Abstract
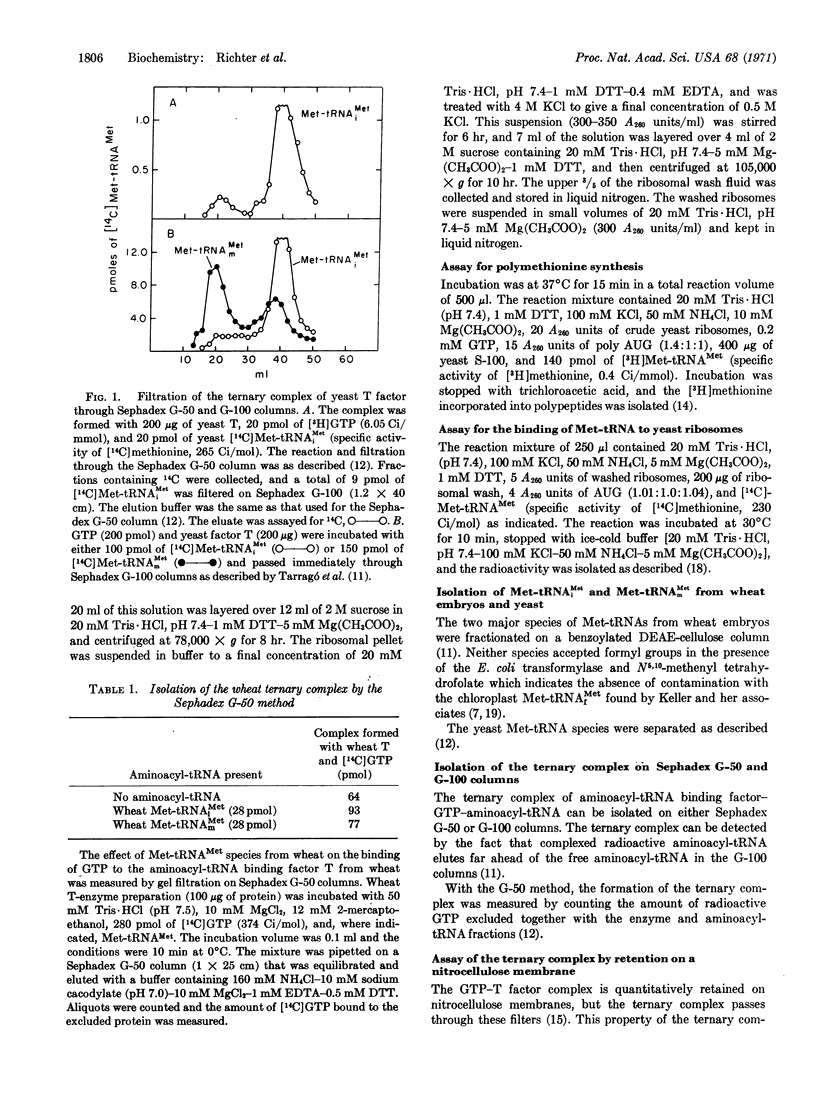
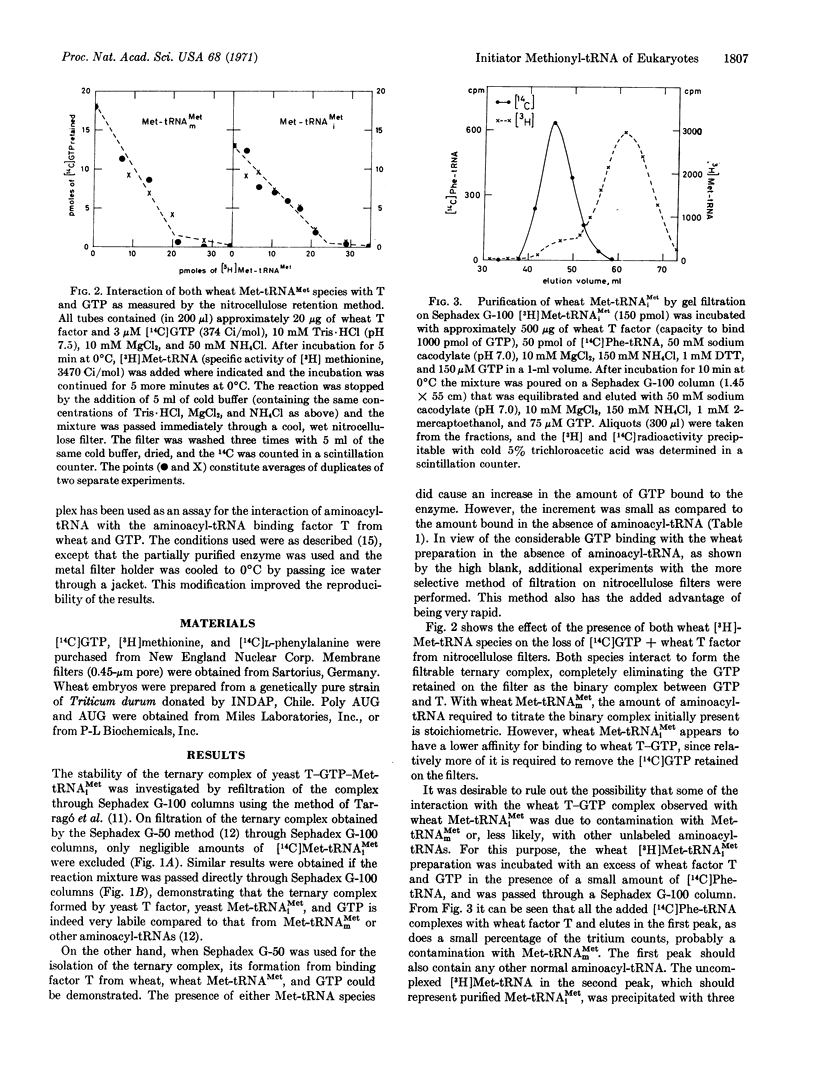
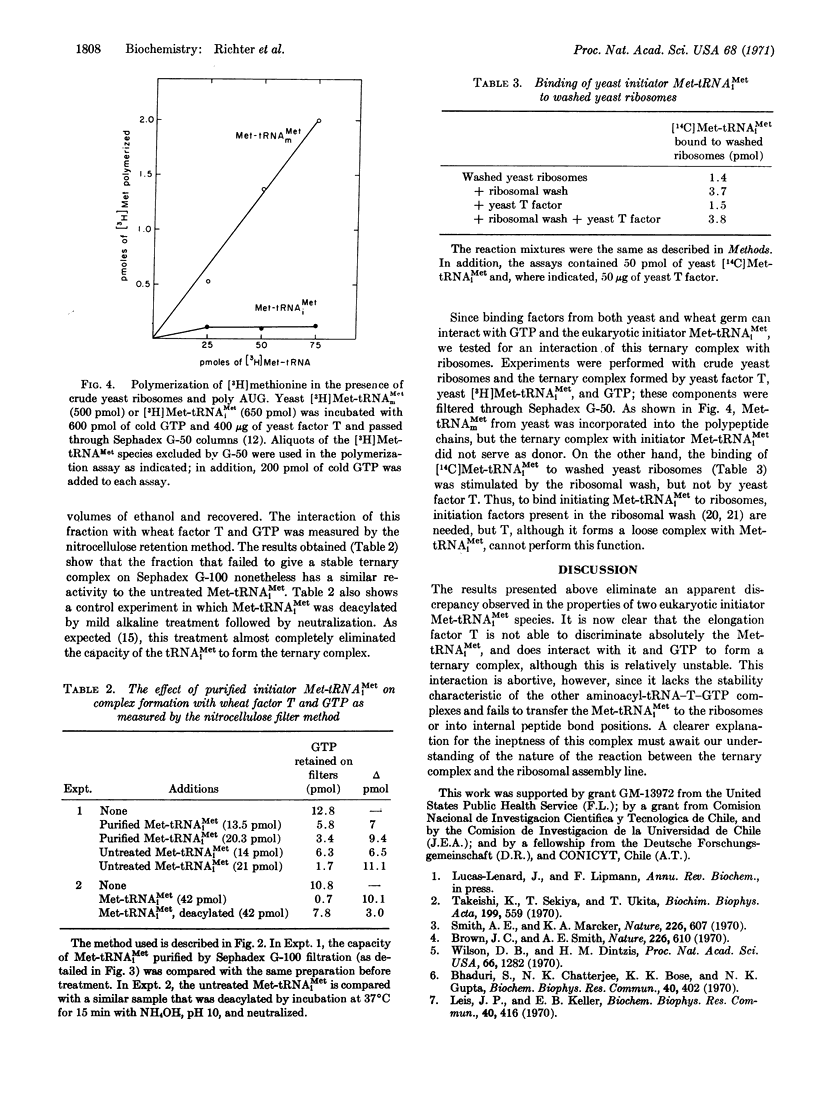
The initiator tRNA, methionyl-tRNAiMet, of yeast and wheat germ forms relatively unstable ternary complexes with their corresponding elongation factors T and GTP. Such complexes can be demonstrated only with fast separation techniques such as Sephadex G-50 and Millipore filtration, but not with the slow Sephadex G-100 method, although both techniques yield stable ternary complexes with all other aminoacyl-tRNAs, including the internal Met-tRNAmMet. To bind yeast-initiating Met-tRNAiMet to ribosomes, initiation factors present in a ribosomal wash fraction from yeast are needed.
Keywords: yeast, wheat, Millipore filter, Sephadex
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhaduri S., Chatterjee N. K., Bose K. K., Gupta N. K. Initiation of protein synthesis in rabbit reticulocytes. Biochem Biophys Res Commun. 1970 Jul 27;40(2):402–407. doi: 10.1016/0006-291x(70)91023-5. [DOI] [PubMed] [Google Scholar]
- Brown J. C., Smith A. E. Initiator codons in eukaryotes. Nature. 1970 May 16;226(5246):610–612. doi: 10.1038/226610a0. [DOI] [PubMed] [Google Scholar]
- Caskey C. T., Redfield B., Weissbach H. Formylation of guinea pig liver methionyl-sRNA. Arch Biochem Biophys. 1967 Apr;120(1):119–123. doi: 10.1016/0003-9861(67)90605-4. [DOI] [PubMed] [Google Scholar]
- Ghosh K., Grishko A., Ghosh H. P. Initiation of protein synthesis in eukaryotes. Biochem Biophys Res Commun. 1971 Feb 5;42(3):462–468. doi: 10.1016/0006-291x(71)90393-7. [DOI] [PubMed] [Google Scholar]
- Jerez C., Sandoval A., Allende J., Henes C., Ofengand J. Specificity of the interaction of aminoacyl ribonucleic acid with a protein-guanosine triphosphate complex from wheat embryo. Biochemistry. 1969 Jul;8(7):3006–3014. doi: 10.1021/bi00835a049. [DOI] [PubMed] [Google Scholar]
- Leis J. P., Keller E. B. Protein Chain-Initiating Methionine tRNAs in Chloroplasts and Cytoplasm of Wheat Leaves. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1593–1599. doi: 10.1073/pnas.67.3.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leis J. P., Keller E. B. Protein chain initiation by methionyl-tRNA. Biochem Biophys Res Commun. 1970 Jul 27;40(2):416–421. doi: 10.1016/0006-291x(70)91025-9. [DOI] [PubMed] [Google Scholar]
- Marcus A., Bewley J. D., Weeks D. P. Aurintricarboxylic acid and initiation factors of wheat embryo. Science. 1970 Mar 27;167(3926):1735–1736. doi: 10.1126/science.167.3926.1735. [DOI] [PubMed] [Google Scholar]
- NIRENBERG M., LEDER P. RNA CODEWORDS AND PROTEIN SYNTHESIS. THE EFFECT OF TRINUCLEOTIDES UPON THE BINDING OF SRNA TO RIBOSOMES. Science. 1964 Sep 25;145(3639):1399–1407. doi: 10.1126/science.145.3639.1399. [DOI] [PubMed] [Google Scholar]
- RajBhandary U. L., Ghosh H. P. Studies on polynucleotides. XCI. Yeast methionine transfer ribonucleic acid: purification, properties, and terminal nucleotide sequences. J Biol Chem. 1969 Mar 10;244(5):1104–1113. [PubMed] [Google Scholar]
- Richter D. Formation of a ternary complex between yeast aminoacyl-tRNA binding factor, GTP, and aminoacyl-tRNA. Biochem Biophys Res Commun. 1970 Mar 12;38(5):864–870. doi: 10.1016/0006-291x(70)90800-4. [DOI] [PubMed] [Google Scholar]
- Richter D., Lipmann F. Formation of a ternary complex between formylatable yeast Met-tRNA, GTP and binding factor T of yeast and of E. coli. Nature. 1970 Sep 19;227(5264):1212–1214. doi: 10.1038/2271212a0. [DOI] [PubMed] [Google Scholar]
- Richter D., Lipmann F. Separation of mitochondrial and cytoplasmic peptide chain elongation factors from yeast. Biochemistry. 1970 Dec 22;9(26):5065–5070. doi: 10.1021/bi00828a004. [DOI] [PubMed] [Google Scholar]
- Shafritz D. A., Anderson W. F. Isolation and partial characterization of reticulocyte factors M1 and M2. J Biol Chem. 1970 Nov 10;245(21):5553–5559. [PubMed] [Google Scholar]
- Shafritz D. A., Laycock D. G., Anderson W. F. Puromycin-peptide bond formation with reticulocyte initiation factors M1 and M2. Proc Natl Acad Sci U S A. 1971 Feb;68(2):496–499. doi: 10.1073/pnas.68.2.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. E., Marcker K. A. Cytoplasmic methionine transfer RNAs from eukaryotes. Nature. 1970 May 16;226(5246):607–610. doi: 10.1038/226607a0. [DOI] [PubMed] [Google Scholar]
- Takeishi K., Sekiya T., Ukita T. Selective utilization of nonformylatable species of methionyl-tRNA's from Escherichia coli and yeast in a reticulocyte cell-free system. Biochim Biophys Acta. 1970 Feb 18;199(2):559–561. doi: 10.1016/0005-2787(70)90108-5. [DOI] [PubMed] [Google Scholar]
- Takeishi K., Ukita T., Nishimura S. Characterization of two species of methionine transfer ribonucleic acid from bakers' yeast. J Biol Chem. 1968 Nov 10;243(21):5761–5768. [PubMed] [Google Scholar]
- Tarragó A., Monasterio O., Allende J. E. Initiator-like properties of a methionyl-tRNA from wheat embryos. Biochem Biophys Res Commun. 1970 Nov 9;41(3):765–773. doi: 10.1016/0006-291x(70)90079-3. [DOI] [PubMed] [Google Scholar]
- Wilson D. B., Dintzis H. M. Protein chain initiation in rabbit reticulocytes. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1282–1289. doi: 10.1073/pnas.66.4.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]


