Abstract
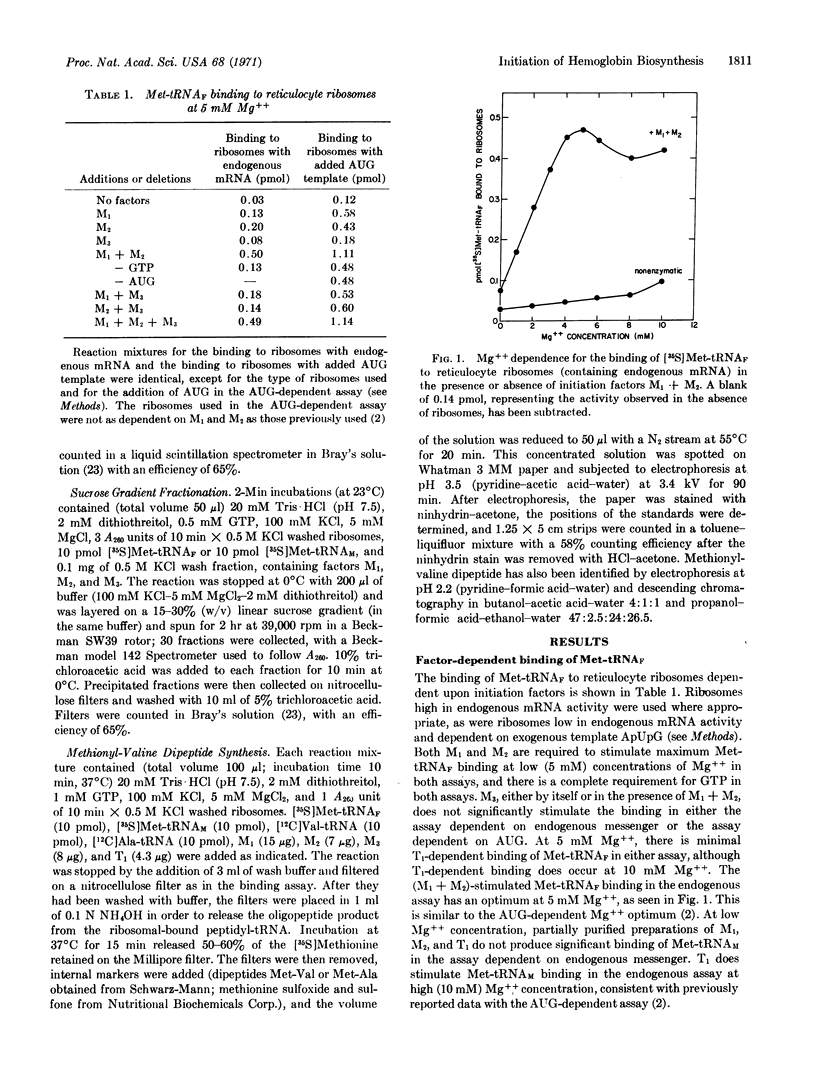
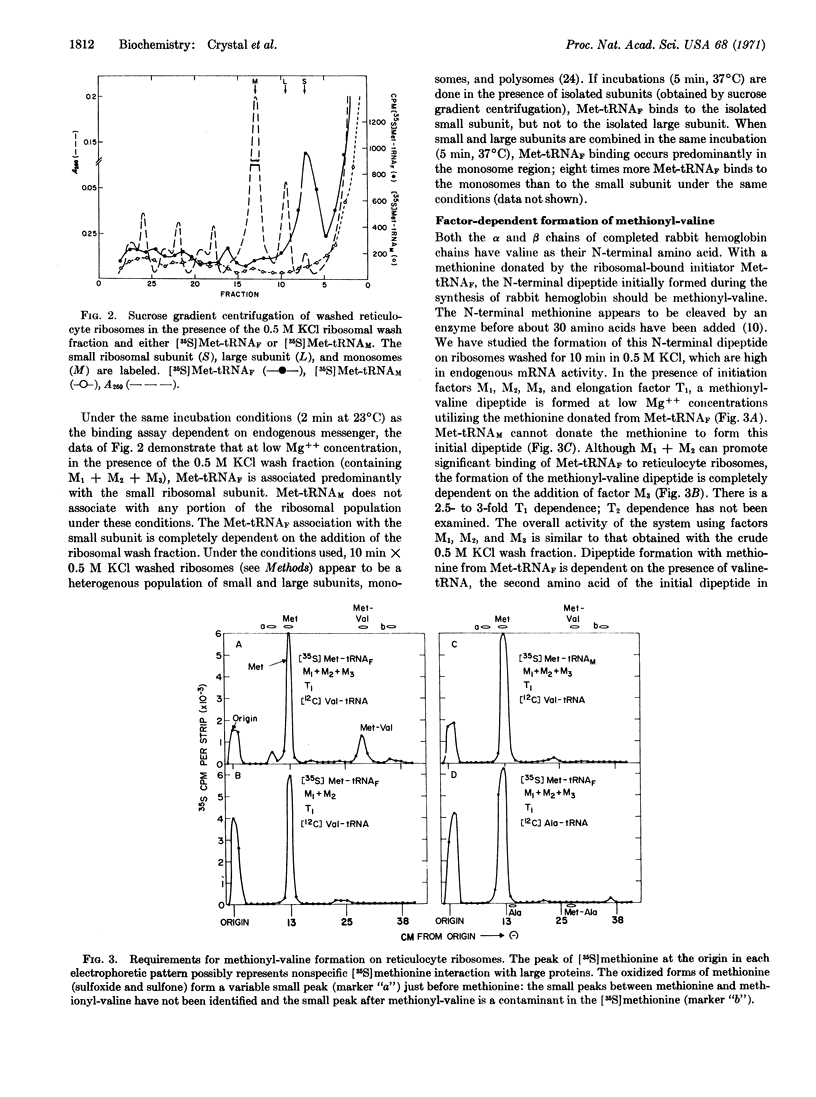
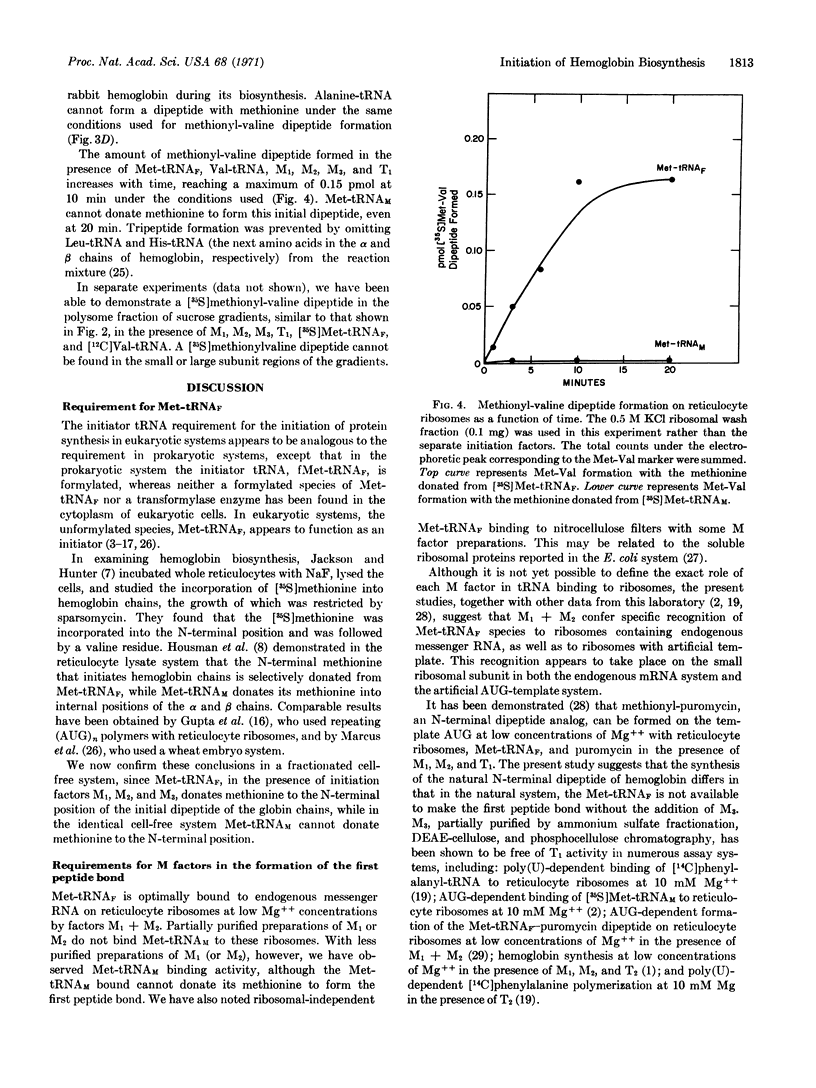
Initiation factors M1 + M2 from reticulocyte ribosomes bind Met-tRNAF to rabbit reticulocyte ribosomes containing endogenous hemoglobin mRNA. The initial binding of Met-tRNAF appears to be to the small ribosomal subunit. The Met-tRNAF is able to participate in what is presumed to be the first peptide bond in the formation of hemoglobin, namely the synthesis of a methionyl-valine dipeptide. The formation of this methionyl-valine dipeptide requires Met-tRNAF, initiation factors M1, M2, and M3, as well as Val-tRNA and T1. No synthesis of methionyl-valine dipeptide takes place if Met-tRNAF is replaced by Met-tRNAM, or if initiation factor M3 is omitted. Thus, Met-tRNAF appears to be the initiator tRNA for hemoglobin biosynthesis and M3, although required for the synthesis of the first peptide bond of hemoglobin, does not appear to be necessary, under the experimental conditions studied, for Met-tRNAF binding.
Keywords: ribosomes, rabbit, initiation factors, elongation factor, endogenous messenger RNA
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhaduri S., Chatterjee N. K., Bose K. K., Gupta N. K. Initiation of protein synthesis in rabbit reticulocytes. Biochem Biophys Res Commun. 1970 Jul 27;40(2):402–407. doi: 10.1016/0006-291x(70)91023-5. [DOI] [PubMed] [Google Scholar]
- Brot N., Yamasaki E., Redfield B., Weissbach H. The binding of aminoacyl-tRNA and poly U to a soluble factor (S) extracted from ribosomes. Biochem Biophys Res Commun. 1970 Aug 11;40(3):698–707. doi: 10.1016/0006-291x(70)90960-5. [DOI] [PubMed] [Google Scholar]
- Brown J. C., Smith A. E. Initiator codons in eukaryotes. Nature. 1970 May 16;226(5246):610–612. doi: 10.1038/226610a0. [DOI] [PubMed] [Google Scholar]
- Caskey C. T., Redfield B., Weissbach H. Formylation of guinea pig liver methionyl-sRNA. Arch Biochem Biophys. 1967 Apr;120(1):119–123. doi: 10.1016/0003-9861(67)90605-4. [DOI] [PubMed] [Google Scholar]
- Culp W., Morrisey J., Hardesty B. Initiator tRNA for the synthesis of globin peptides. Biochem Biophys Res Commun. 1970 Aug 24;40(4):777–785. doi: 10.1016/0006-291x(70)90970-8. [DOI] [PubMed] [Google Scholar]
- Gilbert J. M., Anderson W. F. Cell-free hemoglobin synthesis. II. Characteristics of the transfer ribonucleic acid-dependent assay system. J Biol Chem. 1970 May 10;245(9):2342–2349. [PubMed] [Google Scholar]
- Greenshpan H., Revel M. Initiator protein dependent binding of messenger RNA to ribosomes. Nature. 1969 Oct 25;224(5217):331–335. doi: 10.1038/224331a0. [DOI] [PubMed] [Google Scholar]
- Gupta N. K., Chatterjee N. K., Bose K. K., Bhaduri S., Chung A. Roles of methionine transfer RNA's in protein synthesis in rabbit reticulocytes. J Mol Biol. 1970 Nov 28;54(1):145–154. doi: 10.1016/0022-2836(70)90452-3. [DOI] [PubMed] [Google Scholar]
- Heywood S. M. Specificity of mRNA binding factor in eukaryotes. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1782–1788. doi: 10.1073/pnas.67.4.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerz W., McCarty K. S. Evidence for a proposed initiation complex for protein synthesis in reticulocyte polyribosome profiles. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1206–1213. doi: 10.1073/pnas.63.4.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housman D., Jacobs-Lorena M., Rajbhandary U. L., Lodish H. F. Initiation of haemoglobin synthesis by methionyl-tRNA. Nature. 1970 Aug 29;227(5261):913–918. doi: 10.1038/227913a0. [DOI] [PubMed] [Google Scholar]
- Iwasaki K., Sabol S., Wahba A. J., Ochoa S. Translation of the genetic message. VII. Role of initiation factors in formation of the chain initiation complex with Escherichia coli ribosomes. Arch Biochem Biophys. 1968 May;125(2):542–547. doi: 10.1016/0003-9861(68)90612-7. [DOI] [PubMed] [Google Scholar]
- Jackson R., Hunter T. Role of methionine in the initiation of haemoglobin synthesis. Nature. 1970 Aug 15;227(5259):672–676. doi: 10.1038/227672a0. [DOI] [PubMed] [Google Scholar]
- Kerwar S. S., Spears C., Weissbach H. Studies on the initiation of protein synthesis in animal tissues. Biochem Biophys Res Commun. 1970 Oct 9;41(1):78–84. doi: 10.1016/0006-291x(70)90471-7. [DOI] [PubMed] [Google Scholar]
- Kuechler E., Rich A. Position of the initiator and peptidyl sites in the E. coli ribosome. Nature. 1970 Mar 7;225(5236):920–924. doi: 10.1038/225920a0. [DOI] [PubMed] [Google Scholar]
- Leis J. P., Keller E. B. Protein Chain-Initiating Methionine tRNAs in Chloroplasts and Cytoplasm of Wheat Leaves. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1593–1599. doi: 10.1073/pnas.67.3.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leis J. P., Keller E. B. Protein chain initiation by methionyl-tRNA. Biochem Biophys Res Commun. 1970 Jul 27;40(2):416–421. doi: 10.1016/0006-291x(70)91025-9. [DOI] [PubMed] [Google Scholar]
- Marcus A., Weeks D. P., Leis J. P., Keller E. B. Protein chain initiation by methionyl-tRNA in wheat embryo. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1681–1687. doi: 10.1073/pnas.67.4.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon H. M., Collins J. F., Maxwell E. S. Discrimination by rat liver aminoacyltransferase I against Met-tRNA-F. Biochem Biophys Res Commun. 1970 Oct 9;41(1):170–176. doi: 10.1016/0006-291x(70)90484-5. [DOI] [PubMed] [Google Scholar]
- NIRENBERG M., LEDER P. RNA CODEWORDS AND PROTEIN SYNTHESIS. THE EFFECT OF TRINUCLEOTIDES UPON THE BINDING OF SRNA TO RIBOSOMES. Science. 1964 Sep 25;145(3639):1399–1407. doi: 10.1126/science.145.3639.1399. [DOI] [PubMed] [Google Scholar]
- Prichard P. M., Gilbert J. M., Shafritz D. A., Anderson W. F. Factors for the initiation of haemoglobin synthesis by rabbit reticulocyte ribosomes. Nature. 1970 May 9;226(5245):511–514. doi: 10.1038/226511a0. [DOI] [PubMed] [Google Scholar]
- Revel M., Herzberg M., Becarevic A., Gros F. Role of protein factor in the functional binding of ribosomes to natural messenger RNA. J Mol Biol. 1968 Apr 14;33(1):231–249. doi: 10.1016/0022-2836(68)90291-x. [DOI] [PubMed] [Google Scholar]
- Shafritz D. A., Anderson W. F. Factor dependent binding of methionyl-tRNAs to reticulocyte ribosomes. Nature. 1970 Aug 29;227(5261):918–920. doi: 10.1038/227918a0. [DOI] [PubMed] [Google Scholar]
- Shafritz D. A., Anderson W. F. Isolation and partial characterization of reticulocyte factors M1 and M2. J Biol Chem. 1970 Nov 10;245(21):5553–5559. [PubMed] [Google Scholar]
- Shafritz D. A., Laycock D. G., Anderson W. F. Puromycin-peptide bond formation with reticulocyte initiation factors M1 and M2. Proc Natl Acad Sci U S A. 1971 Feb;68(2):496–499. doi: 10.1073/pnas.68.2.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. E., Marcker K. A. Cytoplasmic methionine transfer RNAs from eukaryotes. Nature. 1970 May 16;226(5246):607–610. doi: 10.1038/226607a0. [DOI] [PubMed] [Google Scholar]
- Takeishi K., Sekiya T., Ukita T. Selective utilization of nonformylatable species of methionyl-tRNA's from Escherichia coli and yeast in a reticulocyte cell-free system. Biochim Biophys Acta. 1970 Feb 18;199(2):559–561. doi: 10.1016/0005-2787(70)90108-5. [DOI] [PubMed] [Google Scholar]
- Wilson D. B., Dintzis H. M. Protein chain initiation in rabbit reticulocytes. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1282–1289. doi: 10.1073/pnas.66.4.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]



