasis has been linked to a wide variety of neurological disorders; overexposure to Mn results in a neurological condition known as manganism, first reported by Couper10. Populations at risk include miners, smelters, and welders, professions where the airborne Mn concentrations are high11–25; patients suffering from cirrhosis of the liver , which is responsible for removal of Mn from the blood26–32; and those who use methcathinone (or ephendrone), an illegal substance produced using potassium permanganate33–35. Symptoms of manganism include tremor, bradykinesia, impaired postural reflexes, dystonia, gait problems, memory loss, apathy, and psychosis, several of these symptoms are also exemplified in Parkinson’s disease17,29,36–38. Unlike those suffering from Parkinson’s disease, patients with manganism do not respond to L-dopa therapy39–40.
Despite being described over 170 years ago, the molecular mechanism(s) resulting in manganism are not well understood. Magnetic resonance imaging (MRI) has provided novel insights; Mn2+ is paramagnetic and therefore Mn exposure results in enhanced contrast of the T1 relaxation time. Increased signal intensity in T1-weighted MRI has been observed in the globus pallidus (GP) of occupationally exposed workers25, 41–45 as well as in substantia nigra reticulate35. Additionally, MRI has been used to study the kinetics of Mn uptake in rodent models46–50. Using this method, it has been shown that Mn enters the cerebral spinal fluid via the choroid plexus in less than 10 minutes following injection, spreading to the sub-structures of the hippocampal formation (HPCf) such as the cornu ammonis 3 (CA3) in 4–24 hours47–48, 50. MRI generally demonstrates Mn accumulation in the HPCf in rodents exposed to Mn but not in the GP and/or the substantia nigra51, in contrast to other techniques such as atomic absorption spectroscopy (AAS)52, inductively coupled plasma mass spectroscopy53, and x-ray fluorescence (XRF) imaging54. This discrepancy has not been resolved, although it has been suggested that the Mn binding environment or speciation can critically affect its MRI T1 relaxation properties55–57.
XRF imaging provides a unique tool for studying metal distribution in situ under the condition of Mn intoxication. Unlike MRI, XRF imaging can simultaneously measure the concentration and distribution of multiple metals in a single scan, regardless of binding environment49, 54, 58–65 and has previously been used to study the distribution of metals within the HPCf49,61–63,66. Furthermore, XRF is regularly performed at resolutions on the order of microns at a ‘typical’ synchrotron facility beamline or down to 30 nanometers at specialized beamlines. Previously we reported quantitative XRF analysis of Mn distribution in thin tissue brain sections from a rodent model of Mn intoxication, demonstrating accumulation in the GP, thalamus, and substantia nigra compact54. While Mn accumulation in the basal ganglia is likely responsible for motor dysfunction, Mn accumulation in HPCf might be related to mood stability, memory loss, and learning disorders67–68. Using XRF imaging and cluster analysis we studied Mn, iron (Fe), copper (Cu) and zinc (Zn) content in the HPCf in a rat model of chronic Mn exposure. XRF imaging allowed us to uncover a strong correlation between Mn and Zn distributions in HPCf. We also report the first single cell XRF imaging of Mn in brain slices, showing a diffuse Mn distribution in CA3 cells.
Experimental Procedures
Animal treatment
Animal selection and treatment closely follow those described in 69. Briefly, 8 week old Sprague-Dawley rats (Harlan, Indianapolis) were randomly divided into two groups (n = 4 per group) and were housed in a temperature controlled, 12/12 hour light/dark room. Not all animals were used for a given imaging experiment. Animals were allowed free access to de-ionized water and food (Purina rodent chow 5001, 70 ppm Mn content). At the beginning of the 9th week, animals received intraperitoneal (i.p.) injections of either 6 mg Mn/kg body weight in solution (treated group) or an equal volume of saline solution (control group). Injections were performed on weekdays for a period of four weeks and rats sacrificed 24 hours after the last injection. Plasma Mn concentrations for control and treated animals following this protocol have been reported to be 3.5 ± 0.3 µg/L and 34.8 ± 2.3 µg/L respectively, measured at 24 hours after the last injection69. Following sacrifice, brains were removed from the skull, blotted to remove excess blood from the exterior of the structure, and snap frozen in liquid nitrogen. Samples were stored at −80 °C. Twelve hours prior to sectioning brains were transferred to a −20 °C freezer; this ensures optimal sample temperature and prevents cracking of the sample during sectioning. All experiments complied with animal rights regulations and were approved by the Institutional Committee on Animal Use at Purdue University.
Preparation of brain sections
Thin tissue brain sections were obtained using a Shandon Cryotome; a frozen sample was attached to a cryocassette at the cerebellum using Tissue-Teck O.C.T. Compound (metal free) and mounted to the cryotome cutting head. The cutting chamber temperature was maintained at −12 °C during the cutting process. Tissue sections (10 µm and 30 µm thick) were thawed on 4 µm thick polypropylene film secured to plastic frames; 10 µm thick sections were also collected on glass slides (for immunohistochemistry). Bregma locations of −2.64 mm and −5.20 mm were chosen as they displayed the HPCf, as well as other brain structures of interest for a different study. Locations were confirmed by cresyl violet staining of adjunct sections and visual identification of structures. Once placed on the substrate, sections were covered and returned to −80 °C for storage. To avoid modification of metal distribution chemical fixation was not applied to the brain tissue.
Synchrotron based XRF
XRF imaging was performed at the Advanced Photon Source (APS); large area scans (~mm2) of coronal sections were taken at Sector 18, the Biophysics Collaborative Access Team (BioCAT) facilities70–71 and single cell imaging (~100 µm2) was performed at Sector 2-ID-D72. Unless otherwise noted, 30 µm thick sections were used for XRF imaging; samples were removed from dry ice, mounted to the beamline, and imaged at room temperature in air (BioCAT measurements) or in helium (Sector 2-ID-D measurements). Samples generally air dry within 2 minutes of removal from dry ice. Spectra were collected on a pixel by pixel basis at a 10 keV incident energy and the MAPS program73 was used for fitting. Thin film standards (NBS-1832/33) allow for metal quantification. Table 1 lists the XRF imaging parameters used for this study and Table S1 lists the minimum detection and analyzable limits for the respective data collections. Data collected at BioCAT had the low Z element contributions to the spectrum suppressed by placing two, 8 µm aluminium filters over the detector collimator affording a larger collection solid angle while preventing detector saturation. The detector to sample distance was determined by optimizing the ratio of signal to total counts of the unfitted Mn Kα peak thereby minimizing the background contribution to the spectrum. Single cell measurements taken at Sector 2-ID-D were performed in a helium bag to record the low Z element contributions to the spectrum.
Table 1. XRF imaging parameters.
| Beamline | Beam size (µm × µm) |
Pixel size (µm × µm) |
Dwell (s) |
Energy (keV) |
Flux (photons/s) |
Figure |
|---|---|---|---|---|---|---|
| APS Sector 18 | 7 × 5 | 40 × 40 | 0.8 | 10.0 | 1.8 × 1011 | 1 & 2 |
| 7 × 7 | 0.8 | 10.0 | 3 | |||
| APS Sector 2-ID-D | 0.3 × 0.3 | 15 × 15 | 0.25 – 0.5 | 10.0 | SI 1B | |
| 3 × 3 | 0.5–1.5 | 10.0 | SI 1C | |||
| 0.3 × 0.3 | 1.5 | 10.0 | 4 × 109 | 4, SI 2 & 3 |
Cluster Analysis
The HPCf was visually identified and its extent delineated by hand taking care to avoid the central and lateral ventricles adjacent to the structure. As the substructures of the HPCf are heterogeneous in metal content49,62,66,74, k-means cluster analysis was employed to objectively identify separate regions. Clustering used both the Fe and Zn signals at equal weights; Fe alone failed to identify the CA3 whereas Zn alone did not delineate the dentate gyrus (DG). Contaminants and/or bubbles would result in a small cluster (<10 pixels) being identified; such pixels were deselected and the analysis repeated. Blood vessels were identified by their strong Fe content as compared to surrounding tissue and their circular shape; vessels were removed before analysis. Five clusters were chosen as more clusters did not delineate any additional, unique anatomical structures. As many as 8 clusters were used on any sample that failed to identify clusters consistent with other samples; in this event, clusters were then merged to form the five clusters. Statistical significance between groups was determined by one-way analysis of variance (ANOVA); analysis of covariance (ANCOVA) was used to determine the similarity of linear regression where appropriate. All statistical analysis used α = 0.05 unless otherwise noted.
Cell body identification
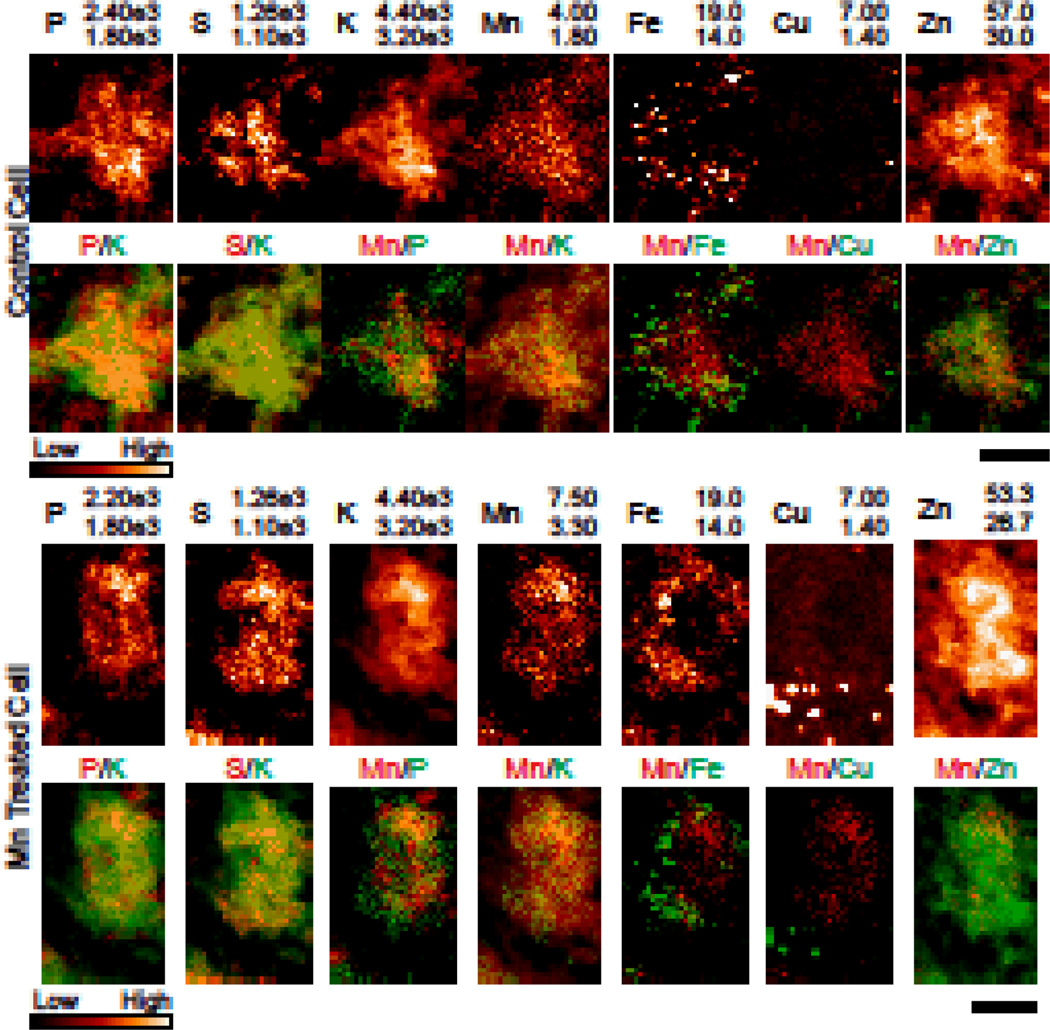
Potassium (K) co-localized with both sulphur (S) and phosphorus (P), indicating that the sample drying did not result in leaching from the cell (Fig 4). Therefore, delineation of the cell was performed using the potassium (K) signal which presented good contrast between the cell body and the surrounding space. Specifically, an exponential filter was applied for contrast enhancement, followed by convolution with a 10 pixel median filter to despeckle the region. The dynamic range of the image was then compressed to 8-bit grayscale and an unsharp mask applied for edge detection. The largest continuous region was then taken to be the cell body.
Fig. 4. XRF images of single cells of the CA3.
Single cell XRF image from control (top) and Mn treated (bottom) samples [n = 4/3 animals (control/treated); n = 11/9 cells (control/treated]. Note that the Mn scale is adjusted between the control and treated samples. Potassium helps to identify the cell body. All numbers are given in µg/g. Scale bar represents a length of 7.5µm.
Results
HPCf substructure delineation by cluster analysis
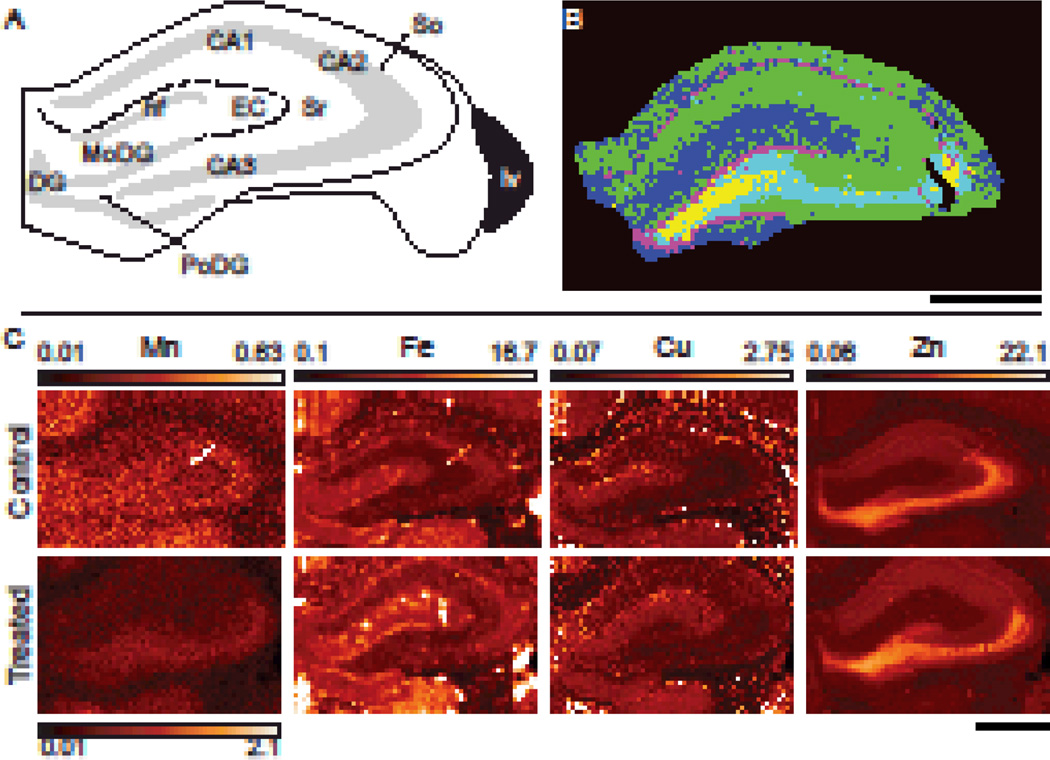
Coronal sections of the rodent brain displaying the HPCf were XRF imaged at 10 keV to obtain Mn, Fe, Cu, and Zn distributions. Comparison with thin film standards allowed for metal quantification. The HPCf is comprised of multiple substructures which demonstrate varying degrees of metal accumulation; Fig. 1A presents a diagram of the HPCf with distinct anatomical regions labeled. To delineate these regions in an objective manner, k-means cluster analysis was performed, resulting in identification of the main anatomical substructures (Fig. 1B). Substructures were grouped as follows: green (HPCf 1) stratum oriens (So) and stratum raditum (Sr); blue (HPCf 2) entorhinal cortex (EC), hippocampal fissure (hf), and molecular layer of the DG (MoDG); cyan (HPCf 3) CA3; magenta (HPCf 4) DG and CA1; yellow (HPCf 5) the polymorphic layer of the DG (PoDG). Fig. 1C shows the distribution patterns of Mn, Fe, Cu and Zn in HPCf of control and treated animals. Mn accumulation is readily seen in the CA2–3 and DG regions of treated samples but is still noticeable in the control section (different intensity scale maximum). Fe was most notably contained within the granular cell layer of the DG and the CA1 whereas Zn was primarily located in the PoDG and the CA3–4. Cu was located along the hf and the upper portion of the So. The circular areas of Fe content predominantly observed in the hf are blood vessels (Fig. 1C); these areas were excluded prior to analysis. Using the aforementioned clustering, average metal concentration of the regions was calculated and compared. This study found statistically significant Mn increase in treated animals across five identified HPCf regions as compared to control (Table 2). The only other statistically significant change in concentration was a 27% decrease in the Fe content of the HPCf 4 in the Mn treated group as compared to control.
Fig. 1. XRF imaging of the hippocampal formation.
(A) Diagram of the HPCf identifying the main anatomical structures present. CA1–3, cornu ammonis 1–3; DG, dentate gyrus; hf, hippocampal fissure; EC, entorhinal cortex; lv, lateral ventricle; MoDG, molecular layer of the DG; PoDG, polymorphic layer of the DG; SO, stratum oriens; SR, stratum radiatum. (B) A typical result of k-mean cluster analysis using the Zn and Fe signals at equal weights where the HPCf is divided into five regions; HPCf1–5 are green, blue, cyan, magenta, and yellow respectively. (C) XRF imaging of coronal sections (Bregma −2.64 mm) of control and Mn treated samples. Note that the maximum Mn concentration for control is 30% of that for the Mn treated. All numbers are given in µg/g. Scale bars represent a length of 1 mm.
Table 2. Average metal concentrations for the HPCf.
| Control (µg/g) | Treated (µg/g) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Region | Study | Method | Mn | Fe | Cu | Zn | Mn | Fe | Cu | Zn |
| HPCfa | Here | XRF | 0.39 ± 0.03 | 10.45 ± 0.99 | 1.41 ± 0.08 | 13.57 ± 0.97 | 1.00 ± 0.19** | 9.28 ± 1.17 | 1.39 ± 0.14 | 12.01 ± 1.71 |
| 75 | AAS | 0.38 ± 0.04 | 12.40 ± 3.44 | 1.91 ± 0.05 | 0.72 ± 0.10** | 19.2 ± 0.88** | 2.26 ± 0.23* | |||
| 76 | AAS | 0.287 ± 0.002 | 13.55 ± 0.26 | 2.122 ± 0.120 | 16.83 ± 0.36 | |||||
| 77 | NAAb/XRF | 0.57 ± 0.17 | 16.42 ± 3.89 | 4.56 ± 0.85 | 2.11 ± 0.44* | 14.01 ± 0.78 | 4.11 ± 0.35 | |||
| 78c | NAA | 0.21 ± 0.04 | 32.0 ± 9.0 | 4.19 ± 1.31 | 15.84 ± 2.78 | 1.10 ± 0.22** | 26 ± 7 | 7.65 ± 0.74* | 14.04 ± 1.87 | |
| 79d | NAA | 0.213 ± 0.060 | 0.360 ± 0.167* | |||||||
| HPCf 1a | Here | XRF | 0.36 ± 0.03 | 8.75± 0.82 | 1.34 ± 0.06 | 12.01± 0.94 | 0.98 ± 0.18** | 8.14 ± 1.09 | 1.34 ± 0.15 | 11.32 ± 1.59 |
| HPCf 2a | Here | XRF | 0.41 ± 0.02 | 12.43 ± 0.86 | 1.67 ± 0.05 | 10.33 ± 0.99 | 0.98 ± 0.08** | 11.84 ± 1.14 | 1.74 ± 0.11 | 9.37 ± 0.70+ |
| Here (7×7 µm2) | XRF | 0.62 ± 0.11 | 8.63 ± 0.70 | 1.11 ± 0.15 | 6.88 ± 0.71 | |||||
| EC | 49 | XRF | 2.0 ±0.9 | 30.2 ±7.5 | 3.5 ±0.7 | 17.3 ±2.9 | 3.0 ±2.7 | 23.5 ±4.0 | 3.9 ±0.4 | 17.3 ±1.7 |
| HPCf 3e | Here | XRF | 0.41 ± 0.05 | 9.97 ± 1.30 | 1.19 ± 0.16 | 20.27 ± 2.43 | 1.21 ± 0.22** | 8.51 ± 1.02 | 1.14 ± 0.09 | 18.71 ± 2.56 |
| CA3/DG | 49 | XRF | 2.5 ±1.6 | 36.4 ±13.7 | 2.9 ±0.4 | 55.0 ±8.6 | 5.0 ±2.4 | 23.8 ±5.9 | 2.4 ±0.7 | 46.9 ±4.4 |
| HPCf 4e | Here | XRF | 0.42 ± 0.05 | 15.14 ± 1.99 | 1.31 ± 0.08 | 17.07 ± 3.38 | 1.26 ± 0.13** | 11.01 ± 1.40** | 1.27 ± 0.17 | 15.63 ± 1.19 |
| Here (7×7 µm2) | XRF | 0.72 ± 0.12 | 10.31 ± 0.94 | 0.81 ± 0.17 | 10.97 ± 1.69 | |||||
| HPCf 5e | Here | XRF | 0.37 ± 0.03 | 9.75 ± 1.60 | 1.27 ± 0.25 | 27.51 ± 4.37 | 1.21 ± 0.08** | 7.68 ± 1.20+ | 1.09 ± 0.09 | 23.45 ± 1.82 |
| Here (7×7 µm2) | XRF | 0.63 ± 0.10 | 6.36 ± 0.88 | 0.95 ± 0.34 | 16.81 ± 1.99 | |||||
p < 0.1,
p < 0.5,
p < 0.01 as compared to control
n = 2/4 animals (control/treated).
NAA used to measure Mn content; XRF was used for Fe and Cu measurements.
Treated rodents exposed to 20 mg Mn/mL drinking water from birth to 120 days.
Treated rodent exposed to 1 mg Mn/mL drinking water from birth to 130 days.
n = 2/3 animals (control/treated).
Manganese correlation with other metals
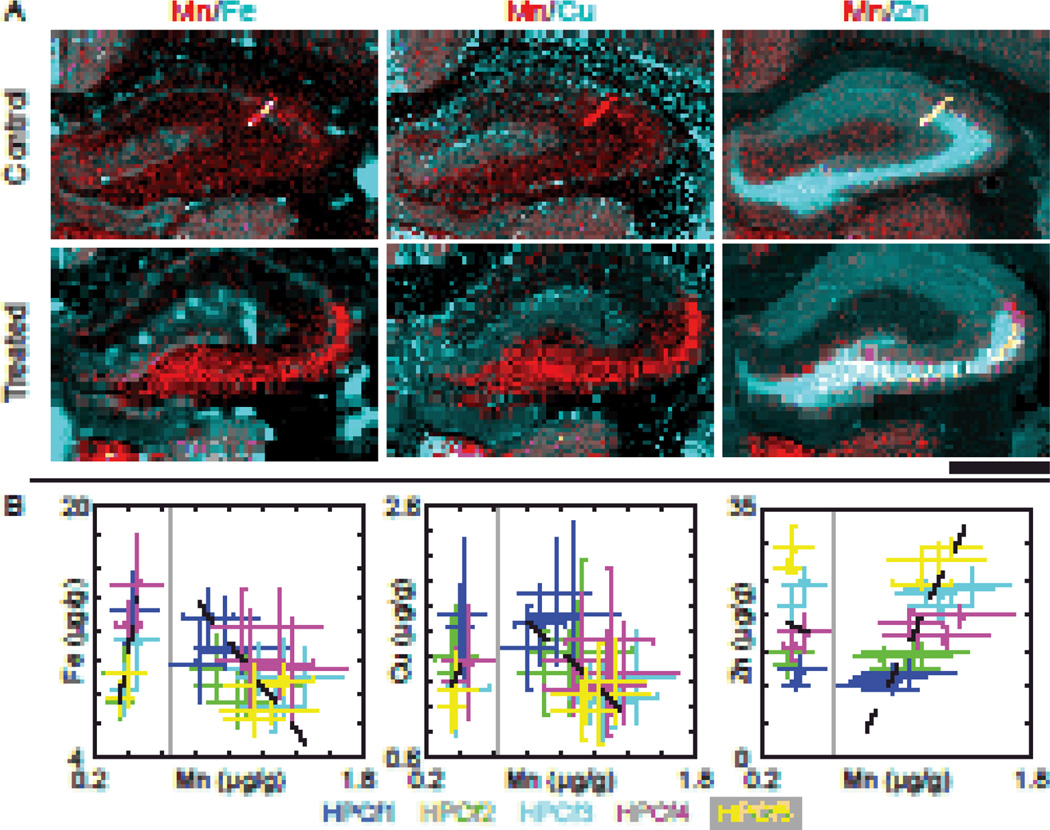
Two color maps of Mn/Fe, Mn/Cu, and Mn/Zn showed that Mn co-localizes with Zn in the CA3 and to a lesser extent with Fe in the DG (Fig. 2A). To further examine Mn co-localization with biologically significant metal ions, scatter plots of the average metal concentration (Fe, Cu, or Zn) in regions identified by cluster analysis versus Mn concentrations were created and fitted with a linear regression weighted by data point variance (Fig. 2B). For treated samples a strong, positive correlation between Zn and Mn (r = 0.68, p <0.01) and a weaker, negative correlation between Cu and Mn (r = −0.52, p = 0.01) were observed. The correlation between Fe and Mn was not statistically significant (r = −0.33, p = 0.14). For control samples, the Cu/Mn and Zn/Mn correlations were not statistically significant (r = 0.39, p = 0.27 and r = −0.06, p = 0.87 respectively), but a strong correlation was observed in Fe/Mn (r = 0.87, p < 0.01). Curiously, correlations seen in one sample group are anti-correlated in the other. Table 3 summarized the fit parameters and correlation results.
Fig. 2. Analysis of the hippocampal formation regions.
(A) Two colored images displaying Fe/Mn, Cu/Mn, and Zn/Mn respectively. Note that Mn and Zn strongly co-localize in the CA3 of the HPCf, while a small portion of Mn also co-localizes with Fe within the DG as indicated by white pixels in the images. Scale bar represents a length of 1 mm. (B) Scatter plots of the mean concentrations of regions identified by clustering. Points are color-matched to the regions specified in Fig. 1B and bars indicate the standard deviation for the given data points. Data points from control/treated samples are to the left/right of the vertical grey line respectively; n = 2/4 animals (control/treated) for HPCf 1&2, and n = 2/3 for HPCf 3–5. One treated animal was measured at both Bregma locations. Linear fit parameters and respective Pearson’s correlation coefficients are given in Table 3. Fits were weighted according to the variance of the data points. All numbers are given in µg/g.
Table 3. Linear fit parameters for HPCf regions.
Fit parameters for scatter plots displayed in Fig. 2B (mean ± std). Fits are based on n = 10/22 points (control/treated).
| Slope | Intercept | Pearson | |||
|---|---|---|---|---|---|
| R | p | ||||
| Mn/Fe | C | 80.49 ± 85.26 | −20.72 ± 32.44 | 0.87 | <0.01 |
| T | −15.62 ± 6.62 | 27.02 ± 7.44 | −0.33 | 0.14 | |
| Mn/Cu | C | 13.41 ± 22.56 | −3.86 ± 8.57 | 0.39 | 0.27 |
| T | −1.43 ± 0.68 | 2.89 ± 0.78 | −0.52 | 0.01 | |
| Mn/Zn | C | −8.94 ± 51.32 | 19.42 ±19.29 | −0.06 | 0.87 |
| T | 45.23 ± 16.16 | −34.62 ±17.99 | 0.68 | <0.01 | |
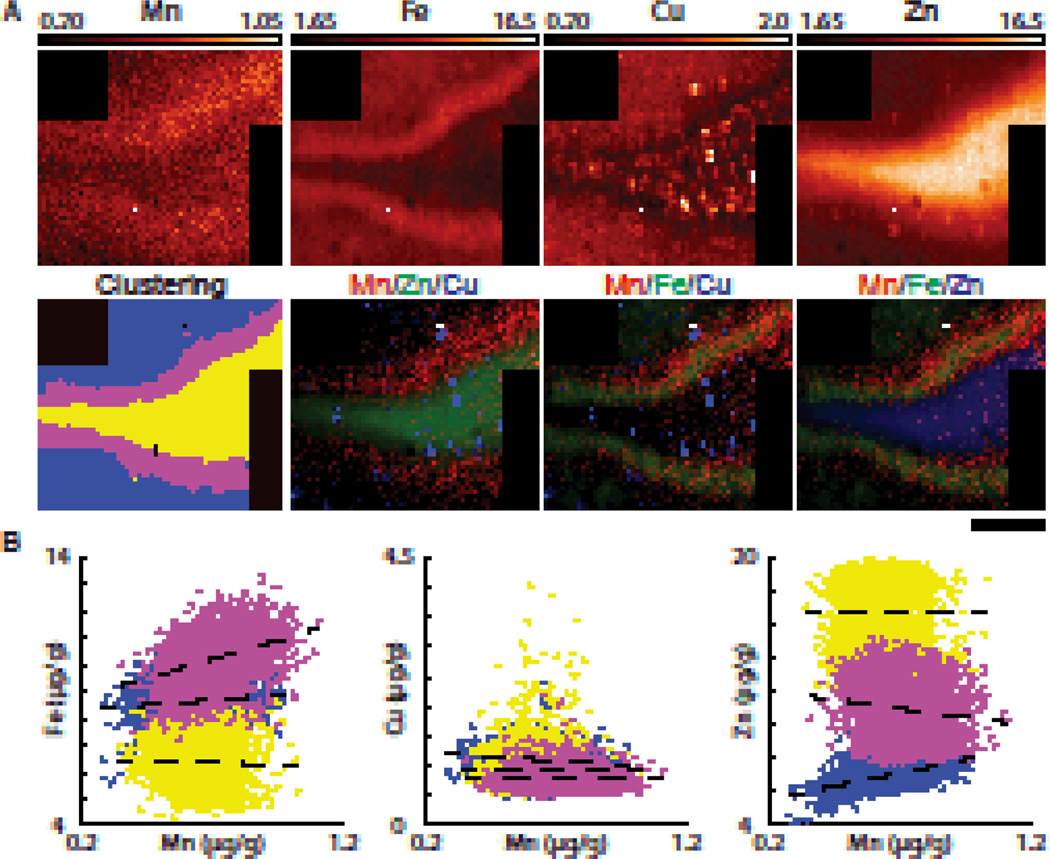
The DG, where our analysis showed co-localization of the Fe and Mn signals (Fig. 2A), was quite small and thus a scan of the crest of the DG was performed in treated rat at increased pixel resolution (7 µm × 7 µm; Fig. 3A). This scan clearly showed Mn accumulation in the blades of the DG which was 17% higher than the Mn concentration in the immediate surrounding area. Fe was also concentrated in this area, although it appeared to be contained within a narrower region. The mean metal concentrations and linear fit parameters are presented in Table 2 and Table 4, respectively. Scatter plots of pixel metal concentrations demonstrated that Mn moderately correlated with Fe (r = 0.35, p < 0.01) within the DG. Cu did not correlate with Mn in any of the regions examined. Small ‘hotspots’ of high Cu concentration were observed within the PoDG which were not visible at lower resolution (40 µm × 40 µm). These hotspots are likely the result of Cu accumulations in astrocytes80. The PoDG region was rich in Zn but did not show a significant correlation with Mn (r = −0.01, p = 0.77). The strongest correlation was observed between Mn and Zn in HPCf2 (blue; r = 0.51, p < 0.01).
Fig. 3. XRF image of the crest of the dentate gyrus.
(A) XRF image of the DG of a Mn treated sample take with 7 × 7 µm2 pixel size. Cluster analysis (k = 3) using Fe and Zn identified HPCf 2 (blue), HPCf 4 (magenta), and HPCf 5 (yellow). Tri-colored images demonstrate an accumulation of Mn in the DG which is enriched with Fe but not in the PoDG. All numbers are given in µg/g. Scale bar represents a length of 200 µm. (B) Scatter plots of pixel data from these three regions are displayed. The strongest correlations observed are Mn/Fe in HPCf4 (r = 0.35, p<0.01) and Mn/Zn in HPCf2 (r = 0.51, p<0.01). A summary of fit parameters are given in Table 4.
Table 4.
Linear fit parameters for DG crest (mean ± std)
| Slope | Int. | Pearson | ANCOVA | ||||
|---|---|---|---|---|---|---|---|
| Region | µg/gmetal:µg/gMn | µg/g | r | p | F | p | |
| Fe/Mn | HPCf2 | 0.79 ± 0.11 | 8.14 ± 0.07 | 0.13 | <0.01 | 92.24a | <0.01 |
| HPCf4 | 2.81 ± 0.17 | 8.29 ± 0.13 | 0.35 | <0.01 | |||
| HPCf5 | −0.24 ± 0.19 | 6.51 ± 0.12 | −0.03 | 0.20 | |||
| Cu/Mn | HPCf2 | −0.28 ± 0.02 | 1.29 ± 0.01 | −0.21 | <0.01 | 13.47b | <0.01 |
| HPCf4 | −0.01 ± 0.03 | 0.82 ± 0.02 | −0.01 | 0.77 | |||
| HPCf5 | −0.06 ± 0.07 | 0.98 ± 0.05 | −0.02 | 0.41 | |||
| Zn/Mn | HPCf2 | 3.22 ± 0.10 | 4.88 ± 0.06 | 0.51 | <0.01 | 112.77a | <0.01 |
| HPCf4 | −2.12 ± 0.33 | 12.48 ± 0.24 | −0.15 | <0.01 | |||
| HPCf5 | −0.12 ± 0.42 | 16.89 ± 0.27 | −0.01 | 0.77 | |||
All slopes statistically different from each other
Slope of this region is statistically different than the slopes of the other regions
Subcellular XRF imaging of Mn
Cells of the CA3 were selected for single cell imaging due to the high Mn concentration in this area and the ease by which the CA3 is identified by its strong Zn content (Fig. S1). Measured cells were likely neurons which demonstrate Zn accumulation within synaptic vesicles62,81. The majority of single cell measurements were performed on 30 µm thick tissue sections to ensure sufficient statistics for fitting; imaging thinner sections was prohibitively long for the available amount of beamtime. Pyramidal cells of the CA3 layer have been reported to be between 17.6 – 31 µm in diameter (soma area between 246 – 750 µm2)82 and therefore, assuming a pyramidal cell has been selected, less than two full cells should be present through the depth of the sample. A total of 11 control and 9 treated cells were measured (n = 4/3 control/treated; Figs. 4, S3, & S4). We do not think that sample drying resulted in significant redistribution of elements. For instance, if such re-distribution would occur, K would be the first element to re-distribute due to its high mobility. However, K co-localizes with both S and P (Fig. 4), elements that are bound to bio-molecules and therefore do not easily diffuse. Thus, K did not leach from the cell due to sample drying and therefore re-distribution of other elements is similarly insignificant. Sample thickness obscured the nucleus/cell body boundary and therefore subcellular divisions were not considered. Nevertheless, localized Fe and Cu hotspots are easily visible; should highly localized Mn accumulations occur they should similarly be detectable. Cellular trace metal concentrations (Table 5) were higher as compared to the images of the bulk structures (i.e. 40 µm × 40 µm pixel size). This is likely due to heterogeneous distribution of metal inside and outside the cells. Chronic Mn exposure resulted in the significant increase of Mn content inside cells. Differences in average elemental content (P, S, K, Cl, Ca, K, Fe, Cu, & Zn) between the control and treated cells were not statistically significant (Table 5 & Fig. S5). The large standard deviations demonstrated a high degree of cell-to-cell variability in intracellular metal contents (Fig. S5A). Potassium is distributed throughout the soma and therefore can be used to delineate the extent of the cell body. From visual inspection, Mn is distributed throughout the cell, although high Mn concentration appears in the areas of high P and K content. The Fe distribution is highly heterogeneous inside the cells with small, localized accumulations that do not co-localize with any other element. Cu hotspots, on the order of microns in size, appeared at the extremities of the cell body; these are likely occurred inside astrocytes or their processes which propagated between the measured cells80(Fig. S6).
Table 5. Average metal concentrations in HPCf CA3 cells.
All values are reported in µg/g (mean ± std); n = 11/9 cells (control/treated) from n = 4/3 animals (control/treated). Three cells from each group were taken on 10µm thick sections.
| P (× 103) | K (× 103) | Mn | Fe | Cu | Zn | |
|---|---|---|---|---|---|---|
| Control | 1.79 ± 0.28 | 3.95 ± 0.50 | 2.31 ± 0.80 | 17.35 ± 5.24 | 2.34 ± 0.39 | 56.01 ± 18.36 |
| Treated | 1.68 ± 0.19 | 3.88 ± 0.14 | 5.62 ± 0.44* | 15.17 ± 2.17 | 2.47 ± 0.63 | 50.29 ± 8.55 |
p < 0.05 as compared to control.
Due to the cell-to-cell variation in metal content, additional data processing was performed to produce pixel scatter plots. For a given cell, the average metal content was subtracted from each pixel value. Data were then binned and plotted as a 2-dimensional histogram (Fig. S5B); linear regression parameters of un-binned data are reported (Table 6). For both control and treated cells, the strongest correlations observed are between Mn/K for both control and treated cells followed by Mn/Zn. Both Mn/K and Mn/Zn have a 60% decrease in scatter plot slope in treated samples which is statistically significant according to ANCOVA.
Table 6. CA3 cell linear fit parameters.
Stronger correlations are K/M and to a lesser extend Zn/Mn (mean ± std); n = 11/9 cells (control/treated) from n = 4/3 animals (control/treated). Three cells from each group were taken on 10µm thick sections.
| Slope | Pearson | ANCOVA | ||||
|---|---|---|---|---|---|---|
| µg/gmetal:µg/gMn | R | p | F | p | ||
| Mn/P | C | 109.5 ± 2.9 | 0.32 | <0.01 | 586 | <0.01 |
| T | 34.8 ± 1.40 | 0.21 | <0.01 | |||
| Mn/K | C | 345.4 ± 4.4 | 0.57 | <0.01 | 1675 | <0.01 |
| T | 149.0 ± 2.2 | 0.50 | <0.01 | |||
| Mn/Fe | C | 1.56 ± 0.09 | 0.15 | <0.01 | 187 | <0.01 |
| T | 0.43 ± 0.02 | 0.15 | <0.01 | |||
| Mn/Cu | C | 0.07 ± 0.03 | 0.02 | 0.02 | 0.23 | 0.29 |
| T | 0.03 ± 0.06 | 0.00 | 0.66 | |||
| Mn/Zn | C | 5.05 ± 0.11 | 0.38 | <0.01 | 687 | <0.01 |
| T | 2.05 ±0.05 | 0.33 | <0.01 | |||
In comparison with cells of the subventricular zone (SVZ) processed in a similar fashion (data not shown), the P and K content were on the same order of magnitude whereas dramatic differences were seen in Fe, Cu, and Zn contents. Astrocytes of the SVZ are known to accumulate unusually high Cu on the order of 100 mM concentrations80. Thus, the average Cu concentration of CA3 cells was 20–30 times lower than that demonstrated for SVZ cells. The average Fe content was a factor of four lower in CA3 cells than SVZ cells; the reason for this difference is unclear. Zn was approximately 60% higher in the CA3 cells likely due to Zn storage in synaptic vesicles. Comparison of Mn content was not possible as SVZ cells were not imaged with sufficient dwell time for proper Mn counting statistics.
Discussion
Mn accumulation and distribution in the hippocampus has been studied previously by MRI47–50,83–84, radiography85, and XRF49. From a survey of literature, it is clear that the amount of Mn retained in HPCf depends on the Mn administration protocol and time between last Mn injection and animal sacrifice. Thus, Mn accumulation and retention in HPCf is presumably a highly dynamic process.
Qualitatively, Mn distribution in the hippocampus has been monitored by increased MRI T1 weighted signal intensity for both acute47–50 and chronic Mn exposure83. The strongest signal enhancements were noted in the CA3 and DG with the weakest being in the CA147–48. Similar enhancement of the CA3 and DG has been noted for neonatal (P4) mice 24 hours post injection84. In agreement with previous studies47–50, our data highlight the CA3 and DG as targets of Mn accumulation in condition of chronic Mn exposure; the PoDG, identified due to increased resolution, can also be included.
Quantitatively, considering the HPCf as a single unit, the average Mn content measured in this study is only 30% higher than reported in75 where quantification was performed by AAS. Both studies used the same animal treatment protocol, however, for our study the post-injection time was only 24 hours rather than 2 weeks. Using neutron activation analysis (NAA), Moldovan, Al-Ebraheem, Miksys, Farquharson and Bock77 reported a Mn content twice as large as this study for the same absolute Mn exposure (120 mg Mn total), however treatment was over a period of 4 days resulting in a higher daily dose. This shows that capacity of the HPCf in accommodating Mn was not saturated in our study. A study using a single dose of 30 mg Mn/kg49 measured Mn concentrations 2–3 times higher than this study. Fe values given in 77 and 75 are up to 2 times higher than reported here; Cu concentrations are 3.25 times higher. For Fe, this discrepancy is due mainly to the precision of tissue sampling; the specified studies dissected the hippocampal region without removal of the blood vessels whereas the current study excluded all nearby blood vessels. It is known that brain capillaries accumulate Fe, in both free and transferrin-bound forms, 108 times more than the capillary-depleted parenchyma86. Hence, the increased Fe in hippocampus observed in the previous study could be contributed by Fe accumulation in hippocampal capillary. Similarly, inadequate separation of the HPCf from the SVZ could result in the inclusion of high Cu accumulations which could skew Cu measurements to higher concentrations. Daoust, Barbier and Bohic49 noted decreased Fe content in the HPCf as a result of Mn exposure, in agreement with this study, although decreased Fe or Zn (Table 2) were generally not significant.
Whether considering the HPCf as a single structure or as the 5 identified regions, Mn concentrations in control samples were comparable to those of the cortex and the G54. Thus, at the normal condition HPCf does not contain more Mn than other major brain structures. For treated samples, Mn content in the HPCf3–5 (1.21 ± 0.22, 1.26 ± 0.13, and 1.21 ± 0.08 µg/g respectively) was approximately equivalent to concentrations measured in the substantia nigra compacta (1.21 ± 0.17 µg/g), thalamus (1.15 ± 0.12 µg/g), and the GP (1.53 ± 0.10 µg/g)54; suggesting that the HPCf3–5 are equivalently susceptible to Mn insult.
The effect of Mn on Fe homeostasis has been implicated as one possible mechanism of Mn induced neurotoxicity69,87. Transferrin receptor mediated endocytosis has been proposed to transport Mn into the cell in the form of Mn3+-transferrin complex88. It has been shown that Fe deficiency results in the up-regulation of transferrin and transferrin receptor expression89–90, and that dietary Mn exposure coupled with Fe deficiency results in an increased Mn uptake into the HPCf90–92. Our earlier studies of other brain areas also had shown positive correlation between Fe content and Mn accumulation54. Mn accumulation in the DG, the area of highest Fe content, suggests that Fe transport may play a role in Mn uptake in this structure. Furthermore, Fe content in this region was observed to decrease significantly, possibly suggesting either competition between Fe and Mn for transport or a saturation of transport into/storage within the cell. The slope of Mn/Fe scatter plot of HPCf regions changes from positive to negative between control and treated brain (Fig. 2B) also suggesting the saturation of Fe pathway for Mn transport in the HPCf. Visualization at single cell resolution presents a picture with defined Fe accumulation inside cells of the CA3 which do not show Mn co-accumulation and are not altered with Mn exposure. Mn distribution inside cell is diffuse whereas Fe is found in small concentrated regions (Fig. 4). Thus, intracellular Fe and Mn are bound/localized differently.
The moderate Mn/Cu anti-correlation supports that sites of Cu accumulation do not correspond to areas of high Mn content. Cu has been shown to accumulate in astrocytes, both in culture93–95 and in brain tissue80. It is therefore plausible that Cu hotspots may indicate, in part, astrocyte location. Astrocytes are thought to be one target of Mn accumulation as the glia-specific enzyme glutamine synthetase96–97 can bind to as many as 8 Mn atoms per octamer5, accounting for roughly 80% of Mn in the healthy brain4. Under normal physiological conditions, only 30% of glutamine synthetase is saturated with Mn, providing capacity for cellular storage. Indeed, Mn has been observed to accumulate in the hf, an area of the HPCf rich in astrocytes, as a result of an acute intracerebral injection49; i.p. injections used in the aforementioned study or our study do not demonstrate a similar accumulation. Immunohistochemical staining of glial fibrillary acidic protein, a marker of astrocytes, demonstrated the most intense expression in the hf where the lowest concentrations of Mn were observed (Fig. S6). The lack of any correlation between Mn and Cu provides further evidence that astrocytes are not the primary target of Mn accumulation in this rodent model in agreement with previous studies49,54.
The strong Mn/Zn correlation (Fig. 2, Table 3) between regions identified by cluster analysis suggests a rapport between the transport and/or accumulation of Mn and Zn in HPCf under condition of Mn intoxication (note that correlations are weaker in control samples). It appears to be unique to HPCf since analysis of other brain structures shown only moderate correlation for group of selected brain structures (see Figure 5 in 54). Two separate families of proteins serve opposing functions for Zn transport in cells; the Zrt- Irt-like protein (ZIP) family serves for cellular Zn influx while the Zn transporter family (ZnT) transports Zn within and out of the cell98–100. The ZIP8 protein is responsible for Zn2+ transport across the cell membrane but has also been established as a cadmium transporter. A competitive inhibition between cadmium and manganese has been observed in cell cultures101–103 and cadmium resistant cells have shown a similar resistance to Mn accumulation103. Lastly, ZIP8 is a symporter of bicarbonate which could explain increased Mn concentrations with increasing extracellular pH levels103–105. A ZIP8 hypomorph model106 could be of use for future investigations with regards to Mn intoxication. In HPCf the Zn transporter 3 (ZnT3) is expressed in hippocampal mossy fibers as identified by Timm’s staining81, suggesting that ZnT3 is responsible for Zn accumulation in synaptic vesicles107. A transgenic mice model with ZnT3 knockout has shown decreased Zn in the HPCf highlighting the role of ZnT362,81,108. Zn ions are released during synaptic transmission, however, their exact physiological role(s) remain unknown. A recent study109 reporting a decrease in spatial memory additionally suggests depletion of Zn in the HPCf may play a role in memory and behaviour. This is in contrast to Cole, et al.110 which supports that spatial learning is not impaired in this rodent model. As Mn distribution shows strong correlation with Zn, one can propose that Mn in HPCf is stored in synaptic vesicles and released during synaptic transmission; presumably interfering with Zn homeostasis, altering synaptic transmission and, thus, contributing to neurological disturbances.
Conflicting reports in literature discuss the preferential targets of Mn location inside the intoxicated cells mainly focusing on mitochondria, the nucleus or the Golgi apparatus58,111–112. Due to mobile nature of the Mn2+ ion in the brain tissue, significant care must be taken for sample preparation. Perfusion of the brain results in approximately a 50% decrease in Mn content111 and any chemical treatment of brain tissue depletes majority of Mn2+ from the samples54. Accordingly, unperfused, chemically untreated samples must be used for imaging of Mn distributions. To the best of our knowledge there is only one imaging study of Mn distribution in cells (PC12 cells culture) prepared by cryofixation58. Cell cultures are quite different from brain cells in situ as the brain barriers that regulate trace metal access are absent. The strongest correlation observed in our single cell study is for Mn/K (Table 6). Our results agree with Ayotte and Plaa113 which reports comparable Mn concentration in the cytoplasm and nucleus up to 3 hours post-injection. However, considering both the sample thickness used for this study and the sample variability observed Fig. S5), additional work is necessary to examine subcellular accumulation in situ using XRF imaging. Clearly no localized Mn accumulations were observed in examined cells.
Conclusion
Here we present the quantitative imaging analysis of the effects of chronic Mn exposure on metal accumulation within the HPCf as measured by XRF. Statistically significant increases in Mn concentration were observed for all substructures of the HPCf as well as a decreased Fe concentration in the DG (p < 0.01). Manganese was confirmed to preferentially accumulate in the DG, PoDG, and CA3 (~1.2 µg/g); this concentration is less than that observed for the GP (~1.5 µg/g) but roughly that of the substantia nigra and thalamus (~1.2 µg/g)54. A strong Mn/Zn positive correlation (r = 0.68, p < 0.01) was found across the five divisions of the HPCf. We suggest that this could be indicative of a global Zn associated mechanism for Mn transport and/or storage within the HPCf, with potential involvements of specifically ZIP8 or ZnT3 (transporter primarily found in mossy fibers of the HPCf). The changed Mn/Fe scatter plot slope for treated samples as compared to control is possibly due to a saturation of a Fe transport/storage mechanism; presumably this mechanism is more strongly expressed in the DG thereby explaining the statistically significant decrease in Fe concentration. Further study is necessary to solidify this inference.
Single cell imaging of CA3 neurons revealed an increased Mn concentration, however cell to cell variability prevents any other meaningful statement regarding other elements. Cellular distribution of Mn was diffuse, confirmed by a strong correlation in with K (control and treated, r > 0.50, p < 0.01) which is known to be present throughout the nucleus and cytoplasm. The areas of higher Mn accumulation do not co-localize with the small, of high Fe or Cu concentrations (r < 0.15).
Supplementary Material
Acknowledgements
We thank Dr. Barry Lai for his assistance in operating Sector 2-ID-D beamline and Dr. Stefan Vogt for his help in using the MAPS program. Use of the Advanced Photon Source, an Office of Science User Facility operated for the U.S. Department of Energy (DOE) Office of Science by Argonne National Laboratory, was supported by the U.S. DOE under Contract No. DE-AC02-06CH11357. BioCAT is a National Institutes of Health-supported Research Center RR-08630. This study was supported by NIH/National Institute of Environmental Health Sciences Grants Numbers R01 ES008146-14, and by Purdue startup funds.
Abbreviation
- ANCOVA
Analysis of covariance
- ANOVA
Analysis of variance
- APS
Advanced Photon Source
- AAS
Atomic absorption spectroscopy
- BioCAT
Biophysics Collaborative Access Team
- CA1–4
Cornus ammonis 1–4
- DG
Dentate gyrus
- EC
Entorhinal cortex
- GP
Globus pallidus
- hf
Hippocampal fissure
- HPCf
Hippocampal formation
- i.p.
Intraperitoneal
- MRI
Magnetic resonance imaging
- MoDG
Molecular layer of the dentate gyrus
- NAA
Neutron activation analysis
- PoDG
Polymorphic layer of the dentate gyrus
- So
Stratum oriens
- Sr
Stratum raditum
- SVZ
Sub-ventricular zone
- XRF
X-ray fluorescence
- ZnT
Zinc transporter family
- ZIP
Zrt- Irt-like protein
Footnotes
Electronic Supplementary Information (ESI) available: Glial fibrillary acidic protein immunohistochemistry protocol and method used for determining the minimum detection and analyzable limites. Fig. S1 HPCf cell scan locations; Fig. S2 HPCf CA3 Control cells; Fig. S3 HPCf CA3 Mn treated cells; HPCf 1-5 pixel scatter plots; Fig. S5 Pixel scatter plots of CA3 cells; Fig. S6 Glial fibrillary acidic protein immunohistochemical staining of the HPCf; Table S1 Minimum detection and analzable limits; Table S2 Linear fit parameters for HPCf pixel scatter plots. See DOI: 10.1039/b000000x/
Notes and references
- 1.Baly DL, Keen CL, Hurley LS. Pyruvate-Carboxylase and Phosphoenolpyruvate Carboxykinase Activity in Developing Rats - Effects of Manganese Deficiency. J. Nutr. 1985;115:872–879. doi: 10.1093/jn/115.7.872. [DOI] [PubMed] [Google Scholar]
- 2.Nowak T. In: Manganese in Metabolism and Enzyme Function. Schramm VL, Wedler FC, editors. Academic Press Inc.; 1986. pp. 165–191. [Google Scholar]
- 3.Akai F, Maeda M, Suzuki K, Inagaki S, Takagi H, Taniguchi N. Immunocytochemical localization of manganese superoxide-dismutase (Mn-SOD) in the hippocampus of the rat. Neurosci. Lett. 1990;115:19–23. doi: 10.1016/0304-3940(90)90510-g. [DOI] [PubMed] [Google Scholar]
- 4.Wedler FC, Denman RB. Glutamin-synthetase - the majory Mn(II) enzyme in mammalian brain. Curr. Top. Cell. Regul. 1984;24:153–169. doi: 10.1016/b978-0-12-152824-9.50021-6. [DOI] [PubMed] [Google Scholar]
- 5.Wedler FC, Denman RB, Roby WG. Glutamine-synthetase from ovine brain is a manganese(II) enzyme. Biochemistry. 1982;21:6389–6396. doi: 10.1021/bi00268a011. [DOI] [PubMed] [Google Scholar]
- 6.Pinkofsky HB, Maurizi MR, Ginsburg A. Binding Mn2+ or Mg2+ to active sites of glutamine-synthetase from bovine brain. Fed. Proc. 1985;44:1807–1807. [Google Scholar]
- 7.Mildvan AS, Scrutton MC. Pyruvate Carboxylase. X. The Demonstration of Direct Coordination of Pyruvate and α-Ketobutyrate by the Bound Manganese and the Formation of Enzyme-Metal-Substrate Bridge Complexes*. Biochemistry. 1967;6:2978–2994. doi: 10.1021/bi00862a003. [DOI] [PubMed] [Google Scholar]
- 8.Scrutton MC. In: Manganese in Metabolism and Enzyme Function. Schramm VL, Wedler FC, editors. Academic Press Inc.; 1986. pp. 147–163. [Google Scholar]
- 9.Reczkowski RS, Ash DE. EPR evidence for binuclear manganese(II) centers in rat liver arginase. J. Am. Chem. Soc. 1992;114:10992–10994. [Google Scholar]
- 10.Couper J. On the effects of black oxide of manganese which inhaled inot the lungs. Brit. Ann. Med. Pharmacol. 1837;1:41–42. [Google Scholar]
- 11.Rybicki BA, Johnson CC, Uman J, Gorell JM. Parkinsons disease mortality and the intdustrial use of heavy metals in Michigan. Mov. Disord. 1993;8:87–92. doi: 10.1002/mds.870080116. [DOI] [PubMed] [Google Scholar]
- 12.Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Kortsha GX, Brown GG, Richardson RJ. Occupational exposures to metals as risk factors for Parkinson's disease. Neurology. 1997;48:650–658. doi: 10.1212/wnl.48.3.650. [DOI] [PubMed] [Google Scholar]
- 13.Lucchini RG, Albini E, Benedetti L, Borghesi S, Coccaglio R, Malara EC, Parrinello G, Garattini S, Resola S, Alessio L. High prevalence of parkinsonian disorders associated to manganese exposure in the vicinities of ferroalloy industries. Am. J. Ind. Med. 2007;50:788–800. doi: 10.1002/ajim.20494. [DOI] [PubMed] [Google Scholar]
- 14.Bouchard M, Mergler D, Baldwin ME, Panisset M. Manganese cumulative exposure and symptoms: A follow-up study of alloy workers. Neurotoxicology. 2008;29:577–583. doi: 10.1016/j.neuro.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 15.Huang CC, Chu NS, Lu CS, Chen RS, Schulzer M, Calne DB. The natural history of neurological manganism over 18 years. Parkinsonism Relat. Disord. 2007;13:143–145. doi: 10.1016/j.parkreldis.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Huang CC, Chu NS, Lu CS, Chen RS, Calne DB. Long-term progression in chronic manganism - Ten years of follow-up. Neurology. 1998;50:698–700. doi: 10.1212/wnl.50.3.698. [DOI] [PubMed] [Google Scholar]
- 17.Cersosimo MG, Koller WC. The diagnosis of manganese-induced parkinsonism. Neurotoxicology. 2006;27:340–346. doi: 10.1016/j.neuro.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Sadek AH, Rauch R, Schulz PE. Parkinsonism due to Manganism in a Welder. Int. J. Toxicol. 2003;22:393–401. doi: 10.1177/109158180302200511. [DOI] [PubMed] [Google Scholar]
- 19.Marsh GM, Gula MJ. Employment as a welder and Parkinson disease among heavy equipment manufacturing workers. J. Occup. Environ. Med. 2006;48:1031–1046. doi: 10.1097/01.jom.0000232547.74802.d8. [DOI] [PubMed] [Google Scholar]
- 20.Cowan DM, Zheng W, Zou Y, Shi XJ, Chen J, Rosenthal FS, Fan QY. Manganese exposure among smelting workers: Relationship between blood manganese-iron ratio and early onset neurobehavioral alterations. Neurotoxicology. 2009;30:1214–1222. doi: 10.1016/j.neuro.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorell JM, Peterson EL, Rybicki BA, Johnson CC. Multiple risk factors for Parkinson's disease. J. Neurol. Sci. 2004;217:169–174. doi: 10.1016/j.jns.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 22.Firestone JA, Lundin JI, Powers KM, Smith-Weller T, Franklin GM, Swanson PD, Longstreth WT, Checkoway H. Occupational Factors and Risk of Parkinson's Disease: A Population-Based Case-Control Study. Am. J. Ind. Med. 2010;53:217–223. doi: 10.1002/ajim.20788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Racette BA, Tabbal SD, Jennings D, Good L, Perlmutter JS, Evanoff B. Prevalence of parkinsonism and relationship to exposure in a large sample of Alabama welders. Neurology. 2005;64:230–235. doi: 10.1212/01.WNL.0000149511.19487.44. [DOI] [PubMed] [Google Scholar]
- 24.Li GJJ, Zhang LL, Lu L, Wu P, Zheng W. Occupational exposure to welding fume among welders: Alterations of manganese, iron, zinc, copper, and lead in body fluids and the oxidative stress status. J. Occup. Environ. Med. 2004;46:241–248. doi: 10.1097/01.jom.0000116900.49159.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang YM, Zheng W, Long LL, Zhao WJ, Li XR, Mo XA, Lu JP, Fu X, Li WM, Liu ST, Long QY, Huang JL, Pira E. Brain magnetic resonance imaging and manganese concentrations in red blood cells of smelting workers: Search for biomarkers of manganese exposure. Neurotoxicology. 2007;28:126–135. doi: 10.1016/j.neuro.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barron TF, Devenyi AG, Mamourian AC. Symptomatic Manganese Neurotoxicity in a Patient with Chronic Liver-Disease = Correlation of Clinical Symptoms with MRI Findings. Pediatr. Neurol. 1994;10:145–148. doi: 10.1016/0887-8994(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 27.Devenyi AG, Barron TF, Mamourian AC. Dystonia, Hyperintense Basal Ganglia, and High Whole-Blood Manganese Leves in Alagilles Syndrome. Gastroenterology. 1994;106:1068–1071. doi: 10.1016/0016-5085(94)90769-2. [DOI] [PubMed] [Google Scholar]
- 28.Hauser RA, Zesiewicz TA, Rosemurgy AS, Martinez C, Olanow CW. Manganese Intoxication and Chronic Liver-Failure. Ann. Neurol. 1994;36:871–875. doi: 10.1002/ana.410360611. [DOI] [PubMed] [Google Scholar]
- 29.Klos KJ, Ahlskog JE, Josephs DA, Fealey RD, Cowl CT, Kumar N. Neurologic spectrum of chronic liver failure and basal ganglia T1 hyperintensity on magnetic resonance imaging - Probable manganese neurotoxicity. Arch. Neurol. 2005;62:1385–1390. doi: 10.1001/archneur.62.9.1385. [DOI] [PubMed] [Google Scholar]
- 30.Krieger D, Krieger S, Jansen O, Gass P, Theilmann L, Lichtnecker H. Manganese and Chronic Heptatic-Encephalopathy. Lancet. 1995;346:270–274. doi: 10.1016/s0140-6736(95)92164-8. [DOI] [PubMed] [Google Scholar]
- 31.Burkhard PR, Delavelle J, Du Pasquier R, Spahr L. Chronic Parkinsonism associated with cirrhosis - A distinct subset of acquired hepatocerebral degeneration. Arch. Neurol. 2003;60:521–528. doi: 10.1001/archneur.60.4.521. [DOI] [PubMed] [Google Scholar]
- 32.Long LL, Li XR, Huang ZK, Jiang YM, Fu SX, Zheng W. Relationship Between Changes in Brain MRI and H-1-MRS, Severity of Chronic Liver Damage, and Recovery After Liver Transplantation. Exp. Biol. Med. 2009;234:1075–1085. doi: 10.3181/0903-RM-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stepens A, Logina I, Liguts V, Aldins P, Eksteina I, Platkajis A, Martinsone I, Terauds E, Rozentale B, Donaghy M. A Parkinsonian syndrome in methcathinone users and the role of manganese. N. Engl. J. Med. 2008;358:1009–1017. doi: 10.1056/NEJMoa072488. [DOI] [PubMed] [Google Scholar]
- 34.Meral H, Kutukcu Y, Atmaca B, Ozer F, Hamamcioglu K. Parkinsonism caused by chronic usage of intravenous potassium permanganate. Neurologist. 2007;13:92–94. doi: 10.1097/01.nrl.0000253089.20746.a8. [DOI] [PubMed] [Google Scholar]
- 35.Levin OS. "Ephedron" encephalopathy. Zhurnal Nevrologii I Psikhiatrii Imeni S S Korsakova. 2005;105:12–20. [PubMed] [Google Scholar]
- 36.Crossgrove J, Zheng W. Manganese toxicity upon overexposure. NMR Biomed. 2004;17:544–553. doi: 10.1002/nbm.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aschner M, Guilarte TR, Schneider JS, Zheng W. Manganese: Recent advances in understanding its transport and neurotoxicity. Toxicol. Appl. Pharmacol. 2007;221:131–147. doi: 10.1016/j.taap.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Josephs KA, Ahlskog JE, Klos KJ, Kumar N, Fealey RD, Trenerry MR, Cowl CT. Neurologic manifestations in welders with pallidal MRI T1 hyperintensity. Neurology. 2005;64:2033–2039. doi: 10.1212/01.WNL.0000167411.93483.A1. [DOI] [PubMed] [Google Scholar]
- 39.Koller WC, Lyons KE, Truly W. Effect of levodopa treatment for parkinsonism in welders - A double-blind study. Neurology. 2004;62:730–733. doi: 10.1212/01.wnl.0000113726.34734.15. [DOI] [PubMed] [Google Scholar]
- 40.Guilarte TR. Manganese and Parkinson's Disease: A Critical Review and New Findings. Environ. Health Perspect. 2010;118:1071–1080. doi: 10.1289/ehp.0901748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dietz MC, Ihrig A, Wrazidlo W, Bader M, Jansen O, Triebig G. Results of magnetic resonance imaging in long-term manganese dioxide-exposed workers. Environ. Res. 2001;85:37–40. doi: 10.1006/enrs.2000.4068. [DOI] [PubMed] [Google Scholar]
- 42.Kim Y, Kim KS, Yang JS, Park IJ, Kim E, Jin YW, Kwon KR, Chang KH, Kim JW, Park SH, Lim HS, Cheong HK, Shin YC, Park J, Moon Y. Increase in signal intensities on T1-weighted magnetic resonance images in asymptomatic manganese-exposed workers. Neurotoxicology. 1999;20:901–907. [PubMed] [Google Scholar]
- 43.Nelson K, Golnick J, Korn T, Angle C. Manganese encephalopathy - utility of early magnetic-resonance-imaging. Br. J. Ind. Med. 1993;50:510–513. doi: 10.1136/oem.50.6.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lucchini R, Albini E, Placidi D, Gasparotti R, Pigozzi MG, Montani G, Alessio L. Brain magnetic resonance imaging and manganese exposure. Neurotoxicology. 2000;21:769–775. [PubMed] [Google Scholar]
- 45.Dydak U, Jiang YM, Long LL, Zhu H, Chen JA, Li WM, Edden RAE, Hu SG, Fu X, Long ZY, Mo XA, Meier D, Harezlak J, Aschner M, Murdoch JB, Zheng W. In Vivo Measurement of Brain GABA Concentrations by Magnetic Resonance Spectroscopy in Smelters Occupationally Exposed to Manganese. Environ. Health Perspect. 2011;119:219–224. doi: 10.1289/ehp.1002192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.London RE, Toney G, Gabel SA, Funk A. Magnetic resonance imaging studies of the brains of anesthetized rats treated with manganese chloride. Brain Res. Bull. 1989;23:229–235. doi: 10.1016/0361-9230(89)90152-4. [DOI] [PubMed] [Google Scholar]
- 47.Aoki I, Wu YJL, Silva AC, Lynch RM, Koretsky AP. In vivo detection of neuroarchitecture in the rodent brain using manganese-enhanced MRI. Neuroimage. 2004;22:1046–1059. doi: 10.1016/j.neuroimage.2004.03.031. [DOI] [PubMed] [Google Scholar]
- 48.Watanabe T, Natt O, Boretius S, Frahm J, Michaelis T. In vivo 3D MRI staining of mouse brain after subcutaneous application of MnCl2. Magn. Reson. Med. 2002;48:852–859. doi: 10.1002/mrm.10276. [DOI] [PubMed] [Google Scholar]
- 49.Daoust A, Barbier EL, Bohic S. Manganese enhanced MRI in rat hippocampus: A correlative study with synchrotron X-ray microprobe. NeuroImage. 2013;64:10–18. doi: 10.1016/j.neuroimage.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 50.Lee JH, Silva AC, Merkle H, Koretsky AP. Manganese-enhanced magnetic resonance imaging of mouse brain after systemic administration of MnCl2: Dose-dependent and temporal evolution of T-1 contrast. Magn. Reson. Med. 2005;53:640–648. doi: 10.1002/mrm.20368. [DOI] [PubMed] [Google Scholar]
- 51.Mok SI, Munasinghe JP, Young WS. Infusion-based manganese-enhanced MRI: a new imaging technique to visualize the mouse brain. Brain. Struct. Funct. 2012;217:107–114. doi: 10.1007/s00429-011-0324-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bonilla E. Flameless atomic absorption spectrophotometric determination of manganese in rat brain and other tissues. Clin. Chem. 1978;24:471–474. [PubMed] [Google Scholar]
- 53.Tarohda T, Ishida Y, Kawai K, Yamamoto M, Amano R. Regional distributions of manganese, iron, copper, and zinc in the brains of 6-hydroxydopamine-induced parkinsonian rats. Anal. Bioanal. Chem. 2005;383:224–234. doi: 10.1007/s00216-005-3423-x. [DOI] [PubMed] [Google Scholar]
- 54.Robison G, Zakharova T, Fu S, Jiang W, Fulper R, Barrea R, Marcus MA, Zheng W, Pushkar Y. X-Ray Fluorescence Imaging: A New Tool for Studying Manganese Neurotoxicity. PLoS One. 2012;7:e48899. doi: 10.1371/journal.pone.0048899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kang YS, Gore JC, Armitage IM. Studies of factors affecting the design of NMR contrast agents - Manganese in blood as a model system. Magn. Reson. Med. 1984;1:396–409. doi: 10.1002/mrm.1910010310. [DOI] [PubMed] [Google Scholar]
- 56.Drahoš B, Lukeš I, Tóth É. Manganese(II) Complexes as Potential Contrast Agents for MRI. Eur. J. Inorg. Chem. 2012;2012:1975–1986. [Google Scholar]
- 57.Koylu MZ, Asubay S, Yilmaz A. Determination of Proton Relaxivities of Mn(II), Cu(II) and CRIII) added to Solutions of Serum Proteins. Molecules. 2009;14:1537–1545. doi: 10.3390/molecules14041537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carmona A, Deves G, Roudeau S, Cloetens P, Bohic S, Ortega R. Manganese Accumulates within Golgi Apparatus in Dopaminergic Cells as Revealed by Synchrotron X-ray Fluorescence Nanoimaging. ACS Chem. Neurosci. 2010;1:194–203. doi: 10.1021/cn900021z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Popescu BFG, George MJ, Bergmann U, Garachtchenko AV, Kelly ME, McCrea RPE, Luning K, Devon RM, George GN, Hanson AD, Harder SM, Chapman LD, Pickering IJ, Nichol H. Mapping metals in Parkinson's and normal brain using rapid-scanning x-ray fluorescence. Phys. Med. Biol. 2009;54:651–663. doi: 10.1088/0031-9155/54/3/012. [DOI] [PubMed] [Google Scholar]
- 60.Yoshida S, Ektessabi A, Fujisawa S. XANES spectroscopy of a single neuron from a patient with Parkinson's disease. J. Synchrotron Radiat. 2001;8:998–1000. doi: 10.1107/s0909049500017726. [DOI] [PubMed] [Google Scholar]
- 61.Miller LM, Wang Q, Telivala TP, Smith RJ, Lanzirotti A, Miklossy J. Synchrotron-based infrared and X-ray imaging shows focalized accumulation of Cu and Zn co-localized with beta-amyloid deposits in Alzheimer's disease. J. Struct. Biol. 2006;155:30–37. doi: 10.1016/j.jsb.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 62.Linkous DH, Flinn JM, Koh JY, Lanzirotti A, Bertsch PM, Jones BF, Giblin LJ, Frederickson CJ. Evidence that the ZNT3 protein controls the total amount of elemental zinc in synaptic vesicles. J. Histochem. Cytochem. 2008;56:3–6. doi: 10.1369/jhc.6A7035.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gu H, Robison G, Hong L, Barrea R, Wei X, Farlow MR, Pushkar YN, Du Y, Zheng W. Increased β-amyloid deposition in Tg-SWDI transgenic mouse brain following in vivo lead exposure. Toxicol. Lett. 2012;213:211–219. doi: 10.1016/j.toxlet.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kosior E, Bohic S, Suhonen H, Ortega R, Deves G, Carmona A, Marchi F, Guillet JF, Cloetens P. Combined use of hard X-ray phase contrast imaging and X-ray fluorescence microscopy for sub-cellular metal quantification. J. Struct. Biol. 2012;177:239–247. doi: 10.1016/j.jsb.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 65.Carmona A, Cloetens P, Deves G, Bohic S, Ortega R. Nano-imaging of trace metals by synchrotron X-ray fluorescence into dopaminergic single cells and neurite-like processes. J. Anal. At. Spectrom. 2008;23:1083–1088. [Google Scholar]
- 66.Leskovjan AC, Kretlow A, Lanzirotti A, Barrea R, Vogt S, Miller LM. Increased brain iron coincides with early plaque formation in a mouse model of Alzheimer's disease. NeuroImage. 2011;55:32–38. doi: 10.1016/j.neuroimage.2010.11.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wasserman GA, Liu XH, Parvez F, Ahsan H, Levy D, Factor-Litvak P, Kline J, van Geen A, Slavkovich V, Lolacono NJ, Cheng ZQ, Zheng Y, Graziano JH. Water manganese exposure and children's intellectual function in Araihazar, Bangladesh. Environ. Health Perspect. 2006;114:124–129. doi: 10.1289/ehp.8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J. Neurol., Neurosurg. Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zheng W, Zhao QQ, Slavkovich V, Aschner M, Graziano JH. Alteration of iron homeostasis following chronic exposure to manganese in rats. Brain Res. 1999;833:125–132. doi: 10.1016/s0006-8993(99)01558-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barrea RA, Fischetti R, Stepanov S, Rosenbaum G, Kondrashkina E, Bunker GB, Black E, Zhang K, Gore D, Heurich R, Vukonich M, Karanfil C, Kropf AJ, Wang S, Irving TC. Biological XAFS at the BioCAT undulator beamline 18ID at the APS. Phys. Scr. 2005;T115:867–869. doi: 10.1107/S0909049504016760. [DOI] [PubMed] [Google Scholar]
- 71.Barrea RA, Gore D, Kujala N, Karanfil C, Kozyrenko S, Heurich R, Vukonich M, Huang R, Paunesku T, Woloschak G, Irving TC. Fast-scanning high-flux microprobe for biological X-ray fluorescence microscopy and microXAS. J. Synchrotron Radiat. 2010;17:522–529. doi: 10.1107/S0909049510016869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cai Z, Lai B, Yun W, McNulty I, Khounsary A, Maser J, Ilinski P, Legnini D, Trakhtenberg E, Xu S, Tieman B, Wiemerslage G, Gluskin E. In: Synchrotron Radiation Instrumentation. Pianetta P, Arthur J, Brennan S, editors. vol. 521. 2000. pp. 31–34. [Google Scholar]
- 73.Vogt S. MAPS: A set of software tools for analysis and visualization of 3D X-ray fluorescence data sets. J. Phys. IV. 2003;104:635–638. [Google Scholar]
- 74.Becker JS. Bioimaging of metals in brain tissue from micrometre to nanometre scale by laser ablation inductively coupled plasma mass spectrometry: State of the art and perspectives. Int. J. Mass Spectrom. 2010;289:65–75. [Google Scholar]
- 75.Zheng W, Jiang YM, Zhang YS, Jiang WD, Wang XQ, Cowan DM. Chelation therapy of manganese intoxication with para-aminosalicylic acid (PAS) in Sprague-Dawley rats. Neurotoxicology. 2009;30:240–248. doi: 10.1016/j.neuro.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Szerdahelyi P, Kása P. Variations in trace metal levels in rat hippocampus during ontogenetic development. Anat Embryol. 1983;167:141–149. doi: 10.1007/BF00304607. [DOI] [PubMed] [Google Scholar]
- 77.Moldovan N, Al-Ebraheem A, Miksys NA, Farquharson MJ, Bock NA. Altered transition metal homeostasis in mice following manganese injections for manganese-enhanced magnetic resonance imaging. BioMetals. 2013;26:179–187. doi: 10.1007/s10534-012-9605-z. [DOI] [PubMed] [Google Scholar]
- 78.Lai JCK, Minski MJ, Chan AWK, Leung TKC, Lim L. Manganese mineral interactions in brain. Neurotoxicology. 1999;20:433–444. [PubMed] [Google Scholar]
- 79.Lai JCK, Minski MJ, Chan AWK, Lim L, Davison AN. Brain regional manganese distribution after chronic manganese treatment. Biochem. Soc. Trans. 1981;9:228. [Google Scholar]
- 80.Pushkar Y, Robison G, Sullivan B, Fu SX, Kohne M, Jiang W, Rohr S, Lai B, Marcus MA, Zakharova T, Zheng W. Aging results in copper accumulations in glial fibrillary acidic protein-positive cells in the subventricular zone. Aging Cell. 2013 doi: 10.1111/acel.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wenzel HJ, Toby BC, Born DE, Schwartzkroin PA, Palmiter RD. Ultrastructural Localization of Zinc Transporter-3 (ZnT-3) to Synaptic Vesicle Membranes within Mossy Fiber Boutons in the Hippocampus of Mouse and Monkey. Proc. Natl. Acad. Sci. U. S. A. 1997;94:12676–12681. doi: 10.1073/pnas.94.23.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ishizuka N, Cowan WM, Amaral DG. A quantitative analysis of the dendritic organization of pyramidal cells in the rat hippocampus. J. Comp. Neurol. 1995;362:17–45. doi: 10.1002/cne.903620103. [DOI] [PubMed] [Google Scholar]
- 83.Grünecker B, Kaltwasser SF, Zappe AC, Bedenk BT, Bicker Y, Spoormaker VI, Wotjak CT, Czisch M. Regional specificity of manganese accumulation and clearance in the mouse brain: implications for manganese-enhanced MRI. NMR Biomed. 2012 doi: 10.1002/nbm.2891. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 84.Wadghiri YZ, Blind JA, Duan X, Moreno C, Yu X, Joyner AL, Turnbull DH. Manganese-enhanced magnetic resonance imaging (MEMRI) of mouse brain development. NMR Biomed. 2004;17:613–619. doi: 10.1002/nbm.932. [DOI] [PubMed] [Google Scholar]
- 85.Takeda A, Akiyama T, Sawashita J, Okada S. Brain uptake of trace metals, zinc and manganese, in rats. Brain Res. 1994;640:341–344. doi: 10.1016/0006-8993(94)91891-0. [DOI] [PubMed] [Google Scholar]
- 86.Deane R, Zheng W, Zlokovic BV. Brain capillary endothelium and choroid plexus epithelium regulate transport of transferrin-bound and free iron into the rat brain. J. Neurochem. 2004;88:813–820. doi: 10.1046/j.1471-4159.2003.02221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Garcia SJ, Gellein K, Syversen T, Aschner M. A manganese-enhanced diet alters brain metals and transporters in the developing rat. Toxicol. Sci. 2006;92:516–525. doi: 10.1093/toxsci/kfl017. [DOI] [PubMed] [Google Scholar]
- 88.Aisen P, Aasa R, Redfield AG. Chromium, manganese, and cobalt complexes of transferrin. J. Biol. Chem. 1969;244:4628-&. [PubMed] [Google Scholar]
- 89.Erikson KM, Pinero DJ, Connor JR, Beard JL. Regional brain iron, ferritin and transferrin concentrations during iron deficiency and iron repletion in developing rats. J. Nutr. 1997;127:2030–2038. doi: 10.1093/jn/127.10.2030. [DOI] [PubMed] [Google Scholar]
- 90.Chua A, Morgan E. Effects of iron deficiency and iron overload on manganese uptake and deposition in the brain and other organs of the rat. Biol. Trace Elem. Res. 1996;55:39–54. doi: 10.1007/BF02784167. [DOI] [PubMed] [Google Scholar]
- 91.Erikson KA, Shihabi ZK, Aschner JL, Aschner M. Manganese accumulates in iron-deficient rat brain regions in a heterogeneous fashion and is associated with neurochemical alterations. Biol. Trace Elem. Res. 2002;87:143–156. doi: 10.1385/BTER:87:1-3:143. [DOI] [PubMed] [Google Scholar]
- 92.Garcia SJ, Gellein K, Syversen T, Aschner M. Iron deficient and manganese supplemented diets alter metals and transporters in the developing rat brain. Toxicol. Sci. 2007;95:205–214. doi: 10.1093/toxsci/kfl139. [DOI] [PubMed] [Google Scholar]
- 93.Qian YC, Tiffanycastiglioni E, Harris ED. Copper transport and kinetics in cultured C6 rat glioma-cells. Am. J. Physiol.: Cell Physiol. 1995;269:C892–C898. doi: 10.1152/ajpcell.1995.269.4.C892. [DOI] [PubMed] [Google Scholar]
- 94.Brown DR. Role of the prion protein in copper turnover in astrocytes. Neurobiol. Dis. 2004;15:534–543. doi: 10.1016/j.nbd.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 95.Scheiber IF, Mercer JFB, Dringen R. Copper accumulation by cultured astrocytes. Neurochem. Int. 2010;56:451–460. doi: 10.1016/j.neuint.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 96.Martinez-Hernandez A, Bell KP, Norenberg MD. Glutamine synthetase - glial localization in brain. Science. 1977;195:1356–1358. doi: 10.1126/science.14400. [DOI] [PubMed] [Google Scholar]
- 97.Norenberg MD, Martinez-Hernandez A. Fine structural localization of glutamine-synthetase in astrocytes of rat brain. Brain Res. 1979;161:303–310. doi: 10.1016/0006-8993(79)90071-4. [DOI] [PubMed] [Google Scholar]
- 98.Liuzzi JP, Cousins RJ. Mammlian Zinc Transporters. Annu. Rev. Nutr. 2004;24:151–172. doi: 10.1146/annurev.nutr.24.012003.132402. [DOI] [PubMed] [Google Scholar]
- 99.Eide DJ. Zinc transporters and the cellular trafficking of zinc. Biochim. Biophys. Acta, Mol. Cell Res. 2006;1763:711–722. doi: 10.1016/j.bbamcr.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 100.Lichten LA, Cousins RJ. Annu. Rev. Nutr. 2009;vol. 29:153–176. doi: 10.1146/annurev-nutr-033009-083312. [DOI] [PubMed] [Google Scholar]
- 101.Fujishiro H, Doi M, Enomoto S, Himeno S. High sensitivity of RBL-2H3 cells to cadmium and manganese: an implication of the role of ZIP8. Metallomics. 2011;3:710–718. doi: 10.1039/c1mt00020a. [DOI] [PubMed] [Google Scholar]
- 102.Rousselet E, Richaud P, Douki T, Chantegrel JG, Favier A, Bouron A, Moulis JM. A zinc-resistant human epithelial cell line is impaired in cadmium and manganese import. Toxicol. Appl. Pharmacol. 2008;230:312–319. doi: 10.1016/j.taap.2008.02.025. [DOI] [PubMed] [Google Scholar]
- 103.Yanagiya T, Imura N, Enomoto S, Kondo Y, Himeno S. Suppression of a high-affinity transport system for manganese in cadmium-resistant metallothionein-null cells. J. Pharmacol. Exp. Ther. 2000;292:1080–1086. [PubMed] [Google Scholar]
- 104.He L, Girijashanker K, Dalton TP, Reed J, Li H, Soleimani M, Nebert DW. ZIP8, member of the solute-carrier-39 (SLC39) metal-transporter family: Characterization of transporter properties. Mol. Pharmacol. 2006;70:171–180. doi: 10.1124/mol.106.024521. [DOI] [PubMed] [Google Scholar]
- 105.Martin P, Fareh M, Poggi MC, Boulukos KE, Pognonec P. Manganese is highly effective in protecting cells from cadmium intoxication. Biochem. Biophys. Res. Commun. 2006;351:294–299. doi: 10.1016/j.bbrc.2006.10.035. [DOI] [PubMed] [Google Scholar]
- 106.Wang B, He L, Dong HB, Dalton TP, Nebert DW. Generation of a Slc39a8 hypomorph mouse: Markedly decreased ZIP8 Zn(2+)/(HCO(3)(−))(2) transporter expression. Biochem. Biophys. Res. Commun. 2011;410:289–294. doi: 10.1016/j.bbrc.2011.05.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Palmiter RD, Cole TB, Quaife CJ, Findley SD. ZnT-3, a putative transporter of zinc into synaptic vesicles. Proc. Natl. Acad. Sci. U. S. A. 1996;93:14934–14939. doi: 10.1073/pnas.93.25.14934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cole TB, Wenzel HJ, Kafer KE, Schwartzkroin PA, Palmiter RD. Elimination of zinc from synaptic vesicles in the intact mouse brain by disruption of the ZnT3 gene. Proc. Natl. Acad. Sci. U. S. A. 1999;96:1716–1721. doi: 10.1073/pnas.96.4.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Martel G, Hevi C, Kane-Goldsmith N, Shumyatsky GP. Zinc transporter ZnT3 is involved in memory dependent on the hippocampus and perirhinal cortex. Behav. Brain Res. 2011;223:233–238. doi: 10.1016/j.bbr.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cole TB, Martyanova A, Palmiter RD. Removing zinc from synaptic vesicles does not impair spatial learning, memory, or sensorimotor functions in the mouse. Brain Res. 2001;891:253–265. doi: 10.1016/s0006-8993(00)03220-0. [DOI] [PubMed] [Google Scholar]
- 111.Morello M, Canini A, Mattioli P, Sorge RP, Alimonti A, Bocca B, Forte G, Martorana A, Bemardi G, Sancesario G. Subcellular localization of manganese in the basal ganglia of normal and manganese-treated rats - An electron spectroscopy imaging and electron energy-loss spectroscopy study. Neurotoxicology. 2008;29:60–72. doi: 10.1016/j.neuro.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 112.Kalia K, Jiang W, Zheng W. Manganese accumulates primarily in nuclei of cultured brain cells. Neurotoxicology. 2008;29:466–470. doi: 10.1016/j.neuro.2008.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ayotte P, Plaa GL. Hepatic subcellular distribution of manganese in manganese and manganese-bilirubin induced cholestasis. Biochem. Pharmacol. 1985;34:3857–3865. doi: 10.1016/0006-2952(85)90435-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.