Abstract
Arterial baroreflex sensitivity is attenuated in chronic heart failure (CHF) state, which is associated with cardiac arrhythmias and sudden cardiac death in the patients with CHF. Our previous study showed that CHF-induced sodium channel dysfunction in the baroreceptor neurons was involved in the blunted baroreflex sensitivity in CHF rats. Mitochondria-derived superoxide overproduction decreased expression and activation of the sodium channels in the baroreceptor neurons from CHF rats. However, the molecular mechanisms responsible for the sodium channel dysfunction in the baroreceptor neurons from CHF rats remain unknown. We tested the involvement of NFκB in the sodium channel dysfunction and evaluated the effects of in-vivo transfection of manganese superoxide dismutase gene and NFκB shRNA on the baroreflex function in CHF rats. CHF was developed at 6–8 weeks after left coronary artery ligation in adult rats. Western bolt and chromatin immunoprecipitation data showed that phosphorylated NFκB p65 and ability of NFκB p65 binding to the sodium channel promoter were increased in the nodose ganglia from CHF rats. In-vivo transfection of adenoviral manganese superoxide dismutase gene or lentiviral NFκB p65 shRNA into the nodose ganglia partially reversed CHF-reduced sodium channel expression and cell excitability in the baroreceptor neurons and improved CHF-blunted arterial baroreflex sensitivity. Additionally, transfection of adenoviral manganese superoxide dismutase also inhibited the augmentation of phosphorylated NFκB p65 in the nodose neurons from CHF rats. The present study suggests that superoxide-NFκB signaling contributes to CHF-induced baroreceptor dysfunction and resultant impairment of baroreflex function.
Keywords: baroreceptor, baroreflex, heart failure, NFκB, sodium channel, superoxide
Introduction
Impairment of arterial baroreflex sensitivity for heart rate and blood pressure has adverse prognostic significance in patients with chronic heart failure (CHF)1–5 and in a variety of experimental models of CHF6, 7. The arterial baroreflex arc consists of an afferent limb (baroreceptor neurons), a central neural component, and autonomic efferent component. Any component of the arterial baroreflex arc could be responsible for the attenuated baroreflex sensitivity in CHF state. Data from one research group have demonstrated that chronic electrical stimulation of the baroreceptors improves the survival rate in dogs with pacing-induced CHF8. Our previous study demonstrated that expression and activation of the voltage-gated sodium (Nav) channels are decreased in the baroreceptor neurons from CHF rats9. The Nav channel dysfunction reduces cell excitability of the baroreceptor neurons and subsequently contributes to the blunted baroreflex sensitivity in the CHF rats9. Our recent study demonstrated that elevation of the mitochondria-derived superoxide lowers expression and activation of the Nav channels in the baroreceptor neurons from CHF rats10. However, it is unclear how the superoxide modulates the expression and activation of the Nav channels in the baroreceptor neurons from CHF rats.
NFκB is a transcription factor that can regulate the expression of a number of genes involved in disease states such as inflammatory disease and heart failure11–14. NFκB consists of five structurally related proteins, namely RelA (p65), RelB, c-Rel, p50, and p52. The p65/p50 heterodimer is the most abundant and widely expressed form of NFκB15. In the resting state, NFκB presents a silent form in the cytosol through tight binding to the specific inhibitor of κBα (IκBα)11, 15. On activation by various stimuli, including reactive oxygen species, IκB kinase-β (IKK-β) induces the phosphorylation and degradation of IκBα16–20. Then the liberated NFκB is phosphorylated, leading to nuclear translocation and binding to specific sites on DNA, finally regulating gene transcription11, 17.
Although many studies have focused on the role of NFκB in target gene transcription, few studies have considered the involvement of NFκB in regulating ion channel gene transcription. Shang et al found that NFκB can directly bind to the SCN5A promoter, which is involved in the Angiotensin II/hydrogen peroxide-induced down-transcription of the Nav1.5 channels21. We hypothesized that NFκB could mediate superoxide-lowered expression and activation of Nav channels in the baroreceptor neurons from CHF rats. In the current study, we first measured expression of the IKK-IκB-NFκB signaling in the nodose ganglia from sham and CHF rats, and then analyzed the effect of in-vivo lentiviral NFκB p65 shRNA or adenoviral manganese superoxide dismutase (Ad.MnSOD) gene transfection on the Nav channels and cell excitability in the baroreceptor neurons from sham and CHF rats. Finally, we evaluated the role of superoxide-NFκB signaling in the baroreflex sensitivity in sham and CHF rats.
Methods
All experimental procedures were approved by the University of Nebraska Medical Center Institutional Animal Care and Use Committee and were carried out in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. Male Sprague-Dawley rats weighing 180–200 g were assigned randomly to one of two groups: sham (n=95) and CHF (n=92). CHF was produced by surgical ligation of the left coronary artery, as previously described9, 10. CHF was confirmed by hemodynamic and morphological characteristics (Table s1). The key experimental procedures included induction of CHF; in-vivo gene or shRNA transfection using adenovirus or lentivirus; western blot analysis; chromatin immunoprecipitation assay; labeling of aortic baroreceptor neurons and isolation of nodose neurons; recording of Nav currents and action potential in aortic baroreceptor neurons; measurement of superoxide production; and measurement of arterial baroreflex sensitivity. The efficacy of the virus infection was confirmed by the expression of enhanced green fluorescent protein (Fig. s1). Detailed procedures are available in the online-only Data Supplement.
Statistical Analysis
All data are presented as means ± SEM. SigmaPlot 1μ was used for data analysis. Student’s unpaired t-test or two-way ANOVA with post hoc bonferroni test was used to determine statistical significance. Statistical significance was accepted when p<0.05.
Results
Expression of IKK-IκB-NFκB signaling cascade
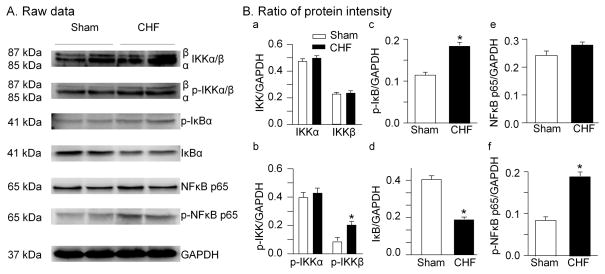
Protein levels of IKK complex, IκBα, and NFκB-p65 in the nodose ganglia from sham and CHF rats are shown in Fig. 1. There is no significant difference in total IKKα and phosphorylated IKKα between sham and CHF rats. Protein level of the phosphorylated IKKβ was increased by CHF although CHF did not affect expression of the total IKKβ. Additionally, CHF increased the phosphorylated IκB protein and decreased the total IκB protein in the nodose ganglia. Furthermore, we found that the phosphorylated NFκB p65 protein was enhanced in the nodose ganglia from CHF rats. However, CHF did not induced alteration of the total NFκB p65 protein in the nodose ganglia (p>0.05 vs. sham).
Figure 1.
Representative (A) and grouped data showing expression and phosphorylation of IKK, IκB, and NFκB proteins in nodose ganglia from sham and CHF rats. IKK: IκB kinase; IκB: NFκB inhibitory protein. Data are mean ± SEM; n=5 rats in each group. *P<0.05 vs. sham rats.
NFκB p65 binding to Nav1.7 promoter, and expression and activation of Nav1.7 channels
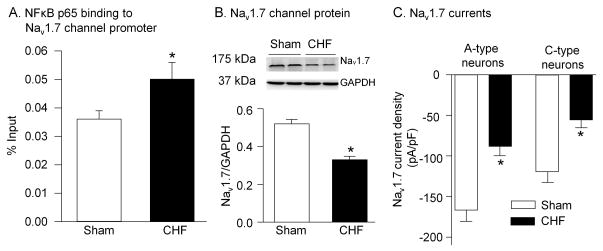
Because NFκB p65 can bind and regulate many gene expressions, we used chromatin immunoprecipitation technique to examine the ability of NFκB p65 binding to the Nav1.7 channel promoter for evaluating the role of NFκB p65 in expression of the Nav1.7 channels. As illustrated in Figs. 2A and s2, the ability of NFκB p65 binding to the Nav1.7 channel promoter was increased in the nodose ganglia from CHF rats. Simultaneously, expression of the Nav1.7 protein and Nav1.7 currents were reduced in the nodose neurons from CHF rats, compared to sham rats (Fig. 2-B and 2-C). These data suggest that enhancement of the phosphorylated NFκB p65 protein might be linked to the lower expression of the Nav1.7 channel protein in the nodose ganglia from CHF rats.
Figure 2.
Ability of NFκB p65 binding to Nav1.7 promoter (A), and expression (B) and currents (C) of Nav 1.7 channels in sham and CHF rats. A, Ability of NFκB p65 binding to Nav1.7 promoter was measured using chromatin immunoprecipitation assay. Nodose ganglia from 4 rats were pooled to obtain sufficient tissue for assay. N=12 rats in each group. B, Nav1.7 channel protein in nodose ganglia was measured by Western blotting. N=5 rats in each group. C, Nav1.7 current density in aortic baroreceptor neurons was measured by whole cell patch-clamp technique. N=12 cells from 5 rats in each group. Data are mean ± SEM. *P<0.05 vs. sham.
Effect of NFκB p65 shRNA transfection on Nav1.7 currents and action potential in the aortic baroreceptor neurons
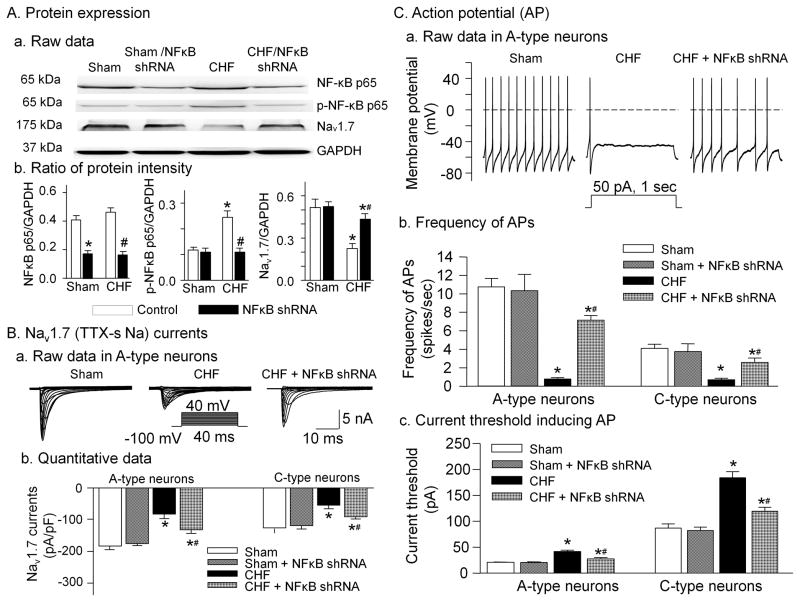
To further confirm the relationship between NFκB p65 and Nav1.7 channels, we in-vivo transfected lentiviral NFκB p65 shRNA into the nodose ganglia and measured the alterations of the NFκB p65 and Nav1.7 channels in the nodose ganglia from sham and CHF rats.
In sham rats, lentiviral NFκB p65 shRNA (2 μl, 3 × 106 ifu/ml) decreased expression of the NFκB p65 protein but did not affect expression of the phosphorylated NFκB p65 and Nav1.7 channel proteins in the nodose ganglia (Fig. 3A). In CHF rats, lentiviral NFκB p65 shRNA significantly reduced level of the NFκB p65 and phosphorylated NFκB p65 proteins. However, it increased expression of the Nav1.7 channel protein in the nodose ganglia, compared with those in the non-infected CHF rats (Fig. 3A).
Figure 3.
Effect of in-vivo lentiviral NFκB p65 shRNA transfection (2 μl, 3 × 106 ifu/ml) on expression of NFκB and Nav1.7 channel proteins in the nodose ganglia (A), and Nav1.7 currents (B) and cell excitability (C) in the aortic baroreceptor neurons from sham and CHF rats with and without NFκB p65 shRNA transfection into nodose ganglia. In panel A, n=5 rats in all experimental groups. In panels B and C, n=10 neurons from 5 rats in each group. Data are mean ± SEM. *P<0.05 vs. sham; #p<0.05 vs. CHF.
The Nav1.7 currents were measured in the aortic baroreceptor neurons from each group (Fig. 3B). The aortic baroreceptor neurons were identified by Dil retrograde-labeling (see Fig. s3 and supplementary data online for detail). The Nav1.7 current was lower in A- and C-type aortic baroreceptor neurons from CHF rats than that in sham rats. Lentiviral NFκB p65 shRNA markedly increased the Nav1.7 currents in A-and C-type aortic baroreceptor neurons from CHF rats but not in sham neurons (Fig. 3B).
We measured current threshold-inducing action potential and frequency of action potentials to investigate the role of NFκB p65 in cell excitability of the aortic baroreceptor neurons from sham and CHF rats. Previously, we showed that cell excitability of the aortic baroreceptor neurons was lower (including higher current threshold-inducing action potential and lower frequency of action potentials) in CHF rats than that in sham rats9, 10. In the present study, we observed similar results (Fig. 3C). In-vivo transfection of lentiviral NFκB p65 shRNA into the nodose ganglia of CHF rats significantly increased frequency of action potentials and decreased current threshold-inducing action potential in A- and C-type aortic baroreceptor neurons, compared to the aortic baroreceptor neurons from non-infected CHF rats. Lentiviral NFκB p65 shRNA had no effect on cell excitability of the A- and C-type aortic baroreceptor neurons from sham rats (Fig. 3C).
We also measured the effect of lentiviral control (scrambled) shRNA (2 μl, 3 × 106 ifu/ml) on above parameters in sham and CHF rats. Lentiviral control shRNA did not affect the protein expression, Nav1.7 currents, and cell excitability in sham and CHF rats (Fig. s4).
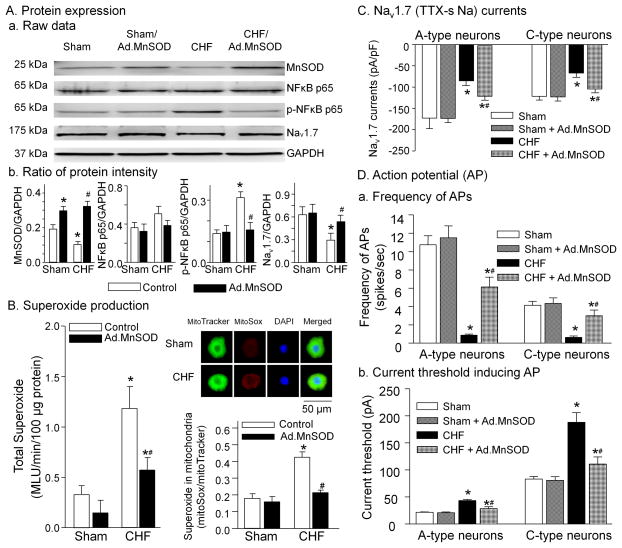
Effect of MnSOD gene transfection on superoxide production, expression of NFκB, and electrophysiological properties
Expression of the MnSOD protein was decreased and the superoxide production was increased in the nodose ganglia from CHF rats compared to sham rats (Fig. 4A and 4B), consistent with data from our previous study10. In-vivo transfection of Ad.MnSOD gene (2 μl, 2 × 1010 pfu/ml) into the nodose ganglia caused an increase in the MnSOD protein in the nodose ganglia from sham and CHF rats, compared with that in the non-infected (control) sham and CHF rats (Fig. 4A). Ad.MnSOD gene transfection decreased total superoxide and mitochondria-derived superoxide levels in the nodose ganglia from CHF rats but not sham rats (Fig. 4B). Additionally, Ad.MnSOD gene transfection also reduced the phosphorylated NFκB p65 protein and enhanced the Nav1.7 protein in the nodose ganglia from CHF rats but not sham rats (Fig. 4A).
Figure 4.
Effect of in-vivo adenoviral manganese superoxide dismutase (Ad.MnSOD, 2 μl, 2 × 1010 pfu/ml) transfection on expression of MnSOD, NFκB and Nav1.7 channel proteins (A) and superoxide production (B) in the nodose ganglia, and Nav1.7 currents (C) and cell excitability (D) in the aortic baroreceptor neurons from sham and CHF rats with and without Ad.MnSOD transfection into nodose ganglia. In panel A, n=5 rats in all experimental groups. In panel B, total superoxide level (left) was measured in 5 rats per group, and mitochondrial-derived superoxide level (right) was analyzed in 20 nodose neurons from 5 rats per group. In panels C and D, n=10 neurons from 5 rats in each group. Data are mean ± SEM. *P<0.05 vs. sham; #p<0.05 vs. CHF.
In-vivo Ad.MnSOD gene transfection markedly augmented the Nav1.7 currents in the A- and C-type aortic baroreceptor neurons from CHF rats but not sham rats (Fig. 4C). Similarly, In-vivo Ad.MnSOD gene transfection also increased the cell excitability (including raising the frequency of action potentials and lowering the current threshold-inducing action potential) in the A- and C-type aortic baroreceptor neurons from CHF rats but not sham rats (Fig. 4D).
However, after in-vivo transfection of Ad.empty (2 μl, 2 × 1010 pfu/ml) into the nodose ganglia, the protein expression, superoxide production, Nav1.7 currents, and cell excitability were not altered in the nodose neurons from sham and CHF rats, compared to the non-infected sham and CHF rats (Fig. s5).
Effect of NFκB p65 shRNA or MnSOD gene transfection on arterial baroreflex sensitivity
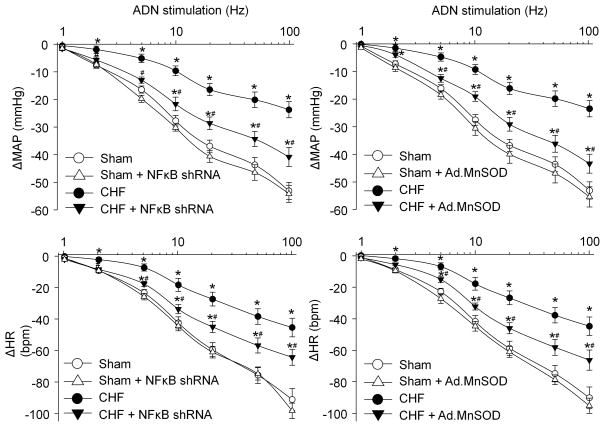
Reflex changes in blood pressure and heart rate in response to different electrical stimulation of the aortic depressor nerve were used as the indices of the aortic baroreflex sensitivity in each experimental group. As indicated in Fig. s6 (representative recordings) and Fig. 5 (mean data), reflex decreases in blood pressure and heart rate were attenuated in CHF rats compared to sham rats. Local transfection of lentiviral NFκB p65 shRNA or Ad.MnSOD gene into the nodose ganglia partially increased the blood pressure and heart rate response to electrical stimulation of the aortic depressor nerve in CHF rats. The lentiviral NFκB p65 shRNA and Ad.MnSOD gene did not show any effect in sham rats (Fig. 5). In-vivo transfection of lentiviral control shRNA or Ad.empty into the nodose ganglia did not affect the aortic baroreflex sensitivity in sham and CHF rats (Fig. s7).
Figure 5.
Effect of in-vivo lentiviral NFκB p65 shRNA (left panel) or Ad.MnSOD (right panel) transfection on reflex ΔMAP (mean arterial pressure) and ΔHR (heart rate) in response to different frequencies of ADN (aortic depressor nerve) stimulation in anesthetized sham and CHF rats with and without lentiviral NFκB p65 shRNA or Ad.MnSOD transfection. Data are mean ± SEM, n=5 rats in each group. *P<0.05 vs. sham; #p<0.05 vs. CHF.
Another measurement for evaluating the arterial baroreflex, arterial blood pressure changes induced by phenylephrine and attendant reflex responses of heart rate and cardiac sympathetic nerve activity are shown in Figs. s8–s9. The reflex responses of heart rate and cardiac sympathetic nerve activity to arterial pressure changes in CHF rats were reduced compared to sham rats. The maximum gain of baroreflex control of heart rate and cardiac sympathetic nerve activity in CHF rats was decreased compared to sham rats (Fig. s9). These results demonstrated that the arterial baroreflex function was attenuated in CHF rats. Local transfection of lentiviral NFκB p65 shRNA or Ad.MnSOD gene into the nodose ganglia partially restored the arterial baroreflex function in CHF rats compared with that in non-infected CHF rats. However, lentiviral NFκB p65 shRNA or Ad.MnSOD gene did not induce the change of the arterial baroreflex function in sham rats compared to the non-infected sham rats (Fig. s9). Local transfection of lentiviral control shRNA or Ad.empty into the nodose ganglia did not alter the baroreflex gain in sham and CHF rats (data not shown).
Discussion
Our present study demonstrates that CHF activates the IKK-IκB-NFκB signaling pathway in the nodose ganglion tissue. The activation of IKK-IκB-NFκB signaling causes reduced Nav channel activity and resultant arterial baroreflex dysfunction in CHF rats. This occurs because NFκB p65 shRNA significantly increases the Nav1.7 current density and cell excitability of the aortic baroreceptor neurons and improves the arterial baroreflex sensitivity in CHF rats through reducing protein level of the NFκB p65. Our experiments also show that superoxide overproduction activates the NFκB p65 and subsequently induces the alterations of Nav1.7 currents, cell excitability of the aortic baroreceptor neurons, and arterial baroreflex sensitivity in CHF rats. Together these results suggest that activation of the superoxide-sensitive transcription factor NFκB is a possible mechanism by which CHF reduces the Nav current density and cell excitability of the aortic baroreceptor neurons and attenuates the arterial baroreflex sensitivity.
NFκB is a family of transcription factors that is normally silent in the cytoplasm through interaction with inhibitory molecules of the IκB family11, 15. In response to multiple stimuli in pathophysiological conditions, the IκB molecules are phosphorylated on Ser32 and Ser36 residues by activation of the IKKβ kinases16, 17. The serine-phosphorylated IκB is ubiquitinated and degraded17, 18. As a consequence, NFκB is activated and translocated from the cytoplasm to the nucleus, and finally induces transcription of numerous target genes11, 17. In our present study, the IKK-IκB-NFκB signaling pathway exists in rat nodose ganglion tissue. CHF increased the phosphorylated IKK, degraded the IκBα, and enhanced the phosphorylated NFκB p65 in the nodose ganglion tissue. Our present study also found that CHF enhanced the ability of NFκB p65 binding to the Nav1.7 promoter in the nodose ganglion tissue. These results provide the molecular evidence that activation of the NFκB p65 might be associated with the changes of the Nav1.7 channel in the nodose neurons (including the aortic baroreceptor neurons) from CHF rats. Compared to the common activation of gene transcription with NFκB binding, however, NFκB overactivation downregulates the Nav1.7 protein expression and current density in the nodose neurons from CHF rats because transfection of NFκB p65 shRNA normalizes the phosphorylated NFκB p65 and partially increased the Nav1.7 protein expression and current density in CHF rats. This is in agreement with another study21 that demonstrated that NFκB can directly bind to the SCN5A promoter, which is involved in down-transcription of the Nav1.5 channel in rat ventricular cells. Our previous study has shown that blunted expression and activation of the Nav channels lower the baroreceptor neuron excitability and contribute to resultant impairment of the arterial baroreflex9. In the current study, NFκB p65 shRNA significantly improves the aortic baroreceptor neuron excitability and arterial baroreflex sensitivity in CHF rats. These data indicate that activation of NFκB signaling is involved in CHF-induced lower expression of the Nav1.7 channels, attenuation of the aortic baroreceptor neuron excitability, and impairment of the arterial baroreflex. However, our present study cannot answer why and how activation of the NFκB downregulates expression of the Nav1.7 channels in the nodose ganglion tissue. Future studies should address these questions.
In our previous study, in-vitro transfection of Ad.MnSOD into the isolated primary nodose neurons reduced CHF-induced elevation of the mitochondrial superoxide level in the nodose neurons and partially reversed CHF-decreased Nav channel activation and cell excitability in the aortic baroreceptor neurons10. In our present study, in-vivo transfection of Ad.MnSOD into the nodose ganglion tissue not only partially increased function of the aortic baroreceptor neurons (including Nav1.7 channel activation and neuron excitability), but also improved the arterial baroreflex sensitivity in CHF rats. These results further confirmed that impairment of the aortic baroreceptor neurons contributes to the arterial baroreflex dysfunction in CHF rats. Additionally, our present study found that in-vivo transfection of Ad.MnSOD also inhibited CHF-induced augmentation of the phosphorylated NFκB p65 in the nodose ganglion tissue (Fig. 4A). Furthermore, in-vivo transfection of NFκB p65 shRNA did not affect the superoxide level in the nodose ganglia from CHF rats (data not shown), which indicates that inhibition of the NFκB p65 improves the aortic baroreceptor function and arterial baroreflex sensitivity even if a high level of the superoxide is preserved in the nodose ganglia from CHF rats. Based on above the results, we consider that superoxide overproduction-induced impairment of the aortic baroreceptor neurons and arterial baroreflex dysfunction in CHF rats is the result of activation of the NFκB p65.
The mechanism by which superoxide induces the activation of NFκB p65 in the nodose neurons from CHF rats is not known. In human endothelial cells, protein kinase C is involved in superoxide-induced activation of the NFκB22. Mendes et al. reported that superoxide mediates interleukin-1β-induced IκBα degradation and consequent NFκB activation in bovine articular chondrocytes23. Superoxide is correlated with NFκB activation also via the IKK pathway17. In our present study, CHF increased the phosphorylated IKKβ, decreased the total IκBα, and enhanced the phosphorylated NFκB p65 in the nodose neurons (Fig. 1). Therefore, it is possible that superoxide regulates activation of the NFκB p65 in the nodose neurons from CHF rats via multiple signal transduction pathways. Further study is needed to clarify this issue.
Our previous study has shown that decreased expression and activation of the Nav channels attenuate the aortic baroreceptor neuron excitability and subsequently impair the arterial baroreflex in CHF rats9. Thus far, nine α subunits (Nav1.1–Nav1.9) of the Nav channels have been functionally characterized. They have also been separated by their sensitivity to tetrodotoxin (TTX) into the low activation threshold, fast activating, and inactivating TTX-sensitive Nav channels (Nav 1.1, Nav1.2, Nav1.3, Nav1.4, Nav1.6, and Nav1.7) and the high activation threshold, slow activating, and inactivating TTX-resistant Nav channels (Nav1.5, Nav1.8, and Nav1.9)24–26. Each Nav channel subunit has particular tissue localization, consistent with a distinct role for each Nav channel subunit in mammalian physiology26. Nav1.7 (TTX-sensitive), Nav1.8 (TTX-resistant), and Nav1.9 (TTX-resistant) are abundantly expressed in the primary sensory neurons such as the nodose neurons9, 25, 27–29. In the present study, we only measured the regulating role of superoxide-NFκB signaling in the Nav1.7 channels in the rat nodose neurons because the Nav1.7 channels are expressed in all nodose neurons (A- and C-type nodose neurons) as a predominant Nav channel α subunit but the Nav1.8 and Nav1.9 channels are located only in C-type nodose neurons9. However, we realize the Nav1.8 and Nav1.9 channels in the nodose neuron may have an important role. Thus future study should also focus on the influence of superoxide-NFκB signaling on the Nav1.8 and Nav1.9 channels in CHF state although there are no reports of the NFκB binding sites on rat Nav1.8 and Nav1.9 channel promoters.
One widely accepted method for evaluation of the arterial baroreflex is to examine the changes of blood pressure and heart rate induced by electrical stimulation of baroreceptor-containing nerve (aortic depressor nerve)30–33. The advantages for this method include: 1) rat aortic depressor nerves do not contain a functionally significant number of chemoreceptor afferent fibers for generation of chemoreflex34 although morphological evidence has demonstrated that about 15% of fibers in rat aortic depressor nerves are chemoreceptor afferent fibers35; 2) direct electrical stimulation of the aortic depressor nerve bypasses the mechano-viscoelastic coupling between changes in the aortic arterial vascular walls and afferent nerve endings; 3) easily changing the frequency and intensity of stimulus can differentiate the reflex responses to activating A- and C- afferent fibers. However, a disadvantage of the electrical stimulation technique is that it does not represent a physiological substrate for baroreceptor activation.
Another method for evaluation of the arterial baroreflex is to measure reflex changes of heart rate and cardiac sympathetic nerve activity in response to changes in arterial blood pressure. This measurement uses a physiological stimulation (changes of arterial blood pressure) to activate the arterial baroreflex. A major limitation to this approach is that possible alterations in the mechanotransduction process at the baro-sensory nerve terminal may also play a role in the baroreflex function in response to blood pressure changes. Thus the results from either of the two measurements need to be tempered by the limitations. Nevertheless, our present study employed the two methods for evaluation of the arterial baroreflex. We are able to more clearly describe a functional role for alterations in superoxide-NFκB-Nav channel in aortic baroreceptor neurons in CHF by combining reflex effects to stimulation of baroafferents in response to both electrical stimulation of aortic depressor nerve and blood pressure change.
Although we measured changes of the Nav1.7 currents and action potentials in the aortic baroreceptor neurons, we did the biochemical analyses in whole nodose ganglia. We found that CHF induced significant biochemical alterations in the nodose ganglia. Because aortic baroreceptor neurons are a small population of the nodose neurons, it is likely that CHF-induced biochemical changes may also occur in other type of nodose neurons. Whether these changes affect other vagal afferent reflexes in CHF rats should be evaluated in future studies.
Perspective
Dysfunction of baroreceptor neurons in the nodose ganglia results in arterial baroreflex impairment, a complication of CHF. Lower expression of the Nav channels causes suppression of the baroreceptor neurons in CHF rats. The precise mechanism about the reduced expression of the Nav channels in the baroreceptor neurons is unclear. In the present study, we found that in-vivo transfection of MnSOD gene or NFκB p65 shRNA into the nodose ganglia increased expression of the Nav channels, enhanced cell excitability of the baroreceptor neurons, and improved the arterial baroreflex function in CHF rats. The data in the present study can further our understanding of the mechanisms responsible for the impaired arterial baroreflex in CHF and unveil pharmacological and genomic targets for improving arterial baroreflex function and reducing mortality in CHF.
Supplementary Material
What Is New?
CHF induces activation of transcription factor NFκB in nodose neurons.
Superoxide-NFκB signaling is involved in CHF-reduced expression of the Nav channels in the baroreceptor neurons.
In-vivo transfection of manganese superoxide dismutase (MnSOD) gene or NFκB shRNA improves baroreflex function in CHF.
What Is Relevant?
Superoxide-NFκB signaling in the baroreceptor neurons plays a major role in the blunted baroreflex in CHF.
This study provides a new strategy to normalize the arterial baroreflex dysfunction and to reduce mortality in CHF.
Summary
Overactivation of superoxide-sensitive NFκB downregulates cell excitability of the aortic baroreceptor neurons and consequently triggers impairment of the baroreflex function in CHF.
Acknowledgments
The authors wish to thank Kaye Talbitzer for their technical assistance.
Sources of Funding
This study was supported by the National Institute of Health NHLBI grant HL-098503 (Y.L. Li).
Footnotes
Disclosures
None.
References
- 1.Ruttanaumpawan P, Gilman MP, Usui K, Floras JS, Bradley TD. Sustained effect of continuous positive airway pressure on baroreflex sensitivity in congestive heart failure patients with obstructive sleep apnea. J Hypertens. 2008;26:1163–1168. doi: 10.1097/HJH.0b013e3282fb81ed. [DOI] [PubMed] [Google Scholar]
- 2.Floras JS. Clinical aspects of sympathetic activation and parasympathetic withdrawal in heart failure. J Am Coll Cardiol. 1993;22:72A–84A. doi: 10.1016/0735-1097(93)90466-e. [DOI] [PubMed] [Google Scholar]
- 3.Frenneaux MP. Autonomic changes in patients with heart failure and in post-myocardial infarction patients. Heart. 2004;90:1248–1255. doi: 10.1136/hrt.2003.026146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinna GD, Maestri R, Capomolla S, Febo O, Robbi E, Cobelli F, La Rovere MT. Applicability and clinical relevance of the transfer function method in the assessment of baroreflex sensitivity in heart failure patients. J Am Coll Cardiol. 2005;46:1314–1321. doi: 10.1016/j.jacc.2005.06.062. [DOI] [PubMed] [Google Scholar]
- 5.Creager MA, Creager SJ. Arterial baroreflex regulation of blood pressure in patients with congestive heart failure. J Am Coll Cardiol. 1994;23:401–405. doi: 10.1016/0735-1097(94)90427-8. [DOI] [PubMed] [Google Scholar]
- 6.Chen JS, Wang W, Bartholet T, Zucker IH. Analysis of baroreflex control of heart rate in conscious dogs with pacing-induced heart failure. Circulation. 1991;83:260–267. doi: 10.1161/01.cir.83.1.260. [DOI] [PubMed] [Google Scholar]
- 7.White CW. Abnormalities in baroreflex control of heart rate in canine heart failure. Am J Physiol. 1981;240:H793–H799. doi: 10.1152/ajpheart.1981.240.5.H793. [DOI] [PubMed] [Google Scholar]
- 8.Zucker IH, Hackley JF, Cornish KG, Hiser BA, Anderson NR, Kieval R, Irwin ED, Serdar DJ, Peuler JD, Rossing MA. Chronic baroreceptor activation enhances survival in dogs with pacing-induced heart failure. Hypertension. 2007;50:904–910. doi: 10.1161/HYPERTENSIONAHA.107.095216. [DOI] [PubMed] [Google Scholar]
- 9.Tu H, Zhang L, Tran TP, Muelleman RL, Li YL. Reduced expression and activation of voltage-gated sodium channels contributes to blunted baroreflex sensitivity in heart failure rats. J Neurosci Res. 2010;88:3337–3349. doi: 10.1002/jnr.22483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tu H, Liu J, Zhu Z, Zhang LB, Pipinos II, Li YL. Mitochondria-derived superoxide and voltage-gated sodium channels in baroreceptor neurons from chronic heart failure rats. J Neurophysiol. 2012;107:591–602. doi: 10.1152/jn.00754.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Israel A. The IKK complex, a central regulator of NF-kappaB activation. Cold Spring Harb Perspect Biol. 2010;2:1–14. doi: 10.1101/cshperspect.a000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frantz S, Fraccarollo D, Wagner H, Behr TM, Jung P, Angermann CE, Ertl G, Bauersachs J. Sustained activation of nuclear factor kappa B and activator protein 1 in chronic heart failure. Cardiovasc Res. 2003;57:749–756. doi: 10.1016/s0008-6363(02)00723-x. [DOI] [PubMed] [Google Scholar]
- 13.Van der Heiden K, Cuhlmann S, Luong lA, Zakkar M, Evans PC. Role of nuclear factor kappaB in cardiovascular health and disease. Clin Sci (Lond) 2010;118:593–605. doi: 10.1042/CS20090557. [DOI] [PubMed] [Google Scholar]
- 14.Valen G. Signal transduction through nuclear factor kappa B in ischemia-reperfusion and heart failure. Basic Res Cardiol. 2004;99:1–7. doi: 10.1007/s00395-003-0442-7. [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann A, Baltimore D. Circuitry of nuclear factor kappaB signaling. Immunol Rev. 2006;210:171–186. doi: 10.1111/j.0105-2896.2006.00375.x. [DOI] [PubMed] [Google Scholar]
- 16.Hacker H, Karin M. Regulation and function of IKK and IKK-related kinases. Sci STKE. 2006;357:re13. doi: 10.1126/stke.3572006re13. [DOI] [PubMed] [Google Scholar]
- 17.Kabe Y, Ando K, Hirao S, Yoshida M, Handa H. Redox regulation of NF-kappaB activation: distinct redox regulation between the cytoplasm and the nucleus. Antioxid Redox Signal. 2005;7:395–403. doi: 10.1089/ars.2005.7.395. [DOI] [PubMed] [Google Scholar]
- 18.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 19.Kim JH, Na HJ, Kim CK, Kim JY, Ha KS, Lee H, Chung HT, Kwon HJ, Kwon YG, Kim YM. The non-provitamin A carotenoid, lutein, inhibits NF-kappaB-dependent gene expression through redox-based regulation of the phosphatidylinositol 3-kinase/PTEN/Akt and NF-kappaB-inducing kinase pathways: role of H(2)O(2) in NF-kappaB activation. Free Radic Biol Med. 2008;45:885–896. doi: 10.1016/j.freeradbiomed.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 20.Takada Y, Mukhopadhyay A, Kundu GC, Mahabeleshwar GH, Singh S, Aggarwal BB. Hydrogen peroxide activates NF-kappa B through tyrosine phosphorylation of I kappa B alpha and serine phosphorylation of p65: evidence for the involvement of I kappa B alpha kinase and Syk protein-tyrosine kinase. J Biol Chem. 2003;278:24233–24241. doi: 10.1074/jbc.M212389200. [DOI] [PubMed] [Google Scholar]
- 21.Shang LL, Sanyal S, Pfahnl AE, Jiao Z, Allen J, Liu H, Dudley SC., Jr NF-kappaB-dependent transcriptional regulation of the cardiac scn5a sodium channel by angiotensin II. Am J Physiol Cell Physiol. 2008;294:C372–C379. doi: 10.1152/ajpcell.00186.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogata N, Yamamoto H, Kugiyama K, Yasue H, Miyamoto E. Involvement of protein kinase C in superoxide anion-induced activation of nuclear factor-kappa B in human endothelial cells. Cardiovasc Res. 2000;45:513–521. doi: 10.1016/s0008-6363(99)00364-8. [DOI] [PubMed] [Google Scholar]
- 23.Mendes AF, Caramona MM, Carvalho AP, Lopes MC. Differential roles of hydrogen peroxide and superoxide in mediating IL-1-induced NF-kappa B activation and iNOS expression in bovine articular chondrocytes. J Cell Biochem. 2003;88:783–793. doi: 10.1002/jcb.10428. [DOI] [PubMed] [Google Scholar]
- 24.Catterall WA, Goldin AL, Waxman SG International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol Rev. 2005;57:397–409. doi: 10.1124/pr.57.4.4. [DOI] [PubMed] [Google Scholar]
- 25.Waxman SG, Dib-Hajj S, Cummins TR, Black JA. Sodium channels and pain. Proc Natl Acad Sci U S A. 1999;96:7635–7639. doi: 10.1073/pnas.96.14.7635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu FH, Catterall WA. Overview of the voltage-gated sodium channel family. Genome Biol. 2003;4:207. doi: 10.1186/gb-2003-4-3-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baker MD, Wood JN. Involvement of Na+ channels in pain pathways. Trends Pharmacol Sci. 2001;22:27–31. doi: 10.1016/s0165-6147(00)01585-6. [DOI] [PubMed] [Google Scholar]
- 28.Cummins TR, Sheets PL, Waxman SG. The roles of sodium channels in nociception: Implications for mechanisms of pain. Pain. 2007;131:243–257. doi: 10.1016/j.pain.2007.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwong K, Carr MJ, Gibbard A, Savage TJ, Singh K, Jing J, Meeker S, Undem BJ. Voltage-gated sodium channels in nociceptive versus non-nociceptive nodose vagal sensory neurons innervating guinea pig lungs. J Physiol. 2008;586:1321–1336. doi: 10.1113/jphysiol.2007.146365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fan W, Andresen MC. Differential frequency-dependent reflex integration of myelinated and nonmyelinated rat aortic baroreceptors. Am J Physiol. 1998;275:H632–H640. doi: 10.1152/ajpheart.1998.275.2.H632. [DOI] [PubMed] [Google Scholar]
- 31.Li YL. Elevated angiotensin II in rat nodose ganglia primes diabetes-blunted arterial baroreflex sensitivity: involvement of NADPH oxidase-derived superoxide. J Diabetes Metab. 2011;2:1000135. doi: 10.4172/2155-6156.1000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salgado HC, Barale AR, Castania JA, Machado BH, Chapleau MW, Fazan R., Jr Baroreflex responses to electrical stimulation of aortic depressor nerve in conscious SHR. Am J Physiol. 2007;292:H593–H600. doi: 10.1152/ajpheart.00181.2006. [DOI] [PubMed] [Google Scholar]
- 33.Tang X, Dworkin BR. Baroreflexes of the rat. V. Tetanus-induced potentiation of ADN A-fiber responses at the NTS. Am J Physiol. 2007;293:R2254–R2259. doi: 10.1152/ajpregu.00143.2007. [DOI] [PubMed] [Google Scholar]
- 34.Kobayashi M, Cheng ZB, Tanaka K, Nosaka S. Is the aortic depressor nerve involved in arterial chemoreflexes in rats? J Auton Nerv Syst. 1999;78:38–48. doi: 10.1016/s0165-1838(99)00054-5. [DOI] [PubMed] [Google Scholar]
- 35.Cheng Z, Powley TL, Schwaber JS, Doyle FJ., III A laser confocal microscopic study of vagal afferent innervation of rat aortic arch: chemoreceptors as well as baroreceptors. J Auton Nerv Syst. 1997;67:1–14. doi: 10.1016/s0165-1838(97)00085-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.