Abstract
Hypothalamic pituitary adrenal (HPA) axis responses to change and social challenges during adolescence can influence mental health and behavior into adulthood. To examine how HPA tone in adolescence may contribute to psychopathology, we challenged male adolescent (5 wk) and adult (16 wk) BTBR T+tf/J (BTBR) and 129S1/SvImJ (129S) mice with novelty in sociability tests. In prior studies these strains had exaggerated or altered HPA stress responses and low sociability relative to C57BL/6J mice in adulthood. In adolescence these strains already exhibited similar or worse sociability deficits than adults or age-matched C57 mice. Yet BTBR adolescents were less hyperactive and buried fewer marbles than adults. Novelty-induced corticosterone (CORT) spikes in adolescent BTBR were double adult levels, and higher than 129S or C57 mice at either age. Due to their established role in HPA feedback, we hypothesized that hippocampal Gαi/o-coupled serotonin 5-HT1A and cannabioid CB1 receptor function might be upregulated in BTBR mice. Adolescent BTBR mice had higher hippocampal 5-HT1A density as measured by [3H]8-hydroxy-2-(di-n-propylamino) tetralin (8-OH-DPAT) binding than C57 mice, and adult BTBR 8-OH-DPAT-stimulated GTPγS binding was higher than in either C57 or 129S mice in this region. Further, BTBR hippocampal CB1 density measured by [3H]CP55,940 binding was 15-20% higher than in C57. CP55,940-stimulated GTPγS binding in adult BTBR dentate gyrus was 30% higher then 129S (p<0.05), but was not a product of greater neuronal or cell density defined by NeuN and DAPI staining. Hence hyperactive HPA responsiveness during adolescence may underlie 5-HT1A and CB1 receptor up-regulation and behavioral phenotype of BTBR mice.
Keywords: 129S1/SvImJ, adolescent, BTBR, C57BL, cannabinoid, GTPγS autoradiography, hippocampus, HPA feedback, novelty stress, serotonin, social behavior
1. INTRODUCTION
Exposure to severe stressors during adolescence can persistently alter hypothalamic pituitary adrenal (HPA) axis response, perception, cognition and mood into adulthood (McCormick and Mathews, 2007; Stevens et al., 2009). For example, unstable childhood home environments are associated with exaggerated HPA responses to stress, particularly among males (Hackman et al., 2012; Brenner et al., 2013). However, teenage boys with autism, depression or other psychiatric disorders often exhibit exaggerated HPA responses (e.g. higher cortisol peaks) following social or novel stimui than their ‘normotypic’ peers, paralleling individuals severely stressed in youth, even without a prior history of severe stress exposure (Lopez-Duran et al., 2009; Corbett et al., 2010; Spratt et al., 2012; Schupp et al., 2013). This low HPA axis resilience can have profound long-term impacts on social behavior, and may also increase the risk of suicide (Sher, 2006; Sunnqvist et al., 2008; Garlow et al., 2008).
Paralleling this, in male BTBR T+tf/J (BTBR) and 129S1/SvImJ (129S) mice exhibiting low social interaction (Gould et al., 2011; 2012), stressors induce exaggerated peak cortiscosterone (CORT) levels. For example, stressed BTBR released more CORT, but had paradoxically higher hippocampal glucocorticoid receptor (GR) mRNA levels than C57BL/6 mice (Benno et al., 2009). 129S mice also had higher post-stress peak CORT, but had lower hippocampal GR mRNA than C57BL/6 mice (Camp et al., 2012). All mice in these studies were adults. Since murine stress reactivity appears to be exaggerated prior to puberty (Romeo et al., 2013), yet adolescent and adult hippocampal GR expression are similar (Pryce, 2008), adolescent HPA axis tone was of great interest in these strains. Hence we compared adolescent and adult CORT responses to mild stressors, specifically novel social interaction and novel object exposure.
Corticosteroids bind to hippocampal GRs, the activation of which promotes serotonin transmission that in turn attenuates CORT release (Lanfumey et al., 2008; Pompili et al., 2010). CORT binding to GR also increases hippocampal endocannabinoid levels, and this likewise suppresses CORT release (Cota, 2008; McLaughlin et al., 2009; Atsak et al., 2012). This HPA axis suppression is likely mediated via inhibitory metabotrophic Gαi/o coupled serotonin 5-HT1A and CB1 receptors. When direct HPA axis feedback by circulating CORT via GRs is impaired, 5-HT1A and CB1 receptors may be up-regulated in compensation (Lanfumey et al., 2008; Pompili et al., 2010; Hensler et al., 2010). We hypothesized that this type of compensation may occur to a greater extent during adolescence, particularly if the HPA stress-response is exaggerated.
We have found that in adult BTBR mice hippocampal 5-HT1A receptor function was enhanced relative to C57BL/10 mice (Gould et al., 2011). We hypothesized that exaggerated stress-evoked CORT release during adolescence could underlie this 5-HT1A up-regulation, and may likewise up-regulate hippocampal CB1 receptors. Since higher binding could stem from increases either in receptor expression or neuronal density, we compared hippocampal neuronal cell density in adults of each strain. Further, receptor up-regulation might be more pronounced in adolescent mice with HPA axis hyperactivity. Thus, we compared adolescent to adult 5-HT1A and CB1 receptor densities and agonist-stimulated G-protein coupling in these strains.
2. METHODS
2.1. Mouse Subjects
BTBR T+tf/J, 129S1/SvImJ, C57BL/10J and C57BL/6J colony founders were from Jackson Laboratory (Bar Harbor, ME, USA). The mice were second or greater generation offspring bred in the laboratory animal facilities at William Paterson University, Wayne, NJ for quantitative autoradiography, or at The University of Texas Health Science Center at San Antonio (UTHSCSA), San Antonio, TX for autoradiography, immunostaining, behavior and corticosterone measures. Mice at both facilities were maintained at 20–22°C on 12:12 light dark cycles, with lights on at 0700 h, and ad-libitum access to food and water in cages lined with wood chip bedding (at UTHSCSA) or shaved wood bedding (at William Patterson University) that was changed bi-weekly. Mice were weaned at around postnatal day 21 and were housed in same-sex littermate groups of 2–5 per cage. All procedures involving mice were approved by Institutional Animal Care and Use Committees, and were consistent with current NIH guidelines.
2.2. Exposure to Novelty in Sociability and Marble Burying Tests and Serum CORT Levels
Mice used for novelty exposures and corticosterone (CORT) measures were 5 or 16 week-old male C57BL/6, 129S and BTBR. Prior to trunk blood collection, half of the mice were subjected to three-chamber sociability tests (40 min), immediately followed by marble burying (30 min), as in Gould et al. (2011; 2012). Briefly, each mouse subject was individually placed in a novel three-chamber arena for sociability testing. First subjects explored their arena for 20 min of preconditioning, then an unfamiliar object (wire cup) and ‘stranger 1’ a 129S male mouse (4 – 5 weeks old, in wire cup) was introduced at either end for 10 min, and finally a second novel ‘stranger 2’ mouse under a wire cup was introduced for 10 min, replacing the empty cage. Self-grooming was only scored during the sociability test and was not scored in an independent task. Afterward each subject mouse was transferred for 30 min to a 51 × 28 × 23 cm box filled with 10 cm of wood-chip bedding topped with 15 blue marbles in a grid pattern. The untested group of mice remained in their home cages until sacrifice. All mice were humanely sacrificed by decapitation between 1500 and 1650 h CST, and trunk blood was collected into tubes containing 25 μl of 20 mM ethylenediaminetetraacetic acid (Sigma, St Louis, MO).
Mouse blood was centrifuged at 4°C for 10 min at 2600 RPM, and serum collected and frozen at −80°C. Serum CORT levels were measured colorometrically on a plate reader (Molecular Devices, Sunnyvale, CA) after using an enzyme immunoassy kit (#ADI-900-097, Enzo, Plymouth Meeting, PA), following the manufacturer’s small sample volume protocol.
2.3. Brain Tissue Preparation
Mice used for quantitative autoradiography and immunostaining were 5 and/or 16 week old naïve males. The mice were sacrificed by decapitation and their brains were frozen on powdered dry ice and stored at −80°C. Coronal sections (20 μm) were collected from 0.90 to 0.60 mm and −1.80 to −2.00 mm relative to Bregma on a cryostat (Leica, Buffalo Grove, IL), and were thaw mounted onto chilled gelatin coated microscope slides. The sections were desiccated for 12–24h under vacuum at 4°C and then stored at −80°C until use in autoradiography or immunostaining.
2.4. Quantitative Autoradiography
Binding assays with [3H] 8-OH-DPAT (2 nM) for serotonin 5-HT1A, with 1 μM WAY 100,635 (Tocris, Ellisville, MO) to define non-specific binding, and [3H] CP55,940 (5 nM) for cannabinoid CB1 receptors, with 200 μM WIN,55-212-2 (Ascent Scientific, Princeton NJ) for non-specific binding, were performed on brain sections as per Gould et al. (2012). [35S] GTPγS binding in absence or presence of 1 μM of agonists 8-OH-DPAT or CP 55,940 was performed as described in Gould et al. (2011; 2012). Radioligands were from Perkin-Elmer (Boston, MA), and Kodak Biomak MR film (ThermoFisher, Waltham, MA) was used for all experiments.
Digital images were captured on a camera (1612M, Scion Corp., Frederick, MD) with a 60 mM lens onto a Macintosh (OS 10), with Image J software (NIH, Bethesda, MD) for density measures. Grey scale units were converted to fmol/mg protein or nCi/mg using calibration standards (American Radiolabeled Chem., St. Louis, MO), as per Gould et al. (2011). Brain regions measured included the hippocampus CA1, CA3 and dentate gyrus regions, frontal and parietal cortex, caudate putamen, nucleus acumbens (CB1 only), amygdala and hypothalamus. The amygdala and hypothalamus were not measured for GTPγS due to high basal binding.
2.5. Immunohistochemical Labeling
Brain sections on slides were thawed, rinsed with Dulbecco’s phosphate buffered saline (DPBS, Life Technologies, Grand Island, NY) and fixed with 4% paraformaldehyde at 4°C for 1 hour. They were rinsed in DPBS 3 times for 5 min each, and permeablized in 0.5% triton x-100 in DPBS at 26°C for 1 hour. The tissue was blocked for 1 hour at 26°C, anti-NeuN (rabbit polyclonal, Millipore, Billerica, MA) 1:500 was added for an overnight incubation at 4°C. The sections were washed 3 times with DPBS for 5 min each, and Alexa Fluor 488 goat anti-rabbit (Millipore) 1:500 secondary antibody was added before incubating for 2 hours at 26°C. After a final round of 3, 5 min DPBS washes, sections were cover slipped with vectashield mounting medium containing 4′, 6-Diamino-2-phenylindole dihydrochloride (DAPI, Vector Labs, Burlingame, CA). Sections were digitally captured on an Eclipse TE-2000-E (T-HUBC) microscope with a digital camera (Model: DXM1200C, Nikon, Melville, NY). Staining intensity in the dentate gyrus of hippocampus was measured using Nikon NIS-Elements AR 3.0 software.
2.6. Statistical Analysis and Sample Sizes
Three-way (strain x age x treatment) analysis of variance (ANOVA) was used to compare mean CORT levels, with Fisher’s least significant difference (LSD) post-hoc tests performed when significant main effects occurred. Repeated measures ANOVA was used to compare time in chamber and sniffing time in sociability tests, significant effects were further resolved by post-hoc ANOVA and/or t-test comparisons. Two-way ANOVA was used to compare mean chamber entries, grooming times, and marbles buried, with Fisher’s LSD post-hoc tests performed to resolve significant effects. There were 6–9 mice per strain/age/treatment group. For autoradiography two-way (strain x age) multivariate (several brain regions measured) ANOVA was used to compare mean binding density for several different brain regions, with Newman Keuls post-hoc tests performed, there were 8 mice per group. ANOVA was used to compare immunostaining intensity in the dentate gyrus, there were 7–8 mice per strain. All analyses were performed using Statistica software (StatSoft, Tulsa, OK).
3. RESULTS
3.1. Effects of Mouse Strain, Age and Behavior Testing on Serum CORT
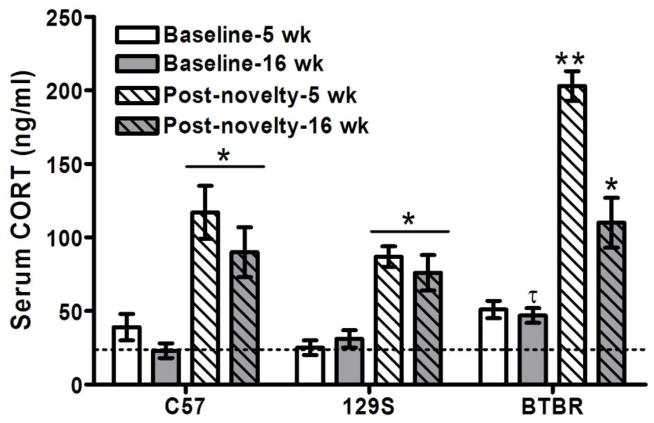
Corticosterone levels increased significantly after 70 min of novelty exposure in behavior tests (F1,66 = 177, p < 0.0001), and differed in magnitude among mouse strains (F2,66 =24, p < 0.0001) and ages (F1,66 = 17, p < 0.0001), with significant interactions (p < 0.017). The post-novelty increase in CORT was two-fold greater in 5 week-old BTBR mice than other strains, and was also greater than in adult BTBR mice (p < 0.0001) (Figure 1). Baseline CORT levels tended to be higher in adult BTBR than C57BL/6, and this difference was nearly significant (p = 0.06).
Figure 1. Novelty exposure raised serum CORT levels the most in adolescent BTBR mice.
CORT levels increased above mean baseline levels in adolescent (5 wk) and adult (16 wk) male mice of C57BL/6, 129S1/SvImJ and BTBR strains after 70 min of novelty exposure in behavior tests (*p<0.05). However, CORT levels in novelty-exposed 5 week-old BTBR mice were far higher than all of the other strains and ages (**p < 0.0001). Baseline CORT levels tended to be significantly higher in BTBR than in C57BL/6 adults (τp = 0.06). The dashed horizontal line illustrates the mean CORT level found in adult C57BL/6 mice to facilitate visual reference.
3.2. Comparison of Social and Repetitive Behaviors in Adolescent and Adult Male Mice
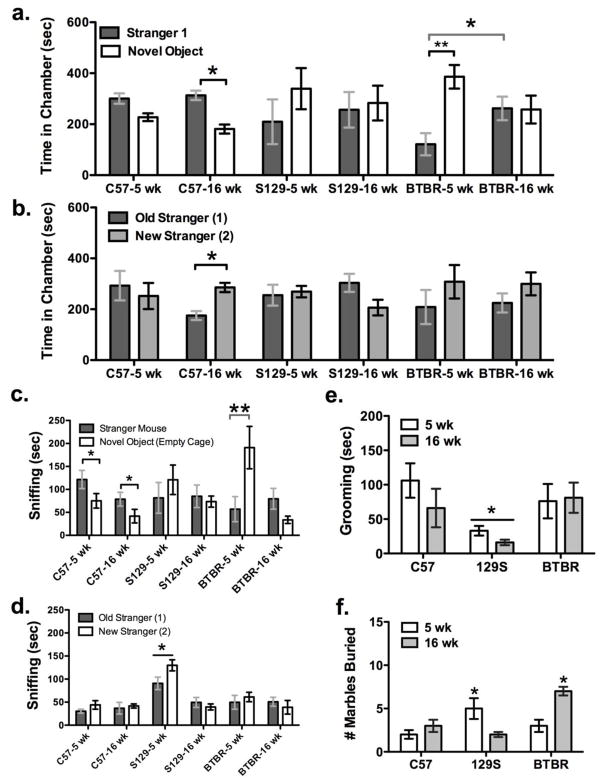
In global analysis of the three-chamber sociability tests, there was a significant interaction between mouse strain and age for time spent in either end chamber of the test arena (F2,30 = 3.23, p < 0.05). In the social interaction phase, C57 adults spent significantly more time by the stranger mouse vs. novel object (t = −3.8, p <0.05), while C57 adolescents also tended to, this trend was not significant (t = −2.14, p = 0.09). Five week-old BTBR mice spent less time in chambers with stranger mice and more time in chambers with novel objects (t = 3.2, p < 0.02), and they differed in comparison to BTBR adults with respect to this measure (F1,10 = 4.95, p<0.05, Fisher’s LSD p <0.05, Figure 2a). In the second phase of the test, only C57BL/6 adult mice showed a significant preference for social novelty based on time spent in the chamber with the new stranger mouse (p <0.05, Figure 2b).
Figure 2. Behavior of adolescent and adult BTBR mice in sociability and repetitive tests.
(a) A significant preference for social interaction was only found in C57 adults (*p < 0.05). Adolescent BTBR mice spent more time in chambers with novel objects than with stranger mice in the social interaction test (**p < 0.001), in contrast to adults (*p < 0.01). (b) Only adult C57 mice spent significantly more time in chambers with new strangers than with the ‘old’ original ones (*p<0.05). (c) Both C57 adolescent and adult mice spent more time sniffing stranger mice than novel objects (*p<0.05), however BTBR adolescents spent significantly more time sniffing novel objects than all other mice (**p<0.001). (d) Time spent sniffing new and old stranger mice during the social novelty test was greatest in 129S adolescents (*p <0.05). (e) Self-grooming among C57 and BTBR mice was comparable, but was lower in 129S mice during sociability testing (*p < 0.05). (f) BTBR adults and 129S adolescents buried more marbles over 30 min after sociability testing than C57BL/6 mice (*p < 0.05).
A global repeated measures analysis of social sniff time during sociability tests revealed significant differences between ages (F1,30 = 15, p < 0.001), test phase (F1, 30 = 25, p < 0.0001), with significant interactions among all main effects. With the finding of a significant age effect in the repeated measures ANOVA (F1,30 = 9.45, p < 0.01), in post-hoc ANOVAs performed on the social interaction phase, there was a significant effect of age for the amount of time spent sniffing empty boxes (F1,30 =15, p < 0.001), and an interaction between strain and age (F2,30 = 3.9, p < 0.32). Specifically BTBR adolescents spent more time sniffing the empty cages than all other groups except 5 week-old 129S mice. There was no significant difference in social sniff of stranger mice between strain (p = 0.4), or age (p = 0.7), as shown in figure 2c. However only C57 adults and adolescents had a significant preference for stranger mice (t > 3.4, p < 0.05). In the social novelty phase, there was a significant effect of strain (F1,30 = 15, p <0.0001) and age (F2,30 = 0.001), with interactions among factors (F2,30 = 12, p <0.0001). This was due to an increased amount of sniffing by 129S adolescents of either stranger relative to all other strains (F2, 30 > 5, p <0.05, Fisher’s LSD p <0.05), and more sniffing of the new strangers in comparison to 129S adults (F1, 30 = 22, p < 0.0001, Fisher’s LSD p <0.05), shown in figure 2d.
During social interaction tests, BTBR adults were hyperactive, making more chamber entries than other groups (F2,30 = 4.7, p < 0.02; Fisher’s p <0.05), yet their self-grooming duration across both sociability test phases combined was similar to other groups (F2,30 = 0.87, p = 0.42, Figure 2e). In contrast, 129S mice spent significantly less time self-grooming than C57BL/6 mice during the sociability tests (F2,30 = 5.1, p<0.01; p <0.05, Figure 2e), while C57BL/6 and BTBR self-grooming was similar. Immediately after sociability tests, adult BTBR and 5-week 129S mice buried more marbles than C57BL/6 mice (F2,30 = 13, p < 0.001, Fisher’s LSD p< 0.05, Figure 2f).
3.2. Serotonin 5-HT1A Receptor Density and 8-OH-DPAT-Stimulated GTPγS Binding
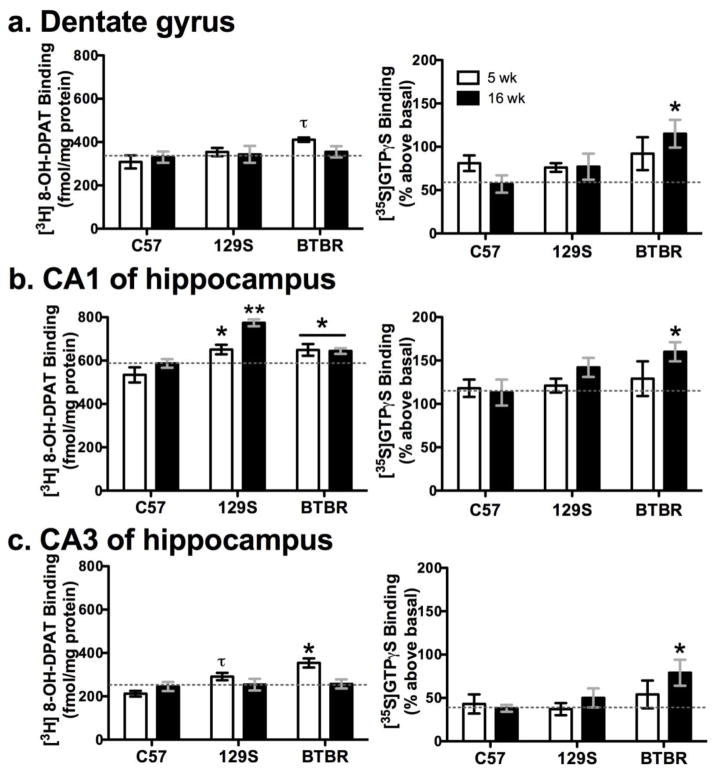
There were significant differences in [3H] 8-OH-DPAT binding density among strains (Wilks’ λ16,70 = 0.17, p<0.0001) and ages (λ8,35 = 0.32, p<0.0001), with interactions among these factors (λ16,70 = 0.51, p = 0.05) in the hippocampus and in other brain areas. Among 16 week-old mice, 129S [3H] 8-OH-DPAT binding was higher in the CA1 of hippocampus (Figure 3b) and cingulate cortex (by roughly 30%) than either BTBR or C57BL/10 (F2,42 > 8, p< 0.01; Newman-Keuls p < 0.05). Further, 5-week old 129S and BTBR mice had higher [3H] 8-OH-DPAT binding in the CA1 of hippocampus than C57BL/10 at either age (F2,42 = 21, p<0.001; p < 0.01, Figure 3b). Five-week old BTBR mice also had higher [3H] 8-OH-DPAT binding in the CA3 of hippocampus (Figure 3c, F2,42 =7.1, p < 0.002; p < 0.05). Also, 16-week old BTBR mice had lower [3H] 8-OH-DPAT binding than 5-week olds in the basolateral amygdala (108 ± 14 vs. ≈ 165 fmol/mg protein) and ventromedial hypothalamus (129 ± 11 vs. ≈ 200 fmol/mg protein) (F1,42 or F 2,42 > 3.15, p < 0.05). Non-specific was 8–10% of total binding.
Figure 3. Specific [3H] 8-OH-DPAT binding and 8-OH DPAT-stimulated [35S] GTPγS binding to serotonin 5-HT1A receptors in the hippocampus of adolescent and adult mice.
In the left-side panels [3H] 8-OH-DPAT binding was higher in 5-week old BTBR and/or 129S mice in (b) the CA1 and (c) CA3 of hippocampus than age-matched C57 mice (*p < 0.05; ** p <0.01; τp = 0.067). In (a) the dentate gyrus, there was also a non-significant trend toward BTBR mice at 5 weeks having greater [3H] 8-OH-DPAT binding (τp = 0.085). In the right-side panels 8-OH-DPAT stimulated [35S] GTPγS binding was enhanced in adult BTBR mice in (a) dentate gyrus (b) CA1 and (c) CA3 of hippocampus as compared to adult C57 (*p ≤ 0.05). Dashed horizontal lines are a reference marker for mean density in adult C57 mice. Thus, in 5-week old BTBR mice hippocampal 5-HT1A density was high, whereas in adults 5-HT1A function was enhanced.
8-OH-DPAT-stimulated [35S] GTPγS binding tended to differ among strains (λ8,78 = 0.11, p = 0.067), but not among ages (λ5,38 = 0.87, p = 0.38), with no interaction (λ10,76 = 0.84, p > 0.7). Specifically, agonist-stimulated 5-HT1A binding in 16 week-old BTBR mice was higher in all hippocampal regions measured (F2,42 >3, p ≤ 0.05), as shown in the plots on the right side in Figure 3a–c. Basal binding ranged from a mean of 249±14 to 435±31 nCi/mg in the brain regions measured, and non specific binding was roughly 40% of basal and 10% of stimulated binding.
Taken together, the age-dependent density and functional relationship between 5-HT1A and 8-OH-DPAT stimulated GTPγS binding in the hippocampus of BTBR mice (Figure 3a–c) is of particular interest, given this receptor’s proposed modulatory role in HPA axis feedback. Specifically [3H] 8-OH-DPAT binding density in 5-week old mice was higher in BTBR than in C57BL/10 mice in the CA1 (p < 0.01) and CA3 (p < 0.005) regions of the hippocampus, yet at 16 weeks of age, [35S]GTPγS binding was significantly higher in BTBR mice than in C57BL/10 mice in the CA1 (p = 0.05) and dentate gyrus (p < 0.05) regions of the hippocampus.
3.3. Cannabinoid CB1 Receptor Density and CP55,940-Stimulated GTPγS Binding
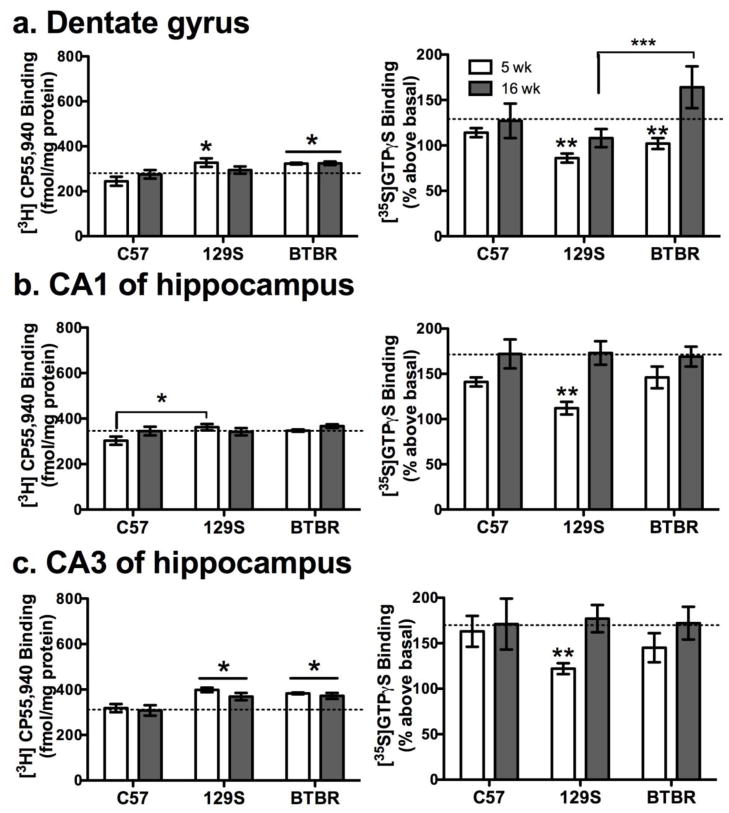
There were differences in [3H] CP55,940 binding density among strains (λ18,68 = 0.1, p < 0.001), specifically C57BL/10 had lower density than BTBR and/or 129S mice in dentate gyrus, CA1, CA3 of hippocampus and in the parietal cortex (175 vs. >202 fmol/mg protein) at 5 weeks, and in the dentate gyrus and hippocampal CA3 at 16 weeks of age (F2,42 >3, p < 0.05, Figure 4a–c, left panels). While a significant age effect was detected (λ9,34 = 0.29, p<0.001), post-hoc ANOVA failed to reveal age differences in the regions measured (F1,42 < 2, p > 0.15), and there were no interactions (λ18,68 = 0.5, p = 0.19). Non-specific binding was 40–50% of total.
Figure 4. Specific [3H] CP55,940 binding and CP55940-stimulated [35S] GTPγS binding to hippocampal cannabinoid CB1 receptors in adolescent and adult mice.
As shown in the left-side panels [3H] CP55,940 binding was greater in the (a) dentate gyrus and (c) CA3 of BTBR vs. C57 mice (*p<0.05). Adolescent 129S mice had significantly higher [3H] CP55,940 binding in the (a) dentate gyrus, (b) CA1 and (c) CA3 than adolescent C57 mice (*p<0.05). On the other hand, CP55,940 stimulated [35S] GTPγS binding generally increased with age, as shown in the panels to the right. However, hippocampal CP55,940 stimulated [35S] GTPγS binding was consistently lower in 129S adolescents (**p<0.05, a–c). Also agonist stimulated GTPγS binding was higher in adult BTBR than in 129S mice (*** p< 0.05, a). Dashed horizontal lines are a reference marker for mean density in adult C57 mice. Thus CB1 receptor coupling capacity is reduced, despite elevated CB1 receptor density in the adolescent 129S hippocampus.
CB1 receptor CP55,940-stimulated [35S] GTPγS binding differed among strains (λ10,76 = 0.55, p<0.01) and ages (λ5,38 = 0.65, p<0.01) without interaction (λ10, 76 = 0.67, p = 0.1). In all strains, CP55,940-stimulated binding increased with age in the hippocampus, as shown in the right panels of figure 4, and also in the cingulate cortex (18–40% greater in adults). In 16 week-old BTBR mice CP55,940-stimulated [35S] GTPγS binding was higher in dentate gyrus than in the other strains (F2,42 = 3.7, p < 0.05), figure 4a, left. We also observed in the hippocampal CA1 and CA3 of 5 week-old 129S mice that CP55,940-stimulated [35S] GTPγS binding was lower (p <0.05) than in C57BL/10 mice of the same age. This corresponded with modest increases in 129S CB1 receptor density in the 5-week old hippocampus, as measured by [3H] CP55,940, which may compensate for impaired CB1 receptor functionality in adolescent 129S mice.
3.4. C57BL/10 vs. C57BL/6 Binding Comparison
Because C57BL/6 mice are more commonly used than C57BL/10, we compared [3H] 8-OH-DPAT, [3H] CP55,940, and agonist stimulated [35S] GTPγS binding density in them. We did not observe any significant differences in 5-HT1A (λ8,7 = 0.28, p = 0.15) or CB1 (λ9,6 = 0.44, p = 0.61) binding site density among these strains. Further, agonist-stimulated [35S] GTPγS binding did not reveal any real differences among C57BL/6 and C57BL/10 adults (λ5,2 = 0.05, p = 0.12).
3.5. Immunostaining for Neuronal Density in the Dentate Gyrus
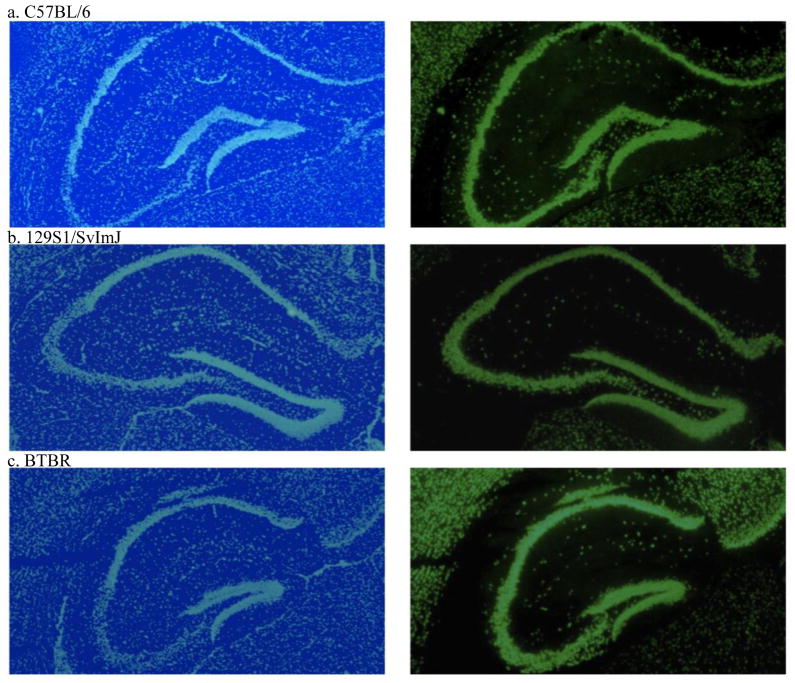
There were no differences in stain intensity for DAPI (F 2,19 = 2.8, p = 0.09) or NeuN (F 2,19 =1.0, p = 0.38) in the dentate gyrus among adult male 129S, BTBR or C57BL/6 mice. NeuN stain intensity ranged from a mean ± S.E.M. of 43 ± 9 to 64 ± 11 optical density units, while DAPI ranged from 72 ± 7 to 92 ± 5 density units. Representative images are shown in Figure 5.
Figure 5. DAPI nuclear (left) and NeuN neuronal (right) staining in the adult mouse hippocampus.
(a) C57BL/6, (b) 129S1/SvImJ and (c) BTBR mouse hippocampi had similar cell (in light blue) and neuron (in light green) density in the dentate gyrus. Representative sections from each strain are shown. For interpretation of the references to color in the figure legend, the reader is referenced to the web version of this article.
4. DISCUSSION
Herein we provide evidence of greater novelty-induced increases in serum CORT in adolescent male BTBR mice, accompanied by greater hippocampal serotonin 5-HT1A and cannabinoid CB1 receptor density relative to C57 mice. In BTBR adults, agonist-stimulated GTPγS binding to hippocampal 5-HT1A receptors was augmented, but this was not accompanied by higher receptor, cellular or neuronal density. Enhanced hippocampal 5-HT1A Gαi/o-coupled receptor activation capacity could facilitate inhibition of CORT release, and as such it may be a key compensatory response in adult BTBR HPA axis feedback. While hippocampal CB1 receptor density was greater in BTBR and 129S mice, CP55,940 stimulated GTPγS binding was no higher than, and in adolescents it was reduced relative to C57 mice. These factors may contribute to the age-dependent patterns in sociability deficits and hyperactivity we observed in BTBR males.
4.1. Social behavior and CORT level comparisons across age and strains
A preference for chambers with novel objects over stranger mice--that might arguably be interpreted as social avoidance--was evident in adolescent male BTBR relative to C57BL/6 mice. This behavioral pattern was pronounced, even as compared to BTBR adults that generally lack preference for social interaction (Moy et al., 2007; Silverman et al., 2010). It also corresponded with higher levels of CORT in serum after behavior testing. No similar age-dependent sociability patterns occurred in either C57 or 129S mice. Further, adult, but not adolescent BTBR mice entered the chambers in the sociability testing arena more frequently than other strains, consistent with earlier findings of hyperactivity in this strain (Silverman et al., 2010).
Based on prior studies, we predicted that CORT spikes in response to novel stimuli, at least in the adolescent C57BL/6 males, might be exaggerated compared to those in adults (Romeo et al., 2013), and that even higher levels might be found in BTBR mice (Benno et al., 2009). Indeed, we saw an exaggerated CORT response to novelty in adolescent BTBR after 70 min of sociability testing and marble burying. Our CORT level measures were also consistent with earlier reports of higher baseline and hyperactive HPA axis responsiveness in BTBR relative to C57BL/6 mice (Benno et al., 2009; Silverman et al., 2010). In contrast, CORT levels in 129S mice were no higher than C57BL/6 following novelty exposure, although restraint stress produced greater initial increases in them in a prior study (Camp et al., 2012). Since we did not measure CORT levels at earlier time points (e.g. at 30 min intervals) during our behavior tests, we suspect that we did not capture peak CORT levels induced by handling and novelty exposure. However, higher peak levels and/or extended release of CORT was nonetheless evident in BTBR adolescents after novelty exposure under our testing paradigm, and we also saw higher CORT levels in all strains and ages after behavior testing as compared to baseline values.
However, elevated CORT levels, either at baseline or in response to novelty, may not necessarily be indicative of a heightened anxiety state in BTBR mice. Five weeks is just prior to puberty onset at around postnatal day 40 for most male mice, including strains such as C57BL/6 and 129S (Wisniewski et al., 2005; Qiu et al., 2013). Male puberty has not been characterized in BTBR mice, so it could occur earlier, as in strains such as CD1 or C3H/HeJ (Divall et al., 2010; Zhou et al., 2012), or later. Yet it is unlikely that any differences in puberty onset alone could have so distinguished the BTBR behavioral and neuroendocrine phenotype. Other endocrine abnormalities are more likely responsible, for example in BTBR mice serum levels of insulin, testosterone, and progesterone are also relatively high (Flowers et al., 2007, Frye and Llaneza et al., 2010). Taken together with their elevated baseline CORT levels and exaggerated response, there is evidence of pituitary over-activity, possibly due to feedback disruption in BTBR mice, and these conditions can adversely impact cognition and behavior (Stranahan et al., 2008).
4.2 Age-dependent patterns of 5-HT1A receptor regulation in BTBR mice
5-HT1A receptors in the ventral and dorsal hippocampus shape social behavior, since 5-HT1A agonists and antagonists affecting anxiety state were found to bi-directionally alter social interactions in rodent open-field tests (File et al., 1996; File and Seth, 2003). Indeed in a prior study we found that a low dose of buspirone (2 mg/kg) improved sociability in adult male BTBR mice (Gould et al., 2011), while a higher dose (10 mg/kg) worsened it (unpublished data). We also saw that 8-OH-DPAT stimulated GTPγS binding in the CA1 of hippocampus was higher in BTBR than in C57BL/10 adult male mice. Hence we sought to determine if our finding of enhanced hippocampal agonist-stimulated 5-HT1A G-protein coupling would generalize to other socially impaired strains such as 129S, and if it was also evident in adolescent BTBR mice.
We found an age-dependent pattern of up-regulation at hippocampal 5-HT1A receptors in BTBR relative to C57 mice. Specifically, higher [3H] 8-OH-DPAT binding density was found in adolescents, while greater 8-OH-DPAT stimulated G-protein coupling was evident in adult BTBR mice as compared to age-matched C57 males. Yet 5-HT1A receptor density did not differ between BTBR and C57 adults. The 129S mice also had higher [3H] 8-OH-DPAT binding density, but only in the CA1 region of the hippocampus, and their 8-OH-DPAT stimulated GTPγS binding was similar to C57 mice.
4.3. Mounting evidence for possible GR dysfunction in BTBR mice
One way by which glucocorticoids modify behavior and HPA feedback response is via changes they generate at the neurotransmitter receptor level (e.g. Schutsky et al., 2011). However, the pattern of hippocampal 5-HT1A expression and function in BTBR mice, taken together with other findings in the literature, indicates that their GR functional integrity may be compromised. For example, adrenalectomized rodents have increased hippocampal 5-HT1A expression without changes in agonist-stimulated [35S] GTPγS binding (Pompili et al., 2010), paralleling our findings in adolescent BTBR mice. Also, hippocampal GR are over-expressed in adult BTBR mice, yet baseline CORT levels are elevated (Silverman et al., 2010; Benno et al., 2009). Further, BTBR and 129S have relatively high thermal pain thresholds compared to C57BL/6 mice (Silverman et al., 2010; Hossain et al., 2004). Yet chronically elevated corticosteroids, as found in BTBR, typically increase pain intensity via GR-induced alterations in cellular signaling (Khasar et al., 2008; McEwen and Kalia et al., 2010; Tramullas et al., 2012). Finally, in GR +/− mice CORT administration enhanced 8-OH-DPAT-stimulated [3H] GTPγS binding in the hippocampal CA1 (Hensler et al., 2010) to a similar extent to what we found in BTBR adults. Thus CORT levels may be higher due to compromised GR function in BTBR mice consequentially leading to increased 5-HT1A function at the G-protein level.
4.3 Up-regulation of CB1 function in the adult male BTBR dentate gyrus
Social behavior in inbred mice is also sensitive to synthetic cannabinoid agonists and manipulation of endogenous cannabinoid levels in frontal cortex and other brain regions by acetaminophen (Umathe et al., 2009; Gould et al., 2012). Given this, we compared CB1 receptor density and G-protein coupling capacity among strains in adolescent and adult male mice. We saw that CP55,940 stimulated G-protein coupling was enhanced in the dentate gyrus of BTBR adults relative to 129S adults and adolescent mice in all strains. We investigated this finding further, since a survey of neuropathological markers in BTBR mice revealed that neurogenesis in their dentate gyrus and glial fiber growth was altered (Stephenson et al., 2011). We saw no differences in neuronal density as quantified by NeuN labeling, DAPI staining, or CB1 receptor expression as quantified by autoradiography with [3H] CP55,940 in the adult BTBR dentate gyrus. However, enhancement of CP55940-stimulated G-protein coupling may yet be due to differences in neural vs. glial cell composition in this brain region (Lopez-Gallardo et al., 2012). More numerous glial cells in BTBR adults, and subsequent increases in the actions of Gαi/o coupled CB2 receptors expressed on them (Brusco et al., 2008) could account for enhanced G-protein coupling capacity in the dentate gyrus of BTBR adults.
4.4 Relative down-regulation of CB1 function in adolescent 129S hippocampi
Agonist-stimulated [35S] GTPγS binding generally increased with age at hippocampal CB1 receptors in all strains, while CB1 receptor density did not. 129S adolescents and BTBR mice had higher [3H] CP55,940 binding density than C57 mice at both ages in the dentate gyrus and CA3 regions. Despite this, 129S adolescent mice had relatively lower CP55,940 stimulated GTPγS binding in all hippocampal areas than C57 or BTBR adolescents. This disconnect between CB1 density and function was not present in adult 129S mice, given our CP55,940 stimulated GTPγS binding data from all three hippocampal subregions, and earlier findings in the cingulate cortex (Gould et al., 2012).
Instead, it appears to be a distinct developmental strain difference in the dentate gyrus that may relate to stress reactivity impairments and fear-related memory impairments that have already been characterized in adult 129S1/SvImJ mice (Camp et al., 2012). Endocannabinoids, via CB1 receptors can prevent the retention of inappropriate generalized fear responses in mice by interfering with hippocampal long-term potentiation and plasticity (Reich et al., 2008; Jacob et al., 2012), and 129S mice exhibit fear overgeneralization and motor activity impairments that have been attributed to elevated anxiety (Kalueff and Tuohimaa, 2004; Camp et al., 2012). It is also possible that the increased 5-HT1A expression we found in the 129S hippocampus might compensate for functional CB1 impairments, but incompletely at the level of G-protein coupling.
4.5. Age-dependent interactions among CB1 and 5-HT1A in BTBR HPA axis regulation
Adolescent rodents might be less sensitive to cannabinoid-induced modulation of 5-HT1A expression and function then adults (Zavitsanou et al., 2010). Hence while in the aggregate the functional G-protein binding response of both CB1 and 5-HT1A receptors is lower in BTBR adolescents than in adults, inhibitory feedback to the HPA axis may be muted to a greater extent. This could be why adolescent, but not adult CORT responses to novelty were exaggerated in BTBR mice. It is plausible that up-regulation of inhibitory coupling capacity at both 5-HT1A and CB1 receptors in these hippocampal regions is required to keep BTBR HPA axis activity in check and dampen the exaggerated adolescent novelty-induced CORT peak in BTBR adults. In turn, up-regulation of hippocampal 5-HT1A and CB1 receptor function or density in BTBR and 129S mice, while it may occurs for different reasons, may similarly contribute to the lack of preference for social interaction in these strains.
4.6 Implications for strain selection in studies of social behavior
Poor emotional resilience in response to stress is associated with impulsive or aggressive behaviors, and psychiatric disorders such as anxiety and depression (Cuomo et al., 2008; Stevens et al., 2009). Impaired HPA axis feedback in susceptible individuals may contribute to this state (Sher et al., 2006; Spijker and van Rossum, 2012). Glucocorticoid tone may be particularly relevant to how the mature BTBR behavioral phenotype develops, since it shapes dendritic spine formation and pruning in the hippocampus (Liston and Gan, 2011, Gourley et al., 2013). This discovery is also potentially relevant for social interactions in teens with autism, since in controlled studies novel social stimuli raised cortisol levels higher in autistic than normotypic adolescents (Corbett et al., 2010; Schupp et al., 2013). In this sense, the BTBR mouse could provide critical mechanistic insight into how glucocorticoids shape adolescent social behavior. Given the variability in CORT response, 5-HT1A and CB1 receptor expression among BTBR, 129S and C57 mice, their use as a combined model system could help to clarify their effects on endpoints such as social behavior.
Supplementary Material
Acknowledgments
This research was supported by the San Antonio Area Foundation, the Morrison Trust, NIH MH086708 (GGG), MH52369 (JGH) and MH071488 (JGH), DA032890 (ESO), the UTHSCSA R25 START UPs program (R25GM097632) and IIMS CTSA grant (UL1RR025767) and by funds from Dr. Sandra DeYoung, Dean of Science and Health at William Paterson University. The authors thank Dr. Lynette C. Daws, Department of Physiology, UTHSCSA for use of her laboratory resources, the UT Medical School Summer Research Program, Catherine Gonzalez of Health Careers High School, and Norman Schanz and Dr. Robert Benno for their administrative and technical assistance.
Role of the funding sources
The funding sources listed in the acknowledgements sup- ported salaries and supplies for the project, they had no vested interest in the outcome of the study.
Conflict of interest statement
The authors have no conflicts of interest to report.
References
- Atsak P, Hauer D, Campolongo P, Schelling G, McGaugh JL, Roozendaal B. Glucocorticoids interact with the hippocampal endocannabinoid system in impairing retrieval of contextual fear memory. Proc Natl Acad Sci. 2012;109:3504–9. doi: 10.1073/pnas.1200742109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benno R, Smirnova Y, Vera S, Liggett A, Schanz N. Exaggerated responses to stress in the BTBR T+tf/J mouse: an unusual behavioral phenotype. Behav Brain Res. 2009;197:462–5. doi: 10.1016/j.bbr.2008.09.041. [DOI] [PubMed] [Google Scholar]
- Brenner AB, Zimmerman MA, Bauermeister JA, Caldwell CH. The physiological expression of living in disadvantaged neighborhoods for youth. J Youth Adolesc. 2013;42:792–806. doi: 10.1007/s10964-012-9838-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusco A, Tagliaferro PA, Saez T, Onaivi ES. Ultrastructural localization of neuronal brain CB2 cannabinoid receptors. Ann N Y Acad Sci. 2008;1139:450–7. doi: 10.1196/annals.1432.037. [DOI] [PubMed] [Google Scholar]
- Camp MC, Macpherson KP, Lederle L, Graybeal C, Gaburro S, Debrouse LM, Ihne JL, Bravo JA, O’Connor RM, Ciocchi S, Wellman CL, Lüthi A, Cryan JF, Singewald N, Holmes A. Genetic strain differences in learned fear inhibition associated with variation in neuroendocrine, autonomic, and amygdala dendritic phenotypes. Neuropsychopharmacology. 2012;37:1534–47. doi: 10.1038/npp.2011.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett BA, Schupp CW, Simon D, Ryan N, Mendoza S. Elevated cortisol during play is associated with age and social engagement in children with autism. Mol Autism. 2010;1:13. doi: 10.1186/2040-2392-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota D. The role of the endocannabinoid system in the regulation of hypothalamic-pituitary-adrenal axis activity. J Neuroendocrinol Suppl. 2008;1:35–8. doi: 10.1111/j.1365-2826.2008.01673.x. [DOI] [PubMed] [Google Scholar]
- Cuomo C, Sarchiapone M, Giannantonio MD, Mancini M, Roy A. Aggression, impulsivity, personality traits, and childhood trauma of prisoners with substance abuse and addiction. Am J Drug Alcohol Abuse. 2008;34:339–45. doi: 10.1080/00952990802010884. [DOI] [PubMed] [Google Scholar]
- Divall SA, Williams TR, Carver SE, Koch L, Brüning JC, Kahn CR, Wondisford F, Radovick S, Wolfe A. Divergent roles of growth factors in the GnRH regulation of puberty in mice. J Clin Invest. 2010;120:2900–9. doi: 10.1172/JCI41069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File SE, Gonzalez LE, Andrews N. Comparative study of pre- and postsynaptic 5-HT1A receptor modulation of anxiety in two ethological animal tests. J Neurosci. 1996;16:4810–5. doi: 10.1523/JNEUROSCI.16-15-04810.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463:35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- Flowers JB, Oler AT, Nadler ST, Choi Y, Schueler KL, Yandell BS, Kendziorski CM, Attie AD. Abdominal obesity in BTBR male mice is associated with peripheral but not hepatic insulin resistance. Am J Physiol Endocrinol Metab. 2007;292:E936–45. doi: 10.1152/ajpendo.00370.2006. [DOI] [PubMed] [Google Scholar]
- Frye CA, Llaneza DC. Corticosteroid and neurosteroid dysregulation in an animal model of autism, BTBR mice. Physiol Behav. 2010;100:264–7. doi: 10.1016/j.physbeh.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlow SJ, Rosenberg J, Moore JD, Haas AP, Koestner B, Hendin H, Nemeroff CB. Depression, desperation, and suicidal ideation in college students: results from the American Foundation for Suicide Prevention College Screening Project at Emory University. Depress Anxiety. 2008;25:482–8. doi: 10.1002/da.20321. [DOI] [PubMed] [Google Scholar]
- Gould GG, Hensler JG, Burke TF, Benno RH, Onaivi ES, Daws LC. Density and function of central serotonin (5-HT) transporters, 5-HT1A and 5-HT2A receptors, and effects of their targeting on BTBR T+tf/J mouse social behavior. J Neurochem. 2011;116:291–303. doi: 10.1111/j.1471-4159.2010.07104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould GG, Seillier A, Weiss G, Giuffrida A, Burke TF, Hensler JG, Rock C, Tristan A, McMahon LR, Salazar A, O’Connor JC, Satsangi N, Satsangi RK, Gu TT, Treat K, Smolik C, Schultz ST. Acetaminophen differentially enhances social behavior and cortical cannabinoid levels in inbred mice. Prog Neuropsychopharmacol Biol Psychiatry. 2012;38:260–9. doi: 10.1016/j.pnpbp.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Swanson AM, Koleske AJ. Corticosteroid-induced neural remodeling predicts behavioral vulnerability and resilience. J Neurosci. 2013;33:3107–12. doi: 10.1523/JNEUROSCI.2138-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman DA, Betancourt LM, Brodsky NL, Hurt H, Farah MJ. Neighborhood disadvantage and adolescent stress reactivity. Front Hum Neurosci. 2012;6:277. doi: 10.3389/fnhum.2012.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensler JG, Vogt MA, Gass P. Regulation of cortical and hippocampal 5-HT(1A) receptor function by corticosterone in GR+/− mice. Psychoneuroendocrinology. 2010;35:469–74. doi: 10.1016/j.psyneuen.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain SM, Wong BK, Simpson EM. The dark phase improves genetic discrimination for some high throughput mouse behavioral phenotyping. Genes Brain Behav. 2004;3:167–77. doi: 10.1111/j.1601-183x.2004.00069.x. [DOI] [PubMed] [Google Scholar]
- Jacob W, Marsch R, Marsicano G, Lutz B, Wotjak CT. Cannabinoid CB1 receptor deficiency increases contextual fear memory under highly aversive conditions and long-term potentiation in vivo. Neurobiol Learn Mem. 2012;98:47–55. doi: 10.1016/j.nlm.2012.04.008. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Tuohimaa P. Contrasting grooming phenotypes in C57Bl/6 and 129S1/SvImJ mice. Brain Res. 2004;1028:75–82. doi: 10.1016/j.brainres.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Khasar SG, Burkham J, Dina OA, Brown AS, Bogen O, Alessandri-Haber N, Green PG, Reichling DB, Levine JD. Stress induces a switch of intracellular signaling in sensory neurons in a model of generalized pain. J Neurosci. 2008;28:5721–30. doi: 10.1523/JNEUROSCI.0256-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanfumey L, Mongeau R, Cohen-Salmon C, Hamon M. Corticosteroid-serotonin interactions in the neurobiological mechanisms of stress-related disorders. Neurosci Biobehav Rev. 2008;32:1174–84. doi: 10.1016/j.neubiorev.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Liston C, Gan WB. Glucocorticoids are critical regulators of dendritic spine development and plasticity in vivo. Proc Natl Acad Sci U S A. 2011;108:16074–9. doi: 10.1073/pnas.1110444108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Duran NL, Kovacs M, George CJ. Hypothalamic-pituitary-adrenal axis dysregulation in depressed children and adolescents: a meta-analysis. Psychoneuroendocrinology. 2009;34:1272–83. doi: 10.1016/j.psyneuen.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Gallardo M, López-Rodríguez AB, Llorente-Berzal Á, Rotllant D, Mackie K, Armario A, Nadal R, Viveros MP. Maternal deprivation and adolescent cannabinoid exposure impact hippocampal astrocytes, CB1 receptors and brain-derived neurotrophic factor in a sexually dimorphic fashion. Neuroscience. 2012;204:90–103. doi: 10.1016/j.neuroscience.2011.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick CM, Mathews IZ. HPA function in adolescence: role of sex hormones in its regulation and the enduring consequences of exposure to stressors. Pharmacol Biochem Behav. 2007;86:220–33. doi: 10.1016/j.pbb.2006.07.012. [DOI] [PubMed] [Google Scholar]
- McLaughlin RJ, Hill MN, Gorzalka BB. Monoaminergic neurotransmission contributes to cannabinoid-induced activation of the hypothalamic-pituitary-adrenal axis. Eur J Pharmacol. 2009;624:71–6. doi: 10.1016/j.ejphar.2009.09.055. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Kalia M. The role of corticosteroids and stress in chronic pain conditions. Metabolism. 2010;59(Suppl 1):S9–15. doi: 10.1016/j.metabol.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, Barbaro JR, Wilson LM, Threadgill DW, Lauder JM, Magnuson TR, Crawley JN. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompili M, Serafini G, Innamorati M, Möller-Leimkühler AM, Giupponi G, Girardi P, Tatarelli R, Lester D. The hypothalamic-pituitary-adrenal axis and serotonin abnormalities: a selective overview for the implications of suicide prevention. Eur Arch Psychiatry Clin Neurosci. 2010;260:583–600. doi: 10.1007/s00406-010-0108-z. [DOI] [PubMed] [Google Scholar]
- Pryce C. Postnatal ontogeny of expression of the corticosterone receptor genes in mammalian brains: Inter-species and intra-species differences. Brain Res Rev. 2008;57:596–605. doi: 10.1016/j.brainresrev.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Qiu X, Dowling AR, Marino JS, Faulkner LD, Bryant B, Brüning JC, Elias CF, Hill JW. Delayed puberty but normal fertility in mice with selective deletion of insulin receptors from Kiss1 cells. Endocrinology. 2013;154:1337–48. doi: 10.1210/en.2012-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich CG, Mohammadi MH, Alger BE. Endocannabinoid modulation of fear responses: learning and state-dependent performance effects. J Psychopharmacol. 2008;22:769–77. doi: 10.1177/0269881107083999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo RD, Kaplowitz ET, Ho A, Franco D. The influence of puberty on stress reactivity and forebrain glucocorticoid receptor levels in inbred and outbred strains of male and female mice. Psychoneuroendocrinology. 2013;38:592–6. doi: 10.1016/j.psyneuen.2012.07.019. [DOI] [PubMed] [Google Scholar]
- Schupp CW, Simon D, Corbett BA. Cortisol responsivity differences in children with autism spectrum disorders during free and cooperative play. J Autism Dev Disord. 2013 doi: 10.1007/s10803-013-1790-2. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutsky K, Ouyang M, Castelino CB, Zhang L, Thomas SA. Stress and glucocorticoids impair memory retrieval via β2-adrenergic, Gi/o-coupled suppression of cAMP signaling. J Neurosci. 2011;31:14172–81. doi: 10.1523/JNEUROSCI.2122-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher L. Combined dexamethasone suppression-corticotropin-releasing hormone stimulation test in studies of depression, alcoholism, and suicidal behavior. Scientific World Journal. 2006;6:1398–404. doi: 10.1100/tsw.2006.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Yang M, Turner SM, Katz AM, Bell DB, Koenig JI, Crawley JN. Low stress reactivity and neuroendocrine factors in the BTBR T+tf/J mouse model of autism. Neuroscience. 2010;171:1197–208. doi: 10.1016/j.neuroscience.2010.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spijker AT, van Rossum EF. Glucocorticoid sensitivity in mood disorders. Neuroendocrinology. 2012;95:179–86. doi: 10.1159/000329846. [DOI] [PubMed] [Google Scholar]
- Spratt EG, Nicholas JS, Brady KT, Carpenter LA, Hatcher CR, Meekins KA, Furlanetto RW, Charles JM. Enhanced cortisol response to stress in children in autism. J Autism Dev Disord. 2012;42:75–81. doi: 10.1007/s10803-011-1214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens HE, Leckman JF, Coplan JD, Suomi SJ. Risk and resilience: early manipulation of macaque social experience and persistent behavioral and neurophysiological outcomes. J Am Acad Child Adolesc Psychiatry. 2009;48:114–27. doi: 10.1097/CHI.0b013e318193064c. [DOI] [PubMed] [Google Scholar]
- Stephenson DT, O’Neill SM, Narayan S, Tiwari A, Arnold E, Samaroo HD, Du F, Ring RH, Campbell B, Pletcher M, Vaidya VA, Morton D. Histopathologic characterization of the BTBR mouse model of autistic-like behavior reveals selective changes in neurodevelopmental proteins and adult hippocampal neurogenesis. Mol Autism. 2011;2:7. doi: 10.1186/2040-2392-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Lee K, Pistell PJ, Nelson CM, Readal N, Miller MG, Spangler EL, Ingram DK, Mattson MP. Accelerated cognitive aging in diabetic rats is prevented by lowering corticosterone levels. Neurobiol Learn Mem. 2008;90:479–83. doi: 10.1016/j.nlm.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunnqvist C, Westrin A, Träskman-Bendz L. Suicide attempters: biological stressmarkers and adverse life events. Eur Arch Psychiatry Clin Neurosci. 2008;258:456–62. doi: 10.1007/s00406-008-0819-6. [DOI] [PubMed] [Google Scholar]
- Tramullas M, Dinan TG, Cryan JF. Chronic psychosocial stress induces visceral hyperalgesia in mice. Stress. 2012;15:281–92. doi: 10.3109/10253890.2011.622816. [DOI] [PubMed] [Google Scholar]
- Umathe SN, Manna SS, Utturwar KS, Jain NS. Endocannabinoids mediate anxiolytic-like effect of acetaminophen via CB1 receptors. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1191–9. doi: 10.1016/j.pnpbp.2009.06.020. [DOI] [PubMed] [Google Scholar]
- Wisniewski AB, Cernetich A, Gearhart JP, Klein SL. Perinatal exposure to genistein alters reproductive development and aggressive behavior in male mice. Physiol Behav. 2005;84:327–34. doi: 10.1016/j.physbeh.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Zavitsanou K, Wang H, Dalton VS, Nguyen V. Cannabinoid administration increases 5HT1A receptor binding and mRNA expression in the hippocampus of adult but not adolescent rats. Neuroscience. 2010;169:315–24. doi: 10.1016/j.neuroscience.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Guan Q, Li K, Tao L, Hu J, Xiao J. Dissection of the maternal effects on puberty onset by embryo transplantation in mouse. J Endocrinol Invest. 2012;35:676–80. doi: 10.3275/8125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.