Abstract
Radioactive fallout from nuclear test detonations during 1946–1958 at Bikini and Enewetak atolls in the Marshall Islands (MI) exposed populations living elsewhere in the archipelago. A comprehensive analysis, presented in seven companion papers, has produced estimates of tissue-specific radiation absorbed dose to MI residents at all historically inhabited atolls from internal (ingested) and external radioactive components of fallout, by calendar year, and by age of the population at time of exposure. The present report deals, for the first time, with the implications of these doses on cancer risk among exposed members of the MI population. Radiation doses differed by geographic location and year of birth, and radiation-related cancer risk depends upon age at exposure and age at observation for risk. Using dose-response models based on committee reports published by the National Research Council and the National Institutes of Health, we project that, during the lifetimes of members of the MI population potentially exposed to ionizing radiation from weapons test fallout deposited during the testing period (1948–1958) and from residual radioactive sources during the subsequent 12 years (1959–1970), perhaps 1.6% (with 90% uncertainty range 0.4% and 3.4%) of all cancers might be attributable to fallout-related radiation exposures. The projected proportion of cancers attributable to radiation from fallout from all nuclear tests conducted in the Marshall Islands is 55% (28%–69%) among 82 persons exposed in 1954 on Rongelap and Ailinginae, 10% (2%–22%) for 157 persons exposed on Utrik, and 2% (0.5%–5%) and 1% (0.2%–2%), respectively, for the much larger populations exposed in mid-latitude locations including Kwajalein and in southern locations including Majuro. By cancer type, point estimates of attributable risk varied by location, between 12% and 95% for thyroid cancer, between 2% and 78% for leukemia, and between 1% and 55% for all cancers combined. The largest projected risks pertain to the Rongelap Island community and the lowest risks pertain to the populations resident on the southern-most atolls. While the projected cancer risks are smaller than those estimated by the National Cancer Institute in simplistic analyses conducted in 2004, these estimates of cancer risk are the best available as they are based on the most detailed dose reconstruction to date and comprehensively include populations at all locations and dose contributions from all nuclear tests.
Keywords: Marshall Islands, cancer risk, fallout, nuclear testing
INTRODUCTION
Previously administered by Japan under a League of Nations mandate, the Marshall Islands was occupied by the United States (U.S.) during World War II. The group of atolls and islands was administered by the U.S. as a United Nations Trust Territory until 1986 when the Republic of the Marshall Islands (RMI) was established as a sovereign nation in free association with the U.S.
After World War II, the U.S. established the Pacific Proving Grounds, essentially the atolls of Bikini and Enewetak and the nearby ocean at the northwestern end of the archipelago, for testing nuclear weapons. The populations of Bikini and Enewetak were relocated to other atolls prior to testing. Between 1946 and 1958, 66 nuclear test detonations were carried out. As discussed in four of the companion papers (Beck et al. 2009; Bouville et al. 2009; Simon et al. 2009a,b), 20 of these tests resulted in varying levels of radiation exposure from radioactive fallout to residents of the inhabited islands in the archipelago. Significant exposures to radioactive fallout began in 1948, and exposures to residual fallout radioactivity continued after the cessation of testing in 1958, until about 1970. The highest exposures by far were from the thermonuclear test (code name Castle BRAVO) on Bikini on March 1, 1954, which unexpectedly resulted in very substantial radiation exposure to 82 members of the Rongelap community who were on Rongelap Island and nearby Sifo Island (Ailinginae atoll) and substantial exposure to 157 members of the Utrik community. Lesser exposures of different degrees affected approximately 13,000 other Marshallese then living elsewhere in the archipelago, including the major population centers of Majuro and Kwajalein.
The purpose of the present paper is to update and replace the cancer risk estimates of an earlier report (DCEG 2004) entitled “Estimation of the Baseline Number of Cancers Among Marshallese and the Number of Cancers Attributable to Exposure to Fallout from Nuclear Weapons Testing Conducted in the Marshall Islands” and to provide thorough documentation of the methods used to estimate cancer risks. Presented here, for the first time, is a comprehensive assessment of cancer risks from exposure to fallout from all nuclear tests for all Marshallese alive during the years 1948 through 1970.
MATERIALS AND METHODS
Ionizing radiation exposure is a known cancer risk factor and, because it is often possible to estimate tissue-specific radiation doses with reasonable precision, the relationship between dose and subsequent cancer risk is probably better quantified than for any other common environmental carcinogen. In the companion papers, estimates of organ-specific radiation absorbed doses from fallout-related internal (ingested) and external radioactive materials are derived for contemporary residents of different atolls affected by different test explosions, by year and age. These values have been summarized to provide yearly radiation doses corresponding to all calendar years from 1948 through 1970, all historically inhabited atolls, and ages when exposures occurred.
Although small-scale medical studies have been reported describing early and late health effects among residents of Rongelap and Utrik in particular (Conard et al. 1970; Hamilton et al. 1987; Cronkite et al 1997; Takahashi et al. 1997, 2001), and records are available concerning compensation claims awarded to RMI residents who developed cancers and other health problems subsequent to fallout-related events (NCT 2004), the infrastructure of medical reporting and records in the RMI is not sufficient to support detailed epidemiological studies such as those carried out among survivors of the atomic bombings of Hiroshima and Nagasaki, Japan (Preston et al. 2003, 2007). However, much of the dose-response information provided by studies of the atomic bomb survivors, and of other populations exposed to medical, occupational and other sources of radiation, is summarized in the recent report of the U.S. National Research Council’s Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation, otherwise known as BEIR VII (NRC 2006). The goal of the present investigation is to estimate the likely consequences of the nuclear tests in terms of cancer risk to the MI population from fallout-related radiation exposures. To do that, we have used (with some modifications) the BEIR VII dose-response models, which are based upon studies of other exposed populations. Cancer risk projections are provided for post-1948 lifetime baseline‡ and radiation-related excess leukemias and cancers of the thyroid, stomach, colon, and all remaining solid cancer sites considered as a group. Lifetime risk is further divided into “past” (from 1948 through 2008) and “future” (after 2008) periods.
Population
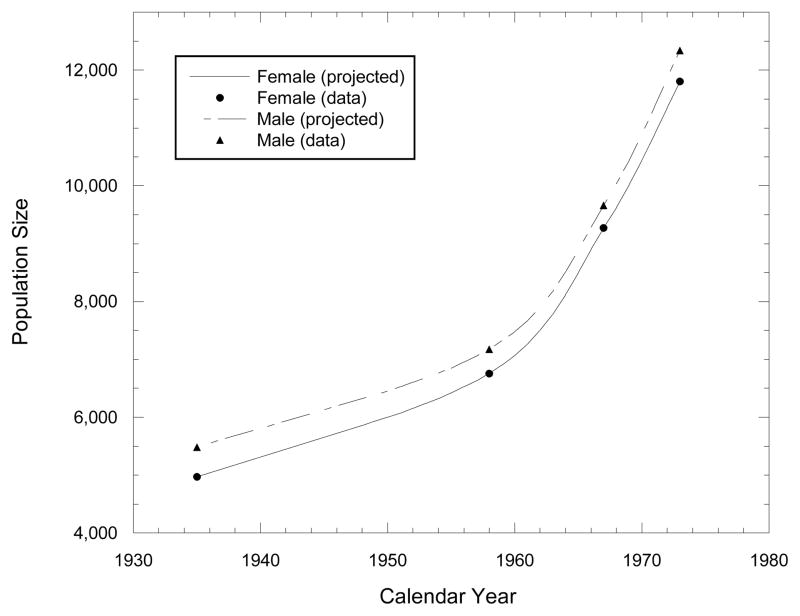
Table 1 shows total MI population numbers by sex, as determined by censuses carried out in 1935, 1958, 1967, and 1973 (RMI 1986, Tables 1.1 and 1.7). These data indicate that the MI population increased by about 14% of the 1935 value between 1935 and 1958, by 36% of the 1958 value between 1958 and 1967 and by another 28% between 1967 and 1973, and that the male/female ratio decreased from 1.10 in 1935 to 1.06 in 1958 and to a little over 1.04 in 1967 and 1973. Fig. 1 shows estimated sex-specific MI population sizes by year, obtained by interpolation using the standard fitted Bezier cubic spline curve algorithm (Foley et al. 1992) as implemented in the Microsoft Excel§ spreadsheet command for XY scatter plot with data points connected by smoothed lines.
Table 1.
Census-based total Marshall Islands population by gender and census year.
| Gender | Census Year
|
|||
|---|---|---|---|---|
| 1935 | 1958 | 1967 | 1973 | |
| Males | 5,480 | 7,175 | 9,658 | 12,335 |
| Females | 4,966 | 6,753 | 9,267 | 11,800 |
Fig. 1.
Fitted Bezier cubic splines expressing yearly interpolated, gender-specific population numbers for the entire Marshall Islands population, based on census results for 1935, 1958, 1967, and 1973.
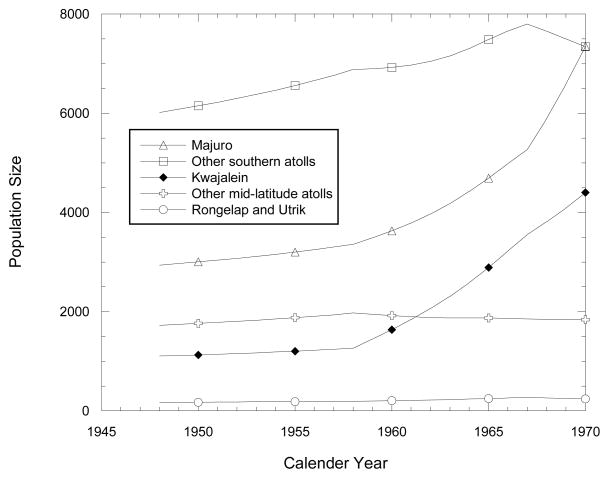
The census reports of 1958, 1967, and 1973 also gave total population numbers by atoll (RMI 1986, Table 1.2), which we used to apportion the total populations for these and other years by atoll. As a general rule, for yearly apportioning of the interpolated yearly total numbers among the various atolls, we used the 1958 proportional allocation for each of the years 1948–1957, and interpolated linearly between 1958 and 1967 for 1959–1966 and between 1967 and 1973 for 1968–1970. Estimated numbers are plotted by year in Fig. 2 for the combined populations of the northern atolls of Rongelap and Utrik, the mid-latitude population center Kwajalein, a group of six other mid-latitude atolls (Ailuk, Likiep, Mejit, Ujelang, Wotho, and Wotje), the southern population center Majuro, and the remaining 13 southern atolls (Ailinglaplap, Arno, Aur, Ebon, Jaluit, Kili, Lae, Lib, Maloelap, Mili, Namorik, Namu, and Ujae). Fig. 2 clearly indicates a substantial trend over time of migration from the nearby as well as remote atolls to two population centers. The populations of Kwajalein and Majuro increased over 1948–1970 by factors of 4 and 2.5, respectively, compared to 1.2 for southern atolls other than Majuro, 1.07 for mid-latitude atolls other than Kwajalein, and 1.05 for Rongelap and Utrik.
Fig. 2.
Estimated population sizes over time for Majuro, 13 other southern atolls, Kwajalein, 6 other mid-latitude atolls, and the Rongelap and Utrik communities including members not present at the time of the Castle BRAVO test on March 1, 1954.
The census reports for 1958 and 1973, but not 1967, gave distributions for the entire MI population by sex and age interval (RMI 2003, Table 2.7) (Table 2). Using what might be termed a “global enumeration algorithm”, we applied the 1958 sex-specific age distributions to all atolls for 1948–1957, and interpolated linearly between the 1958 and 1973 distributions for 1959–1970. Two exceptions were 82 members of the Rongelap community who received very high radiation doses from exposure to fallout from Castle BRAVO on March 1, 1954 while on Rongelap (64) or Ailinginae (18), and 157 members of the Utrik community who received 10-fold lower, but still substantial, doses while exposed on Utrik to fallout from the same event. Both groups were enumerated in historical documents (see for example, BNL 1975) by age and gender when they were evacuated several days later, while the numbers and distribution by age and gender of community members who were not present at the time were estimated using the above-mentioned global enumeration algorithm.
Table 2.
Distribution of Marshall Islands population by age and gender in 1958 and 1973: number of persons and percent (%).
| Age group | 1958: Males (%) | 1958: Females (%) | 1973: Males (%) | 1973: Females (%) |
|---|---|---|---|---|
| <1 | 268 (3.79) | 246 (3.68) | 485.23* (3.94*) | 409.19* (3.47*) |
| 1 to 4 | 1,078 (15.23) | 1,073 (16.06) | 1,951.77* (15.86*) | 1,784.81* (15.15*) |
| 5 to 9 | 1,162 (16.41) | 953 (14.26) | 2,023 (16.44) | 1,876 (15.92) |
| 10 to 14 | 782 (11.05) | 703 (10.52) | 1,550 (12.60) | 1,538 (13.05) |
| 15–19 | 452 6.38) | 476 (7.12) | 1,379 (11.21) | 1,385 (11.75) |
| 20–24 | 411 (5.81) | 426 (6.38) | 1,070 (8.70) | 975 (8.27) |
| 25–29 | 462 (6.53) | 443 (6.63) | 741 (6.02) | 770 (6.53) |
| 30–34 | 421 (5.95) | 390 (5.84) | 489 (3.97) | 446 (3.78) |
| 35–39 | 386 (5.45) | 387 (5.79) | 429 (3.49) | 432 (3.67) |
| 40–44 | 294 (4.15) | 291 (4.36) | 427 (3.47) | 362 (3.07) |
| 45–49 | 317 (4.48) | 274 (4.10) | 358 (2.91) | 369 (3.13) |
| 50–54 | 201 (2.84) | 231 (3.46) | 357 (2.90) | 359 3.05) |
| 55–59 | 201 (2.84) | 220 (3.29) | 328 (2.67) | 312 (2.65) |
| 60–64 | 231 (3.26) | 178 (2.66) | 263 (2.14) | 249 (2.11) |
| 65–69 | 151 (2.13) | 112 (1.68) | 159 (1.29) | 189 (1.60) |
| 70–74 | 120 (1.69) | 102 (1.53) | 113 (0.92) | 139 (1.18) |
| 75–79 | 50 (0.71) | 53 (0.79) | 63.64* (0.52*) | 56.91* (0.48*) |
| 80–84 | 50 (0.71) | 57 (0.85) | 63.64* (0.52*) | 61.21* (0.52*) |
| 85+ | 43 (0.61) | 66 (0.99) | 54.73* (0.44*) | 70.88* (0.60*) |
|
| ||||
| Total | 7,080 (100.00) | 6,681 (100.00) | 12,305 (100.00) | 11,784 (100.00) |
1973 age detail apportioned according to 1958 detail.
Radiation dose
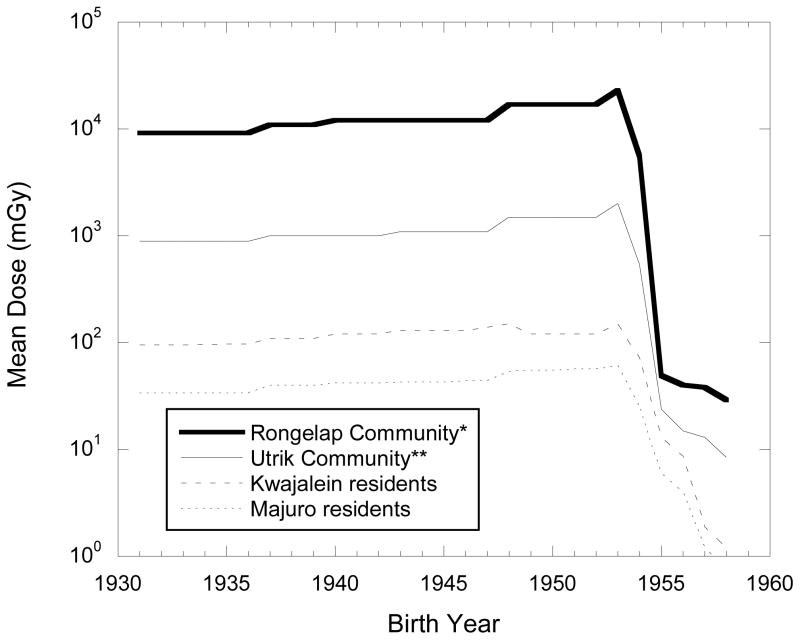
Estimation of tissue-specific radiation absorbed doses to bone marrow, thyroid gland, stomach, colon, and other organs and tissues from fallout-related internal (ingested) and external radioactive sources is discussed in the companion papers (Bouville et al. 2009; Simon et al. 2009a). External doses were assumed to be essentially the same for all organs, but separate internal doses were estimated for colon, bone marrow, thyroid gland and stomach. Total site-specific radiation dose estimates accumulated over time varied substantially by geographical location (atoll) and year of birth. Fig. 3, drawn from Table 6 in Simon et al (2009b) illustrates the 10-fold differences in overall dose between Rongelap and Utrik and between Utrik and Kwajalein (representative of the other mid-latitude islands and atolls), and the two-fold difference between Kwajalein and Majuro (representative of the other southern-latitude communities). Fig. 3 also gives some idea of the overwhelming significance of fallout from the 1954 Castle series of tests (see Table 1 of Simon et al. 2009b), with a steep drop of 1–2 orders of magnitude in dose corresponding to birth dates before and after 1954.
Fig. 3.
Estimated cumulative thyroid doses for different communities, by year of birth, drawn from Table 6 in Simon et al., 2009b.
*Rongelap community members exposed to BRAVO fallout on Rongelap Island and on Ailinginae on March 1, 1954.
** Dose estimates for persons born in 1931 also pertain to persons born earlier.
For risk projection purposes, colon dose estimates were used for organs other than bone marrow, thyroid, and stomach. The exposures associated with any one fallout event were considered to be continuous (and, in general, decreasing over time until 1970, after which they were considered to be negligible), as distinguished from the acute (i.e., near-instantaneous), direct external radiation exposures experienced by persons exposed to the Hiroshima and Nagasaki atomic bombings, which form the primary basis for current dose-response estimates of radiation-related cancer risk (Preston et al. 2007, NRC 2006) on which the risk projections presented later in this report are based. Estimated dose varied by atoll, fallout event, calendar year, and age at exposure. As discussed in the companion papers, the precision of the dose reconstruction data was considered to be better for exposures on Rongelap and Ailinginae than for those on Utrik, and both were judged to be more precise than the estimates of exposures on the mid-latitude and southern atolls. Accordingly, the subjective, lognormal uncertainty distributions for estimated doses were assigned geometric standard deviations (GSD) of 2.0 for exposure on Rongelap and Ailinginae, 2.5 for exposure on Utrik, and 3.0 for exposures on the other atolls.
Also, each dose estimate was assumed to represent the mean of its lognormal uncertainty distribution, which implies that the median of that uncertainty distribution therefore equals the point estimate divided by exp{0.5 × ln2 (GSD) }. For example, an estimated radiation dose, in 1954, of 0.04 Gy to the thyroid gland at a mid-latitude atoll would be assigned a lognormal subjective uncertainty distribution with mean of 0.04 and GSD of 3. The corresponding geometric mean (GM) is therefore
| ** |
Estimation of baseline cancer rates
In the absence of comprehensive cancer incidence data for the Marshall Islands, approximate tissue-specific, baseline cancer rates were calculated by age and gender, using incidence rates reported by the Surveillance, Epidemiology, and End Results (SEER) registry of the U.S. National Cancer Institute (NCI) for all ethnic groups combined (NCI 1997). These rates were adjusted to reflect the ratio of site-specific, age-standardized (world) rates for ethnic Hawaiians from the Hawaii Tumor Registry (which is a part of the SEER registry) to the corresponding age-standardized (world), or ASW, rates for the SEER registry as a whole. ASW rates are weighted averages of age-specific rates, with the weights determined by the estimated sex-specific age distribution of the entire world population, which is somewhat younger than those for most developed countries (Parkin et al. 2002). For example, the 1973–1998 SEER baseline rate per 100,000 per year for thyroid cancer among U.S. females (all races) at age 62 is 12.85 (SEER-Stat 2008). After multiplying by the ratio of the Hawaii Tumor Registry ASW rate for native Hawaiian females, divided by the corresponding ASW rate for U.S. females, the corresponding adjusted rate per 100,000 per at age 62 was calculated as
which we used as the projected baseline rate per 100,000 per year for a MI woman at age 62.
The computation of baseline rates in this paper is based upon a number of assumptions, the uncertainties of which are difficult to quantify, even subjectively. However, it can be argued that, when dealing with a past radiation event attributable risk, i.e., the proportion of total cancer cases related to exposure, has more practical significance than the total number of radiation-related cancers. For example, attributable risk is the primary basis in the U.S. for evaluating compensation claims for possible radiation-related cancer (NIH 2003, Kocher et al 2008). Projection of attributable risk is also less sensitive to uncertainties in baseline risk.
Models for Estimation of Radiation-Related Cancer Risk
BEIR VII linear dose-response models (NRC 2006) for estimating the excess relative risk (ERR) per unit dose for radiation-related leukemia, cancers of the thyroid gland, stomach, and colon, and solid cancers other than thyroid and non-melanoma skin cancer, are shown in Table 3. Age-specific and lifetime risks for a “residual” category of solid cancers, leaving out stomach and colon as well as thyroid and non-melanoma skin cancer, were obtained by subtraction.
Table 3.
Percentiles 50 and (in parentheses) 2.5 and 97.5 of the uncertainty distributions for parameters of the BEIR VII (NRC 2006) linear dose-response models for cancer-specific ERR Gy−1 at attained age a following exposure at age e to protracted radiation dose D.†† The gender-specific parameters βM and βF have lognormal uncertainty distributions and the gender-neutral parameters γ, η, δ, and ϕ are assumed to be normally distributed. Parameters with missing values for percentiles 2.5 and 97.5 in the table were considered in BEIR VII to make negligible contributions to error in the risk estimates and are therefore treated as constants.
| Associated parameter | Radiation dose (D) | Exposure age (e) | Attained age (a) | time since exposure (t=a−e). | ||
|---|---|---|---|---|---|---|
|
| ||||||
| (males) | (females) | f(e)¶ | g(a)¶ | h(t)¶ | f(e) × h(t) | |
| βM | βF | γ | η | δ | ϕ | |
| All solid cancers‡‡ | 0.33 (0.24, 0.47) | 0.57 (0.44, 0.74) | −0.30 (−0.51, −0.10) | −1.4 (−2.2, −0.7) | 0 | 0 |
| Thyroid | 0.53 (0.14, 2.0) | 1.05 (0.28, 3.9) | −0.83§§ | 0 | 0 | 0 |
| Stomach | 0.21 (0.11, 0.40) | 0.48 (0.31, 0.73) | −0.30§§ | −1.4§§ | 0 | 0 |
| Colon | 0.63 (0.37, 1.1) | 0.43 (0.19, 0.96) | −0.30§§ | −1.4§§ | 0 | 0 |
| Leukemia*** | 1.1 (0.10, 2.6) | 1.2 (0.10, 2.9) | −0.4 (−0.78, 0.0) | 0 | −0.48 (−1.1, 0.20) | 0.42 (0.0, 0.96) |
ERR Gy−1 = β × exp{γ × f(e) + η × g(a) + δ × h(t) + ϕ × f(e) × h(t)}, where f(e) = max{0, (e − 30)/10}, g(a) = loge(a/60), t = a − e, and h(t) = loge(t/25.
Except thyroid cancer and non-melanoma skin cancer
Error contribution assumed to be negligible, following BEIR VII (NRC 2006)
Except chronic lymphocytic leukemia (CLL)
The BEIR VII algorithms express cancer-specific ERR as a gender-specific, parametric function linear in radiation dose, with dose coefficients βM for males and βF for females. Gender-specific dose response was modified by gender-independent functions of age at exposure (e), attained age (a), and/or time since exposure (t). These estimates pertain to the population of atomic bomb survivors studied by the Radiation Effects Research Foundation (RERF) in Hiroshima and Nagasaki, Japan (Preston et al 2007). The general form of the expression for ERR per unit dose (i.e., per Gy) for specified solid cancers, as derived from the BEIR VII report (NRC 2006, Table 12–2), is
| (1) |
Here, β, γ, and η are uncertain parameters that have been estimated from epidemiological data, while e is age at exposure and a is attained age, both expressed in years:
| (2) |
and
| (3) |
Parameter β is sex specific (βM for males and βF for females) whereas parameters γ and η are gender neutral.
For leukemia other than chronic lymphocytic leukemia (CLL), the dose response is a quadratic function of dose for acute exposures but is linear in dose for exposures protracted in time such as those from radioactive fallout. From BEIR VII (NRC 2006), the ERR for protracted exposure is assumed to be proportional to radiation dose in Gy, and the ERR per Gy is expressed as:
| (4) |
Here, δ and ϕ are additional uncertain parameters, t = a e (time since exposure), and
| (5) |
The uncertain parameters are assumed to be distributed as random variables (lognormal for βM and βF and normal for γ, η, δ, and ϕ) with 50th and, in parentheses, 2.5th, and 97.5th percentiles as presented in Table 3 for all solid cancers as a group (other than thyroid cancer and non-melanoma skin cancer) and for leukemia and cancers of the thyroid gland, stomach, and colon in particular.
While the approach used in the present analysis uses BEIR VII dose-response estimates, it differs from BEIR VII with respect to modification of dose response at low doses and low dose rates (as discussed below under “Adjustment for protracted exposures”), transfer between the Japanese A-bomb survivors and the exposed Marshall Islands population (as discussed above under “Estimation of baseline cancer rates” and below under “Transfer of estimated excess risk to the exposed MI populations”), and to treatment of latent period between radiation exposure and diagnosis of cancer. In these matters, we followed an earlier NIH approach (NIH 2003) to evaluating the extent to which a given cancer diagnosis might be attributable to a given prior history of exposure to ionizing radiation.
Example
From Table 3, the estimated ERR per Gy, according to BEIR VII, for radiation-related thyroid cancer in a woman at age 62, following exposure at age 12, is
(Note that the ERR for excess thyroid cancer, unlike that for other cancers, does not depend upon attained age.) We treat this estimate as an uncertain value distributed as approximately lognormal with geometric mean GM = 4.68 and GSD = 1.96 (as indicated by the 95% uncertainty bounds for the parameter βF in Table 3). The arithmetic mean of this distribution is
For an uncertain dose estimate of 0.04 Gy, e.g., from an exposure in 1954 on a mid-latitude atoll, the estimated excess relative risk (ERR) must reflect the statistically independent uncertainties of both the estimated ERR per Gy and the estimated dose in Gy. Given a lognormal uncertainty distribution for the estimated dose with mean 0.04 Gy and GSD 3, the GM of that uncertainty distribution would be 0.04 Gy × exp{−0.5 × ln2(3)} = 0.0219 Gy. The estimated ERR at 0.04 Gy, then, is considered to be approximately lognormal with
and
Adjustment for protracted exposures
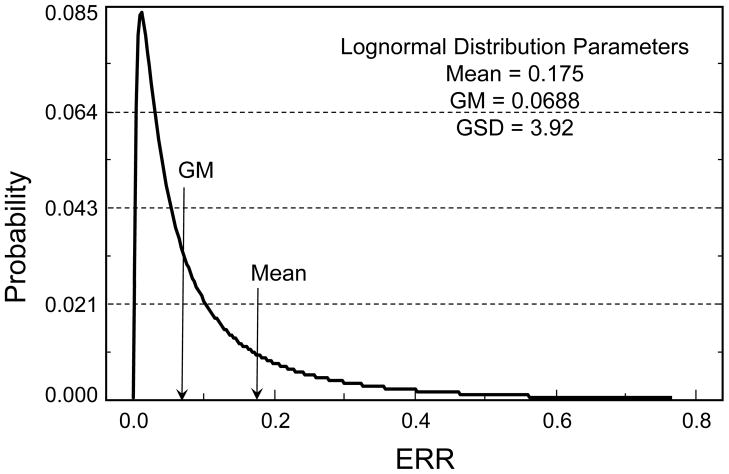
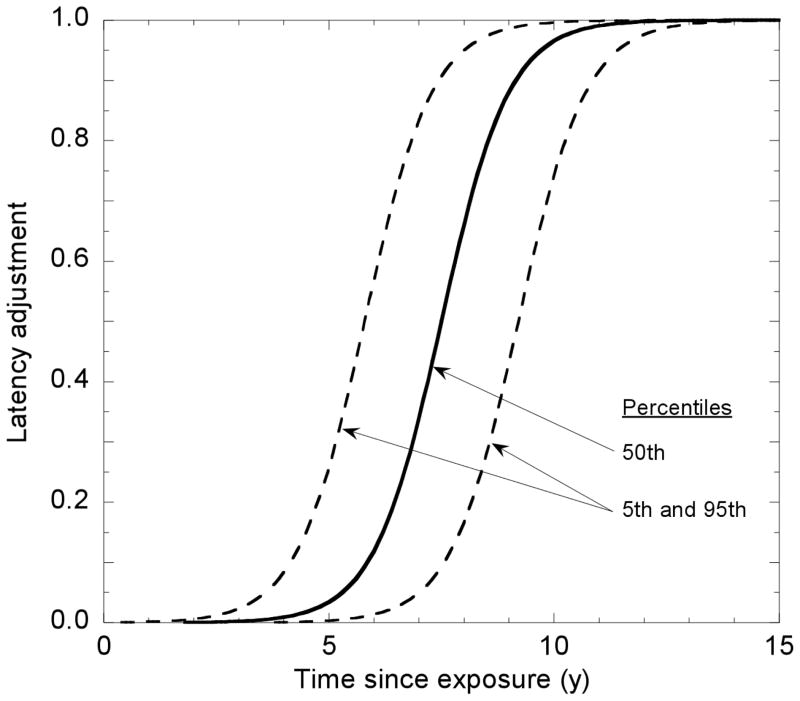
In the BEIR VII report (NRC 2006), as elsewhere, a linear-quadratic dose-response model is used for leukemia ERR associated with an acute radiation dose, but for protracted doses the coefficient for dose-squared is set equal to zero, giving a linear dose-response model for leukemia. For solid cancer risk following protracted or very low-dose exposures, the ERR is divided by a dose-and-dose-rate effectiveness factor (DDREF). The present calculations involve a different DDREF, shown in Fig. 4 (panel 1), which was developed for the “Interactive Radio-Epidemiological Program” (IREP) used to facilitate adjudication of compensation claims against the U.S. government for radiation-related cancers (NIH 2003, Kocher 2008). When applied to the thyroid cancer example introduced under Models for Estimation of Radiation-Related Cancer Risk and, evaluated by Monte Carlo simulation, the uncertainty distribution for the ERR estimate divided by the DDREF corresponds closely to a lognormal distribution with GM = 0.0688 and GSD = 3.92 (mean = 0.175) (Fig. 5).
Fig. 4.
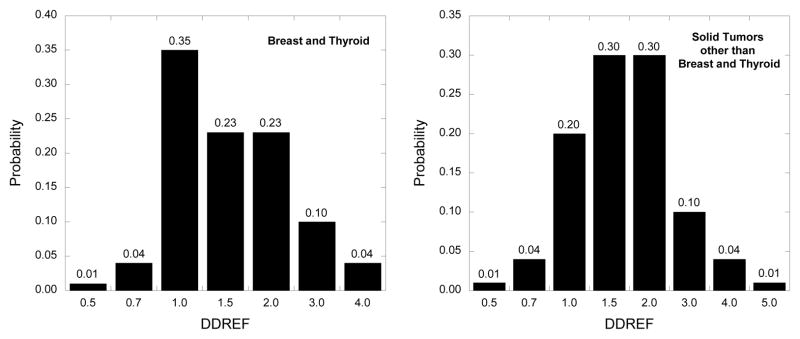
IREP uncertainty distributions for the dose and dose rate effectiveness factors (DDREF) to be applied at low doses and low dose rates to risk estimates for (a) breast and thyroid cancer and (b) solid cancers other than breast and thyroid.
Fig. 5.
Results of a Monte Carlo simulation to evaluate the effects of adjusting an uncertain thyroid cancer excess relative risk (ERR) projection distributed as lognormal with geometric mean (GM) = 0.1023 and geometric standard deviation (GSD) = 3.625, by the DDREF with uncertainty distribution shown in Figure 4, panel 1. The simulated uncertainty distribution is approximately lognormal with GM = 0.0688 and GSD = 3.92.
Latent period
As shown in Table 3, the dose-specific ERR may depend upon attained age and/or time following exposure, but experimental studies at the cellular and animal level strongly suggest that the process of radiation carcinogenesis requires time; i.e., there is a minimum latent period of uncertain duration that is superimposed upon the age/time dependence of the ERR. This is an important consideration for compensation claims adjudication in cases where the claim involves a cancer diagnosed within a few years after exposure, but it has relatively little importance for estimates of lifetime risk. In the present analysis, we follow IREP (NIH 2003; Kocher 2008), using a sigmoid function multiplier like that used in the report of the NIH Ad Hoc Working Group to Develop Radioepidemiological Tables (NIH 1985), which is discussed in Appendix 1. For thyroid cancer, the latent period is somewhat shorter than that for other solid cancers, increasing from zero at age 1 to its full value at 8 years and older (NIH 2003).
Transfer of estimated excess relative risk to the exposed MI populations
The tissue-specific BEIR VII parametric models for ERR (Table 3) apply mainly to the Japanese A-bomb survivor Life Span Study (LSS) cohort studied by the RERF (Preston et al. 2007), which we will denote as ERRLSS. Two simple approaches can be used to transfer estimated ERRLSS from the population of Hiroshima/Nagasaki A-bomb survivors to the MI population. One, called multiplicative transfer, involves assuming that dose-specific ERR values for the MI population (ERRMI) are the same as those for the LSS population even though the two populations may have different baseline cancer rates, i.e., ERRMI(mult) = ERRLSS. The other, called additive transfer, involves the assumption that the excess absolute risk, EAR = ERR times the age-specific baseline rate (B), does not vary by population:
| (6) |
and thus,
| (7) |
The BEIR VII approach uses multiplicative transfer for thyroid cancer, but uses a weighted average, on the log scale, with weights of 0.7 on multiplicative transfer and 0.3 on additive transfer for leukemia and for cancers of the stomach, colon, and solid cancers other than thyroid, lung, and female breast, for which the multiplicative model weights are 1.0, 0.3, and 1.0, respectively. The approach used in the present analysis is similar except that the weighted average is in the arithmetic, rather than the logarithmic, scale and the weights of 0.7 and 0.3 for multiplicative and additive transfer, respectively, are applied to all solid cancers as a group, excluding thyroid, stomach, and colon. Compared to BEIR VII, the present approach to transfer between populations, yields slightly higher overall risk estimates, with wider uncertainty bounds.
For thyroid cancer, the estimated ERR (ERRLSS) obtained above for thyroid cancer is directly applicable to the MI population (i.e., ERRMI = ERRLSS). Thus, ERRMI has a lognormal uncertainty distribution with GM = 0.0688 and GSD = 3.92 (mean = 0.175) (Fig. 5). For leukemia, stomach cancer, colon cancer, and solid cancers as a group, both additive and multiplicative models are required, and in the present calculations the transfer models used in IREP were employed (NIH 2003).
Life tables
Until this point, the narrative has concerned only estimates of ERR at specific ages. Projection of lifetime risk requires a differentially-weighted summation of age-specific absolute risks (EAR) using weights that reflect that the likelihood of reaching a given age is inversely related to the numerical value of that age. For this purpose we used a 1989–1991 life table for the U.S. (NCHS 1997) (Fig. 6) to adjust for competing, age-specific mortality in estimating cumulative baseline and radiation-related excess risk for exposure to a given radiation dose at a given age. This life table, based on sex- and age-specific mortality rates for the U.S. in 1989–1991, provides one-year survival data for persons alive at any given age during that period, i.e., the proportion of persons of a given age that survived until the next year of life. However, it is often used, as in Fig. 6, to show the average likelihood that a newborn person would survive until one, two, three, etc. years of age provided that the age-specific mortality rates observed in 1989–1991 also were to hold for every other calendar year (which, of course, may not really be the case). If we multiply age-specific baseline rates by the life table survival probability for that age, and sum over the different ages, the “life-table-weighted sum” is an estimate of lifetime cancer risk, here also assuming that age-specific baseline cancer rates, as well as survival probabilities, do not change over time. With the same assumptions, we can calculate the lifetime excess cancer risk for each year following exposure to a given dose at a given age, as the life-table-weighted sum of estimated age-specific excess risks. Of course, in order to be exposed at a given age one must have survived until that age, so a modified life table is required, conditional on survival to the specified exposure age.
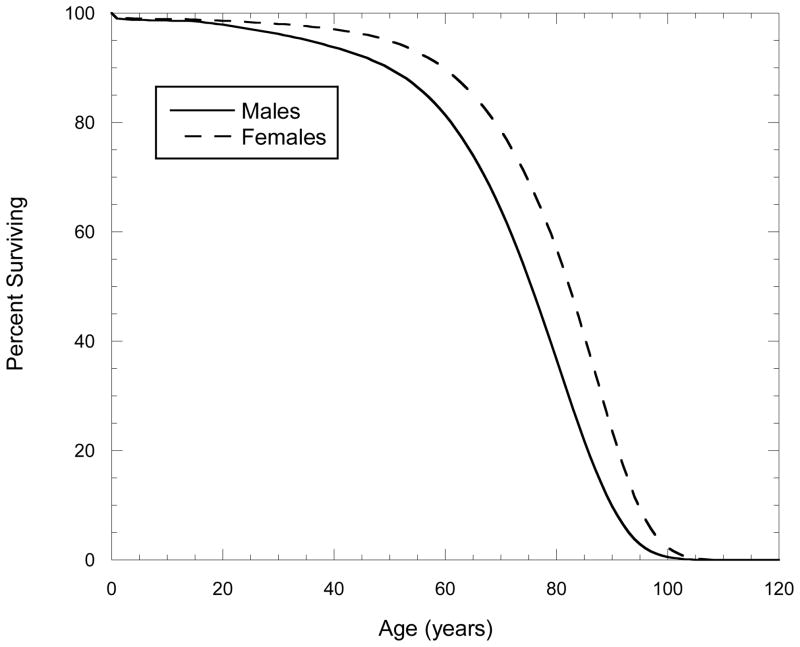
Fig. 6.
U.S. male and female life tables used in the preparation of this report. Drawn from U.S. Decennial Life Tables for 1989–1991, Vol. 1, Number 1, United States Life Tables. Hyattsville, MD. U.S. Dept. of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics (PHS) 97-110-1; 1997.
As noted several times in the previous paragraph, life tables are not perfect, but they do provide a standard way of accounting for competing mortality risks and when estimating future and lifetime risk associated with a particular exposure of interest. For computational convenience, we used a simple life table for projecting lifetime radiation-related excess risk rather than a doubly-decremented life table which takes account of additional mortality due to radiation-related cancer. This likely resulted in a slight overestimation of excess cancer risks for most of the communities, and probably more so for risks associated with the higher-dose exposures experienced on Rongelap, Ailinginae, and Utrik in 1954. However, the uncertainties in radiation doses and year-to-year variation in population sizes by atoll likely vastly outweigh any likely reduction in bias obtainable by using the more complex life table approach.
Calculation of projected lifetime baseline and excess risks
In the present analysis, lifetime baseline risk is calculated from birth or from 1948, whichever occurred first. Thus, for someone born in 1950, the sex-specific life table survival probabilities in Fig. 6 would be used, but for someone born in 1942, and known (or assumed) to have been alive in 1948, an adjusted life table would be used, with probabilities of survival to ages one, two, …, six each set equal to one and probabilities of survival until ages seven, eight, etc. (from Fig. 6) each divided by the Fig. 6 probability of survival to age six (i.e., survival until 1948). Denoting the adjusted life table probability to age a by L(a) and the age-specific baseline cancer rate by BMI(a), the lifetime baseline rate from age six is calculated as the life table-weighted sum over ages a from seven through 120 of BMI(a), i.e., . In the case of thyroid cancer, that sum is 1.1% (the value for the U.S. SEER population) times the ratio of the ASW rate per 100,000 for native Hawaiians to that for the U.S. SEER population: 1.177% × 11.0/7.5 = 1.726%.
Supposing an exposure at age 12 and, therefore, survival until that age, the appropriate U.S. life table for calculating lifetime excess risk is obtained from Fig. 6 by setting to one the probabilities of survival to ages one through 12 and dividing each of the Fig. 6 life table probabilities of survival to ages 13, 14, etc. by the Fig. 6 probability of survival to age 12. Then, using the revised life table survival probabilities for subsequent ages, L(13), L(14), etc., the lifetime excess rate associated with exposure at age 12 is calculated as the sum over a = 13 through 110,
| (8) |
Here, the notation ERRMI(12, a) is required because of the time-dependent, uncertain latent period discussed above under Latent period. (In fact, the notation above is somewhat overly simplified because radiation dose varied by calendar year in each location; the above formulation should be understood as corresponding to a given calendar year as well.)
In contrast to the BEIR VII dose-response models for leukemia, stomach cancer, colon cancer, and the group of solid cancers other than thyroid and non-melanoma skin cancer, ERR for thyroid cancer does not depend upon attained age (Table 3). It does, however, depend upon latent period, increasing from zero within the first two years after exposure to its full value 8 years after exposure. A reasonable rough calculation, used here for illustration purposes, of lifetime EAR associated with a 0.04 Gy thyroid dose at age 12 is obtained by multiplying ERRMI(12, 20), which is distributed as lognormal with GM = 0.0688 and GSD = 3.92 (mean = 0.175), by the lifetime baseline risk at age 16, which is 1.172%. The product is lognormal with GM = 0.00081, GSD = 3.92, and mean = 0.0021.
Computational approach
Each factor that is part of the calculation of the excess number of cancers has an associated uncertainty, including radiation doses, parameter values of dose-response models, DDREF values and other adjustments of dose-related risk. The uncertainty of each component was described using probability distribution functions (e.g., in Table 3) and Monte-Carlo methods were used to propagate these uncertainties.
Monte Carlo methods use pseudo-random numbers to generate realizations from each of the assumed uncertainty distributions describing particular uncertain components. The randomly sampled values are introduced in the excess risk equations and realizations of the excess number of cancers are produced. The collection of values for the excess number of cancers obtained by repeating the process for many iterations is analyzed to estimate the mean, median and uncertainty interval for the excess number of cancers. The uncertainty distributions of several input parameters used in the present analysis (e.g., DDREF) are based partly on expert judgment regarding the appropriateness of the available data about that parameter for the radiation exposures in the Marshall Islands, rather than strictly on statistical analysis of those data, and therefore the term “uncertainty interval” is used instead of “confidence interval” which involves only statistical uncertainty. Given a sufficient number of iterations, Monte Carlo methods are accurate and, compared to first-order analytical methods like those used by the BEIR VII committee, have definite practical advantages for handling any magnitude of uncertainty, distributions of any shape, and for dealing with large numbers of correlated, uncertain parameters.
For this report, two hundred estimates of radiation doses were generated for each of 25 populations (including a Rongelap control population, see BNL 1958 for a discussion of control subjects), 24 atolls and islands where exposure took place, 23 calendar years of exposure, 100 possible exposure ages (treating anyone exposed at ages 100 or older as having been exposed at age 99), and 5 target organs or groups of organs. (The details of the populations, atolls and islands, and exposure years are given in Simon et al. 2009b.) Similarly, two hundred realizations were generated for the risk per unit dose for each possible combination of gender, exposure age, and cancer type. The Monte-Carlo estimated doses and risks per unit dose were combined together with the number of individuals in each exposure age group, each gender and each atoll to obtain two hundred estimates of the predicted number of cancers, from which means, medians and 90% uncertainty intervals were generated for each cancer type and selected population. The estimated numbers of cancers were then summed to obtain totals for desired groups of atolls and for the entire MI population. The realizations used for each of the risk calculations were obtained by Median Latin Hypercube sampling (Iman and Shortencarier 1984). For a given sample size, this method provides more precise estimates of the mean, median, and 90% uncertainty limits than are obtainable through simple random sampling.
RESULTS
Cancer risk estimates are based on 12,175 MI residents projected to have been born before 1948 and another 12,608 projected to have been born in the years 1948 through 1970, giving a total exposed population of 24,783. Projected lifetime numbers of baseline and radiation-related cancers are shown in Table 4 by organ site, population, and calendar time period. Reflecting the general decreasing trend in exposure levels from the northern atolls of Ailinginae, Rongelap, and Utrik to more southern latitudes, the atolls of Ailuk, Kwajalein, Likiep, Mejit, Wotho, Wotje, Ujelang (the relocated Enewetak population) were grouped together in Table 4 as the “mid-latitude” group, while the atolls of Ailinglaplap, Arno, Aur, Ebon, Jaluit, Kili (the relocated Bikini population), Lae, Lib, Majuro, Maloelap, Mili, Namorik, Namu, and Ujae constitute the “southern-latitude” group.
Table 4.
Projected numbers of baseline cancers and radiation-related (excess) cancers by population group, cancer type, and time-period (all values rounded to two significant digits).
|
|
|
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Population group and cancer type | Projected lifetime cancers from 1948
|
Projected cancers 1948–2008
|
Projected cancers from 2009
|
|||||||||
| Baseline | Excess | Baseline | Excess | Baseline | Excess | |||||||
|
|
|
|
||||||||||
| Mean | 5% | 95% | Mean | 5% | 95% | Mean | 5% | 95% | ||||
| Rongelap Island community (cohort exposed on Rongelap Island in 1954) | ||||||||||||
| Leukemia | 0.45 | 1.6 | 0.30 | 4.3 | 0.27 | 1.3 | 0.2 | 3.7 | 0.17 | 0.3 | 0.072 | 0.59 |
| Thyroid | 0.6 | 12 | 4.2 | 21 | 0.48 | 9.5 | 2.8 | 18 | 0.12 | 2.3 | 1.2 | 3.6 |
| Stomach | 1.8 | 1.7 | 0.23 | 4.9 | 1 | 0.79 | 0.1 | 2.5 | 0.8 | 0.87 | 0.12 | 2.7 |
| Colon | 3 | 5.4 | 1.7 | 11 | 1.7 | 2.5 | 0.77 | 5.1 | 1.4 | 2.9 | 0.9 | 5.9 |
| Other solid | 28 | 21 | 7 | 33 | 18 | 16 | 5.2 | 27 | 10 | 4.9 | 2.2 | 7.9 |
| Total | 34 | 41 | 13 | 74 | 21 | 30 | 9 | 57 | 13 | 11 | 4.5 | 21 |
| Utrik community | ||||||||||||
| Leukemia | 1.4 | 0.34 | 0.062 | 1.2 | 0.77 | 0.28 | 0.044 | 1 | 0.63 | 0.062 | 0.016 | 0.13 |
| Thyroid | 1.8 | 4.4 | 0.85 | 11 | 1.3 | 3 | 0.56 | 8.2 | 0.5 | 1.4 | 0.27 | 3.3 |
| Stomach | 5.9 | 0.3 | 0.038 | 0.99 | 2.8 | 0.14 | 0.018 | 0.39 | 3 | 0.16 | 0.02 | 0.61 |
| Colon | 9.6 | 1 | 0.32 | 2.3 | 4.6 | 0.42 | 0.13 | 0.93 | 5.1 | 0.58 | 0.16 | 1.3 |
| Other solid | 91 | 6.5 | 1.4 | 14 | 49 | 3.6 | 0.89 | 8.7 | 42 | 2.9 | 0.46 | 6.8 |
| Total | 110 | 12 | 2.6 | 30 | 58 | 7.4 | 1.6 | 19 | 51 | 5.1 | 0.93 | 12 |
| Kwajalein and other mid-latitude atolls* | ||||||||||||
| Leukemia | 35 | 3.2 | 0.59 | 8.8 | 14 | 2.5 | 0.41 | 7.8 | 21 | 0.62 | 0.15 | 1.2 |
| Thyroid | 46 | 15 | 3 | 38 | 27 | 10 | 2 | 26 | 19 | 5 | 1 | 12 |
| Stomach | 140 | 2.7 | 0.36 | 8.5 | 44 | 1.3 | 0.16 | 4.1 | 96 | 1.5 | 0.2 | 4.3 |
| Colon | 230 | 5.3 | 1.7 | 12 | 69 | 2.3 | 0.77 | 4.8 | 160 | 3 | 0.9 | 6.3 |
| Other solid | 2,200 | 31 | 7.4 | 66 | 790 | 18 | 4.7 | 38 | 1,400 | 13 | 2.8 | 28 |
| Total | 2,600 | 58 | 13 | 130 | 940 | 35 | 8.1 | 80 | 1,700 | 23 | 5.1 | 52 |
| Majuro and other southern-latitude atolls,** including Rongelap control population | ||||||||||||
| Leukemia | 100 | 2.4 | 0.43 | 6.5 | 44 | 1.9 | 0.3 | 5.8 | 59 | 0.45 | 0.13 | 0.85 |
| Thyroid | 140 | 20 | 3.5 | 52 | 84 | 13 | 2.3 | 34 | 54 | 6.7 | 1.2 | 18 |
| Stomach | 420 | 2 | 0.29 | 5.7 | 140 | 0.89 | 0.13 | 2.6 | 280 | 1.1 | 0.16 | 3.2 |
| Colon | 690 | 4.8 | 1.6 | 9.8 | 220 | 2 | 0.68 | 4.5 | 460 | 2.8 | 0.92 | 5.7 |
| Other solid | 6,500 | 31 | 6.9 | 68 | 2,500 | 17 | 4.6 | 35 | 4,000 | 15 | 2.8 | 33 |
| Total | 7,800 | 60 | 13 | 140 | 3,000 | 34 | 8 | 82 | 4,800 | 26 | 5.2 | 60 |
| Entire Marshall Islands population exposed between 1948 and 1970 | ||||||||||||
| Leukemia | 140 | 7.4 | 1.3 | 20 | 59 | 6 | 0.94 | 17 | 81 | 1.4 | 0.37 | 2.6 |
| Thyroid | 190 | 51 | 12 | 120 | 110 | 35 | 7.9 | 85 | 74 | 15 | 3.9 | 36 |
| Stomach | 570 | 6.6 | 0.94 | 20 | 190 | 3.1 | 0.43 | 9.4 | 380 | 3.6 | 0.51 | 11 |
| Colon | 930 | 16 | 5.5 | 33 | 300 | 7.2 | 2.4 | 15 | 630 | 9.3 | 0.9 | 5.9 |
| Other solid | 8,800 | 90 | 24 | 180 | 3,400 | 54 | 16 | 110 | 5,400 | 36 | 8.6 | 71 |
| Total | 11,000 | 170 | 44 | 380 | 4,000 | 110 | 27 | 240 | 6,600 | 65 | 14 | 130 |
Ailuk, Kwajalein, Likiep, Mejit, Ujelang (population relocated from Enewetak), Wotho, and Wotje
Ailinglaplap, Arno, Aur, Ebon, Jaluit, Kili (population relocated from Bikini), Lae, Lib, Majuro, Maleolap, Mili, Namorik, Namu, and Ujae
The lifetime projection for baseline cancers of all sites, excepting non-melanoma skin cancer which is not covered by the SEER registries, totals 10,500 or a little over 40% of the exposed population, similar to all other countries worldwide, whereas the projected number of radiation-related cancers is only 170, or 1.6% of the projected total cancer risk (excess plus baseline), with 90% uncertainty range 0.4% to 3.4% and about 0.7% of the population, with an upper 95% uncertainty limit of 378 or 1.5% of the population. By population group, the projected number of radiation-related cancers for the heavily exposed Rongelap Island community (those exposed in 1954 on Rongelap Island and on Ailinginae) is about 50% of the number exposed, while the projected number is about 5% of the population for Utrik, 0.9% for the mid-latitude group and about 0.3%.for southern-latitude group, which is estimated to have received the lowest radiation doses.
Except for thyroid cancer, which tends to be diagnosed at younger ages than the majority of cancers, most of the baseline (i.e., non-radiation related) cancers are projected to occur after 2008. An exception to this finding is for the Rongelap Island exposed community which does not include anyone born after 1954. For that cohort, the projected number of lifetime, radiation-related thyroid cancers (Table 4) is 11.8, about 14% of the number of exposed members (uncertainty limits 5% to 25%), and 20 times the 0.6 baseline cases projected in the absence of exposure. The projected lifetime number of excess leukemia cases is 1.6, or 4 times the projected baseline of 0.4. About 80% of both the excess and baseline thyroid cancers and leukemias are projected to have been diagnosed by the end of 2008. For stomach, colon, and remaining solid cancers respectively, the excess cancers are estimated to equal 94%, 180%, and 74% of projected baseline values.
In the results for Utrik, the projected numbers of excess cancers for the relatively small percentage of community members who were not present on the atoll at the time of the BRAVO test have been included in the total. In contrast to the Rongelap Island community and, to a lesser extent the Utrik exposed community, we estimate that, among the members of the mid- and southern-latitude populations alive at some time during 1948–1970, about 20% were born after 1954. This difference in age distribution is reflected in the fact that proportionally fewer baseline cancers and, except for leukemia, proportionally fewer radiation-related cancers among the mid- and southern-latitude populations are projected to have been diagnosed in 2008 or earlier (Table 4).
In Table 5, the values in Table 4 have been converted to estimates of the percentage of cancers attributable to fallout-related radiation dose. These values are considered to be the main result of our analysis.
Table 5.
Projected proportion (in %) of total cancer risk attributable to radioactive fallout, by population, cancer site, and time period. Uncertainty distributions represented by their means and 90% uncertainty bounds.
| Population group and cancer type | Lifetime Attributable Risk (%)
|
Attributable Risk (%) 1948–2008
|
Attributable Risk (%) from 2009
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | 5% | 95% | Mean | 5% | 95% | Mean | 5% | 95% | |
| Rongelap Island community (cohort exposed on Rongelap Island in 1954) | |||||||||
| Leukemia | 78 | 39 | 91 | 83 | 43 | 93 | 63 | 29 | 77 |
| Thyroid | 95 | 87 | 97 | 95 | 85 | 97 | 95 | 91 | 97 |
| Stomach | 48 | 11 | 73 | 44 | 9.2 | 71 | 52 | 13 | 77 |
| Colon | 64 | 36 | 78 | 60 | 32 | 75 | 68 | 40 | 81 |
| Other solid | 43 | 20 | 54 | 48 | 23 | 61 | 32 | 17 | 43 |
| Total | 55 | 28 | 69 | 59 | 30 | 73 | 47 | 26 | 62 |
| Utrik community | |||||||||
| Leukemia | 19 | 4.3 | 45 | 26 | 5.4 | 57 | 9.0 | 2.5 | 17 |
| Thyroid | 71 | 32 | 86 | 69 | 29 | 86 | 74 | 35 | 87 |
| Stomach | 4.8 | 0.64 | 14 | 4.5 | 0.63 | 12 | 5.0 | 0.67 | 17 |
| Colon | 9.4 | 3.2 | 19 | 8.4 | 2.8 | 17 | 10 | 3.1 | 21 |
| Other solid | 6.7 | 1.5 | 14 | 6.8 | 1.8 | 15 | 6.5 | 1.1 | 14 |
| Total | 10 | 2.4 | 22 | 11 | 2.7 | 25 | 9.0 | 1.8 | 19 |
| Kwajalein and other mid-latitude atolls | |||||||||
| Leukemia | 8.4 | 1.7 | 20 | 15 | 2.9 | 36 | 2.9 | 0.75 | 5.5 |
| Thyroid | 25 | 6.1 | 45 | 28 | 7.0 | 49 | 21 | 5.1 | 39 |
| Stomach | 1.9 | 0.26 | 5.7 | 2.8 | 0.37 | 8.5 | 1.5 | 0.20 | 4.3 |
| Colon | 2.3 | 0.73 | 4.8 | 3.3 | 1.1 | 6.6 | 1.8 | 0.57 | 3.8 |
| Other solid | 1.4 | 0.34 | 2.9 | 2.3 | 0.60 | 4.6 | 0.96 | 0.20 | 2.0 |
| Total | 2.2 | 0.50 | 4.8 | 3.5 | 0.86 | 7.9 | 1.4 | 0.30 | 3.0 |
| Majuro and other southern-latitude atolls, including Rongelap control population | |||||||||
| Leukemia | 2.2 | 0.41 | 6.0 | 4.2 | 0.67 | 12 | 0.76 | 0.22 | 1.4 |
| Thyroid | 12 | 2.5 | 27 | 13 | 2.7 | 29 | 11 | 2.2 | 25 |
| Stomach | 0.47 | 0.069 | 1.3 | 0.63 | 0.089 | 1.8 | 0.39 | 0.058 | 1.2 |
| Colon | 0.69 | 0.23 | 1.4 | 0.90 | 0.31 | 2.0 | 0.59 | 0.20 | 1.2 |
| Other solid | 0.48 | 0.11 | 1.0 | 0.65 | 0.18 | 1.4 | 0.37 | 0.071 | 0.81 |
| Total | 0.76 | 0.16 | 1.8 | 1.1 | 0.27 | 2.7 | 0.53 | 0.11 | 1.2 |
| Entire Marshall Islands population exposed between 1948 and 1970 | |||||||||
| Leukemia | 5.1 | 0.96 | 12 | 9.3 | 1.6 | 23 | 1.7 | 0.46 | 3.1 |
| Thyroid | 21 | 6.0 | 39 | 24 | 6.5 | 43 | 17 | 5.0 | 33 |
| Stomach | 1.2 | 0.17 | 3.4 | 1.6 | 0.23 | 4.7 | 0.94 | 0.14 | 2.8 |
| Colon | 1.7 | 0.59 | 3.4 | 2.4 | 0.80 | 4.9 | 1.4 | 0.14 | 0.93 |
| Other solid | 1.0 | 0.27 | 2.0 | 1.6 | 0.46 | 3.2 | 0.66 | 0.16 | 1.3 |
| Total | 1.6 | 0.41 | 3.4 | 2.6 | 0.67 | 5.6 | 0.99 | 0.22 | 1.9 |
DISCUSSION
The dose-response relationship between ionizing radiation and subsequent cancer risk is among the best quantified for any common environmental carcinogen, and we feel reasonably confident about our risk projections, with a few caveats. First, there is some evidence that Micronesians, including Marshallese, may share similar cancer patterns, including high thyroid cancer rates, with native Hawaiians (Henderson et al. 1985). However, an extensive review of published reports of cancer surveillance studies and epidemiological and clinical cancer studies in the Native Hawaiian and Pacific Islander populations (Hughes et al. 2000) found a lack of systematic data collection on cancer incidence and mortality in Pacific Islanders, with wide variations in the status of cancer research among ethnic groups. Thus, baseline cancer rates and life tables used in our analysis, which were constructed to be representative of the native population of Hawaii, are not necessarily representative of the MI population. The second caveat is that any static or time-specific life table, like the U.S. Decennial Life Tables for 1999–2001 used here (NCHS 1997), corresponds to a snapshot in time and reflects current mortality rates when the life table was constructed which may differ from those (say) 30 years ago or 30 years from now.
These considerations aside, our calculations project a substantial burden of radiation-related cancer in the more heavily-exposed Marshallese population groups, and a correspondingly lighter burden in the more populous but less exposed atolls in the mid-latitude and southern-latitude regions of the MI. We project that over half (55%, with 90% uncertainty limits 28%–69%) of the cancers (since 1948) that have already been diagnosed or may be diagnosed in the future among members of the Rongelap cohort are attributable to their fallout exposure, whereas radiation exposure accounts for less than 2% (1.6% with limits 0.4%–3.4%) of past and future cancer diagnoses among the exposed MI population as a whole. The reader is also referred to Fig. 6 of Simon et al. (2009b) which presents the ratio of the excess cancers to baseline number of cancers in the four geographic areas of the MI (southern atolls, mid-latitude atolls, Utrik, and Rongelap). That presentation underscores the relatively small excess in the mid- and southern-latitude atolls.
In the exposed MI population, and in all population subsets represented in Table 4, the residual category, “other solid cancers”, which makes up 83% of baseline risk, is projected to account for the largest number of lifetime radiation-related cancers. However, in terms of “attributable risk”, or the fraction attributable to radiation exposure, the thyroid gland is the single organ projected to develop the largest attributable fraction of cancers. In the exposed population as a whole, 21% (6%–39%) of thyroid cancers are projected to be radiation-related compared to 95% (87%–97%) among members of the Rongelap exposed cohort, 70% (32%–86%) of those in the Utrik population, 25% (6%–45%) in the mid-latitude atoll populations, and 12% (2%–27%) of those in the southern atoll populations (Table 5). These numbers reflect the large effect of exposure to radioactive iodine in fallout, primarily due to the active uptake of ingested or inhaled iodine by the thyroid gland for the production of thyroid hormone. From another perspective, the 50.5 projected lifetime excess of thyroid cancers in the exposed MI population is 30% of the total projected excess of 171 total lifetime cancers, while the corresponding proportion of projected baseline cancers, 187 thyroid cancers out of 10,589 baseline cancers of all types, is less than 2% of the total (Table 4). The same ratios hold approximately for each of the population subsets represented in Table 4.
If not for the large contribution to total cancers due to exposure to radioiodines in fallout, the fraction of leukemia risk (excluding chronic lymphocytic leukemia, or CLL, which is not included in the BEIR VII model) attributable to radiation exposure might be expected to dominate, as it does, for example, in the Life Span Study cohort of atomic bomb survivors (Preston et al. 2003) for whom radioactive fallout was at most a very minor contributor to total radiation dose (Young and Kerr 2005). Overall, non-CLL leukemia accounts for about 4% of total radiation-related risk with some variation by sub-population, compared to 1.3% of projected baseline risk. Attributable risk for leukemia is high for the Rongelap exposed cohort (78% with 90% uncertainty limits 39%–91%), but 19% (4%–45%) for Utrik, 8% (2%–20%) for Kwajalein and the mid-latitude atolls, and 2% (0.4%–6%) for Majuro and the southern atolls.
In conclusion, the reader is reminded that the present analysis is not an epidemiological study but, instead, an application of existing information, gained in recent years from epidemiological studies of other exposed populations, about the relationship between radiation dose and subsequent cancer risk. This information has been combined with new estimates of radiation doses to the populations of different atolls in the Marshall Islands, as discussed in the companion papers. Our conclusions are as follows: (1) substantial numbers of cancers have already occurred or are projected to occur in the future (about 170 but perhaps as many as 380 or as few as 40) that would not have occurred in the absence of fallout exposure from nuclear testing in the Marshall Islands; (2) about half of projected past and future cancers among members of the Rongelap Island community (i.e., those exposed to BRAVO fallout on Rongelap Island and Ailinginae in 1954) are radiation-related; and (3) with the exception of thyroid cancer, the overwhelming majority of cancers that have occurred or will occur among persons exposed only on atolls and islands in the mid- and southern-latitudes are likely to be baseline cancers unrelated to radiation exposure.
Acknowledgments
This work was supported by the Intra-Agency Agreement between the National Institute of Allergy and Infectious Diseases and the National Cancer Institute, NIAID agreement #Y2-A1-5077 and NCI agreement #Y3-CO-5117.
Appendix 1. Minimum latent period of specific cancer types
The models developed by the BEIR VII committee to estimate ERR and EAR for solid cancers and leukemia do not explicitly account for effects of the time delay between exposure to ionizing radiation and the earliest diagnosis of a radiation-induced cancer. Thus, for calculations of lifetime risk, the risk models need to be modified by a function that is assumed to represent the effect of a minimum latent period on reducing risk at early times since exposure.
In their calculations of lifetime risk, the BEIR VII committee assumed that the risk is equal to zero at times since exposure less than 5 years for solid cancers and less than 2 years for leukemia. No uncertainty was associated with this threshold function.
In this study, to avoid an abrupt increase in risk from zero at times since exposure less than a minimum latency period to their maximum values at times when the minimum latent period has been exceeded, the effect of latency was represented by a sigmoid (“S-shaped”) function
| (A.1) |
where t is the time since exposure in years, μ is the value of t corresponding to the inflection point where Flatency = 0.5, and S is a shape parameter that defines the steepness of the function as it increases from values near zero to values near the maximum of 1.0.
For stomach, colon and all solid cancers as a group, μ is assumed to be 7.5 years and the shape parameter S is set so that the latent period adjustment in equation (A.1) attains values of approximately 0.01 and 0.99 at t = 4 and 11 years, respectively. Thus, risk is assumed to be very small (close to zero) at t < 4 years and to attain its full value at t > 11 years. This adjustment, to represent the effect of the minimum latent period on reducing ERR for most solid cancers, is given by the solid curve in Fig. A1.
Fig. A1 .
(Appendix). Sigmoid (S-shaped) function representing the multiplicative adjustment factor (and its uncertainty) applied to the risk of stomach cancer, colon cancer and all solid cancers (less thyroid and non-melanoma) as group, due to the effect of minimum latency period at early times since exposure.
Thyroid cancer is assumed to have a shorter minimum latent period than all other solid cancers, with a nominal value of μ equal to 5 y. In this case, the latent period adjustment attains values of approximately 0.01 and 0.99 at t = 2.5 and 7.6 y, respectively.
Leukemia is assumed to have a shortest minimum latent period with nominal value of μ set to 2.25 y. The latent period adjustment for leukemia attains values of approximately 0.01 and 0.99 at t = 0.4 and 4.1 y, respectively.
To represent uncertainty in the effects of latency on risk estimates, the midpoint, μ, is described by the following triangular probability distributions: stomach, colon and all solid cancers as a group, T(5, 7.5, 10); thyroid, T(3, 5, 7); and leukemia, T(2, 2.25, 2.5). The effect of uncertainty in μ on the adjustment for minimum latency for all solid cancers except thyroid cancer is indicated by the various percentiles of the latency adjustment shown in Fig. A1.
Footnotes
Cancers that presumably would have occurred in the absence of exposure.
Provided for informational purposes only. Identification of software does not imply any endorsement.
Here and elsewhere, results of intermediate calculations are given to more than two significant digits as needed for subsequent calculations.
References
- Beck HL, Bouville A, Moroz BE, Simon SL. Fallout deposition in the Marshall Islands from Bikini and Enewetak nuclear weapons tests. Health Phys. 2009 doi: 10.1097/HP.0b013e3181bbbfbd. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouville A, Beck HL, Simon SL. Estimation of Doses from External Irradiation to Marshall Islanders from Nuclear Testing. Health Phys. 2009 doi: 10.1097/HP.0b013e3181dc521d. this issue. [DOI] [PubMed] [Google Scholar]
- Brookhaven National Laboratory. A twenty-year review of medical findings in a Marshallese population accidentally exposed to radioactive fallout. Upton, NY: Brookhaven National Laboratory; 1973. BNL 50424. [Google Scholar]
- Brookhaven National Laboratory. March 1957 medical survey of Rongelap and Utirik people three years after exposure to radioactive fallout. Upton, NY: Brookhaven National Laboratory; 1958. [Google Scholar]
- Conard RA, Dobyns BM, Sutow WW. Thyroid neoplasia as a late effect of active exposure to radioactive iodine in fallout. J Amer Med Assoc. 1970;214:316–324. [PubMed] [Google Scholar]
- Cronkite EP, Conard RA, Bond VP. Historical events associated with fallout from BRAVO shot-Operation Castle and 25 y of medical findings. Health Phys. 1997;73:176–186. doi: 10.1097/00004032-199707000-00014. [DOI] [PubMed] [Google Scholar]
- DCEG. Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department of Health and Human Services. Report prepared for the Senate Committee on Energy and Natural Resources. Sep, 2004. Estimation of the baseline number of cancers among Marshallese and the number of cancers attributable to exposure to fallout from nuclear weapons testing conducted in the Marshall Islands. [Google Scholar]
- Foley J, van Dam A, Feiner S, Hughes J. Computer Graphics Principles and Practice. New York: Addison-Wesley; 1992. [Google Scholar]
- Hamilton TE, van Belle G, LoGerfo JP. Thyroid neoplasia in Marshall Islanders exposed to nuclear fallout. J Amer Med Assoc. 1987;258:629–636. [PubMed] [Google Scholar]
- Henderson BE, Kolonel LN, Dworsky B, Kerford D, Mori E, Thevenot H. Cancer incidence in the islands of the Pacific. Nat Cancer Inst Monogr. 1985;69:73–81. [PubMed] [Google Scholar]
- Hughes CK, Tsark JU, Kenni CK, Alexander GA. Cancer research studies in native Hawaiians and Pacific Islanders. Ann Epidemiol. 2000;10(8 Suppl):S49–60. doi: 10.1016/s1047-2797(00)00193-9. [DOI] [PubMed] [Google Scholar]
- Iman RL, Shortencarier MJ. Fortran 77 program and user’s guide for the generation of Latin hypercube and random samples for use with computer models. Sandia National Labs; Albuquerque, NM: 1984. NUREG/CR-3624; SAND-83-2365. [Google Scholar]
- Kocher D, Apostoaei, Henshaw RW, Hoffman FO, Schubauer-Berigan MK, Stancescu DO, Thomas BA, Trabalka JR, Gilbert ES, Land CE. Interactive Radioepidemiological Program (IREP): A web-based tool for estimating probability of causation/assigned share for radiogenic cancers. Health Phys. 2008;95:119–47. doi: 10.1097/01.HP.0000291191.49583.f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCHS. National Center for Health Statistics. US Decennial Life Tables for 1989–1991, Vol. 1, Number 1, United States Life Tables. Hyattsville, MD: U.S. Dept. of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics (PHS); 1997. 97-110-1. [Google Scholar]
- Ries LAG, Kosary CL, Hankey BF, Miller BA, Harras A, Edwards BK, editors. NCI. National Cancer Institute. SEER Cancer Statistics Review, 1973–1994. Bethesda, MD: 1997. NIH Pub. No. 97-2789. [Google Scholar]
- NCT. Nuclear Claims Tribunal. Annual Report to the Nitijelã for the calendar year 2003. Majuro: Republic of the Marshall Islands; 2004. [Google Scholar]
- NIH. National Institutes of Health. Report of the National Institutes of Health Ad Hoc Working Group to Develop Radioepidemiological Tables. Bethesda, MD: National Institutes of Health; 1985. [Google Scholar]
- NIH. National Institutes of Health. Report of the NCI-CDC Working Group to revise the 1985 NIH Radioepidemiological Tables. Washington, DC: 2003. NIH Publication No. 03-5387. [Google Scholar]
- NRC. National Research Council. Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2. Washington, DC: National Academy Press; 2006. [PubMed] [Google Scholar]
- Parkin DM, Whelan SL, Ferlay J, Teppo L, Thomas DB, editors. Cancer Incidence in Five Continents. VIII. Lyon: International Agency for Research in Cancer; 2002. IARC Scientific Publication No. 155. [Google Scholar]
- Preston DL, Ron E, Tokuoka S, Funamoto S, Nishi N, Soda M, Mauchi K, Kodama K. Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat Res. 2007;168:1–64. doi: 10.1667/RR0763.1. [DOI] [PubMed] [Google Scholar]
- Preston DL, Shimizu Y, Pierce DA, Suyama A, Mabuchi K. Studies of mortality of atomic bomb survivors. Report 13: Solid cancer and non-cancer disease mortality: 1950–1997. Radiat Res. 2003;160:381–407. doi: 10.1667/rr3049. [DOI] [PubMed] [Google Scholar]
- RMI. Republic of the Marshall Islands 1986 Statistical Abstract. Majuro: RMI Office of Planning and Statistics; 1987. (reproduced in April, 2003 by the Insular Areas Statistical Enhancement Program) [Google Scholar]
- RMI. Republic of the Marshall Islands 2001 RMI Statistical Abstract. 14. Majuro: Economic Planning and Statistics Office; 2003. [Google Scholar]
- SEER-Stat 2003–2005 [database online] Bethesda, MD: National Cancer Institute; 2008. ( http://seer.cancer.gov/faststats/) [Google Scholar]
- Simon SL, Beck HL, Land CE, Bouville A. Radiation doses and cancer risks in the Marshall Islands associated with exposure to fallout from Bikini and Enewetak nuclear weapons tests: Summary. Health Physics. 2009b doi: 10.1097/HP.0b013e3181dc523c. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SL, Bouville A, Melo D, Beck HL, Weinstock RM. Acute and chronic intakes of fallout radionuclides by Marshallese from nuclear weapons testing at Bikini and Enewetak, and related internal radiation doses. Health Physics. 2009a doi: 10.1097/HP.0b013e3181dc4e51. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Trott KR, Fujimori K, Nakashima N, Ohtomo H, Schoemaker MJ, Simon SL. Thyroid Disease In The Marshall Islands, Findings from 10 Years of Study. Sendai: Tohoku University Press; 2001. [Google Scholar]
- Takahashi T, Trott KR, Fujimori K, Simon SL, Ohtomo H, Nakashima N, Takaya K, Kimura N, Konno T, Satomi S, Shoemaker M. An investigation into the prevalence of thyroid nodules and thyroid cancer on Kwajalein Atoll, Marshall Islands. Health Phys. 1997;73:199–213. doi: 10.1097/00004032-199707000-00017. [DOI] [PubMed] [Google Scholar]
- Young RW, Kerr GD, editors. Reassessment of the Atomic Bomb Radiation Dosimetry for Hiroshima and Nagasaki: Dosimetry System 2002. Hiroshima: Radiation Effects Research Foundation; 2005. [Google Scholar]