Abstract
Objective
Concerns of breast cancer risk in postmenopausal women taking combined estrogen+progestin therapy have generated interest in the use of selective estrogen receptor modulators (SERMs) as potential progestin alternatives. Endometrial proliferation and cancer risk are major concerns, however, for estrogens and certain types of SERMs when given alone. The primary aim of this study was to evaluate the endometrial profile of bazedoxifene acetate (BZA), a third-generation SERM, alone and in combination with conjugated equine estrogens (CEE) in a postmenopausal primate model.
Methods
Ninety-eight ovariectomized cynomolgus monkeys (Macaca fascicularis) were randomized to receive no hormone treatment (control), BZA 20 mg, CEE 0.45 mg, or the combination of BZA 20 mg + CEE 0.45 mg once daily for 20 months in a parallel-arm study design. The primary outcome measure was endometrial epithelial proliferation.
Results
BZA+CEE and BZA treatment resulted in significantly less endometrial epithelial area and Ki67 expression compared to CEE (P < 0.001 for all). The prevalence of endometrial hyperplasia and other estrogen-induced morphologic changes in the BZA+CEE and BZA groups were not significantly different from control. The addition of BZA to CEE completely inhibited the expression of ERα-regulated genes (TFF1 and PGR), while BZA alone had no effect. BZA+CEE and BZA treatment also resulted in lower ERα protein expression in the endometrium compared to control and CEE (P < 0.05 for all).
Conclusions
BZA given at a clinically relevant dose inhibits estrogen effects on the endometrium and lacks uterotropic effects when given alone.
Keywords: Estrogens, menopause, hormone therapy, selective estrogen receptor modulator, bazedoxifene acetate, endometrial cancer
INTRODUCTION
Estrogen-alone therapy (ET) alleviates menopausal symptoms and reduces osteoporosis and fracture risk in aging women.1-3 However, long-term use of ET has been associated with an increased risk of endometrial hyperplasia and endometrial cancer, even when given at low doses.4-7 To date, progestin co-therapy has been the only clinical strategy to effectively prevent estrogen-induced endometrial proliferation and lower cancer risk.6, 8 Historically, the most commonly prescribed estrogen + progestin co-therapy (EPT) in the United States has been conjugated equine estrogens (CEE) given with medroxyprogesterone acetate (MPA).9 However, primary results from the Women’s Health Initiative (WHI) randomized clinical trial10 and prior observational studies11 have associated long-term CEE+MPA co-therapy with an increased risk of breast cancer as well as a higher incidence of gynecological surgeries (e.g. hysterectomies) due to increased uterine bleeding.12 Consequently, there has been increased interest in new menopausal therapies that provide comparable efficacy to ET without adverse proliferative effects on the breast and endometrium.
Recently, selective estrogen receptor modulators (SERMs) have been proposed as an alternative to the progestin component in EPT.13 As a class of non-steroidal compounds, SERMs bind to estrogen receptors (ERs) alpha and beta and induce a mixed pattern of ER agonist and antagonist responses depending on the particular SERM and the target tissue.14 Current SERMs include tamoxifen and raloxifene, which are widely used in the prevention of breast cancer.15 Similar to ET, most SERMs increase bone mineral density and improve lipid profiles, but in contrast to ET, current SERMs given alone do not treat menopausal symptoms and in some cases worsen them.16, 17, 18 The ideal clinical goal of combining a SERM with ET would be to selectively retain the benefits of both agents while reducing the adverse effects of either agent alone. The estrogen component of such a combination would relieve hot flushes, improve urogenital atrophy, and prevent bone loss, while the SERM would help maintain bone mass and provide anti-estrogenic effects in the breast and endometrium. Information regarding the uterotropic effects of the SERM component is critical considering that SERMs such as tamoxifen have been associated with an increased incidence of endometrial cancer and other adverse morphologic changes such as stromal fibrosis, cystic change, and polyp formation.16, 19-22
Bazedoxifene acetate (BZA; 20 mg/day) is an indole-based SERM currently under evaluation for the treatment of osteoporosis and, in combination with CEE (0.45 and 0.625 mg/day), for the reduction of menopausal symptoms and the prevention of osteoporosis.23-26 The purpose of this study was to investigate the endometrial safety profile of BZA alone and in combination with CEE in a randomized multisystem nonhuman primate translational trial. We hypothesized that BZA would antagonize the proliferative and transcriptional effects of CEE on the endometrium, while BZA would have minimal estrogen agonist effects when administered alone. Effects on other systems including breast, bone, and the cardiovascular system will be reported elsewhere.
METHODS
Study design and treatments
This preclinical trial followed a parallel-arm study design in which ninety-eight ovariectomized adult cynomolgus macaques (Macaca fascicularis) were randomized by social group to receive one of the following four treatments for 20 months: (i) no treatment (control (CTL), n=23), (ii) BZA 20 mg (n=24), (iii) CEE 0.45 mg (n=24), or (iv) the combination of BZA 20 mg + CEE 0.45 mg (n=27). An important feature of this design is the inclusion of a CEE-alone group as a positive control, which was not feasible in previous clinical trials since all participating women did not have a prior hysterectomy.26
All monkeys were imported from the Indonesian Primate Center (Pusat Studi Satwa Primata) at the Institut Pertanian Bogor in Bogor, Indonesia. Following quarantine, all monkeys were placed in stable social groups consisting of two to five animals; the groups were approximately equivalent in body weight. Social groups were then randomly assigned a treatment condition using a simple randomization procedure. To achieve adequate power for the cardiovascular endpoints (published elsewhere) in this preclinical trial, each group originally consisted of 25 animals; however 2 animals were added to the BZA and BZA+CEE groups to account for any exclusions or deaths during the study. Subsequently, 2 animals from the control and 3 animals from the BZA groups were excluded due to elevated serum ovarian hormone levels indicating the presence of ectopic ovarian tissue (follicles, luteal tissue, and/or stroma found outside the ovary).27 Another monkey from the CEE group was euthanized due to an intussusception.
An important rationale for the use of the macaque model in this trial is the high degree of similarity in pathophysiology28 and responses to hormonal agents29 between the human and macaque endometrium. All monkeys were considered monoparous or multiparous based on clinical records from the original breeding colony. Procedures involving these animals were approved by the Institutional Animal Care and Use Committee of Wake Forest University and conducted in accordance with federal, state, and institutional guidelines. The facilities and animal resources program of Wake Forest University are fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC).
Hormone treatments were administered in a standard isoflavone-free casein + lactalbumin control diet and fed once daily for 20 months. The standard control diet was formulated to model the high cholesterol (0.29 mg/Cal) and high fat (35% of calories from fat) diets typically consumed by postmenopausal women in the United States. Studies conducted to determine CEE and BZA doses for female macaques that were equivalent to a woman’s daily dose of CEE 0.45 mg and BZA 20 mg have been published elsewhere.30 Briefly, drug doses were determined based on caloric intake to account for differences in metabolic rates between female monkeys and women as well as plasma concentrations measured in postmenopausal women receiving BZA 20 mg/day.31 Animals in the CEE-treated groups were given 0.03 mg/kg body weight/day of CEE while those animals in the BZA-treated groups received 2.5 mg/kg body weight/day of BZA.
Circulating estrogen and BZA concentrations
As part of the main study, plasma estrone (E1), 17β-estradiol (E2), and BZA concentrations were obtained at 4 hours post-prandial and following an overnight fast to confirm adequate dosing and dietary intake. These results have been reported elsewhere.30 Repeat measurements of E1 and E2 were also done in a randomly selected subset of animals (n=47) following an 18 hour fast on the day of necropsy, and these values are reported here. E1 and E2 concentrations were measured at the Wake Forest University Primate Center (WFUPC) Clinical Laboratory using commercially available radioimmunoassay kits (Siemens/DPC), while plasma BZA concentrations were measured at Pfizer using high-performance liquid chromatography (HPLC) with fluorescence detection. The lower limit of quantification was 2.5 pg/mL for E1 and E2, and 1.0 ng/mL for BZA. Serum used to measure E2 concentrations were first extracted with ethyl ether, and extracts were then dried and reconstituted with zero-standard serum.
Uterine Area via Uterine Ultrasounds
Trans-abdominal ultrasounds were conducted using a Sonosite MicroMaxx portable ultrasound machine with a 6.0-MHz linear transducer (Sonosite, Bothell, WA) at baseline (4 weeks after ovariectomy) and following 6, 12, and 20 months of treatment. For each timepoint, at least 3 static digital images were captured at the maximal transverse cross-sectional area of the uterus. Measurements of uterine area (cm2) were made using public domain software (National Institutes of Health ImageJ 1.34, available at http://rsb.info.nih.gov/ij/upgrade/).32 All sonographic images were obtained and measured by the same person blinded to the treatment groups.
Histomorphometry and histopathology
At necropsy, uteri were collected, weighed, and prepared for histology.32 Percent epithelial area and mean endometrial thickness were quantified using hematoxylin and eosin (H&E) stained slides and morphometry techniques similar to those described previously.22, 33 Briefly, epithelial area was determined by manual tracing of glandular units (minus the luminal areas) within the superficial and basal endometrium and expressed as a percentage of the total area examined (Image-Pro Plus software, Media Cybernetics, Silver Spring, MD). Mean endometrial thickness (superficial + basal) was measured in a similar manner. H&E-stained slides were also evaluated for evidence of glandular hyperplasia (simple and complex), stromal hyperplasia, stromal edema, cystic dilation, and other histological lesions by a board-certified veterinary pathologist (CEW). Stromal edema and cystic dilation are considered low-risk morphologic changes associated with exogenous estrogen exposure.29 Endometrial stromal fibrosis (collagen content) was quantified from slides stained with Masson’s trichrome (containing Weigert’s iron hematoxylin, Crocein Scarlet MOO, 5% aqueous phosphomolybdic acid, and aniline blue; Fisher Scientific and Sigma) using a selective color-based analysis in the Image Pro-Plus Software.22 Blue-stained areas in the superficial endometrial stroma represented collagen, while red area represented stromal cell nuclei and cytoplasm. Area of stromal edema and undefined (pale blue) ground substance was estimated by subtracting total area examined by the blue and red-strained areas. All histomorphometry and histopathological evaluations were completed by persons blinded to the treatment groups.
Immunohistochemistry
Immunohistochemistry (IHC) was performed on fixed endometrial sections using commercially-available primary monoclonal antibodies for the proliferation marker Ki67 (Ki67SP6; Thermo Scientific, Fremont, CA) and the sex steroid receptors ERα (NCL-ER-6F11, Novocastra Reagents, Leica Microsystems Inc., Buffalo, NY) and progesterone receptor (PGR) (NCL-PGR-312, Novocastra Reagents, Leica Microsystems Inc., Buffalo, NY) as described previously.30 Nuclear immunolabeling within the superficial and basal endometrium was quantified using a computer-assisted manual counting technique with a grid filter to select cells for counting (Image-Pro Plus software, Media Cybernetics, Silver Spring, MD). An H score was then calculated using the equation (3 x % of strongly stained nuclei) + (2 x % of moderately stained nuclei) + (% of weakly stained nuclei).34 All IHC quantification was performed by a technician blinded to the treatment groups.
Quantitative real-time PCR
Expression of genes related to cell proliferation (MKI67, Ki67 antigen) and estrogen receptor activity (ESR1, ERα; ESR2, ERβ; PGR, progesterone receptor; TFF1, trefoil factor 1 [pS2]) was determined by quantitative real-time reverse transcription-PCR (qRT-PCR) using techniques described elsewhere.30 Briefly, all qRT-PCR reactions were run with Applied Biosystems (ABI) Taqman primer-probe sets and performed on an ABI 7500 Fast Real-Time PCR System (Applied Biosystems, Carlsbad, CA). β-actin was used as the endogenous control while reference endometrial tissue cDNA was run in parallel for plate-to-plate calibration.32 The reliability of β-actin as an internal control was evaluated using analysis of variance, analyses of the standard deviation of Ct, and change of Ct of β-actin between control and treatment samples. Relative expression of each target gene was calculated using ABI Relative Quantification 7500 Software v2.0.1.
Statistical analyses
All variables were evaluated for their distribution and equality of variance. Data not normally distributed were transformed (log10 or square root) to improve normality for analysis and then reverse transformed to the original scale for display in the results. The following data violated the Levene’s test for equality of variance and were analyzed using the nonparametric Kruskal-Wallis and post hoc Wilcoxon (rank sums) tests: uterine weights, E2 concentrations, (superficial) epithelial area, (superficial) luminal area, all qRT-PCR assays except for ESR1 and ESR2, and IHC for Ki67 (superficial glands and stroma) and ERα (superficial glands). Significance levels were then adjusted for multiple pair-wise comparisons using a Bonferroni correction. Differences in uterine area and body weight among the treatment groups were determined using a mixed model approach with baseline values as a covariate. For each variable, this model allowed for a within-group comparison at each post-treatment time period and a between time point comparison within each group (e.g. 20 months post-treatment values compared to baseline). A Fisher’s exact test was used to evaluate treatment group differences in the prevalence of histopathological findings. The remaining data were assessed using analysis of variance (ANOVA) and the Tukey HSD post hoc test for multiple pair-wise comparisons. A two-tailed significance level of 0.05 was selected for all comparisons and all analyses were done using JMP statistical software (version 9.0.2; SAS Institute, Inc, Cary, NC).
RESULTS
Treatment group characteristics
Treatment group characteristics including age, body weights, and hormone concentrations are summarized in Table, Supplemental Digital Content 1, http://links.lww.com/MENO/A44. At baseline, the mean estimated age of all animals was 12.7 (range 9 - 18) years with no between-group differences (P > 0.1). All groups showed a small increase in body weight from baseline to 20 months post-treatment. This gain in body weight reached significance only in the control group (P < 0.001) with a trend towards significance in the BZA group (P = 0.06). After 20 months of treatment, the BZA+CEE group weighed significantly less than the control group (P < 0.01); however, no significant differences in body weight and plasma estrogen (E1 and E2) concentrations were noted between the BZA+CEE and CEE groups (P > 0.1 for all).
Uterine area via trans-abdominal ultrasound
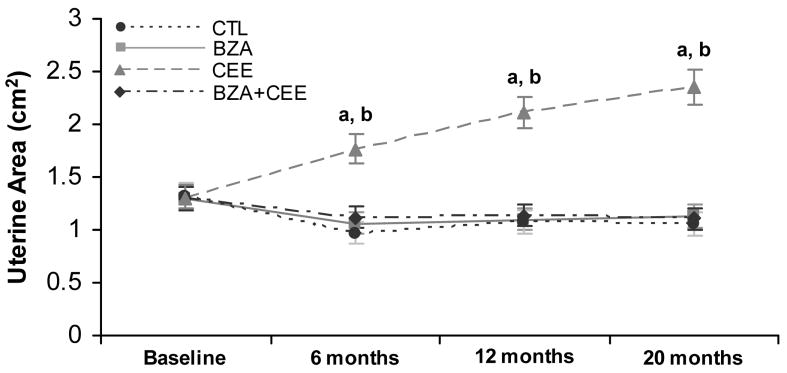
Uterine area among CEE-treated animals increased progressively from baseline to 20 months post-treatment (P < 0.0001) and was significantly greater than the control group at 6, 12, and 20 months of treatment (P < 0.0001 for all, Fig. 1). The BZA+CEE and BZA groups had significantly smaller uterine areas relative to CEE (P < 0.0001 for all) and similar uterine areas compared to control at each post-treatment time point (Fig. 1).
FIG. 1.
Ultrasonographic measurements of uterine area in postmenopausal macaques receiving no hormone therapy (n=23), BZA (n=24), CEE (n=24), and BZA+CEE co-therapy (n=27). Uterine area values among the control, BZA, and BZA+CEE groups were comparable and significantly smaller than the CEE group at 6, 12, and 20 months of treatment. a P < 0.0001 compared to respective control and BZA+CEE groups. b P < 0.0001 compared to baseline values. Values represent means ± 95% confidence interval (CI). CTL = control (no hormone treatment).
Endometrial thickness, epithelial area, and proliferation
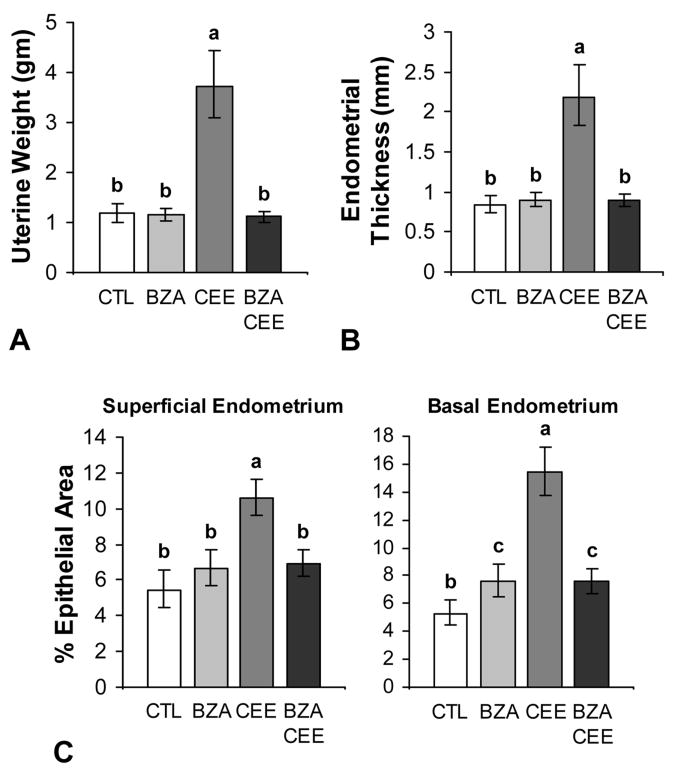
Following 20 months of treatment, uterine weight and endometrial thickness among the control, BZA, and BZA+CEE groups were comparable and 2.5 to 3.5-fold lower than the CEE group (P < 0.0001 for all compared to CEE, Fig. 2A and 2B). Similarly, epithelial area within the superficial endometrium was not significantly different among the control, BZA, and BZA+CEE groups and measured 1.5 to 2.0-fold less than the CEE group (P < 0.0001 for all compared to CEE, Fig. 2C). Epithelial area specifically within the basal endometrium was also significantly less in BZA+CEE and BZA groups compared to the CEE group (P < 0.0001 for both) but 44% higher than control (P < 0.01 for both).
FIG. 2.
Effects of BZA with and without CEE on uterine weight, endometrial thickness, and epithelial area in postmenopausal macaques (A-C). The addition of BZA to CEE significantly inhibited the agonistic effects of CEE on uterine weight (A), endometrial thickness (B), and epithelial area (C) (P < 0.0001 to P < 0.05 for all). n = 23, 24, 24, and 27 for control (CTL), BZA, CEE, and BZA+CEE co-therapy, respectively, for all measures. Treatment groups not connected by the same letter are significantly different. Values represent means ± 95% CI.
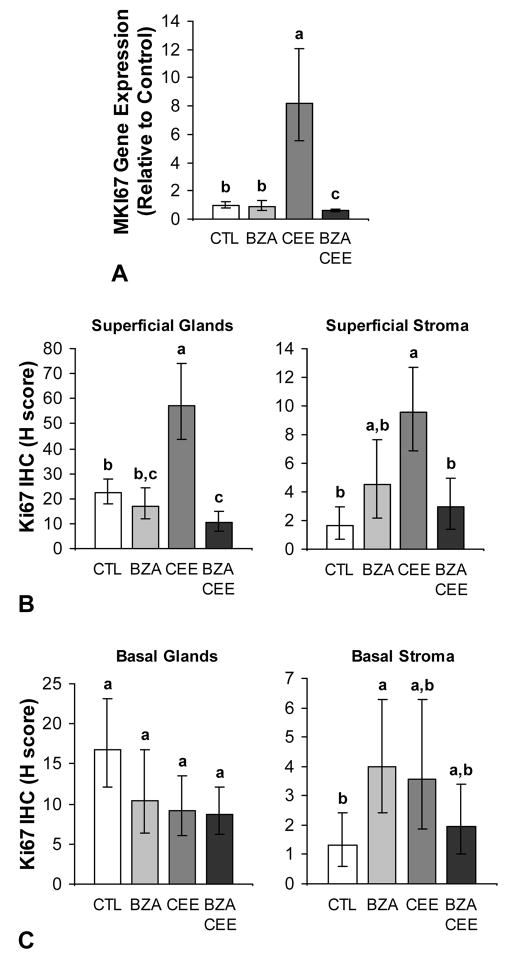
The addition of BZA to CEE significantly inhibited CEE-induced endometrial proliferation, indicated by lower MKI67 gene expression and Ki67 immunolabeling in the superficial glands and stroma for BZA+CEE compared to CEE (P < 0.001 for all) (Fig. 3A and 3B). Proliferation in the superficial glands was also lower for BZA+CEE compared to control (P < 0.01) (Fig. 3B). Treatment with BZA alone did not induce MKI67 expression (Fig. 3A) or Ki67 immunolabeling in the superficial or basal endometrial glands (Fig. 3B and 3C) but did result in 3-fold higher Ki67 immunoreactivity in the basal stroma compared to control (P = 0.04) (Fig. 3C). Representative photomicrographs of Ki67 immunolabeling for BZA, CEE, BZA+CEE, and no hormone treatment are displayed in Supplemental Digital Content 4, http://links.lww.com/MENO/A47.
FIG. 3.
Effects of BZA given alone and in combination with CEE on endometrial proliferation in postmenopausal macaques (A-C). Treatment with BZA+CEE resulted in lower MKI67 expression and Ki67 immunolabeling in the superficial endometrium compared to CEE alone (P < 0.001 for all). BZA given with CEE significantly inhibited the gene and protein expression of the proliferation marker Ki67 in the superficial endometrium to the level of control or beyond (A and B). No treatment effect was observed in the basal endometrial glands (C) however treatment with BZA induced greater Ki67 immunolabeling in the basal stroma compared to control (P = 0.04). n = 23, 24, 24, and 27 for control (CTL), BZA, CEE, and BZA+CEE co-therapy, respectively, for all measures. Treatment groups not connected by the same letter are significantly different. Values represent means ± 95% CI.
Endometrial morphology
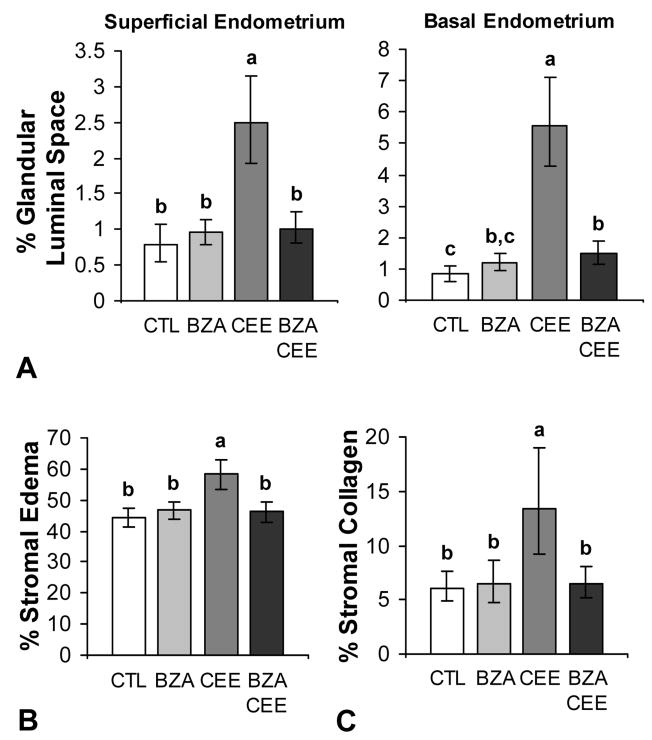
The addition of BZA to CEE also inhibited estrogen-induced changes in endometrial morphology. The prevalence of simple glandular and stromal hyperplasia was higher in the CEE group (P < 0.0001 compared to control and BZA+CEE) but not in BZA and BZA+CEE groups (Table 1). Similarly, the prevalence of stromal edema and cystic dilation in the endometrial glands was most evident in the CEE group (Table 1). These findings were confirmed using quantitative morphometric measurements, shown in Figs. 4A and 4B. Treatment with BZA+CEE and BZA had no significant effect on endometrial collagen content compared to control, but treatment with CEE resulted in significantly more collagen compared to the other groups (P < 0.01 for all) (Fig. 4C). Incidental histological findings included vascular remodeling (adventitial expansion) associated with prior pregnancy. No evidence of endometrial polyps, complex or atypical hyperplasia, or neoplasia was observed.
TABLE 1.
Histopathological Findings
| Control | BZA 20 mg/d | CEE 0.45 mg/d | BZA 20 mg/d + CEE 0.45 mg/d | |
|---|---|---|---|---|
| Simple Glandular Hyperplasia | 1 (4%) | 4 (17%) | 23 (96%) | 2 (7%) |
| Mild | 3 | 4 | 1 | 2 |
| Moderate | 0 | 0 | 15 | 0 |
| Marked | 0 | 0 | 7 | 0 |
| P value vs. Control | NA | 0.35 | <0.0001 | 1.0 |
| Stromal Hyperplasia | 1 (4%) | 4 (17%) | 22 (92%) | 3 (11%) |
| Mild | 1 | 4 | 0 | 3 |
| Moderate | 0 | 0 | 15 | 0 |
| Marked | 0 | 0 | 7 | 0 |
| P value vs. Control | NA | 0.35 | <0.0001 | 0.61 |
| Stromal Edema | 3 (13%) | 1 (4%) | 23 (96%) | 0 (0%) |
| Mild | 3 | 1 | 1 | 0 |
| Moderate | 0 | 0 | 14 | 0 |
| Marked | 0 | 0 | 8 | 0 |
| P value vs. Control | NA | 0.35 | <0.0001 | 0.09 |
| Cystic Dilation | 0 (0%) | 1 (4%) | 9 (38%) | 0 (0%) |
| Mild | 0 | 1 | 4 | 0 |
| Moderate | 0 | 0 | 4 | 0 |
| Marked | 0 | 0 | 1 | 0 |
| P value vs. Control | NA | 1.0 | 0.006 | 1.0 |
| Number Examined | 23 | 24 | 24 | 27 |
NOTE: All lesions demonstrate evidence of an estrogenic effect but are not considered high-risk. Neither complex nor atypical endometrial hyperplasia was observed. All cases had evidence of vascular remodeling associated with past pregnancy. All P values were determined using a Fisher’s Exact Test.
FIG. 4.
Effects of BZA, CEE, and BZA+CEE on endometrial morphology in postmenopausal macaques (A-C). Within the superficial endometrium, only treatment with CEE induced change in luminal space (cystic dilation) (A), stromal edema (B), and collagen content (C) compared to control (P < 0.01 for all). In the basal endometrium, treatment with BZA+CEE induced a modest but significant increase in luminal space (A) of the endometrial glands (P < 0.05). n = 23, 24, 24, and 27 for control (CTL), BZA, CEE, and BZA+CEE co-therapy, respectively, for all measures. Treatment groups not connected by the same letter are significantly different. Values represent means ± 95% CI.
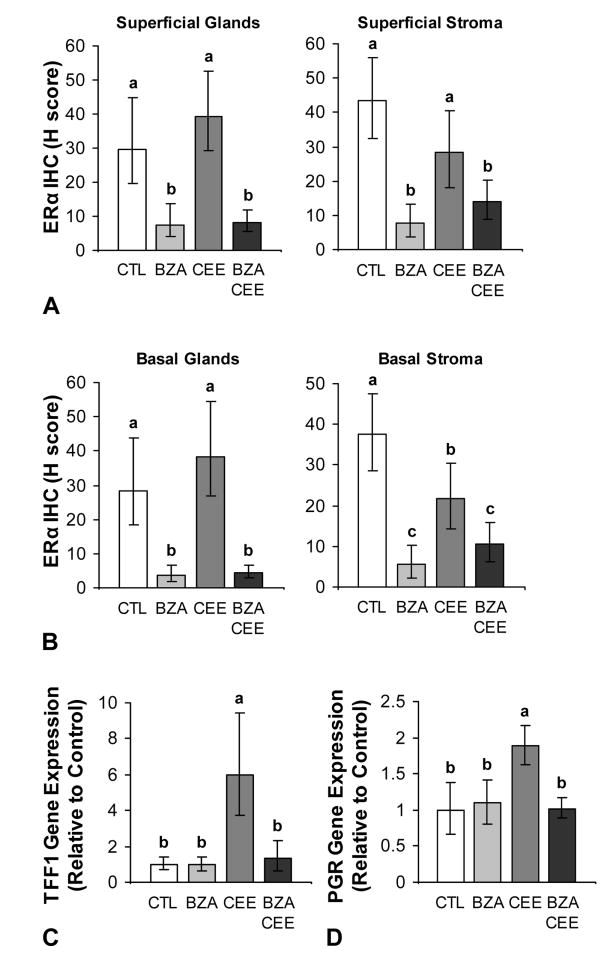
ERα expression and transcriptional activation
Treatment with BZA, CEE, and BZA+CEE altered endometrial ERα immunolabeling (Fig. 5A and 5B) but not gene expression (see graph A, Supplemental Digital Content 2, http://links.lww.com/MENO/A45). Groups treated with BZA+CEE and BZA had significantly less glandular and stromal ERα immunolabeling in the superficial and basal endometrium compared to control and CEE groups (P < 0.0001 to P < 0.05 for all compared to control and CEE). Treatment with CEE resulted in significantly lower ERα immunolabeling in the basal stroma compared to control (P < 0.05) but greater ERα immunoreactivity relative to BZA+CEE (P < 0.05). Unlike ERα gene expression, ERβ mRNA expression was significantly decreased by CEE treatment (P < 0.0001 compared to control, see graph B, Supplemental Digital Content 2, http://links.lww.com/MENO/A45).
FIG. 5.
Effects of BZA given alone and in combination with CEE on ERα expression and markers of ERα activity in the postmenopausal macaque endometrium (A-D). BZA treatment with and without CEE decreased ERα immunolabeling in the superficial and basal endometrium (A and B) compared to control and CEE alone (P < 0.0001 to P < 0.05 for all). The concomitant use of BZA and CEE significantly attenuated ERα-mediated expression of TFF1 and PGR (C and D) compared to CEE alone (P < 0.001 for all). n = 23, 24, 24, and 27 for control (CTL), BZA, CEE, and BZA+CEE co-therapy, respectively, for all measures. Treatment groups not connected by the same letter are significantly different. Values represent means ± 95% CI.
As expected, CEE treatment significantly induced the expression of the estrogen response genes TFF1 and PGR compared to control (P < 0.001 for both, Fig. 5C and 5D). The addition of BZA to CEE significantly attenuated these effects (P < 0.001 for both), while BZA alone had no effect. Glandular and stromal PGR immunolabeling within the endometrium showed a similar pattern (see graphs A and B, Supplemental Digital Content 3, http://links.lww.com/MENO/A46).
DISCUSSION
Traditional hormone therapies are associated with increased cancer risk in the endometrium (ET) and breast (EPT) in postmenopausal women. Estrogen + SERM co-therapies are emerging as potential alternatives to these traditional therapies. Endometrial stimulation and cancer risk are major concerns, however, for both estrogens and certain types of SERMs when given alone and limited data exist regarding the endometrial safety of estrogen + SERM co-therapies. In this preclinical trial, we investigated the endometrial risk profile of a new SERM, BZA, given alone and with CEE, the most widely prescribed ET in the United States.9 Treatment with CEE increased uterine size, endometrial thickness, epithelial area, proliferation, and gene markers of ERα activity, while the addition of BZA to CEE significantly antagonized these effects. Treatment effects of BZA alone were comparable to control. These findings show for the first time that BZA is a clear estrogen antagonist in the endometrium at clinically relevant doses.
The full inhibition of CEE effects by BZA shown here supports data from phase III clinical trials suggesting that BZA+CEE co-therapy does not have tamoxifen-like uterotropic effects in postmenopausal women.35, 36 In a 2-year study of osteoporotic postmenopausal women (SMART-1: Selective estrogens, Menopause, And Response to Therapy), endometrial hyperplasia (evaluated via biopsy) and uterine bleeding incidences with BZA 20 mg combined with either CEE 0.45 mg or 0.625 mg were not significantly different from placebo.35, 36 Similarly, preliminary data from another smaller 1-year trial (SMART-5) reported comparable incidences of endometrial hyperplasia among those women receiving BZA 20 mg + CEE 0.45 mg (0.3%) and BZA 20 mg + CEE 0.625 mg (0.27%) relative to MPA 1.5 mg + CEE 0.45 mg (0%), BZA 20 mg monotherapy (0%), and placebo (0%).37
Evidence from prior reports, however, suggests that the inhibitory effects of BZA on ER-activity may differ according to the dose and/or type of estrogen used in BZA+estrogen therapies. For example, cell culture studies showed that 10 nM of BZA completely inhibits the growth of E2-stimulated MCF-7 breast cancer cells, while 1.0 pM of BZA results in negligible inhibition.38 Similarly, in the 2-year SMART-1 trial, the uterotropic effects of CEE (0.45 mg/d or 0.625 mg/d) were effectively antagonized with 20 mg/d but not 10 mg/d of BZA.35 To date, the interactive effects of estrogens and BZA on the breast and endometrium have only been evaluated with oral CEE as the primary ET;30, 35-37, 39 therefore, it is not known whether the target dose of BZA (20 mg/d) would prevent endometrial and breast proliferation if co-administered with standard doses of other ETs such as oral and transdermal E2.
Few prior studies have evaluated endometrial effects of estrogen + SERM therapies. In one small study evaluating the combination of raloxifene (60 mg/d) and oral E2 (1 mg/d) in postmenopausal women transitioning from EPT, the frequency of vasomotor symptoms, hot flushes, and night sweats were significantly reduced compared to baseline and raloxifene monotherapy.40 However, this combination was associated with increased endometrial thickness and two cases of atypical endometrial hyperplasia. Similarly, another pilot study found significantly less vasomotor events but increased endometrial thickness with the concomitant use of oral raloxifene and a transdermal E2 patch.41 Results from a rodent study suggested that BZA has greater ER-antagonist activity than raloxifene in the endometrium and may inhibit proliferation if co-administered with E2,42 but this observation has not been tested in a randomized clinical trial or at clinically relevant doses of BZA and E2. It is also worth noting that some estrogen + SERM combinations have shown mixed agonist and antagonist effects in the endometrium. In a prior macaque study, the addition of tamoxifen to low-dose E2 therapy inhibited E2-induced proliferation and expression of genes related to cell cycle progression, but still induced stromal fibrosis, cystic change, and increases in endometrial thickness similar to tamoxifen-alone therapy.22
We have previously reported that BZA with and without CEE reduces ERα immunolabeling in the breast.30 As reported here, a similar observation was noted in the endometrium in which both glandular and stromal ERα immunolabeling were significantly less with BZA and BZA+CEE treatments compared to control and CEE, while ERα gene expression was not affected. These unanticipated results suggest that BZA may increase ERα turnover in addition to blocking estrogen binding. This hypothesis is supported by a recent breast cancer cell study that showed proteasome-mediated degradation of the ERα by BZA.43 However, further studies should be conducted to confirm that BZA may increase ERα ubiquitation and degradation in normal endometrial cells.
CONCLUSIONS
Exposure to estrogens is a key risk factor for endometrial cancer. In postmenopausal women, exogenous ET leads to increased endometrial proliferation, hyperplasia, and up to a 5-fold higher incidence of cancer.1, 44, 45 Our results show that BZA at the target human equivalent dose fully antagonized the proliferative and transcriptional effects of CEE on the macaque endometrium while having no estrogen agonist activity when given alone. This information should be useful in the planning of future SERM+estrogen clinical trials and to symptomatic postmenopausal women seeking alternatives to traditional estrogen + progestin therapies.
Supplementary Material
Acknowledgments
We thank the following: Dewayne Cairnes, Debbie Golden, Margaret (Chrissy) May, Margaret Mehaffey, Edison Floyd, Joseph Finley, Lisa O’Donnell, Hermina Borgerink, Jean Gardin, and Maryanne Post for their outstanding technical skills; Dr. Haiying Chen for statistical advice; and Dr. Jay Kaplan for assistance with study design.
This work was supported by grants from Pfizer, Inc. (an investigator initiated grant to Wake Forest School of Medicine, principal investigator TBC); National Center of Research Resources (NCRR) (5T32 RR07009-32 to KE and K01 RR 021322-05 to CEW). The contents are solely the responsibility of the authors and do not necessarily represent the view of the NIH, NCRR, or Pfizer.
Footnotes
Conflicts of Interests: Wake Forest School of Medicine has received an investigator (TBC) initiated grant (Bazedoxifene acetate) from Pfizer. TBC, CEW, SEA were paid co-investigators and KFE was a paid research fellow on this grant. JMC and TCR were unpaid co-investigators. TBC and JMC have been paid consultants for Pfizer and JMC is the principal investigator on a pending investigator initiated proposal to Pfizer related to Bazedoxifene acetate.
Disclaimers: None to report.
Contributor Information
Kelly F. Ethun, Wake Forest University Primate Center and the Department of Pathology/Comparative Medicine, Wake Forest School of Medicine
Charles E. Wood, Wake Forest University Primate Center and the Department of Pathology/Comparative Medicine, Wake Forest School of Medicine
J. Mark Cline, Wake Forest University Primate Center and the Department of Pathology/Comparative Medicine, Wake Forest School of Medicine
Thomas C. Register, Wake Forest University Primate Center and the Department of Pathology/Comparative Medicine, Wake Forest School of Medicine
Susan E. Appt, Wake Forest University Primate Center and the Department of Pathology/Comparative Medicine, Wake Forest School of Medicine
Thomas B. Clarkson, Wake Forest University Primate Center and the Department of Pathology/Comparative Medicine, Wake Forest School of Medicine
References
- 1.The Writing Group for the PEPI Trial. Effects of hormone therapy on bone mineral density: results from the Postmenopausal Estrogen/Progestin Interventions (PEPI) trial. JAMA. 1996;276:1389–1396. [PubMed] [Google Scholar]
- 2.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 3.Brunner RL, Aragaki A, Barnabei V, et al. Menopausal symptom experience before and after stopping estrogen therapy in the Women’s Health Initiative randomized, placebo-controlled trial. Menopause. 2010;17:946–954. doi: 10.1097/gme.0b013e3181d76953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ziel HK, Finkle WD. Increased risk of endometrial carcinoma among users of conjugated estrogens. N Engl J Med. 1975;293:1167–1170. doi: 10.1056/NEJM197512042932303. [DOI] [PubMed] [Google Scholar]
- 5.Cushing KL, Weiss NS, Voigt LF, McKnight B, Beresford SA. Risk of endometrial cancer in relation to use of low-dose, unopposed estrogens. Obstet Gynecol. 1998;91:35–39. doi: 10.1016/s0029-7844(97)00577-2. [DOI] [PubMed] [Google Scholar]
- 6.Weiderpass E, Adami HO, Baron JA, et al. Risk of endometrial cancer following estrogen replacement with and without progestins. J Natl Cancer Inst. 1999;91:1131–1137. doi: 10.1093/jnci/91.13.1131. [DOI] [PubMed] [Google Scholar]
- 7.Pickar JH, Yeh IT, Wheeler JE, Cunnane MF, Speroff L. Endometrial effects of lower doses of conjugated equine estrogens and medroxyprogesterone acetate: two-year substudy results. Fertil Steril. 2003;80:1234–1240. doi: 10.1016/s0015-0282(03)01167-1. [DOI] [PubMed] [Google Scholar]
- 8.Pickar JH. The endometrium--from estrogens alone to TSECs. Climacteric. 2009;12:463–477. doi: 10.3109/13697130903042790. [DOI] [PubMed] [Google Scholar]
- 9.Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: annual trends and response to recent evidence. JAMA. 2004;291:47–53. doi: 10.1001/jama.291.1.47. [DOI] [PubMed] [Google Scholar]
- 10.Chlebowski RT, Hendrix SL, Langer RD, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative Randomized Trial. JAMA. 2003;289:3243–3253. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- 11.Schairer C, Lubin J, Troisi R, Sturgeon S, Brinton L, Hoover R. Menopausal estrogen and estrogen-progestin replacement therapy and breast cancer risk. JAMA. 2000;283:485–491. doi: 10.1001/jama.283.4.485. [DOI] [PubMed] [Google Scholar]
- 12.Barnabei VM, Cochrane BB, Aragaki AK, et al. Menopausal symptoms and treatment-related effects of estrogen and progestin in the Women’s Health Initiative. Obstet Gynecol. 2005;105:1063–1073. doi: 10.1097/01.AOG.0000158120.47542.18. [DOI] [PubMed] [Google Scholar]
- 13.Komm BS. A new approach to menopausal therapy: the tissue selective estrogen complex. Reprod Sci. 2008;15:984–992. doi: 10.1177/1933719108325759. [DOI] [PubMed] [Google Scholar]
- 14.Palacios S. The future of the new selective estrogen receptor modulators. Menopause Int. 2007;13:27–34. doi: 10.1258/175404507780456791. [DOI] [PubMed] [Google Scholar]
- 15.Visvanathan K, Chlebowski RT, Hurley P, et al. American society of clinical oncology clinical practice guideline update on the use of pharmacologic interventions including tamoxifen, raloxifene, and aromatase inhibition for breast cancer risk reduction. J Clin Oncol. 2009;27:3235–3258. doi: 10.1200/JCO.2008.20.5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 17.Davies GC, Huster WJ, Lu Y, Plouffe L, Jr, Lakshmanan M. Adverse events reported by postmenopausal women in controlled trials with raloxifene. Obstet Gynecol. 1999;93:558–565. doi: 10.1016/s0029-7844(98)00476-1. [DOI] [PubMed] [Google Scholar]
- 18.Vardy MD, Lindsay R, Scotti RJ, et al. Short-term urogenital effects of raloxifene, tamoxifen, and estrogen. Am J Obstet Gynecol. 2003;189:81–88. doi: 10.1067/mob.2003.374. [DOI] [PubMed] [Google Scholar]
- 19.Fisher B, Costantino JP, Redmond CK, Fisher ER, Wickerham DL, Cronin WM. Endometrial cancer in tamoxifen-treated breast cancer patients: findings from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14. J Natl Cancer Inst. 1994;86:527–537. doi: 10.1093/jnci/86.7.527. [DOI] [PubMed] [Google Scholar]
- 20.Deligdisch L, Kalir T, Cohen CJ, de Latour M, Le Bouedec G, Penault-Llorca F. Endometrial histopathology in 700 patients treated with tamoxifen for breast cancer. Gynecol Oncol. 2000;78:181–186. doi: 10.1006/gyno.2000.5859. [DOI] [PubMed] [Google Scholar]
- 21.Cohen I. Endometrial pathologies associated with postmenopausal tamoxifen treatment. Gynecol Oncol. 2004;94:256–266. doi: 10.1016/j.ygyno.2004.03.048. [DOI] [PubMed] [Google Scholar]
- 22.Wood CE, Kaplan JR, Fontenot MB, Williams JK, Cline JM. Endometrial profile of tamoxifen and low-dose estradiol combination therapy. Clin Cancer Res. 2010;16:946–956. doi: 10.1158/1078-0432.CCR-09-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pinkerton JV, Utian WH, Constantine GD, Olivier S, Pickar JH. Relief of vasomotor symptoms with the tissue-selective estrogen complex containing bazedoxifene/conjugated estrogens: a randomized, controlled trial. Menopause. 2009;16:1116–1124. doi: 10.1097/gme.0b013e3181a7df0d. [DOI] [PubMed] [Google Scholar]
- 24.Bachmann G, Bobula J, Mirkin S. Effects of bazedoxifene/conjugated estrogens on quality of life in postmenopausal women with symptoms of vulvar/vaginal atrophy. Climacteric. 2010;13:132–140. doi: 10.3109/13697130903305627. [DOI] [PubMed] [Google Scholar]
- 25.Utian W, Yu H, Bobula J, Mirkin S, Olivier S, Pickar JH. Bazedoxifene/conjugated estrogens and quality of life in postmenopausal women. Maturitas. 2009;63:329–335. doi: 10.1016/j.maturitas.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Lobo RA, Pinkerton JV, Gass ML, et al. Evaluation of bazedoxifene/conjugated estrogens for the treatment of menopausal symptoms and effects on metabolic parameters and overall safety profile. Fertil Steril. 2009;92:1025–1038. doi: 10.1016/j.fertnstert.2009.03.113. [DOI] [PubMed] [Google Scholar]
- 27.Kuwamura Y, Kakehi K, Hirakawa K, Miyajima H. Ectopic uterine ovarian tissue in cynomolgus monkeys. Toxicol Pathol. 2006;34:220–222. doi: 10.1080/01926230600695482. [DOI] [PubMed] [Google Scholar]
- 28.Van Esch E, Cline JM, Buse E, Weinbauer GF. The macaque endometrium, with special reference to the cynomolgus monkey (Macaca fascicularis) Toxicol Pathol. 2008;36:S67–S100. [Google Scholar]
- 29.Cline JM, Söderqvist G, Register TC, Williams JK, Adams MR, Von Schoultz B. Assessment of hormonally active agents in the reproductive tract of female nonhuman primates. Toxicol Pathol. 2001;29:84–90. doi: 10.1080/019262301301418883. [DOI] [PubMed] [Google Scholar]
- 30.Ethun KF, Wood CE, Register TC, Cline JM, Appt SE, Clarkson TB. Effects of bazedoxifene acetate with and without conjugated equine estrogens on the breast of postmenopausal monkeys. Menopause. 2012 Jul 9; doi: 10.1097/gme.0b013e318252e46d. pubmed ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ermer J, McKeand W, Sullivan P, Parker V, Orczyk G. Bazedoxifene acetate dose proportionality in healthy postmenopausal women. Clin Pharmacol Ther. 2003;73:46. [Google Scholar]
- 32.Wood CE, Lees CJ, Cline JM. Mammary gland and endometrial effects of testosterone in combination with oral estradiol and progesterone. Menopause. 2009;16:466–476. doi: 10.1097/gme.0b013e318191747a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cline JM, Register TC, Clarkson TB. Comparative effects of tibolone and conjugated equine estrogens with and without medroxyprogesterone acetate on the reproductive tract of female cynomolgus monkeys. Menopause. 2002;9:242–252. doi: 10.1097/00042192-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Goulding H, Pinder S, Cannon P, et al. A new immunohistochemical antibody for the assessment of estrogen receptor status on routine formalin-fixed tissue samples. Hum Pathol. 1995;26:291–294. doi: 10.1016/0046-8177(95)90060-8. [DOI] [PubMed] [Google Scholar]
- 35.Pickar JH, Yeh IT, Bachmann G, Speroff L. Endometrial effects of a tissue selective estrogen complex containing bazedoxifene/conjugated estrogens as a menopausal therapy. Fertil Steril. 2009;92:1018–1024. doi: 10.1016/j.fertnstert.2009.05.094. [DOI] [PubMed] [Google Scholar]
- 36.Archer DF, Lewis V, Carr BR, Olivier S, Pickar JH. Bazedoxifene/conjugated estrogens (BZA/CE): incidence of uterine bleeding in postmenopausal women. Fertil Steril. 2009;92:1039–1044. doi: 10.1016/j.fertnstert.2009.05.093. [DOI] [PubMed] [Google Scholar]
- 37.Archer DF, Lobo RA, Pan K, Chines AA, Mirkin S. Safety and tolerability of bazedoxifene/conjugated estrogens in postmenopausal women: findings from a 1-year, randomized, placebo- and active-controlled, phase 3 trial. NAMS 22nd Annual Meeting; Washington, DC: The North American Menopause Society; 2011. Program No. P-25. [Google Scholar]
- 38.Komm BS, Kharode YP, Bodine PV, Harris HA, Miller CP, Lyttle CR. Bazedoxifene acetate: a selective estrogen receptor modulator with improved selectivity. Endocrinology. 2005;146:3999–4008. doi: 10.1210/en.2005-0030. [DOI] [PubMed] [Google Scholar]
- 39.Pinkerton JV, Taylor H, Pan K, Chines A, Mirkin S. Breast parameters with bazedoxifene/conjugated estrogens in randomized, controlled trials of postmenopausal women. NAMS 21st Annual Meeting Abstract Viewer; Chicago, IL: The North American Menopause Society; 2010. Program No. S-21. [Google Scholar]
- 40.Stovall DW, Utian WH, Gass ML, et al. The effects of combined raloxifene and oral estrogen on vasomotor symptoms and endometrial safety. Menopause. 2007;14:510–517. doi: 10.1097/GME.0b013e318031a83d. [DOI] [PubMed] [Google Scholar]
- 41.Davis SR, O’Neill SM, Eden J, et al. Transition from estrogen therapy to raloxifene in postmenopausal women: effects on treatment satisfaction and the endometrium-a pilot study. Menopause. 2004;11:167–175. doi: 10.1097/01.gme.0000087981.28957.cf. [DOI] [PubMed] [Google Scholar]
- 42.Crabtree JS, Peano BJ, Zhang X, Komm BS, Winneker RC, Harris HA. Activity of three selective estrogen receptor modulators on hormone-dependent responses in the mouse uterus and mammary gland. Mol Cell Endocrinol. 2008;287:40–46. doi: 10.1016/j.mce.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 43.Lewis-Wambi JS, Kim H, Curpan R, Grigg R, Sarker MA, Jordan VC. The selective estrogen receptor modulator bazedoxifene inhibits hormone-independent breast cancer cell growth and down-regulates estrogen receptor {alpha} and cyclin D1. Mol Pharmacol. 2011;80:610–620. doi: 10.1124/mol.111.072249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klaassens AH, van Wijk FH, Hanifi-Moghaddam P, et al. Histological and immunohistochemical evaluation of postmenopausal endometrium after 3 weeks of treatment with tibolone, estrogen only, or estrogen plus progestagen. Fertil Steril. 2006;86:352–361. doi: 10.1016/j.fertnstert.2005.12.077. [DOI] [PubMed] [Google Scholar]
- 45.Grady D, Gebretsadik T, Kerlikowske K, Ernster V, Petitti D. Hormone replacement therapy and endometrial cancer risk: a meta-analysis. Obstet Gynecol. 1995;85:304–313. doi: 10.1016/0029-7844(94)00383-O. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.