Abstract
Purpose
While there has been increasing interest in the use of preoperative breast magnetic resonance imaging (MRI) for women with breast cancer, little is known about trends in MRI use, or the association of MRI with surgical approach among older women.
Methods
Using the SEER–Medicare database, we identified a cohort of women diagnosed with breast cancer from 2000-2009 who underwent surgery. We used Medicare claims to identify preoperative breast MRI and surgical approach. We evaluated temporal trends in MRI use according to age and type of surgery, and identified factors associated with MRI. We assessed the association between MRI and surgical approach: breast conserving surgery (BCS) vs. mastectomy, bilateral vs. unilateral mastectomy, and use of contralateral prophylactic mastectomy.
Results
Among the 72,461 women in our cohort, 10.1% underwent breast MRI. Preoperative MRI use increased from 0.8% in 2000-2001 to 25.2% in 2008-2009 (p<.001). Overall, 43.3% received mastectomy and 56.7% received BCS. After adjustment for clinical and demographic factors, MRI was associated with an increased likelihood of having a mastectomy compared to BCS (adjusted odds ratio [AOR]=1.21, 95% CI: 1.14-1.28). Among women who underwent mastectomy, MRI was significantly associated with an increased likelihood of having bilateral cancer diagnosed (9.7%) and undergoing bilateral mastectomy (12.5%) compared to women without MRI (3.7% and 4.1% respectively, p <.001 for both).
Conclusion
The use of preoperative breast MRI has increased substantially among older women with breast cancer and is associated with an increased likelihood of being diagnosed with bilateral cancer, and more invasive surgery.
Keywords: Imaging, Magnetic Resonance Imaging, Preoperative Care, Surgery
Introduction
The use of preoperative breast magnetic resonance imaging (MRI) for newly diagnosed breast cancer patients is controversial. Advocates for incorporating this imaging modality into the surgical management of women with newly diagnosed breast cancer suggest that the extent of disease can be more accurately assessed and additional mammographically and/or sonographically occult lesions can be detected with MRI.[1-3] Yet there is increasing evidence that the use of breast MRI in newly diagnosed patients confers no advantage with respect to attainment of negative margins, or lower rates of reoperation.[4, 5]
Despite the paucity of evidence and the high cost of the test, the number of women who undergo MRI prior to surgical resection is increasing. [6-9] Between 2005 and 2008, preoperative MRI use among women younger than 64 years of age who were undergoing breast cancer surgery increased from 22.8% to 52.9%.[9] As increasing age is inversely related to time at risk for disease progression and recurrence, cancer management strategies must be carefully scrutinized across all age strata.[10, 11] That is, with increasing age and shorter life expectancy, the clinical benefit of detecting occult lesions on MRI is likely to diminish. Hence, while the benefits of preoperative MRI are still being determined, it is particularly important to understand MRI use among older women with breast cancer. Earlier studies have found that the use of preoperative MRI among Medicare beneficiaries increased from 3.9% in 2003 to 10.1% in 2005.[8] In addition to assessing MRI use in the Medicare program using more recent data, several knowledge gaps regarding the clinical impact of MRI use remain.
Breast MRI has high sensitivity for detecting breast abnormalities, including additional loci of invasive disease. In prior studies, largely including younger patients, preoperative MRI detected additional foci of mammographically occult disease in the ipsilateral breast in 11-31% of newly diagnosed breast cancer patients[12] with approximately 3% diagnosed with additional breast cancer in the contralateral breast.[13] However, the impact of MRI on contralateral disease detection at the population level remains to be assessed. Preoperative MRI might alter surgical management strategies in part through detecting additional invasive lesions on the contralateral breast. As a result, concern has been expressed that the use of breast MRI is contributing to rising mastectomy rates.[14] Mastectomy is not without potential complications, especially when immediate reconstruction is performed.[15] A national UK audit of over 3,000 women found a 16% readmission rate for complications and a 10% implant loss rate.[16] Yet little is known about how the diffusion of MRI is affecting mastectomy use among Medicare beneficiaries with breast cancer. Further, given that MRI can increase detection of lesions in the contralateral breast, it is important to determine the relation between MRI use and receipt of bilateral mastectomy.
Amidst uncertainty about how new technologies are affecting patient outcomes, and concerns about rising cancer care costs, understanding the clinical implications of new imaging strategies is crucial. We therefore assessed the use of breast MRI among female Medicare beneficiaries who were diagnosed with breast cancer in 2000 through 2009 to describe imaging and surgical trends for the treatment of unilateral and bilateral breast cancer over the same time period, and to assess the association between receipt of preoperative MRI and the extent of surgical treatment.
Methods
Overview
Among older women who underwent surgery for breast cancer, we used Medicare claims to identify the use of preoperative breast MRI within 6 months prior to surgery. We observed temporal trends and factors associated with the use of MRI and assessed the relation between preoperative MRI and surgery type.
Data Source
The Surveillance, Epidemiology and End Results (SEER)-Medicare database provides sociodemographic and cancer characteristics for patients residing in SEER regions linked with Medicare claims. The registry covers approximately 28% of the US population. The Yale Human Investigation Committee determined that this study did not constitute human subjects research.
Study Sample
We identified all women diagnosed with stage I-III invasive breast cancer during 2000-2009 who underwent surgery and were at least 67 years old at the time of breast cancer diagnosis. We excluded patients if: 1) breast cancer was not the first tumor diagnosis reported to SEER, or Medicare claims indicated a history of cancer in the two years before diagnosis; 2) the tumor was reported by autopsy or death certificate only; 3) tumor histology was not of epithelial origin; 4) month or stage of diagnosis was missing; or 5) patients did not have continuous fee-for-service Medicare Part A and Part B coverage from two years before diagnosis through death or December 31, 2011, whichever occurred first. We also excluded women with breast cancer diagnosed in the Greater Georgia registry before 2004, as we did not have complete claims to assess their MRI use and comorbidity, and women with no Medicare claims in the 24 months before through 12 months after cancer diagnosis, as these women were likely receiving cancer treatment outside the Medicare system.
Exposure and Outcome Ascertainment
We identified preoperative breast MRI according to Healthcare Common Procedure Coding System (HCPCS) codes (Appendix 1). Type of surgery was identified using HCPCS codes and their modifiers, as well as International Classification for Diseases, Ninth Revision (ICD-9) procedure codes. Breast surgery was classified into breast conserving surgery (BCS) or mastectomy, with further subdivision of mastectomy according to unilateral or bilateral mastectomy.[17] We defined bilateral breast cancer as a SEER report where laterality indicated bilateral involvement or a diagnosis of breast cancer in the contralateral breast between the month of diagnosis and breast cancer surgery. Women who received bilateral mastectomy, but were not identified as having bilateral breast cancer, were classified as receiving contralateral prophylactic mastectomy.
Covariate Creation and Selection
Covariates included age, race, marital status, year of diagnosis, median household income at the zip code level and SEER region. We used Elixhauser comorbid conditions, adapting an approach which requires the diagnosis code to appear on an inpatient claim or two or more physician or outpatient claims greater than 30 days apart for the condition to be considered present (Appendix 1).[18] We also assessed stage, grade, tumor size, hormone receptor status, and number of positive lymph nodes as reported by SEER.
Statistical Analysis
We used chi-squared tests to evaluate the association between demographic and clinical characteristics and MRI. We evaluated trends in MRI use over time by age group and in combination with the type of mastectomy (bilateral vs. unilateral) using Cochran-Armitage and Jonckheere-Terpstra tests of trend. We identified factors associated with undergoing preoperative MRI using multivariable logistic regression.
We used multivariable logistic regression to assess the association between preoperative MRI and the extent of the surgery (BCS vs. mastectomy). Among women who underwent mastectomy, we then evaluated the association between MRI and type of mastectomy (unilateral or bilateral). Finally, we used multinomial logistic regression to assess the association between preoperative MRI and the following surgery types: (1) bilateral mastectomy for the treatment of unilateral breast cancer (i.e., contralateral prophylactic mastectomy), (2) unilateral mastectomy for the treatment of bilateral breast cancer, (3) bilateral mastectomy for the treatment of bilateral breast cancer, and (4) unilateral mastectomy for the treatment of unilateral breast cancer (reference). All analyses were conducted usingSAS (version 9.2, SAS Institute, Inc., Cary, NC).Tests were two-sided with an alpha value of 0.05.
Results
There were a total of 72,461 women in the analysis. The majority was white, had early stage disease, and had estrogen receptor (ER) positive tumors (Table 1). Overall, 10.1% (n=7,333) underwent preoperative breast MRI (Table 2). Women who underwent MRI were more likely to be younger, white, of higher median income, and have less comorbidity compared to those who did not (p≤.001 for all).
Table 1. Demographic and cancer characteristics of sample.
| Characteristic | N | % | ||
|---|---|---|---|---|
| Total | 72,461 | |||
| Age group | ||||
| 67-69 | 11,790 | 16.3% | ||
| 70-74 | 19,563 | 27.0% | ||
| 75-79 | 18,695 | 25.8% | ||
| 80-84 | 13,559 | 18.7% | ||
| 85+ | 8,854 | 12.2% | ||
| Race | ||||
| White | 65,190 | 90.0% | ||
| Black | 4,596 | 6.3% | ||
| Other | 2,675 | 3.7% | ||
| Marital Status | ||||
| Married | 30,983 | 42.8% | ||
| Unmarried | 38,688 | 53.4% | ||
| Other | 2,790 | 3.9% | ||
| Median income of zip code | ||||
| Less than $33,000 | 14,850 | 20.5% | ||
| $33,000-40,000 | 10,925 | 15.1% | ||
| $40,000-50,000 | 15,090 | 20.8% | ||
| $50,000-63,000 | 14,102 | 19.5% | ||
| More than $63,000 | 17,464 | 24.1% | ||
| Unknown | 30 | 0.0% | ||
| Comorbidity | ||||
| None | 33,462 | 46.2% | ||
| 1 to 2 | 27,851 | 38.4% | ||
| 3 or more | 11,148 | 15.4% | ||
| Stage | ||||
| I | 40,631 | 56.1% | ||
| II | 25,427 | 35.1% | ||
| III | 6,403 | 8.8% | ||
| Tumor size | ||||
| <2.0 cm | 44,076 | 60.8% | ||
| 2.0-<=5.0 cm | 24,133 | 33.3% | ||
| >5.0 cm | 3,669 | 5.1% | ||
| Missing | 583 | 0.8% | ||
| Grade | ||||
| Well differentiated | 17,255 | 23.8% | ||
| Moderately differentiated | 31,095 | 42.9% | ||
| Poorly differentiated | 18,561 | 25.6% | ||
| Undifferentiated | 716 | 1.0% | ||
| Unknown | 4,834 | 6.7% | ||
| Number positive lymph nodes | ||||
| No positive nodes | 45,679 | 63.0% | ||
| 1-3 positive nodes | 12,376 | 17.1% | ||
| 4+ positive modes | 5,713 | 7.9% | ||
| No nodes Examined | 8,374 | 11.6% | ||
| Unknown # Positive Nodes | 319 | 0.4% | ||
| Hormone receptors | ||||
| ER+ or PR+ | 56,114 | 77.4% | ||
| ER– and PR– | 9,063 | 12.5% | ||
| Missing | 7,284 | 10.1% | ||
| Bilateral Breast Cancer | ||||
| Yes | 1,951 | 2.7% | ||
| None | 70,510 | 97.3% | ||
Table 2. Factors associated with receipt of preoperative breast MRI.
| MRI | No MRI | Crude | Adjusted OR (95% CI) |
Adjusted P-value | ||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | P-value | ||||
| Total | 7,333 | 10.1% | 65,128 | 89.9% | ||||
| Age group | ||||||||
| 67-69 | 1,864 | 15.8% | 9,926 | 84.2% | <.001 | Reference | <.001 | |
| 70-74 | 2,506 | 12.8% | 17,057 | 87.2% | 0.82 (0.76-0.88) | |||
| 75-79 | 1,706 | 9.1% | 16,989 | 90.9% | 0.59 (0.55-0.64) | |||
| 80-84 | 898 | 6.6% | 12,661 | 93.4% | 0.40 (0.36-0.44) | |||
| 85+ | 359 | 4.1% | 8,495 | 95.9% | 0.25 (0.22-0.29) | |||
| Race | ||||||||
| White | 6,764 | 10.4% | 58,426 | 89.6% | <.001 | Reference | .001 | |
| Black | 279 | 6.1% | 4,317 | 93.9% | 0.79 (0.69-0.91) | |||
| Other | 290 | 10.8% | 2,385 | 89.2% | 0.87 (0.75-1.00) | |||
| Marital Status | ||||||||
| Married | 3,877 | 12.5% | 27,106 | 87.5% | <.001 | Reference | <.001 | |
| Unmarried | 3,148 | 8.1% | 35,540 | 91.9% | 0.85 (0.80-0.90) | |||
| Other | 308 | 11.0% | 2,482 | 89.0% | 1.14 (0.99-1.31) | |||
| Median income of zip code | ||||||||
| Less than $33,000 | 826 | 5.6% | 14,024 | 94.4% | <.001 | Reference | <.001 | |
| $33,000-40,000 | 779 | 7.1% | 10,146 | 92.9% | 1.21 (1.08-1.35) | |||
| $40,000-50,000 | 1,387 | 9.2% | 13,703 | 90.8% | 1.48 (1.34-1.63) | |||
| $50,000-63,000 | 1,587 | 11.3% | 12,515 | 88.7% | 1.79 (1.62-1.98) | |||
| More than $63,000* | >2,743 | >15.7% | <14,721 | <84.3% | 2.68 (2.43-2.96) | |||
| Unknown* | <11 | <36.7% | >19 | >63.3% | 2.88(0.74-11.23) | |||
| Comorbidity | ||||||||
| None | 3,900 | 11.7% | 29,562 | 88.3% | <.001 | Reference | <.001 | |
| 1 to 2 | 2,739 | 9.8% | 25,112 | 90.2% | 0.84 (0.79-0.89) | |||
| 3 or more | 694 | 6.2% | 10,454 | 93.8% | 0.54 (0.49-0.59) | |||
| Stage | ||||||||
| I | 4,160 | 10.2% | 36,471 | 89.8% | .17 | Reference | <.001 | |
| II | 2,503 | 9.8% | 22,924 | 90.2% | 1.25 (1.11-1.40) | |||
| III | 670 | 10.5% | 5,733 | 89.5% | 1.00 (0.81-1.24) | |||
| Tumor size | ||||||||
| <2.0 cm | 4,729 | 10.7% | 39,347 | 89.3% | <.001 | Reference | <.001 | |
| 2.0-<=5.0 cm | 2,186 | 9.1% | 21,947 | 90.9% | 0.87 (0.79-0.95) | |||
| >5.0 cm | 353 | 9.6% | 3,316 | 90.4% | 1.12 (0.95-1.32) | |||
| Missing | 65 | 11.1% | 518 | 88.9% | 1.41 (1.03-1.91) | |||
| Grade | ||||||||
| Well differentiated | 1,894 | 11.0% | 15,361 | 89.0% | <.001 | Reference | <.001 | |
| Moderately differentiated | 3,403 | 10.9% | 27,692 | 89.1% | 1.03 (0.96-1.10) | |||
| Poorly differentiated | 1,625 | 8.8% | 16,936 | 91.2% | 0.82 (0.76-0.90) | |||
| Undifferentiated | 38 | 5.3% | 678 | 94.7% | 0.78 (0.54-1.11) | |||
| Unknown | 373 | 7.7% | 4,461 | 92.3% | 1.13 (0.99-1.28) | |||
| Number positive lymph nodes | ||||||||
| No positive nodes | 5,096 | 11.2% | 40,583 | 88.8% | <.001 | Reference | <.001 | |
| 1-3 positive nodes | 1,355 | 10.9% | 11,021 | 89.1% | 1.01 (0.92-1.12) | |||
| 4+ positive modes | 549 | 9.6% | 5,164 | 90.4% | 1.18 (0.98-1.42) | |||
| No nodes Examined | 304 | 3.6% | 8,070 | 96.4% | 0.52 (0.45-0.59) | |||
| Unknown # Positive Nodes | 29 | 9.1% | 290 | 90.9% | 1.15 (0.74-1.77) | |||
| Hormone receptors | ||||||||
| ER+ or PR+ | 6,121 | 10.9% | 49,993 | 89.1% | <.001 | Reference | .002 | |
| ER– and PR– | 900 | 9.9% | 8,163 | 90.1% | 1.02 (0.93-1.11) | |||
| Missing | 312 | 4.3% | 6,972 | 95.7% | 0.80 (0.70-0.91) | |||
| Bilateral Breast Cancer | ||||||||
| Yes (bilateral) | 443 | 22.7% | 1,508 | 77.3% | <.001 | Not Included | ||
| No (unilateral) | 6,890 | 9.8% | 63,620 | 90.2% | ||||
Values expressed as a range to conform to SEER-Medicare policy of not publishing cell sizes <11. Odds ratios adjusted for all variables in table as well as year of diagnosis and SEER region.
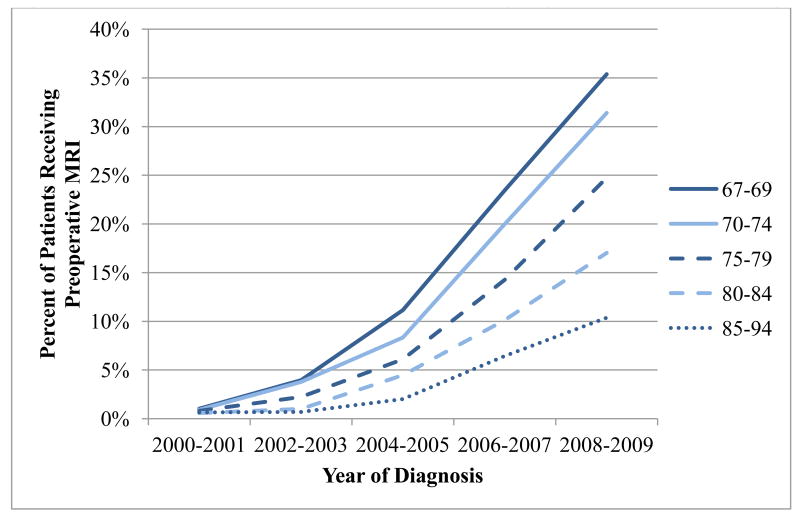
The use of breast MRI increased steadily over the study period, from 0.8% in 2000-2001 to 25.2% in 2008-2009 (p<001 for trend; Figure 1). The proportion of women who underwent MRI varied according to age group; throughout the study period, the youngest women (67-69 years) were most likely to undergo preoperative breast MRI (p-value for trend<.001), with approximately 35% of women receiving an MRI in 2008-2009 (Table 2). Nonetheless, among the oldest women (84-94 years) approximately 10% underwent a preoperative breast MRI in 2008-2009.
Figure 1. Percent of women with breast cancer undergoing preoperative MRI by age.
Overall 43.3% of women in the study underwent mastectomy and 56.7% received BCS (Table 3). In bivariate analysis, preoperative MRI was associated with a decreased likelihood of mastectomy compared to BCS (odds ratio [OR]=0.85, 95% CI: 0.80-0.89, p<.001). However, after adjusting for demographic and cancer characteristics, preoperative MRI was associated with a significantly higher likelihood of mastectomy compared to BCS (adjusted OR [AOR]=1.21, 95% CI: 1.14-1.28, p<.001).
Table 3. Association between preoperative MRI and surgery type.
| All Patients | MRI | No MRI | Adjusted OR* | ||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | (95% CI) | |
| Mastectomy vs. Breast Conserving Surgery (All Patients N=72,461) | |||||||
| BCS | 41,088 | 56.7% | 4,428 | 60.4% | 36,660 | 56.3% | Reference |
| Mastectomy | 31,373 | 43.3% | 2,905 | 39.6% | 28,468 | 43.7% | 1.21 (1.14-1.28) |
| Bilateral vs. Unilateral Mastectomy (Mastectomy Patients N=31,373) | |||||||
| Unilateral Mastectomy | 29,858 | 95.2% | 2,543 | 87.5% | 27,315 | 95.9% | reference |
| Bilateral Mastectomy | 1,515 | 4.8% | 362 | 12.5% | 1,153 | 4.1% | 1.98 (1.72-2.29) |
| Presence of Bilateral Cancer and Use of Bilateral Mastectomy (Mastectomy Patients N=31,373) | |||||||
| Unilateral Mastectomy-Unilateral Cancer | 29,341 | 93.5% | 2,425 | 83.5% | 26,916 | 94.6% | reference |
| Contralateral Prophylactic Mastectomy (Bilateral Mastectomy-Unilateral Cancer) | 701 | 2.2% | 200 | 6.9% | 501 | 1.8% | 2.52 (2.08-2.68) |
| Bilateral Mastectomy-Bilateral Cancer | 814 | 2.6% | 162 | 5.6% | 652 | 2.3% | 2.20 (1.81-2.68) |
| Unilateral Mastectomy-Bilateral Cancer | 517 | 1.6% | 118 | 4.1% | 399 | 1.4% | 2.97 (2.35-3.75) |
Adjusted for demographic characteristics (age, race, marital status, median income of zip code, comorbidity, year of diagnosis and SEER region) and cancer characteristics (stage, grade, tumor size, number positive lymph nodes, hormone receptor status).
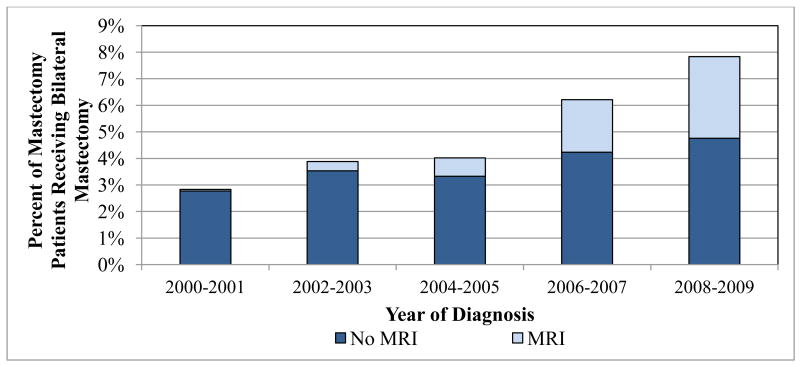
Of the 31,373 women who underwent mastectomy, 4.8% had a bilateral mastectomy (Table 3). The use of bilateral mastectomy almost doubled over the study period, from 2.8% of those undergoing mastectomy in 2000-2001 to 7.8% in 2008-2009 (p for trend <.001, Figure 2). While there was a significant increase in bilateral mastectomy among both women who did and did not receive preoperative breast MRI over time (p for trend both <.001), women who had an MRI were more likely to have a bilateral procedure than those who did not (12.5% vs. 4.1%, p<.001, Table 3). After adjusting for patient and clinical factors, preoperative MRI was associated with a significantly increased likelihood of having bilateral vs. unilateral mastectomy (AOR=1.98, 95% CI: 1.72-2.29).
Figure 2. Bilateral mastectomy rates among women undergoing mastectomy according to MRI use by diagnosis year, 2000-2009.
Receipt of MRI was also associated with diagnosis of bilateral disease among women who underwent a mastectomy. While 3.7% of women who did not receive MRI were diagnosed with bilateral breast cancer, 9.7% of women who received an MRI were diagnosed with bilateral breast cancer (p<.001). Accordingly, as MRI use increased over time, the percentage of mastectomy patients diagnosed with bilateral breast cancer increased from 3.6% in 2000 to 5.2% in 2009 (p for trend <.001).
We then distinguished bilateral mastectomy performed in the setting of bilateral breast cancer, from bilateral mastectomy performed in the setting of unilateral breast cancer (contralateral prophylactic mastectomy). Preoperative breast MRI use was significantly associated with use of contralateral prophylactic mastectomy. Among women who underwent mastectomy, 6.9% of women who had an MRI underwent contralateral prophylactic mastectomy, compared to 1.8% in women who did not have an MRI (Table 3). In multivariable analysis, MRI use was associated with an increased rate of contralateral prophylactic mastectomy (AOR=2.52, 95% CI: 2.08-2.68), as well as bilateral mastectomy for bilateral cancer (AOR=2.20, 95% CI: 1.81-2.68), and unilateral mastectomy for bilateral cancer (AOR=2.97, 95% CI: 2.35-3.75), compared to unilateral mastectomy for unilateral cancer.
Discussion
We observed a significant increase in the use of preoperative breast MRI among Medicare beneficiaries with early stage breast cancer from 2000-2009. The use of preoperative MRI was more prevalent across the study period among women in younger age groups, with one in three women age 75 and under receiving an MRI by the end of the study period, despite the lack of evidence linking MRI use to superior outcomes. By 2009, 10% of women in the oldest age category (85-94 years old) were receiving preoperative MRI. This study builds upon prior work in several ways. We not only demonstrated that preoperative MRI has diffused rapidly into the care of older women with breast cancer, but have also found that this imaging strategy is associated with important changes in management. Women who received an MRI were more likely to subsequently undergo more aggressive surgical treatment, such as mastectomy (compared to BCS), or bilateral (compared to unilateral) mastectomy. Notably, the majority of the increase in use of bilateral mastectomy during the study period was in women who had undergone preoperative MRI.
The association between MRI use and mastectomy is concerning for several reasons. First, evidence does not support mastectomy as a superior strategy. Six prospective randomized trials have proven BCS to be no less effective than mastectomy for breast cancer treatment.[19-24] Ten-year local recurrence rates after BCS in trials conducted in the 1970s ranged from 3.5% to 6.5% and are considerably less than 5% when adjuvant hormone and radiotherapy are given. This is particularly true for the well differentiated, hormone receptor positive cancers commonly seen among elderly women. Second, research has shown improved body image and lower rates of depression among women of all ages with BCS compared to mastectomy.[25] Third, potential complications and length of hospital stay associated with mastectomy are more numerous than for BCS, especially among older women with multiple comorbidities, and especially when reconstruction is performed. [16] BCS and sentinel lymph node biopsy with or without axillary dissection can usually be performed as outpatient surgery and are associated with less time under anesthesia and fewer complications than mastectomy. Lastly, BCS is less costly to the healthcare system than mastectomy at 5 years.[26] In light of these compelling reasons to pursue BCS when feasible, it is difficult to appreciate an added value of more extensive surgery brought about by the use of preoperative MRI.
While there are cases where mastectomy is necessary for early stage disease, it is likely that many of the women in this study would have been well served with BCS and appropriate adjuvant treatment. Fear of recurrence and poorer survival may in part be driving this trend toward MRI use and more extensive surgery, but this is difficult to measure. A recent study by Fisher et al reported that approximately 40% of women elected mastectomy when BCS was an option, with fear of recurrence and perceived improvement in survival cited as the most common reasons.[27] It also possible that some women elected to undergo a mastectomy to avoid radiotherapy. However, assuming that a similar percentage would have needed radiotherapy at the beginning of the study, this trend toward more aggressive surgery is likely related to preoperative MRI, rather than avoidance of radiation.
Among those undergoing mastectomy, we also observed an increased rate of bilateral mastectomy, regardless of whether bilateral cancer was present. The increased use of contralateral prophylactic mastectomy is provocative, as the risk of developing a contralateral breast cancer is only 0.5% - 1.0% per year among women treated for a unilateral cancer.[28] It is possible that additional suspicious areas seen on MRI which were not biopsied prior to surgery contributed to increased patient anxiety and the desire for contralateral prophylactic mastectomy. A recent analysis concluded that contralateral prophylactic mastectomy was not cost effective for women over the age of 70; this group represented about 80% of our sample.[29-31]
While we have demonstrated that MRI is associated with higher rates of detection of contralateral cancers, the impact that these contralateral foci of disease have on survival is unknown at the population level.[32] It is possible, that hormone therapy, which is very effective against the ER positive tumors that older women are likely to present with, may be effective treatment. Clearly further research is needed to evaluate the long term outcomes and the survival benefit, if any, with regard to small contralateral cancer detection. This is particularly important, as bilateral mastectomy does confer an even greater risk of adverse events than unilateral mastectomy[33-36].
The use of a large, population based database allows us to report on national trends in the use of MRI and breast cancer surgery; however, we are limited by a lack of clinical variables that will be important to include in future analyses. We were not able to link pathologic information to suspicious MRI findings, cannot identify multicentric disease, assess family history of breast cancer or presence BRCA mutations, or evaluate breast density (a factor which renders mammography less sensitive).[37, 38] In addition, we were unable to measure the effect that patient anxiety associated with MRI had on the decision to undergo mastectomy.
It is unclear why the use of MRI has increased so rapidly. Clinicians are not responding to a new groundswell of medical evidence; neither of the two large, prospective randomized trials to evaluate preoperative breast MRI use demonstrated a clinical benefit. [39, 40] We can only speculate that patient preference to have an MRI, providers' concerns about performing surgery without a “complete” evaluation, and reimbursement incentives may have played a role in the rapid dissemination of breast MRI. Provider practice style is likely a driving factor: a recent survey of practicing surgeons reveals wide variation in breast MRI recommendation for recently diagnosed patients—with about one in five recommending it to <10% of their patients, and about one in ten surgeons recommending it to >95% of their patients.[41] Forty-one percent of surgeons routinely (≥75% of the time) recommended the test, with these proportions varying by practice volume, specialization, and practice type. With “personal experience” being the leading influence on its utilization, the trend is likely to increase.[41]
In summary, the use of preoperative breast MRI has increased dramatically among older women and is associated with an increased rate of mastectomy among those undergoing surgery. In order to inform decision-making, the benefits and harms of MRI – and the accompanying change in surgical approach – need to be established. Recent studies have also demonstrated the adoption of newer and more expensive breast cancer screening and treatment approaches in the Medicare population, with scant evidence to support them.[42-44] Our study suggests that breast imaging may be considered in a similar category: it is expensive, affects clinical care, yet has little evidence to supports its use. It is time for policy makers to invest in a comprehensive approach to studying breast cancer management among older women with breast cancer.
Acknowledgments
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The collection of the California cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute's Surveillance, Epidemiology and End Results Program under contract N01-PC-35136 awarded to the Northern California Cancer Center, contract N01-PC-35139 awarded to the University of Southern California, and contract N02-PC-15105 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention's National Program of Cancer Registries, under agreement #U55/CCR921930-02 awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Public Health the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
Drs. Ross and Gross receive support from Medtronic, Inc. to develop methods of clinical trial data sharing. Drs. Ross and Gross are members of a scientific advisory board for FAIR Health, Inc. Dr. Ma receives support from Celgene Corporation. No other authors have relationships to disclose.
Funding: This work was supported by the National Cancer Institute (5R01CA149045) and the P30 Cancer Center Support Grant at the Yale Comprehensive Cancer Center (P30CA016359).
Appendix 1.
Administrative codes used for assessing exposure and outcomes.
| Breast MRI | HCPCS: C8903-C8908, 76093-76094, 77058-77059 | |
|
| ||
| Breast Cancer Surgery | Breast Conserving Surgery | HCPCS: 19110, 19120, 19125, 19126, 19160, 19162, 19301, 19302 ICD-9 Procedure: 85.20-85.23, 85.25 |
|
| ||
| Mastectomy | HCPCS: 19180, 19182, 19200, 19220, 19240, 19303, 19304, 19305, 19306, 19307 ICD-9 Procedure: 85.41-48 |
|
|
| ||
| Elixhauser Comorbidity Conditions Assessed | Congestive Heart Failure, Cardiac Arrhythmia, Valvular Disease, Pulmonary Circulation Disorders, Peripheral Vascular Disorders, Paralysis, Other Neurological Disorders, Chronic Pulmonary Disease, Diabetes, Renal Failure, Liver Disease, AIDS/HIV, Rheumatoid Arthritis, Coagulopathy, Weight Loss, Fluid and Electrolyte Disorders, Blood Loss Anemia, Deficiency Anemia, Alcohol Abuse, Drug Abuse, Psychoses, Depression | |
References
- 1.Hollingsworth AB, Stough RG, O'Dell CA, Brekke CE. Breast magnetic resonance imaging for preoperative locoregional staging. American journal of surgery. 2008;196(3):389–397. doi: 10.1016/j.amjsurg.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Schnall MD, Blume J, Bluemke DA, Deangelis GA, Debruhl N, Harms S, Heywang-Kobrunner SH, Hylton N, Kuhl CK, Pisano ED, et al. MRI detection of distinct incidental cancer in women with primary breast cancer studied in IBMC 6883. Journal of surgical oncology. 2005;92(1):32–38. doi: 10.1002/jso.20381. [DOI] [PubMed] [Google Scholar]
- 3.Lehman CD, Blume JD, Thickman D, Bluemke DA, Pisano E, Kuhl C, Julian TB, Hylton N, Weatherall P, O'Loughlin M, et al. Added cancer yield of MRI in screening the contralateral breast of women recently diagnosed with breast cancer: results from the International Breast Magnetic Resonance Consortium (IBMC) trial. Journal of surgical oncology. 2005;92(1):9–15. doi: 10.1002/jso.20350. discussion 15-16. [DOI] [PubMed] [Google Scholar]
- 4.Young P, Kim B, Malin JL. Preoperative breast MRI in early-stage breast cancer. Breast cancer research and treatment. 2012;135(3):907–912. doi: 10.1007/s10549-012-2207-1. [DOI] [PubMed] [Google Scholar]
- 5.Weber JJ, Bellin LS, Milbourn DE, Verbanac KM, Wong JH. Selective preoperative magnetic resonance imaging in women with breast cancer: no reduction in the reoperation rate. Arch Surg. 2012;147(9):834–839. doi: 10.1001/archsurg.2012.1660. [DOI] [PubMed] [Google Scholar]
- 6.Dinan MA, Curtis LH, Hammill BG, Patz EF, Jr, Abernethy AP, Shea AM, Schulman KA. Changes in the use and costs of diagnostic imaging among Medicare beneficiaries with cancer, 1999-2006. JAMA. 2010;303(16):1625–1631. doi: 10.1001/jama.2010.460. [DOI] [PubMed] [Google Scholar]
- 7.Houssami N, Hayes DF. Review of preoperative magnetic resonance imaging (MRI) in breast cancer: should MRI be performed on all women with newly diagnosed, early stage breast cancer? CA: a cancer journal for clinicians. 2009;59(5):290–302. doi: 10.3322/caac.20028. [DOI] [PubMed] [Google Scholar]
- 8.Sommer CA, Stitzenberg KB, Tolleson-Rinehart S, Carpenter WR, Carey TS. Breast MRI utilization in older patients with newly diagnosed breast cancer. The Journal of surgical research. 2011;170(1):77–83. doi: 10.1016/j.jss.2011.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breslin TM, Banerjee M, Gust C, Birkmeyer NJ. Trends in advanced imaging use for women undergoing breast cancer surgery. Cancer. 2013;119(6):1251–1256. doi: 10.1002/cncr.27838. [DOI] [PubMed] [Google Scholar]
- 10.Komoike Y, Akiyama F, Iino Y, Ikeda T, Akashi-Tanaka S, Ohsumi S, Kusama M, Sano M, Shin E, Suemasu K, et al. Ipsilateral breast tumor recurrence (IBTR) after breast-conserving treatment for early breast cancer: risk factors and impact on distant metastases. Cancer. 2006;106(1):35–41. doi: 10.1002/cncr.21551. [DOI] [PubMed] [Google Scholar]
- 11.Mellemkjaer L, Friis S, Olsen JH, Scelo G, Hemminki K, Tracey E, Andersen A, Brewster DH, Pukkala E, McBride ML, et al. Risk of second cancer among women with breast cancer. International journal of cancer Journal international du cancer. 2006;118(9):2285–2292. doi: 10.1002/ijc.21651. [DOI] [PubMed] [Google Scholar]
- 12.Morrow M, Freedman G. A clinical oncology perspective on the use of breast MR. Magnetic resonance imaging clinics of North America. 2006;14(3):363–378. doi: 10.1016/j.mric.2006.07.006. vi. [DOI] [PubMed] [Google Scholar]
- 13.Lehman CD, Gatsonis C, Kuhl CK, Hendrick RE, Pisano ED, Hanna L, Peacock S, Smazal SF, Maki DD, Julian TB, et al. MRI evaluation of the contralateral breast in women with recently diagnosed breast cancer. N Engl J Med. 2007;356(13):1295–1303. doi: 10.1056/NEJMoa065447. [DOI] [PubMed] [Google Scholar]
- 14.Katipamula R, Degnim AC, Hoskin T, Boughey JC, Loprinzi C, Grant CS, Brandt KR, Pruthi S, Chute CG, Olson JE, et al. Trends in mastectomy rates at the Mayo Clinic Rochester: effect of surgical year and preoperative magnetic resonance imaging. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(25):4082–4088. doi: 10.1200/JCO.2008.19.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeevan RCD, Browne J, Caddy C, Pereira J, Sheppard C, van der Meulen J. Complication Rates Associated with Mastectomy and Breast Reconstruction Procedures. The Royal College of Surgeons of England: Nov 30 2011 - Dec 2 2011 (Abstract) 2011 (Abstract) 2011 (Abstract) [Google Scholar]
- 16.Fourth Annual Report of the National Mastectomy and Breast Reconstruction Audit. http://www.ic.nhs.uk/mbr.
- 17.Tuttle TJS, Durham S, et al. Use of Preoperative MRI Among Older Women with Ductal Carcinoma in Situ (DCIS) and Early Invasive Breast Cancer: Use of Preoperative Breast MRI. Data Points #13 (prepared by the University of Minnesota DEcIDE Center, under Contract No. HHSA290201000131.) Rockville, MD: Agency for Healthcare Research and Quality; Aug, 2012. AHRQPublication No. 12-EHC086-EF. [Google Scholar]
- 18.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Medical care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, Jeong JH, Wolmark N. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 20.Jacobson JA, Danforth DN, Cowan KH, d'Angelo T, Steinberg SM, Pierce L, Lippman ME, Lichter AS, Glatstein E, Okunieff P. Ten-year results of a comparison of conservation with mastectomy in the treatment of stage I and II breast cancer. N Engl J Med. 1995;332(14):907–911. doi: 10.1056/NEJM199504063321402. [DOI] [PubMed] [Google Scholar]
- 21.van Dongen JA, Bartelink H, Fentiman IS, Lerut T, Mignolet F, Olthuis G, van der Schueren E, Sylvester R, Winter J, van Zijl K. Randomized clinical trial to assess the value of breast-conserving therapy in stage I and II breast cancer, EORTC 10801 trial. Journal of the National Cancer Institute Monographs. 1992;(11):15–18. [PubMed] [Google Scholar]
- 22.Blichert-Toft M, Nielsen M, During M, Moller S, Rank F, Overgaard M, Mouridsen HT. Long-term results of breast conserving surgery vs. mastectomy for early stage invasive breast cancer: 20-year follow-up of the Danish randomized DBCG-82TM protocol. Acta oncologica. 2008;47(4):672–681. doi: 10.1080/02841860801971439. [DOI] [PubMed] [Google Scholar]
- 23.Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, Aguilar M, Marubini E. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347(16):1227–1232. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 24.Sarrazin D, Le MG, Arriagada R, Contesso G, Fontaine F, Spielmann M, Rochard F, Le Chevalier T, Lacour J. Ten-year results of a randomized trial comparing a conservative treatment to mastectomy in early breast cancer. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 1989;14(3):177–184. doi: 10.1016/0167-8140(89)90165-5. [DOI] [PubMed] [Google Scholar]
- 25.Rowland JH, Desmond KA, Meyerowitz BE, Belin TR, Wyatt GE, Ganz PA. Role of breast reconstructive surgery in physical and emotional outcomes among breast cancer survivors. Journal of the National Cancer Institute. 2000;92(17):1422–1429. doi: 10.1093/jnci/92.17.1422. [DOI] [PubMed] [Google Scholar]
- 26.Barlow WE, Taplin SH, Yoshida CK, Buist DS, Seger D, Brown M. Cost comparison of mastectomy versus breast-conserving therapy for early-stage breast cancer. Journal of the National Cancer Institute. 2001;93(6):447–455. doi: 10.1093/jnci/93.6.447. [DOI] [PubMed] [Google Scholar]
- 27.Fisher CS, Martin-Dunlap T, Ruppel MB, Gao F, Atkins J, Margenthaler JA. Fear of recurrence and perceived survival benefit are primary motivators for choosing mastectomy over breast-conservation therapy regardless of age. Ann Surg Oncol. 2012;19(10):3246–3250. doi: 10.1245/s10434-012-2525-x. [DOI] [PubMed] [Google Scholar]
- 28.Rosen PP, Groshen S, Kinne DW, Hellman S. Contralateral breast carcinoma: an assessment of risk and prognosis in stage I (T1N0M0) and stage II (T1N1M0) patients with 20-year follow-up. Surgery. 1989;106(5):904–910. [PubMed] [Google Scholar]
- 29.Zendejas B, Moriarty JP, O'Byrne J, Degnim AC, Farley DR, Boughey JC. Cost-effectiveness of contralateral prophylactic mastectomy versus routine surveillance in patients with unilateral breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(22):2993–3000. doi: 10.1200/JCO.2011.35.6956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.King TA, Sakr R, Patil S, Gurevich I, Stempel M, Sampson M, Morrow M. Clinical management factors contribute to the decision for contralateral prophylactic mastectomy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(16):2158–2164. doi: 10.1200/JCO.2010.29.4041. [DOI] [PubMed] [Google Scholar]
- 31.Stucky CC, Gray RJ, Wasif N, Dueck AC, Pockaj BA. Increase in contralateral prophylactic mastectomy: echoes of a bygone era? Surgical trends for unilateral breast cancer. Ann Surg Oncol. 2010;17(Suppl 3):330–337. doi: 10.1245/s10434-010-1259-x. [DOI] [PubMed] [Google Scholar]
- 32.Lostumbo L, Carbine N, Wallace J, Ezzo J. Prophylactic mastectomy for the prevention of breast cancer. Cochrane database of systematic reviews. 2004;(4):CD002748. doi: 10.1002/14651858.CD002748.pub2. [DOI] [PubMed] [Google Scholar]
- 33.Osman FSF, Corrigan M, Jackson T, Cil T. Increased postoperative complications in bilateral mastectomy patients compared to unilateral mastectomy: An analysis of NSQIP data. Ann Surg Oncol Presented. 2013 May 3;20:2. doi: 10.1245/s10434-013-3116-1. [DOI] [PubMed] [Google Scholar]
- 34.Gahm JWM, Brandberg Y. Bilateral prophylactic mastectomy in women with inherited risk of breast cancer - Prevalence of pain and discomfort, impact on sexuality, quality of life and feelings of regret two years after surgery. The Breast. 2010;19(6):462–469. doi: 10.1016/j.breast.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 35.Goldflam K, Hunt KK, Gershenwald JE, Singletary SE, Mirza N, Kuerer HM, Babiera GV, Ames FC, Ross MI, Feig BW, et al. Contralateral prophylactic mastectomy. Predictors of significant histologic findings. Cancer. 2004;101(9):1977–1986. doi: 10.1002/cncr.20617. [DOI] [PubMed] [Google Scholar]
- 36.Woerdeman LA, Hage JJ, Smeulders MJ, Rutgers EJ, van der Horst CM. Skin-sparing mastectomy and immediate breast reconstruction by use of implants: an assessment of risk factors for complications and cancer control in 120 patients. Plastic and reconstructive surgery. 2006;118(2):321–330. doi: 10.1097/01.prs.0000234049.91710.ba. discussion 331-322. [DOI] [PubMed] [Google Scholar]
- 37.Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology. 2002;225(1):165–175. doi: 10.1148/radiol.2251011667. [DOI] [PubMed] [Google Scholar]
- 38.Mandelson MT, Oestreicher N, Porter PL, White D, Finder CA, Taplin SH, White E. Breast density as a predictor of mammographic detection: comparison of interval- and screen-detected cancers. Journal of the National Cancer Institute. 2000;92(13):1081–1087. doi: 10.1093/jnci/92.13.1081. [DOI] [PubMed] [Google Scholar]
- 39.Turnbull L, Brown S, Harvey I, Olivier C, Drew P, Napp V, Hanby A, Brown J. Comparative effectiveness of MRI in breast cancer (COMICE) trial: a randomised controlled trial. Lancet. 2010;375(9714):563–571. doi: 10.1016/S0140-6736(09)62070-5. [DOI] [PubMed] [Google Scholar]
- 40.Peters NH, van Esser S, van den Bosch MA, Storm RK, Plaisier PW, van Dalen T, Diepstraten SC, Weits T, Westenend PJ, Stapper G, et al. Preoperative MRI and surgical management in patients with nonpalpable breast cancer: the MONET - randomised controlled trial. European journal of cancer. 2011;47(6):879–886. doi: 10.1016/j.ejca.2010.11.035. [DOI] [PubMed] [Google Scholar]
- 41.Parker ASA, Brenin DR. MRI Utilization in Nwly Diagnosed Bresat Cancer: A Survey of Practicing Surgeons. Ann Surg Oncol. 2013 doi: 10.1245/s10434-013-2934-5. [DOI] [PubMed] [Google Scholar]
- 42.Presley CJ, Soulos PR, Herrin J, Roberts KB, Yu JB, Killelea B, Lesnikoski BA, Long JB, Gross CP. Patterns of use and short-term complications of breast brachytherapy in the national medicare population from 2008-2009. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 30(35):4302–4307. doi: 10.1200/JCO.2012.43.5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gross CP, Long JB, Ross JS, Abu-Khalaf MM, Wang R, Killelea BK, Gold HT, Chagpar AB, Ma X. The cost of breast cancer screening in the Medicare population. JAMA Intern Med. 173(3):220–226. doi: 10.1001/jamainternmed.2013.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roberts KB, Soulos PR, Herrin J, Yu JB, Long JB, Dostaler E, Gross CP. The adoption of new adjuvant radiation therapy modalities among Medicare beneficiaries with breast cancer: clinical correlates and cost implications. Int J Radiat Oncol Biol Phys. 85(5):1186–1192. doi: 10.1016/j.ijrobp.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]