Abstract
Thin sheets of nanofibrous (NF) poly(L-lactic acid) (PLLA) matrix were fabricated using a novel phase separation method, mimicking the structure of natural collagen fibers. In this study, the cell morphology, cytoskeleton and adhesion structure, proliferation and differentiation were investigated on NF PLLA matrix using an osteoblast cell line model. Scanning electron microscopy revealed that the MC3T3-E1 cells took a more rounded shape on NF matrix, with abundant interactions with nanofibers. There were no long dense stress fibers or typical focal adhesion structures formed on NF matrix. In consistence with the flat cell morphology and abundant focal adhesions, the cell proliferation was faster on flat PLLA films. With the addition of ascorbic acid (AA), cells were induced to differentiate both on NF matrix and flat films. Cells on NF matrix exhibited an enhanced osteoblast differentiation phenotype, with dramatically higher bone sialoprotein (BSP) gene expression (2 orders of magnitude higher) and significantly higher alkaline phosphatase (ALP) activities. Strikingly, even without the addition of AA and thus no natural collagen fibers deposited into the matrix, the BSP gene expression was still highly up-regulated on NF matrix, showing a direct effect of PLLA nanofibers on BSP gene expression. Enhanced BSP gene expression was correlated with the down-regulation of the small GTPase RhoA activities. Inhibition of RhoA effector ROCK induced BSP gene expression of cells in AA-free medium on flat PLLA films. These results suggest that the nanofibers promote the differentiation of osteoblasts likely through RhoA-Rock signaling pathway.
Keywords: polylactic acid, osteoblast, bone tissue engineering, nanofiber, differentiation
Introduction
Osteoblasts secrete abundant extracellular matrix (ECM) during the process of differentiation. The ECM of osteoblasts contains collagenous and noncollagenous proteins. In the absence of ascorbic acid (AA) which is necessary for post-translational modification of proline to hydroxyproline, or through the use of specific inhibitors to collagen fiber assembly, the formation of collagen fibers can be inhibited, and the differentiation of osteoblasts can thus be blocked, suggesting the important role of collagenous ECM on osteoblast differentiation [1, 2].
Collagen matrices have been used for cell cultures, and it was found that differentiation of bone marrow stem cells into osteoblastic lineage was induced when cells were embedded in collagen gel but not in Petri dishes [3], providing strong evidence that cells require matrix environments to achieve specific phenotype. To mimic the morphological structure of natural collagen matrix with fiber diameters in the range of 50–500 nm, a novel phase separation technique has been developed to fabricate nanofibrous (NF) matrices in our laboratory [4, 5]. To enhance cell seeding capacity and vascularization, highly porous and interconnected NF scaffolds have also been developed in our laboratory by combining the phase separation technique with new porogen leaching techniques that can better control pore size and inter-pore connectivity [6–8]. Previously, it was found that with a high surface/volume ratio and enhanced adsorption of cell adhesion proteins such as fibronectin and vitronectin, cell adhesion to NF scaffold was enhanced [9]. Further investigation found that the differentiation of MC3T3-E1 pre-osteoblastic cells was also enhanced on NF scaffolds [8]. Since cell differentiation was also affected by 3D structures of scaffolds including pore size and connectivity, and mass transfer conditions [5], to investigate the direct interactions between osteoblasts and the PLLA nanofibers, and the related signaling pathways, thin sheets of NF matrix were fabricated and cell behavior on the NF matrix sheets was investigated in the present study.
Materials and methods
Reagents
PLLA with an inherent viscosity of approximately 1.6 dl/g was purchased from Boehringer Ingelheim (Ingelheim, Germany). Ascorbic acid-free α-modified essential medium (α-MEM) was obtained from Quality Biological (Gaithersburg, MD, USA). Fetal bovine serum (FBS), penicillin-streptomycin, Dulbecco’s phosphate-buffered saline (PBS), and trypsin-EDTA were purchased from Gibco BRL Products, Life Technologies (Grand Island, NY, USA). Gelatin, glutaraldehyde, osmium tetraoxide, ascorbic acid, β -glycerophosphate, neutral-buffered formalin, Triton X-100, mouse anti-vinculin antibody were obtained form Sigma Chemical Co. (St. Louis, MO, USA). Fluorescein isothiocyanate (FITC)-conjugated secondary antibody was obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). Tetramethyl rhodamine isothiocyanate (TRITC) conjugated phalloidin was obtained from Invitrogen Co. (Carlsbad, CA, USA). Rho-associated kinase (ROCK) inhibitor Y-27632 was from Calbiochem (Darmstadt, Germany). Mitogen-activated protein kinase kinase (MEK) inhibitor U0126 was from Promega (Madison, WI, USA).
Fabrication of PLLA NF matrix and flat films
PLLA was dissolved in tetrahydrofuran (THF) (10 % wt/v) at 60ºC and cast into a preheated glass mold. The mold was quickly sealed using a cover glass. The PLLA solution was phase separated at −20 ºC for 2 h and then immersed into ice/water mixture to exchange THF for 24 h. The matrix was washed with distilled water at room temperature for 24 h. The obtained thin sheets of NF matrix (thickness~40mu;m) were then vacuum-dried for 2 days. Flat films were fabricated in a similar manner excluding the phase separation step. Instead, the solvent was evaporated at room temperature in a fume hood.
Surface modification with gelatin
Surface modification of PLLA NF matrix was carried out as previously reported [10]. Briefly, PLLA matrix was activated in aqueous poly(diallyldimethylammonium chloride) (PDAC) solution at the concentration of 1 mg/mL for 1 h. After washed in water for 1 min, the matrix was immersed in 1 mg/mL gelatin solution (pH=7.68) for 20 min and then washed with water. The PDAC/gelatin assembled layers were fixed by crosslinking gelatin with 1-ethyl-3-(3-dimethyl aminopropyl) carbodiimide (EDC) and N-hydroxysuccinimide (NHS) in morpholineethanesulfonic acid (MES) buffer at 4ºC for 24 h. The crosslinked matrix was washed with distilled water and vacuum-dried.
Cell culture and seeding
MC3T3-E1 subclone 4 (MC-4) cells were a gift from Dr. Renny Franceschi (University of Michigan, Ann Arbor, MI, USA). The cells were cultured in ascorbic acid-free α-MEM supplemented with 10% FBS, 1% penicillin/streptomycin in a humidified incubator at 37ºC, with 5% CO2. For differentiation study, 3.5cm × 3.5cm square PLLA NF matrix thin sheets and PLLA flat films were sterilized by soaking in 70% ethanol for 30 min, washed three times with PBS for 30 min each, and twice in cell culture medium for 1 h each on an orbital shaker. To avoid NF matrix floating in the medium and cell growth on the downside of the matrix or films, NF matrix and flat films were placed on 60mm Petri dishes, with the four edges covered and sealed by strips of Parafilm, leaving 3cm × 3cm area exposed for cell seeding. Cells were plated at a density of 10,000 cells/cm2 with a medium volume of 1 ml. The cell suspension was limited on the exposed NF matrix or flat films due to the hydrophobic property of Parafilm placed on the four edges. After 2 h, most cells had adhered, and 4 ml more medium was added to each Petri dish. Medium was changed after the initial 24 h of seeding and culture, and then was changed every 2 days using either AA-containing or AA-free medium as specified in the text. For ALP activity assay, 50 mu;g/ml AA and 10 mM β-glycerophosphate were added to the culture medium. For proliferation assay, smaller (1.5cm × 1.5cm) NF matrix and flat films were used. NF matrix or flat films were placed in the wells of 12 well plates, sealed with Parafilm, leaving 1cm × 1cm area for cell seeding. Cells were plated at the same density of 10,000 cells/cm2 in 100 mu;l medium. After the initial 2 h of cell seeding, 900 mu;l more medium was added to each well.
SEM observation
Cells cultured on PLLA NF matrix and flat films were rinsed in PBS, fixed in 2.5% glutaraldehyde, and post-fixed in 1% osmium tetroxide. Samples were dehydrated in a series of increasing concentrations of ethanol in distilled water and hexamethyldisilizane (HMDS). The samples were then sputter-coated with gold and observed under a SEM (Philips XL30 FEG) at 10 kV.
Immunofluoresence microscopy
Samples were rinsed with PBS, fixed with 3.7% formaldehyde in PBS for 25 min, washed three times with PBS, then treated in blocking solution (0.1% TritonX-100, 10% goat serum in PBS) for 1 h. Mouse anti-vinculin antibody was diluted at 1:100 in blocking solution and added to samples, incubated overnight at 4ºC. After being washed for three times with PBS solution, FITC-conjugated secondary antibody was added at 1:100 dilutions and incubated for 1 h. After being washed for three times with PBS solution, samples were incubated with TRITC-conjugated phalloidin for 20 min. After being washed with PBS solution for 3 more times, they were mounted on glass slides using mounting medium containing 4'-6-Diamidino-2-phenylindole (DAPI) and observed under a confocal microscope (Olympus FluoView 500).
Proliferation assay
Cell proliferation was examined using the CellTiter 96 Aqueous One Solution Cell Proliferation Assay kit (Promega, Madison, WI, USA) according to the manufacturer’s protocol. Briefly, the 1x1 cm NF matrix or flat films with cells were moved to a new 24- well cell culture plate, 200 mu;l fresh medium and 40 mu;l CellTiter 96 Aqueous One Solution Reagent were added to each well, and the plate was incubated in the incubator for 1.5 h. Absorbance was read at 490 nm.
RNA analysis
Samples were homogenized with a Tissue-Tearor. Total RNA was isolated using RNeasy Mini Kit (Qiagen, Valencia, CA, USA), and DNA was digested by RNase-free DNase set (Qiagen) according to the manufacturer’s protocol. The cDNA was reverse-transcribed with TaqMan reverse transcription reagents (Applied Biosystems, Foster City, CA, USA). Real time PCR was carried out using TaqMan Universal PCR Master Mix (Applied Biosystems) and specific primers for OCN (5-CCGGGAGCAGTGTGAGCTTA-3 and 5-TAGATGCGTTTGTAGGCGGTC-3) and BSP (5-CAGAGGAGGCAAGCGTCACT-3 and 5-CTGTCTGGGTGCCAACACTG-3) on an ABI Prism 7500 Real time PCR system (Applied Biosystems) [8]. Gene expression levels were normalized against glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression.
Alkaline phosphatase (ALP) assay
ALP was extracted and detected with EnzoLyte pNPP Alkaline Phosphatase Assay Kit (AnaSpec, San Jose, CA, USA) according to the manufacturer’s protocol. Briefly, cells on NF matrix or flat films were homogenized in 400 mu;l lysis buffer provided in the kit. Lysate was centrifuged for 15 min at 10,000 g at 4 ºC. Supernatant was collected for ALP assay using p-nitrophenyl phosphate (p-NPP) as a phosphatase substrate and alkaline phosphatase provided in the kit as a standard. Amount of ALP in the cells was normalized against total DNA content [9].
Western blotting
Cells were plated at a density of 5x104 cells/cm2 and incubated for 6 and 12 h. Cells were washed twice with cold PBS buffer. Cells were scraped off and centrifuged. The collected cell pellets were resuspended in ice-cold lysis buffer. Protein concentrations were determined from cell lysates clarified by centrifugation at 14,000 rpm for 10 min. 30 mu;g protein lysates were resuspended in 3x loading buffer (0.0625 M Tris-HCl pH 6.8, 25% glycerol, 2% sodium dodecyl sulfate, 0.01% bromophenol blue, 5% β-mercaptoethanol) and were subjected to electrophoresis and transferred to polyvinylidene difluoride membranes. Blots were probed with antibody to active extracelluar signal regulated kinese 1/2 (ERK1/2) or α-tubulin (both from Cell signaling Technology, Danvers, MA, USA) at 1:1000 dilution, followed by 1:1000 dilution of horseradish peroxidase- conjugated (HRP) goat anti-rabbit IgG antibody and an enhanced chemiluminescent (ECL) substrate. The house-keeping gene α-tubulin was used as control.
RhoA pull down assay
Cells were plated at a higher density of 5x104 cells/cm2 and incubated for 12 h. Activation of RhoA was determined with Rho activation assay biochem kit from Cytoskeleton (Denver, CO, USA). Briefly, cells were washed with cold PBS, scraped, centrifuged and then suspended in lysis buffer provided by the supplier. Part of the cell lysate was used for the amount of total RhoA by western blot experiments. Pull down experiments were performed following the instruction provided by the supplier. Western blot was performed according to the ECL protocol.
Statistical analysis
For all experiments, values were reported as mean ± S.D. based on triplicate cell cultures. The experiments were performed twice to ensure reproducibility. To test the significance of observed differences between the study groups, an unpaired Student’s t-test was applied. A value of p < 0.05 was considered to be statistically significant.
Results
Morphology of cells on NF matrix and flat films
The average diameter of the fibers of PLLA NF matrix was between 100 and 200 nm, which is in the same diameter range as that of natural collagen fibers (Fig.1A, B). The fiber diameters of the PLLA NF matrix did not change significantly with the concentration of PLLA solution that was used to prepare the NF matrix from 5% to 10% (Data not shown). For all cell culture experiments, 10% PLLA solution was used to fabricate PLLA NF matrix. The morphology of cells on the two different surfaces was examined using SEM and fluorescence confocal microcopy. After 24 h of culture in AA-free medium, the cells on NF matrix showed a more “rounded” phenotype with small processes interacting with PLLA nanofibers (Fig. 1C). In contrast, the cells on flat films were spread over larger areas (Fig. 1D, note the magnification difference from Fig.1C). Cytoskeleton structure was examined using F-actin stain. It was shown that few stress fibers formed in the cells cultured on PLLA NF matrix (Fig. 2A); in contrast, abundant long actin stress fibers formed in cells cultured on PLLA flat films (Fig.2B).
Figure 1.
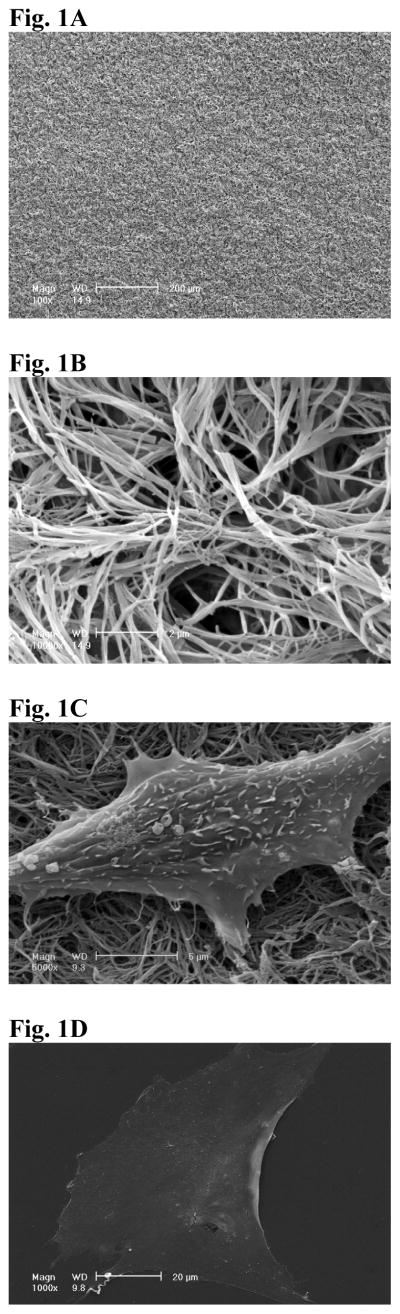
SEM view of NF matrix at low magnification (A) and high magnification (B). The average diameter of the fibers of PLLA NF matrix was between 100 and 200 nm. SEM view of MC-4 cells cultured on NF matrix (C) and flat films (D) for 24hr. The cells on NF matrix were more rounded, with small processes interacting with PLLA nanofibers.
Figure 2.

Cytoskeleton and adhesion structures of MC-4 cells cultured on different surfaces. F-actin and vinculin stain of cells cultured on NF matrix (A), flat films (B), gelatin coated NF matrix (C). Vinculin was stained green, F-actin was stained red and nucleus was stained blue. Scale bar: 50 mμ.
Modification of PLLA nanofibers with gelatin had little effect on stress fiber formation (Fig.2C). There were no typical focal adhesion structures in cells, with low vinculin stain on either NF matrix (Fig.2A) or gelatin-modified NF matrix (Fig.2C). On PLLA flat films, there were abundant spot-like focal adhesion structures (Fig.2B), especially at the ends of long stress fibers.
Proliferation of cells on NF matrix
Viable cells were counted using CellTiter 96 Aqueous One Solution Cell Proliferation Assay. Cells were cultured in AA-free or AA-supplemented medium and the proliferation assays were performed on days 1, 4 and 7. Cells proliferated faster on PLLA flat films than on PLLA NF matrix. The number of cells cultured in AA-containing and AA-free media on PLLA flat films were not statistically significantly different. However, there were significantly more cells in the AA-containing medium than in AA-free medium after being cultured on NF matrix for 7 days (Fig. 3).
Figure 3.
Proliferation of cells on PLLA NF matrix and flat films for 1 day,4 days and 7days in AA-free medium or AA-supplemented medium. A significant increase in cell number was observed between day 4 and day 1 on all surfaces (day 4 versus day 1); between day 7 and day 4 on flat films and NF matrix in the presence of AA (day 7 versus day 4) but not on NF matrix in the absence of AA (* p<0.05, **p<0.01,*** p<0.001 ). At day 7, cell number on NF matrix in the presence of AA were significantly higher than that in AA-free medium (#p<0.05).
Gene expression of bone differentiation markers
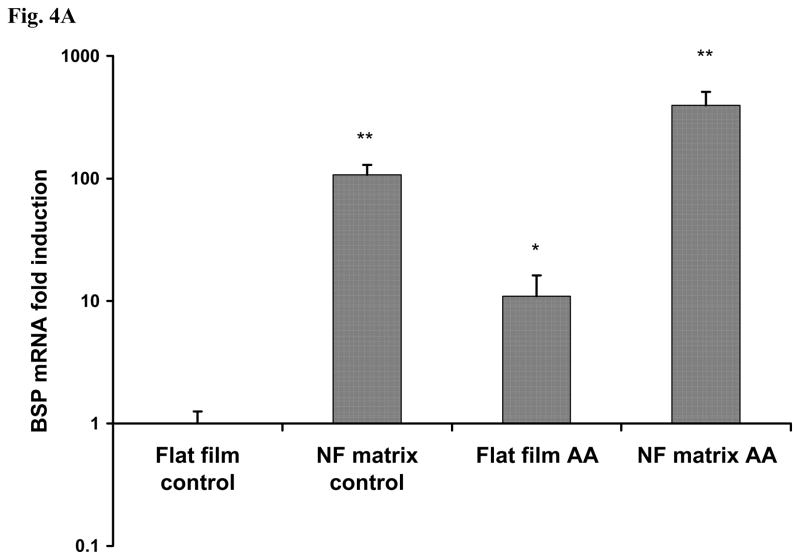
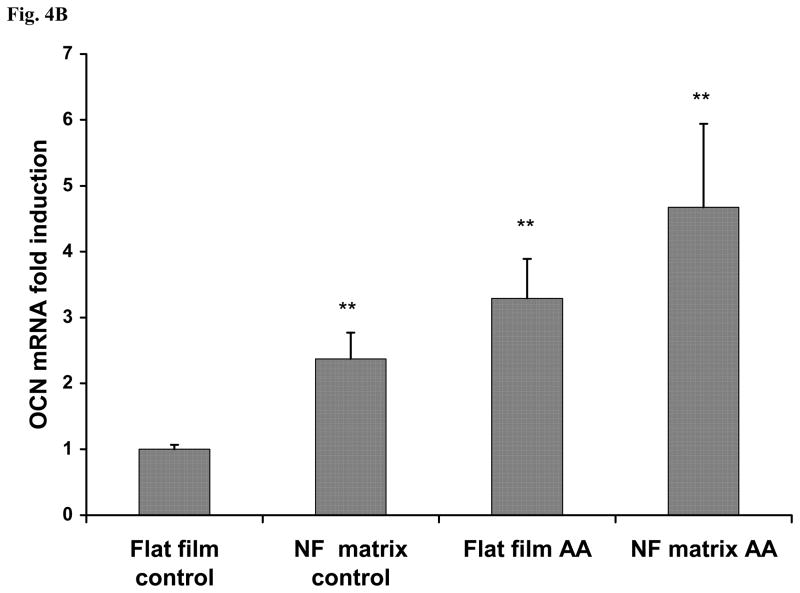
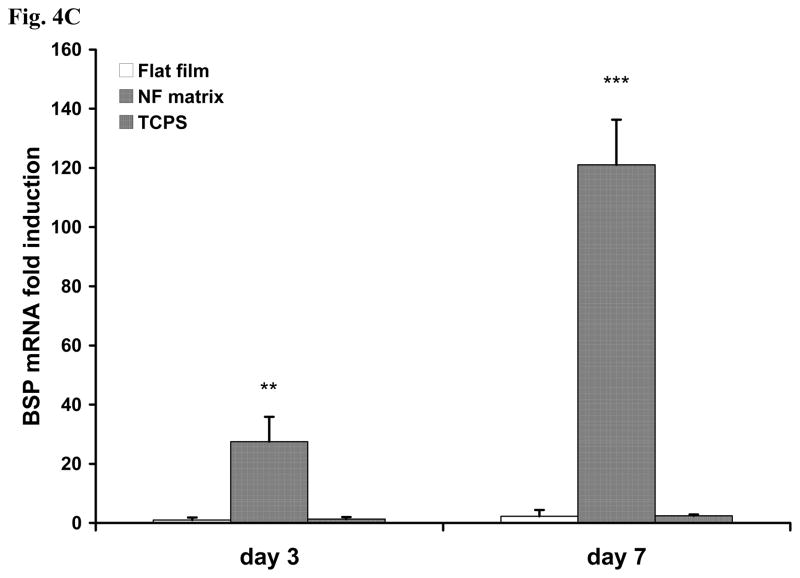
After cell seeding and culture in the AA-free medium on NF matrix and flat films for 24 h, either AA-containing or AA-free medium was used for subsequent cell cultures. After 6 days of culture following the 1 day cell seeding and initial culture, the gene expression of bone sialoprotein (BSP) and osteocalcin (OCN) was assayed using real time PCR. The expression levels of both BSP and OCN genes were significantly higher in the AA-containing medium than in the AA-free medium. The expression level of BSP was orders of magnitude higher in cells cultured on the NF matrix than on the flat films (Fig.4A). OCN gene was also expressed at a significantly higher level on the NF matrix than on the flat films (Fig.4B). Strikingly, even without the supplement of AA, BSP gene expression level was very high on the NF matrix: two orders of magnitude higher than that on the flat films without AA, and even an order of magnitude higher than that in cells cultured in AA-containing medium on the flat films. This result suggests that there is a direct inductive effect of PLLA nanofibers on BSP gene expression. Similarly, the PLLA nanofibrous matrix also enhanced the OCN gene expression in cells cultured either with or without AA supplement although at a less dramatic degree compared to the effect on BSP gene expression. To further probe the underlying signaling pathway, we focused on BSP gene expression because of its high responsiveness to both substrate structure and the inductive agent (AA) based on the afore-mentioned results. To verify that PLLA flat films can serve as reasonable control surfaces, the BSP gene expression on both PLLA flat films and NF matrix sheets were compared with the conventional cell culture surface -- tissue culture polystyrene (TCPS) for 3 and 7 days of cell culture in the AA-free medium. It was found that there was no significant difference in BSP expression level between cells cultured on TCPS and PLLA flat films at both days 3 and 7, while the BSP gene expression was dramatically enhanced on the NF matrix at both of these time points (Fig. 4C).
Figure 4.
BSP (A) and OCN (B) gene expression of cells cultured on PLLA NF matrix and flat films with or without AA supplement for 6 days. (C) BSP gene expression of cells on TCPS, flat films and NF matrix for day 3 and day 7 without AA supplement (* p<0.05, ** p<0.01, *** p<0.001 versus flat films control).
ALP activities of cells on NF matrix
Consistent with higher BSP gene expression, ALP content of cells on NF matrix was much higher than that on flat films after 6 days of culture in complete induction medium (supplemented with 50 mu;g/ml AA, 10mM β -Glycerophosphate): 6.42±0.65 ng/mu;g DNA on NF matrix versus 1.78±0.41 ng/mu;g DNA on flat films, p<0.001.
Effect of ERK activity on BSP gene expression
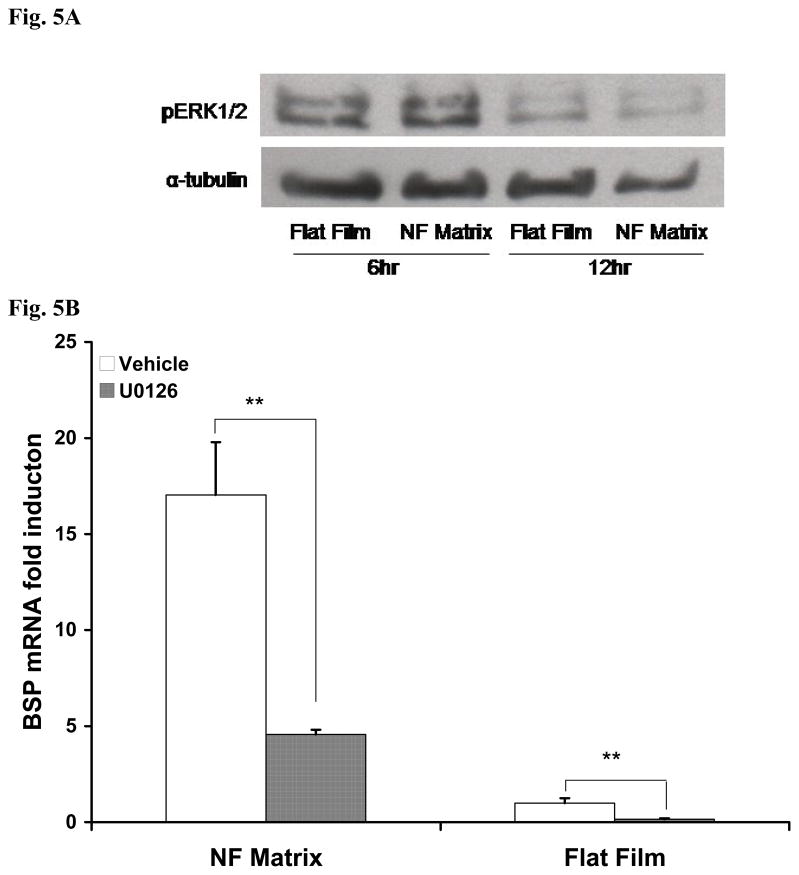
The phosphorylation of ERK1/2 was detected via western blotting. Following the high activity at 6 h after seeding and initial culture, the active form of ERK1/2 was decreased on both PLLA NF matrix and PLLA flat films at 12 h (Fig. 5A). However, there was no substantial difference in the ERK1/2 phosphorylation activity between PLLA NF matrix and flat films at either 6 or 12 h. 50 mu;M U0126 was added to the culture medium at the beginning of cell seeding to inhibit ERK1/2 activity, and it was found that the BSP gene expression was down regulated on both PLLA NF matrix and PLLA flat films at day 3 (Fig. 5B).
Figure 5.
Effect of ERK activity on BSP gene expression. ERK1/2 phosphorylation was down-regulated both on NF matrix and flat film after initial seeding (A). BSP gene expression was significantly inhibited both on NF matrix and flat film in the presence of MEK inhibitors U0126 for 3 days (**p<0.01).
Role of RhoA-ROCK signaling pathway on BSP gene expression
To further probe the signaling pathways related to BSP gene up-regulation on the PLLA NF matrix, the activity of small GTPase RhoA was also determined using a pull down assay. As shown in Fig. 6, after 12 h of culture, RhoA activity was lower on NF matrix compared to flat films. To find out the role of RhoA on BSP gene expression, 15 mu;M Y-27632, the inhibitor of ROCK, was added to flat film cultures for 3 days. It was found the inhibition of RhoA-ROCK pathway significantly enhanced BSP gene expression in cells cultured on PLLA flat films. The relative BSP gene expression was enhanced to 3.92 ±0.36 with Y-27632 treatment, compared to 1.00±0.20 with vehicle control (p<0.001).
Figure 6.

Pull-down experiments for GTP-bound RhoA in MC-4 cells cultured for 12 hr on flat film or NF matrix. Rho-GTP content was lower for cells cultured on NF matrix compared to cells on flat films.
Discussion
MC3T3-E1 preosteoblast cells require only serum and AA to express a fully differentiated phenotype [11]. The AA acts on collagen hydroxylation, which is necessary for subsequent triple-helix formation and fibril assembly. Fibrillar collagen interacts further with osteoblasts via α2β1 integrins to initiate a Runx2 dependent response of gene expression and mineralization [1].
In order to mimic the nanofibrous structure of the ECM collagen, a novel phase separation technique has been developed in our laboratory to fabricate NF materials from polymers. The new phase-separation technology allows for further design of 3D pore network to generate advantageous 3D scaffolds for tissue engineering and regeneration [5]. These highly porous and interconnected NF materials have been found to be excellent scaffolds for bone tissue engineering [8, 12]. Other groups have generated fibrous matrices with fiber diameters at the nanoscale or sub-micrometer scale using an electrospinning technique, and reported that these matrices could support cell cultures [13–15]. Since electrospinning technique has not generated scaffolds with designed pore shape and size, its application in engineering tissues of a large size scale may be limited. Since cell phenotype could also be affected by 3D pore structures, to investigate the direct effects of cell-matrix interactions on osteoblast growth and differentiation, we used a thin PLLA NF matrix fabricated by the new phase separation process in the present study.
MC3T3-E1 cells (MC-4) cultured on PLLA NF matrix assumed a less spread shape compared to those on PLLA flat films, and showed abundant interactions with the PLLA nanofibers. In addition, there were few stress fibers assembled in the cytoskeletons and no typical focal adhesion structures formed on the PLLA NF matrix, while there were abundant stress fibers and typical focal adhesion structure formation on the flat PLLA films. Although surface modification using gelatin is an effective way to enhance cell adhesion and spreading [10], it had no effect on stress fiber and focal adhesion formation on the PLLA NF matrix, supporting our hypothesis that the morphological change of cells result from the physical structure of the nanofibers rather than the surface chemistry of the matrix. It should be noted that the typical focal adhesion structures are commonly artificial results when cells respond to in vitro cultures on flat TCPS (for review, see [16]). There was no significant focal adhesion formation in vivo [17]. In this regard, the NF matrix may provide cells with an environment closer to ECM than the flat films. The growth rate of MC-4 cells on NF matrix was slower than on flat films. This may be related to the different degrees of cell spreading on these two types of surface. Supplementation of AA increased cell growth rate on NF matrix, possibly due to further ECM deposition into NF matrix. We previously found that cell growth rate on porous 3D NF scaffolds was faster than on solid walled (SW) scaffolds [8]. The “solid walls” of those porous 3D scaffolds did not have the nanofibrous features, but they are not truly flat or smooth at the micrometer level. Since cell behavior on 3D scaffolds is also affected by other structural parameters of the scaffolds [18], mass transfer conditions, cell migration and ECM deposition, more studies are needed to understand different cell responses in different porous 3D scaffolds.
After 6 days of culture in AA-containing medium compared to AA-free control, both BSP and OCN gene expression levels were up-regulated on both types of surface (PLLA NF matrix and flat films), which is consistent with the cell response to AA found in cell culture plates [1]. The BSP gene expression on NF matrix was 2 orders of magnitude higher than that on flat films. Even without the supplement of AA, thus no natural collagen fiber formation, the BSP gene expression was still greatly enhanced on PLLA NF matrix, suggesting that there is a direct interaction between PLLA nanofibers and osteoblasts. BSP gene is the key regulator for bone mineralization and is crucial for differentiated osteoblast phenotype [19]. Consistence with the up-regulation of BSP gene expression, the cells cultured on PLLA NF matrix showed much higher ALP content. It has been reported that ECM-dependent OCN gene expression can be completely inhibited with the MEK1/2 inhibitor, and the BSP gene expression is only partially blocked and not well correlated with changes of ERK phosphorylation [2], suggesting that BSP gene is subject to multiple signaling pathways. To investigate the signaling pathway related to BSP gene up-regulation on NF matrix, ERK1/2 activity was examined. Although inhibition of ERK1/2 activity partially inhibited BSP gene expression on NF matrix, there were no significant difference in ERK1/2 phosphorylation between the cultures on PLLA NF matrix and flat films, indicating that BSP gene expression was dependent on ERK1/2 activity, but the up-regulation of BSP gene expression on NF matrix was not through ERK1/2 activation.
It was reported in the literature that inhibition of ROCK, the effector of RhoA, could enhance BSP gene expression in osteoblast cells [20]. Since RhoA activity was also important for stress fiber formation and cell spreading [21] and we observed that stress fiber formation and cell spreading were substantially inhibited on the PLLA NF matrix, we hypothesized that RhoA activity plays an important role in cell phenotype on NF matrix. Thus RhoA pull down experiments were performed and it was found that RhoA activity was lower on PLLA NF matrix compared to that on flat films, supporting our hypothesis. To test whether the difference in BSP gene expression on the two different surfaces (NF matrix and flat films) was related to RhoA activity, the cells cultured on the PLLA flat films were treated with a well established ROCK inhibitor, Y-27632. For the same cell type, similar dosage of Y-27632 to that we used has been reported to inhibit RhoA activity [22]. The inhibition of ROCK activity by Y-27632 treatment significantly enhanced BSP gene expression of MC-4 cells cultured on flat films, suggesting that the up-regulation of BSP gene expression on NF matrix may also be related to the observed down-regulation of RhoA-Rock signaling pathway.
Conclusions
Cells (MC3T3-E1 preosteoblasts) took a more rounded shape on PLLA NF matrix with abundant cell protrusions interacting with the nanofibers, while cells assumed a more spread morphology on PLLA flat films. Cells on NF matrix exhibited an enhanced osteoblast differentiation phenotype, with 2 orders of magnitude higher BSP gene expression and significantly higher levels of other osteogenic markers (osteocalcin expression and alkaline phosphatase activities). The BSP gene expression was still highly up-regulated on NF matrix even without ascorbic acid and thus no natural collagen fiber deposition into the matrix, suggesting a direct effect of PLLA nanofibers on BSP gene expression. The enhanced BSP gene expression on the nanofibrous PLLA matrix was correlated with the down-regulation of the small GTPase RhoA activities.
Acknowledgments
The authors would like to acknowledge the financial support from the National Institutes of Health (Research Grants DE015384, GM075840 and DE017689: PXM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Xiao GZ, Wang D, Benson MD, Karsenty G, Franceschi RT. Role of the alpha(2)-integrin in osteoblast-specific gene expression and activation of the Osf2 transcription factor. Journal of Biological Chemistry. 1998;273(49):32988–32994. doi: 10.1074/jbc.273.49.32988. [DOI] [PubMed] [Google Scholar]
- 2.Xiao GZ, Gopalakrishnan R, Jiang D, Reith E, Benson MD, Franceschi RT. Bone morphogenetic proteins, extracellular matrix, and mitogen-activated protein kinase signaling pathways are required for osteoblast-specific gene expression and differentiation in MC3T3-E1 cells. Journal of Bone and Mineral Research. 2002;17(1):101–110. doi: 10.1359/jbmr.2002.17.1.101. [DOI] [PubMed] [Google Scholar]
- 3.Mizuno M, Kuboki Y. Osteoblast-related gene expression of bone marrow cells during the osteoblastic differentiation induced by type I collagen. Journal of Biochemistry. 2001;129(1):133–138. doi: 10.1093/oxfordjournals.jbchem.a002824. [DOI] [PubMed] [Google Scholar]
- 4.Ma PX, Zhang RY. Synthetic nano-scale fibrous extracellular matrix. Journal of Biomedical Materials Research. 1999;46(1):60–72. doi: 10.1002/(sici)1097-4636(199907)46:1<60::aid-jbm7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 5.Ma PX. Biomimetic Materials for Tissue Engineering. Advanced Drug Delivery Reviews. 2008;60(2):184–198. doi: 10.1016/j.addr.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei G, Ma PX. Macroporous and nanofibrous polymer scaffolds and polymer/bone-like apatite composite scaffolds generated by sugar spheres. Journal of Biomedical Materials Research: Part A. 2006;78(2):306–315. doi: 10.1002/jbm.a.30704. [DOI] [PubMed] [Google Scholar]
- 7.Chen VJ, Ma PX. Nano-fibrous poly(L-lactic acid) scaffolds with interconnected spherical macropores. Biomaterials. 2004;25(11):2065–2073. doi: 10.1016/j.biomaterials.2003.08.058. [DOI] [PubMed] [Google Scholar]
- 8.Chen VJ, Smith LA, Ma PX. Bone regeneration on computer-designed nano-fibrous scaffolds. Biomaterials. 2006;27(21):3973–3979. doi: 10.1016/j.biomaterials.2006.02.043. [DOI] [PubMed] [Google Scholar]
- 9.Woo KM, Chen VJ, Ma PX. Nano-fibrous scaffolding architecture selectively enhances protein adsorption contributing to cell attachment. Journal of Biomedical Materials Research Part A. 2003;67A(2):531–537. doi: 10.1002/jbm.a.10098. [DOI] [PubMed] [Google Scholar]
- 10.Liu XH, Won YJ, Ma PX. Surface modification of interconnected porous scaffolds. Journal of Biomedical Materials Research Part A. 2005;74A(1):84–91. doi: 10.1002/jbm.a.30367. [DOI] [PubMed] [Google Scholar]
- 11.Wang D, Christensen K, Chawla K, Xiao GZ, Krebsbach PH, Franceschi RT. Isolation and characterization of MC3T3-E1 preosteoblast subclones with distinct in vitro and in vivo differentiation mineralization potential. Journal of Bone and Mineral Research. 1999;14(6):893–903. doi: 10.1359/jbmr.1999.14.6.893. [DOI] [PubMed] [Google Scholar]
- 12.Woo KM, Jun JH, Chen VJ, Seo J, Baek JH, Ryoo HM, et al. Nano-fibrous scaffolding promotes osteoblast differentiation and biomineralization. Biomaterials. 2007;28(2):335–343. doi: 10.1016/j.biomaterials.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 13.Li WJ, Tuli R, Huang XX, Laquerriere P, Tuan RS. Multilineage differentiation of human mesenchymal stem cells in a three-dimensional nanofibrous scaffold. Biomaterials. 2005;26(25):5158–5166. doi: 10.1016/j.biomaterials.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Ma ZW, He W, Yong T, Ramakrishna S. Grafting of gelatin on electrospun poly(caprolactone) nanofibers to improve endothelial cell spreading and proliferation and to control cell orientation. Tissue Engineering. 2005;11(7–8):1149–1158. doi: 10.1089/ten.2005.11.1149. [DOI] [PubMed] [Google Scholar]
- 15.Yoshimoto H, Shin YM, Terai H, Vacanti JP. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials. 2003;24(12):2077–2082. doi: 10.1016/s0142-9612(02)00635-x. [DOI] [PubMed] [Google Scholar]
- 16.Wozniak MA, Modzelewska K, Kwong L, Keely PJ. Focal adhesion regulation of cell behavior. Biochimica Et Biophysica Acta-Molecular Cell Research. 2004;1692(2–3):103–119. doi: 10.1016/j.bbamcr.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294(5547):1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 18.O'Brien FJ, Harley BA, Yannas IV, Gibson LJ. The effect of pore size on cell adhesion in collagen-GAG scaffolds. Biomaterials. 2005;26(4):433–441. doi: 10.1016/j.biomaterials.2004.02.052. [DOI] [PubMed] [Google Scholar]
- 19.Mizuno M, Imai T, Fujisawa R, Tani H, Kuboki Y. Bone sialoprotein (BSP) is a crucial factor for the expression of osteoblastic phenotypes of bone marrow cells cultured on type I collagen matrix. Calcified Tissue International. 2000;66(5):388–396. doi: 10.1007/s002230010078. [DOI] [PubMed] [Google Scholar]
- 20.Chaplet ML, Detry D, Deroanne C, Fisher LW, Castronovo V, Bellahcene A. Zoledronic acid up-regulates bone sialoprotein expression in osteoblastic cells through Rho GTPase inhibition. Biochemical Journal. 2004;384:591–598. doi: 10.1042/BJ20040380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katoh K, Kano Y, Ookawara S. Rho-kinase dependent organization of stress fibers and focal adhesions in cultured fibroblasts. Genes to Cells. 2007;12(5):623–638. doi: 10.1111/j.1365-2443.2007.01073.x. [DOI] [PubMed] [Google Scholar]
- 22.Tokuda H, Hanai Y, Matsushima-Nishiwaki R, Yamauchi J, Doi T, Harada A, et al. Rho-kinase regulates endothelin-1-stimulated IL-6 synthesis via p38 MAP kinase in osteoblasts. Biochem Biophys Res Commun. 2007;362(4):799–804. doi: 10.1016/j.bbrc.2007.08.018. [DOI] [PubMed] [Google Scholar]