Abstract
Circulating total free fatty acids (FFA) are elevated early in myocardial infarction (MI) and are associated with an increase in mortality. We investigated the association of serum unbound free fatty acids (FFAu) levels with mortality,in patients presenting with ST elevation myocardial infarction (STEMI) in the Thrombolysis in Myocardial Infarction (TIMI) II trial.TIMI II enrolled patients within 4 hours of chest pain. Patients were treated with recombinant tissue plasminogen activator within 1 hour of enrollment. The concentration of FFAu was evaluated in serum samplesfrom 1834 patients obtained at baseline, before therapy.FFAu was an independent risk factor for death as early as one day of hospitalization and continued to be an independent risk factor for the more than 3·8 years of follow up. When adjusted for other cardiovascular risk factors FFAu levels in the fourth as compared to the first quartile remained an independent risk factor for death due to MI (hazard ratio, 5.0; 95 % confidence interval, 1.9-13.0), to all cardiac death (hazard ratio, 2.4; confidence interval, 1.3-4.4) and to all cause death (hazard ratio, 1.9, confidence interval, 1.2-3.1).Females were twice as likely to be in the upper two FFAu quartiles and had approximately twice the rate of death as males. In conclusion, increased levels of FFAu are one of the earliest molecular biomarkers of mortality in STEMI and are independent of other risk factors known to affect outcomes in STEMI.
Keywords: unbound free fatty acids, mortality, myocardial infarction, risk factors
Plasma free fatty acid (FFA) levels are elevated early after acute myocardial infarction (MI) and correlate with increased rates of arrhythmias and mortality, particularly within the first 12 hours.1-4 The MI-associated FFA increase occurs primarily through catecholamine activation of adipose tissue lipolysis rather than FFA release from the ischemic cardiac tissue.4 Although most plasma FFA is bound to albumin, a small fraction (< 10−4) is unbound FFA (FFAu). FFAu levels increase exponentially with increasing ratio of total FFA to albumin and thus FFAu are more sensitive to physiologic changes than total FFA.5;6FFAulevels increase rapidly, within 30 minutes of cardiac ischemia induced by balloon angioplasty.7;8We investigated whether FFAulevels from patients in the Thrombolysis in Myocardial Infarction (TIMI)Phase II trial of STEMI provide an independent assessment of risk for poor outcomes at timesas early as 24 hours after symptoms.
METHODS
The TIMI II trial treated 3262 patients,who presented within 4 hours of STEMIonset,with intravenous recombinant tissue plasminogen activator plus heparin.9After recombinant tissue plasminogen activatortherapy patients were randomly assigned to either a percutaneous coronary interventionor a conservative strategyin which only patients exhibiting ischemia (13%) received percutaneous coronary intervention. TIMI investigators recorded demographics, medical history and outcomes over a follow up period of 3.8 years. A Limited Access DataSetof patient parameters was available for this study.
Measurements were performed on serum samples (baseline) drawn prior to recombinant tissue plasminogen activator and heparin therapy.10Serum wascollected in STEMI patients by the TIMI investigators 11 and maintained at −70°C by the National Heart Lung Blood Institute blood specimen repository. A subset of these specimens from 2500 patients was provided by the National Heart Lung Blood Institute. The linkage between patient information and blood specimen tube identification was maintained separately by the Maryland Medical Research Institute. After our results were deposited with the National Heart Lung Blood Institute, the linkage was un-blinded. Complete patient information from the Limited Access Data Setwas available for 1834 patients of the 2500 baseline blood specimens and results reported here are for the 1834 patients. This study complies with the Declaration of Helsinki and was approved by the Institutional Review Board committee of the Torrey Pines Institute for Molecular Studies.
Measurements of serum FFAu concentrations were performed using the fluorescent probe ADIFAB2 (FFA Sciences) as described previously in cardiac ischemia and MI patients8;12 but modified for 96-well plate fluorometry using a Flurolog 3 spectrofluorometer with a MicroMax plate reader (J.Y Horiba). Serumsamples were diluted to 1% (v/v) in 200 L of measuring buffer13 in 96-well plates. Fluorescence was measured after addingADIFAB2 (1.5 mol/L) and the intensities were used to determine the ratio (R) of the fluorescence intensitiesat 550 to 457 nm,with background subtracted. Sample FFAu concentrations (nmol/L) were calculated using FFAu=227(R-Ro)/(0.925 – R), where Ro is the ADIFAB2 fluorescence ratio in the absence of FFA and the numeric factors were determined as described previously 6. All samples were measured in duplicate, yielding an average CV of 6.5 %.
FFAu quartiles were determined using all baseline FFAu values (Table 1). Correlations between FFAu and patient baseline characteristics were carried out usingallcardiovascular confounders available in the limited data set. In TIMI II, patients with renal disorders were excluded, Killip class was not recorded and creatine kinase was the only cardiac biomarker measured. Outcomes of death due to STEMI, to cardiac causes and to all causes were determined through committee adjudication by the TIMI investigators. Statistical analyses were performed using XLSTAT (Addinsoft, New York), p values ≤ 0.05 were considered significant.
Table 1.
Baseline parameters for TIMI II patients as a function of unbound free fatty acid quartiles.
| Number in quartiles | Quartile1 458 | Quartile2 458 | Quartile3 459 | Quartile4 459 | p Value |
|---|---|---|---|---|---|
| Unbound free fatty acids (nmol/L) | 1.9 (0.09 – 2.6) | 3.2 (2.6 – 3.9) | 4.9 (3.9 – 6.4) | 10.2 (6.46 – 523) | |
| Age (years) | 53.9 | 53.8 | 55.3 | 55.4 | 0.008 |
| Men | 398(87%) | 389(85%) | 344(75%) | 372(81%) | <0.0001 |
| White | 398(87%) | 398(87%) | 409(89%) | 398(87%) | 0.62 |
| Body Mass Index (kg/m2) | 27 (15-40) | 27 (18-46) | 27 (16-54) | 27 (17-54) | 0.55 |
| Diastolic blood pressure (mm Hg) | 81 (42 -130) | 81 (50 -110) | 80.2 (40-120) | 78.2 (40-118) | 0.053 |
| Systolic blood pressure (mm Hg) | 130 (56 -180) | 130 (60-183) | 129 (80-210) | 126 (50 -190) | 0.044 |
| Creatine Kinase (IU/L) | 105 (5 -1760) | 104 (12 -3590) | 94 (11 -4422) | 99 (11-3654) | 0.080 |
| Diabetes mellitus | 41(9%) | 37(8%) | 73(16%) | 78(17%) | <0.0001 |
| Prior MI | 60(13%) | 64(14%) | 69(15%) | 73(16%) | 0.50 |
| Prior hypertension | 174(38%) | 156(34%) | 188(41%) | 179(39%) | 0.20 |
| β blocker within 24 hours | 87(19%) | 87(19%) | 78(17%) | 73(16%) | 0.68 |
Values for ordinal parameters are percent and p values were calculated by χ2. Continuous parameters are median values and intra-quartile ranges. p values were determined by the Kruskal-Wallis test. Ages were grouped to protect confidentiality and are proportional to the grouped mean for each quartile. Baseline creatine kinase levels were elevated above the upper limit of normal in fewer than 20 % of patients. Mean time from chest pain symptom to treatment initiation was virtually identical (2.6 h) for each FFAu quartile (data not shown). Demographic and clinical parameters in this table are representative of the entire TIMI II population.10
RESULTS
Baseline FFAu levels were measured in serum samples collected within 4 hours of initial symptoms. FFAu concentrations ranged from < 1 nmol/L to > 500 nmol/L (Table 1). This range is larger than we reported previously for a cohort of nominally healthy subjects whose range was 0.6 to 4.5 nmol/L with a mean value of 1.5 nmol/L.12Correlations of FFAu quartiles with all cardiovascular risk factors available from the limited access data set and for which patientswith the indicated risk factors comprised at least 10% of all patients are shown in Table 1.
Of the 1834 patients, 187 died from all causes, 125 from cardiac causes and 76 from MI (Table 2). A positive correlation of death from MI with FFAu quartile was present as early as one day following enrollment and peaked at about 30 days post enrollment. Because most deaths within 30 days were due to MI, cardiac deaths and deaths from all causes also correlated strongly with baseline FFAu. Peak (within 8 hours, Table 2), but not baseline (Table 1) creatine kinase levels,were correlated with FFAu.
Table 2.
Death and outcomes according to baseline unbound free fatty acid quartiles
| Median unbound free fatty acid (nmol/L) | Quartile1 1.9 | Quartile2 3.2 | Quartile3 4.9 | Quartile4 10.2 | Total | p Value | p Trend |
|---|---|---|---|---|---|---|---|
| Death due to: | |||||||
| Myocardial Infarction at 1 day | 3 | 7 | 11 | 12 | 33 (1.8%) | 0.1 | 0.015 |
| Myocardial Infarction at 7 days | 3 | 7 | 19 | 21 | 50 (2.7%) | 0.0002 | <0.0001 |
| Myocardial Infarction at 30 days | 4 | 10 | 21 | 29 | 64 (3.5%) | <0.0001 | <0.0001 |
| Myocardial Infarction at 3.8 years | 6 | 14 | 24 | 32 | 76 (4.1%) | <0.0001 | <0.0001 |
| All cardiac causes at 1 day | 3 | 7 | 11 | 12 | 33 (1.8%) | 0.1 | 0.015 |
| All cardiac causes at 7 days | 3 | 7 | 19 | 21 | 50 (2.7%) | 0.0002 | <0.0001 |
| All cardiac causes at 30 days | 5 | 11 | 21) | 29 | 66 (3.6%) | <0.0001 | <0.0001 |
| All cardiac causes at 3.8 years | 16 | 27 | 38 | 44 | 125 (6.9%) | 0.0006 | <0.0001 |
| All causes at 1 day | 3 | 8 | 13 | 13 | 37 (2.0%) | 0.056 | 0.0095 |
| All causes at 7 days | 3 | 9 | 24 | 23 | 59 (3.2%) | <0.0001 | <0.0001 |
| Al causes at 30 days | 5 | 13 | 26 | 34 | 78 (4.3%) | <0.0001 | <0.0001 |
| All causes at 3.8 years | 29 | 38 | 57 | 63 | 187 (10%) | 0.0004 | <0.0001 |
| Non cardiovascular causes at 3.8 years* | 13 | 9 | 13 | 15 | 50 (2.7%) | 0.67 | 0.530 |
| Other outcomes | |||||||
| Peak Creatine Kinase (8 hours) (IU/L) | 1628 | 1957 | 2039 | 2156 | <0.0001 |
All other outcomes are numbers of patients. The number of deaths from all causes is equal to all cause cardiac plus non-cardiac plus hemorrhage. Values in parenthesis are % of total number of patients (1834). p values were determined by the χ2 test for all but peak creatine kinase for which the Kruskal-Wallis test was used. p trend values were calculated using the Cochran-Armitage trend test.
An additional 12 patients died due to hemorrhage over the 3·8 year follow up period (4 at day 1). Adding all cardiac plus non-cardiovascular deaths, plus one death without cause sums to 187.
Relative to the total TIMI II population FFAu levels correlated positively with female gender, age, and diabetes. Approximately twice as many females and diabetics were in the upper two FFAu quartiles (Table 1). Deaths due to MI in non-diabetic females and males as well as in diabetics increased with increasing FFAu quartile (Table 3). This correlation reached significance in non-diabetic females and males, but not obtained in diabetics. The lack of correlation in diabetics is likely due to small numbers and to two deaths within 5 hours in the first quartile, but no additional deaths in the first quartile over the following 3.8 years. The death rate for females and diabetics was almost 2-fold > for non-diabetic males,except for day 1, mostly due to larger death rates at 30 days in the 3rd and 4th FFAu quartiles. Using subgroup interaction analysis death rates in Q3+Q4 were significantly (p=0.05) higher in non-diabetic females thanmales but not (p=0.07) inall diabetics versus non-diabetics14.
Table 3.
Myocardial infarction deaths in females, males and diabetics relative to baseline unbound free fatty acids.
| Unbound free fatty acid Quartiles | Quartile1 | Quartile2 | Quartile3 | Quartile4 | Total | p Value | p Trend |
|---|---|---|---|---|---|---|---|
| Non Diabetic Females (n=283): | |||||||
| By day 1 | 0 | 1 | 1 | 3 | 5(1.8%) | 0.02 | 0.10 |
| By 7 days | 0 | 1 | 5 | 6 | 12(4.2%) | 0.08 | 0.009 |
| By 30 days | 0 | 2 | 5 | 8 | 15(5.3%) | 0.04 | 0.004 |
| By 3.8 years | 1 | 2 | 6 | 8 | 17(6.0%) | 0.1 | 0.06 |
| Non Diabetic Males (n=1322): | |||||||
| By day 1 | 1 | 6 | 7 | 6 | 20(1.5%) | 0.9 | <0.022 |
| By 7 days | 1 | 6 | 9 | 10 | 26(2.0%) | <0.014 | <0.0018 |
| By 30 days | 2 | 7 | 11 | 14 | 34(2.6%) | <0.003 | <0.0003 |
| By 3.8 years | 3 | 10 | 12 | 17 | 42(3.2%) | <0.002 | <0.0002 |
| Diabetics (n=229): | |||||||
| By day 1 | 2 | 0 | 3 | 3 | 8(3.5%) | 0.11 | 0.86 |
| By 7 days | 2 | 0 | 5 | 5 | 12(5.2%) | 0.44 | 0.37 |
| By 30 days | 2 | 1 | 5 | 7 | 15(6.6%) | 0.57 | 0.23 |
| By 3.8 years | 2 | 2 | 6 | 7 | 17(7.4%) | 0.79 | 0.32 |
Data are numbers of deaths and percent of each subgroup (n). p values were determined by χ2 and p trend by the Cochran-Armitage trend test.
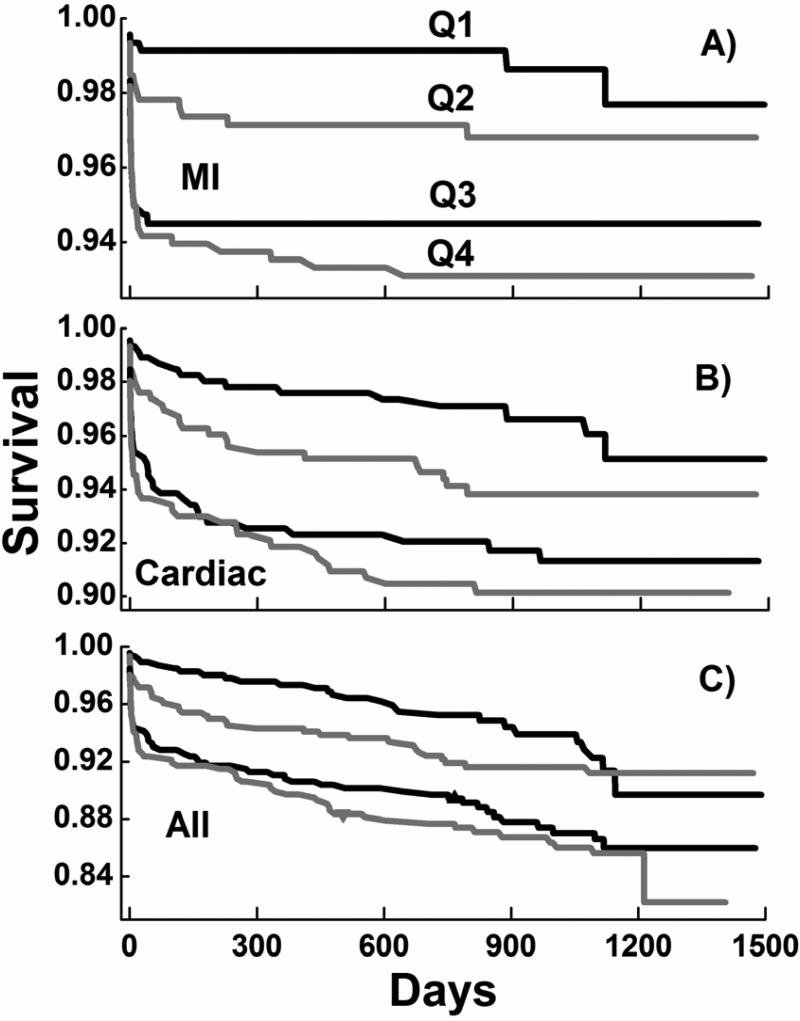
Kaplan-Meier survival curves for deaths due to STEMI, to all cardiac causes and to all causes reveal striking dependencies on FFAu quartiles (Figure 1). Log-rank tests indicate that the risk of death for all three categories increased significantly (p < 0.001) with increasing FFAu quartile. For MI almost all deaths occurred within the first 30 days. For deaths from all causes the survival curve reveals a similar slope after 60 daysfor all 4 quartiles, consistent with a lack of correlation with FFAu for non-cardiac mortality (Table 2). Cox proportional hazard modeling of the three sets of survival curves reveals hazard ratios that increase significantly with FFAu quartile relative to the first quartile and are relatively unaffected by other risk factors (Table 4). The unadjusted hazard ratios for MI deaths increased from 2.3 for Q2 to 5.6 for Q4 and the corresponding adjusted hazard ratios were 2.9 and 5, respectively. Except for the Q1 to Q2 increase, the hazard ratios increases with FFAu quartile were significant (p ≤ 0.03) for all unadjusted and adjusted analyses.
Figure 1. Kaplan-Meier survival curves for death by quartiles of baseline FFAu.
Survival curves for Q1 to Q4 for death due to A) MI, B) all cardiac causes and C) all causes. Log-rank probabilities were < 0·001.
Table 4.
Cox proportional hazard ratios for deaths relative to the first unbound free fatty acid quartile
| Quartilea | Hazard Ratio | CI (95%) | p Value | |
|---|---|---|---|---|
| Myocardial Infarction Deaths | ||||
| Unadjusted | Q2 | 2.3 | (0.9 – 5.9) | 0.094 |
| Q3 | 4 | (1.6 – 9.8) | 0.002 | |
| Q4 | 5.6 | (2.3 – 13) | < 0.001 | |
| Adjusted | Q2 | 2. 9 | (1.0 – 8.2) | 0.043 |
| Q3 | 4.2 | (1.6 – 11) | 0.002 | |
| Q4 | 5 | (1.9 – 13) | 0.001 | |
| All Cause Cardiac Deaths | ||||
| Unadjusted | Q2 | 1.7 | (0.9 – 3.1) | 0.103 |
| Q3 | 2.4 | (1.3 – 4.3) | 0.003 | |
| Q4 | 2.8 | (1.6 – 5.0) | 0.000 | |
| Adjusted | Q2 | 1.7 | (0·9 – 3.2) | 0.108 |
| Q3 | 2.1 | (1.2 – 4.0) | 0.015 | |
| Q4 | 2.4 | (1.3 – 4.4) | 0.004 | |
| All Cause Deaths | ||||
| Unadjusted | Q2 | 1.3 | (0.8 – 2.1) | 0.324 |
| Q3 | 1.9 | (1.2 – 3.0) | 0.004 | |
| Q4 | 2.2 | (1.4 – 3.4) | 0.001 | |
| Adjusted | Q2 | 1.3 | (0.8 – 2.1) | 0.374 |
| Q3 | 1.7 | (1.0 – 2.7) | 0.032 | |
| Q4 | 1.9 | (1.2 – 3.1) | 0.004 |
Cox proportional hazard model generated hazard ratios and corresponding 95 % confidence interval (CI) and Wald's χ2 probabilities. The model was adjusted for age, gender, race, body mass index, diastolic and systolic blood pressure at baseline, history of diabetes, MI and hypertension and use of β blockers within 24 hours of enrollment. This choice of factors was dictated by the limited access data set, known cardiac risk factors associated with more than 10 % of the 1834 patients and previous observed associations with free fatty acids.
DISCUSSION
Oliver and Opie recognized more than 40 years ago that serum levels of FFA increase rapidly following MI and that the elevated FFA levels may, by inducing arrhythmias, contribute to sudden cardiac death and death in MI.1-4Moreover, these studies found that early death in MI was highly correlated with increasing total FFA levels 1. Total FFA levels were also correlated with the long term (6.9 years) increased risk of sudden cardiac death inthe LURIC study.15Our results are consistent with and additive to LURIC.15Both the present and LURIC studies found strong baseline correlations between FFAu or total FFA levels and female gender or a history of diabetes (Table 1).Hazard ratios for MI deaths in TIMI II were larger (≥3 fold) and less sensitive to other risk factors than in the LURIC study. Presumably, this increase in sensitivity is a reflection of the acute presentation of STEMI in TIMI II and the high degree of FFAu sensitivity to cardiac ischemia 7;8.
The lower survival of females and diabetics as compared to non-diabetic men (Table 3) is consistent with other studies in STEMI patients.16;17In TIMI II females had higher FFAu levels than men; median FFAu for non-diabetic men and females were 3·7 nmol/L and 4.5 nmol/L, respectively.The higher FFAu levels for femalesin TIMI II might reflect a gender specific difference in the response to STEMI or might be a related to the older (7 years) age of females in TIMI II, given that FFAu increase with age (Table 1). However, the adjusted (see Table 4 legend) hazard ratio for death due to MI for females in Q4 versus Q1 is 9·7 (95% CI: 0.971 to 100) with p = 0.053. These results, and the higher death rates in Q3+Q4 for females, raise the possibility that FFAu may be a more potent independent risk factor for females than for males.
Although these results demonstrate that FFAu correlate with early death after STEMI, causation is unproven. The increase in circulating FFAu may simply reflect the ischemia-induced activation of adipose lipolysis in proportion to the degree of ischemia and is probably largely generated from adipose tissue lipolysis stimulated by an ischemia-mediated increase in catecholamine levels.3;4In contrast, evidence for FFA having a causal role are studies in nonischemic animals in which increasing circulating FFA adversely affect myocardial metabolism, stimulate insulin resistance, induce arrhythmias and increase cardiac enzyme release.18-21Consistent with a causal role for FFAuin TIMI II are the increase in peak (8 hours) but not baselinecreatine kinase levels with increasing baseline FFAu(Table 3).
If FFA adversely affectmyocardial function, reducing FFAu levels at times early after the ischemic event may reduce deaths and/or arrhythmias in MI.3;4Infusions of glucose-insulin-potassium have been, shown to be protective in the dog 22, were used to treat STEMI patients andreduced circulating total FFA acutely in STEMI patients.23Although the effect on outcome in STEMI patients has been mixed, it has been suggested that GIK treatment would be most effective if given at the earliest possible time.24;25 This concept was implemented in the IMMEDIATE trial by treating STEMI patients with glucose-insulin-potassium in pre-hospital emergency medical settings.25;26The IMMEDIATE trial revealed that glucose-insulin-potassium significantly reduced FFA levels, reduced cardiac arrest plus in-hospital mortality and reduce infarct size as compared to patients not treated with glucose-insulin-potassium. Conceivably, therapeutic efficacy of such interventions, using glucose-insulin-potassium or inhibitors of lipolysis, would be most evident in those MI patients who present with the highest levels of FFAu; for which further studies are necessary.
Evidence-based guidelines currently recommend the rapid application of re-perfusion therapy with primacy given to percutaneous coronary artery intervention, with limited use of tissue plasminogen activator,the therapy in TIMI II. Nevertheless,the30 day death rate in TIMI II (4.2%) was not significantly differentthan the 5.4% in contemporary STEMI patients.16Adjustments for confounders that might affect hazard ratios for FFAuwere limited because important prognostic factors27,includingKillip class, baseline heart rate, number of diseased vessels, ejection fraction,smokingand troponin, were not available or not recorded in the limited access data set. ACE inhibitors and statins were first approved for use in 1981 and 1987, respectively. TIMI II enrollment wasbetween 1986 and 1988 and therefore most patients were unlikely to have been treated with these medications. Catecholamines are associated, weakly, with mortality in STEMI 28 but were not measured. The beneficial effects of glucose-insulin-potassium suggest that FFA not catecholamines areimportant contributors to mortality in STEMI.TIMI II protocols did not anticipate determination of FFA levels and therefore blood samples may have had higher ex vivo than in vivo FFA as a consequence of lipoprotein lipolysis.29However, this ex vivo effect would have obscured rather than enhanced the observed FFAu correlations with outcome. The determination of FFAu concentrations requires knowledge of the relative distribution of the different FFAu present in serum and was estimated from the distribution of total FFA as described in references.6;13Differences in the FFAu distributions such as those that may occur in acute coronary syndromes30 are not expected to alter the levels of total FFAu significantly.6The study was borderline under powered for female patients, suggesting the need for further studies.
ACKNOWLEDGEMENTS
We thank the TIMI investigators, the NHLBI and the Maryland Medical Research Institute.
FUNDING
This work was supported by in part by the National Institutes of Healthgrants DK070314, DK058762 andin part by FFA Sciences LLC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
AMK is the founder of and major stock holder in FFA Sciences LLC. AHH, JPK, TK and BZ either are (AHH) or were employees, are inventors on patents and applications assigned to FFA Sciences LLC and have profit interests in FFA Sciences LLC. JA is an advisor to FFA Sciences LLC.
References
- 1.Oliver MF, Kurien VA, Greenwood TW. Relation between serum-free-fatty acids and arrhythmias and death after acute myocardial infarction. Lancet. 1968;291:710–714. doi: 10.1016/s0140-6736(68)92163-6. [DOI] [PubMed] [Google Scholar]
- 2.Gupta DK, Jewitt DE, Young R, Hartog M, Opie LH. Increased plasma-free-fatty-acid concentrations and their significance in patients with acute myocardial infarction. Lancet. 1969;294:1209–1213. doi: 10.1016/s0140-6736(69)90749-1. [DOI] [PubMed] [Google Scholar]
- 3.Oliver MF, Opie LH. Effects of glucose and fatty acids on myocardial ischaemia and arrhythmias. Lancet. 1994;343:155–158. doi: 10.1016/s0140-6736(94)90939-3. [DOI] [PubMed] [Google Scholar]
- 4.Oliver MF. Sudden cardiac death: the lost fatty acid hypothesis. QJM. 2006;99:701–709. doi: 10.1093/qjmed/hcl084. [DOI] [PubMed] [Google Scholar]
- 5.Richieri GV, Anel A, Kleinfeld AM. Interactions of long chain fatty acids and albumin: Determination of free fatty acid levels using the fluorescent probe ADIFAB. Biochemistry. 1993;32:7574–7580. doi: 10.1021/bi00080a032. [DOI] [PubMed] [Google Scholar]
- 6.Richieri GV, Kleinfeld AM. Unbound free fatty acid levels in human serum. J Lipid Res. 1995;36:229–240. [PubMed] [Google Scholar]
- 7.Kleinfeld AM, Prothro D, Brown DL, Davis RC, Richieri GV, DeMaria A. Increases in serum unbound free fatty acid levels following coronary angioplasty. Am J Cardiol. 1996;78:1350–1354. doi: 10.1016/s0002-9149(96)00651-0. [DOI] [PubMed] [Google Scholar]
- 8.Cantor WJ, Hoe Kim H, Jolly S, Moe G, Burstein JM, Mendelsohn A, Kleinfeld AM, Fitchett D. B-Type Natriuretic Peptide and Serum Unbound Free Fatty Acid Levels after Contemporary Percutaneous Coronary Intervention. J Invasive Cardiol. 2008;20:186–188. [PubMed] [Google Scholar]
- 9.The TIMI Study Group Comparison of invasive and conservative strategies after treatment with intravenous tissue plasminogen activator in acute myocardial infarction. Results of the thrombolysis in myocardial infarction (TIMI) phase II trial. New Engl J Med. 1989;320:618–625. doi: 10.1056/NEJM198903093201002. [DOI] [PubMed] [Google Scholar]
- 10.Passamani E, Hodges M, Herman M, Grose R, Chaitman B, Rogers W, Forman S, Terrin M, Knatterud G, Robertson T. The Thrombolysis in Myocardial Infarction (TIMI) phase II pilot study: tissue plasminogen activator followed by percutaneous transluminal coronary angioplasty. J Am Coll Cardiol. 1987;10:51B–64B. doi: 10.1016/s0735-1097(87)80429-1. [DOI] [PubMed] [Google Scholar]
- 11.Bovill EG, Terrin ML, Stump DC, Berke AD, Frederick M, Collen D, Feit F, Gore JM, Hillis LD, Lambrew CT. Hemorrhagic events during therapy with recombinant tissue-type plasminogen activator, heparin, and aspirin for acute myocardial infarction. Results of the Thrombolysis in Myocardial Infarction (TIMI), Phase II Trial. Ann Intern Med. 1991;115:256–265. doi: 10.7326/0003-4819-115-4-256. [DOI] [PubMed] [Google Scholar]
- 12.Apple FS, Kleinfeld AM, Adams JE. Unbound Free Fatty Acid Concentrations Are Increased in Cardiac Ischemia. Clinical Proteomics. 2004;1:41–44. [Google Scholar]
- 13.Huber AH, Kampf JP, Kwan T, Zhu B, Kleinfeld AM. Fatty acid-specific fluorescent probes and their use in resolving mixtures of different unbound free fatty acids in equilibrium with albumin. Biochemistry. 2006;45:14263–14274. doi: 10.1021/bi060703e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ. 2003;326:219. doi: 10.1136/bmj.326.7382.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pilz S, Scharnagl H, Tiran B, Wellnitz B, Seelhorst U, Boehm BO, Marz W. Elevated plasma free fatty acids predict sudden cardiac death: a 6.85-year follow-up of 3315 patients after coronary angiography. Eur Heart J. 2007;28:2763–2769. doi: 10.1093/eurheartj/ehm343. [DOI] [PubMed] [Google Scholar]
- 16.Donahoe SM, Stewart GC, McCabe CH, Mohanavelu S, Murphy SA, Cannon CP, Antman EM. Diabetes and mortality following acute coronary syndromes. JAMA. 2007;298:765–775. doi: 10.1001/jama.298.7.765. [DOI] [PubMed] [Google Scholar]
- 17.Jneid H, Fonarow GC, Cannon CP, Hernandez AF, Palacios IF, Maree AO, Wells Q, Bozkurt B, Labresh KA, Liang L, Hong Y, Newby LK, Fletcher G, Peterson E, Wexler L. Sex differences in medical care and early death after acute myocardial infarction. Circulation. 2008;118:2803–2810. doi: 10.1161/CIRCULATIONAHA.108.789800. [DOI] [PubMed] [Google Scholar]
- 18.Kurien VA, Yates PA, Oliver MF. Free fatty acids, heparin, and arrhythmias during experimental myocardial infarction. Lancet. 1969;294:185–187. doi: 10.1016/s0140-6736(69)91424-x. [DOI] [PubMed] [Google Scholar]
- 19.de Leiris J, Opie LH, Lubbe WF. Effects of free fatty acid and glucose on enzyme release in experimental myocardial infarction. Nature. 1975;253:746–747. doi: 10.1038/253746a0. [DOI] [PubMed] [Google Scholar]
- 20.Vik-Mo H, Mjos OD. Influence of free fatty acids on myocardial oxygen consumption and ischemic injury. Am J Cardiol. 1981;48:361–365. doi: 10.1016/0002-9149(81)90621-4. [DOI] [PubMed] [Google Scholar]
- 21.Gruzdeva O, Uchasova E, Dyleva Y, Belik E, Kashtalap V, Barbarash O. Relationship between free fatty acids, insulin resistance markers, and oxidized lipoproteins in myocardial infarction and acute left ventricular failure. Diabetes Metab Syndr Obes. 2013;6:103–111. doi: 10.2147/DMSO.S37830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maroko PR, Libby P, Sobel BE, Bloor CM, Sybers HD, Shell WE, Covell JW, Braunwald E. Effect of glucose-insulin-potassium infusion on myocardial infarction following experimental coronary artery occlusion. Circulation. 1972;45:1160–1175. doi: 10.1161/01.cir.45.6.1160. [DOI] [PubMed] [Google Scholar]
- 23.Chaudhuri A, Janicke D, Wilson M, Ghanim H, Wilding GE, Aljada A, Dandona P. Effect of modified glucose-insulin-potassium on free fatty acids, matrix metalloproteinase, and myoglobin in ST-elevation myocardial infarction. Am J Cardiol. 2007;100:1614–1618. doi: 10.1016/j.amjcard.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 24.Kloner RA, Nesto RW. Glucose-insulin-potassium for acute myocardial infarction: continuing controversy over cardioprotection. Circulation. 2008;117:2523–2533. doi: 10.1161/CIRCULATIONAHA.107.697979. [DOI] [PubMed] [Google Scholar]
- 25.Selker HP, Beshansky JR, Sheehan PR, Massaro JM, Griffith JL, D'Agostino RB, Ruthazer R, Atkins JM, Sayah AJ, Levy MK, Richards ME, Aufderheide TP, Braude DA, Pirrallo RG, Doyle DD, Frascone RJ, Kosiak DJ, Leaming JM, Van Gelder CM, Walter GP, Wayne MA, Woolard RH, Opie LH, Rackley CE, Apstein CS, Udelson JE. Out-of-hospital administration of intravenous glucose-insulin-potassium in patients with suspected acute coronary syndromes: the IMMEDIATE randomized controlled trial. JAMA. 2012;307:1925–1933. doi: 10.1001/jama.2012.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grossman AN, Opie LH, Beshansky JR, Ingwall JS, Rackley CE, Selker HP. Glucose-insulin-potassium revived: current status in acute coronary syndromes and the energy-depleted heart. Circulation. 2013;127:1040–1048. doi: 10.1161/CIRCULATIONAHA.112.130625. [DOI] [PubMed] [Google Scholar]
- 27.Morrow DA, Antman EM, Charlesworth A, Cairns R, Murphy SA, de Lemos JA, Giugliano RP, McCabe CH, Braunwald E. TIMI risk score for ST-elevation myocardial infarction: A convenient, bedside, clinical score for risk assessment at presentation: An intravenous nPA for treatment of infarcting myocardium early II trial substudy. Circulation. 2000;102:2031–2037. doi: 10.1161/01.cir.102.17.2031. [DOI] [PubMed] [Google Scholar]
- 28.Ostrowski SR, Pedersen SH, Jensen JS, Mogelvang R, Johansson PI. Acute myocardial infarction is associated with endothelial glycocalyx and cell damage and a parallel increase in circulating catecholamines. Crit Care. 2013;17:R32. doi: 10.1186/cc12532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zambon A, Hashimoto SI, Brunzell JD. Analysis of techniques to obtain plasma for measurement of levels of free fatty acids. J Lipid Res. 1993;34:1021–1028. [PubMed] [Google Scholar]
- 30.Bhardwaj A, Truong QA, Peacock WF, Yeo KT, Storrow A, Thomas S, Curtis KM, Foote RS, Lee HK, Miller KF, Januzzi JL., Jr. A multicenter comparison of established and emerging cardiac biomarkers for the diagnostic evaluation of chest pain in the emergency department. Am Heart J. 2011;162:276–282. doi: 10.1016/j.ahj.2011.05.022. [DOI] [PubMed] [Google Scholar]