Abstract
Objective
To compare temporal order memory in older adults with and without HIV infection.
Background
The frontal and temporal lobes play a key role in temporal order memory for items in a sequence. HIV-associated episodic memory deficits correlate with damage to neocortical interneurons in the fronto-striato-thalamo-cortical pathway and with atypical activation of the medial temporal lobes. Therefore, temporal order memory may be sensitive to neuropathological changes in individuals with HIV.
Methods
In this study, 50 HIV-seropositive individuals aged ≥ 50 years and 50 seronegative controls performed a computerized visuospatial temporal order memory task. During the sample phase of each trial, participants were shown circles presented 1 at a time in a random sequence at the end of each of the 8 arms of a radial maze. During the choice phase, they were shown the maze with a circle at the ends of 2 of the arms and asked which circle had appeared earlier than the other in the original sequence.
Results
Performance in both groups improved as a function of greater temporal separation between circle presentations. However, the HIV group had significantly worse memory impairment across all temporal separations, and the impairment was independently associated with clinical deficits in executive function and delayed retrospective memory.
Conclusions
Our results extend prior findings that HIV is associated with deficits in strategic aspects of memory encoding and retrieval. The neural mechanisms warrant further research, as do potential impacts on everyday function, eg, adherence to antiretroviral drug regimens.
Keywords: HIV, temporal order, visuospatial, episodic memory, executive function
The human immunodeficiency virus (HIV) is neurovirulent, commonly crossing the blood-brain barrier early in the course of infection and altering the structure and function of several neural systems, most notably the fronto-striato-thalamo-cortical (FSTC) loops (Ellis et al, 1997). In about half of HIV-infected (HIV+) individuals, the infection produces a wide range of neuropathological changes, including HIV encephalitis, vasculopathy, and gliosis (Everall et al, 2009). Even in the era of combination retroviral therapy (cART), HIV-associated neurocognitive disorders (HAND) affect 30% to 50% of HIV+ individuals (Heaton et al, 2010). Among those with less severe immunocompromise, even higher numbers may be affected in the cART era than the pre-cART era, particularly in the domains of executive function and episodic memory (Heaton et al, 2011).
Episodic memory is among the most commonly affected neurocognitive domains in HAND in the cART era (Carey et al, 2004; Heaton et al, 2011), and episodic memory impairment strongly predicts a range of functional problems, including nonadherence to medication regimens (Woods et al, 2009). HIV-associated episodic memory deficits are primarily characterized by impairment in the strategic aspects of encoding and retrieval (Gongvatana et al, 2007). HIV+ individuals show a profile of impaired immediate and delayed free recall, with relatively intact recognition (Carey et al, 2006; Delis et al, 1995); they may also show mild forgetting (Cattie et al, 2012; Woods et al, 2006), particularly if they have dementia (Scott et al, 2006). Impaired acquisition is marked by deficits in strategic encoding (eg, semantic clustering); this impairment tends to worsen in step with the severity of HAND (Gongvatana et al, 2007; Peavy et al, 1994).
At the level of neural systems, this profile is consistent with other conditions in which the FSTC circuits are disrupted, eg, Huntington disease. In fact, HIV-associated deficits in episodic memory correlate with damage to neocortical interneurons in the FSTC pathway (eg, calbindin immunoreactive interneurons) and also with atypical activation (eg, hippocampal overactivation during recognition and decreased activation during encoding) of the medial temporal lobes (Chana et al, 2006; Maki et al, 2009). Further supporting the hypothesis that HIV is associated with deficits in the strategic aspects of episodic memory are studies showing moderate serostatus effects on source memory (ie, the context for acquiring specific information) (Morgan et al, 2009) and prospective memory (ie, remembering to perform an intended action in the future) (Carey et al, 2006).
One aspect of episodic memory that might, therefore, be relevant to neuroAIDS is temporal order memory, ie, memory for the order in which stimuli or events have been experienced. As discussed by Tolentino et al (2012), an inability to remember the temporal order of items or events in a sequence may adversely affect daily living skills and cognitive functions (Pirogovsky et al, 2009). One function of the prefrontal cortex may be to integrate temporal information to attain prospective behavioral goals (Fuster, 2001). The prefrontal cortex may temporally organize fragments of information distributed across cortical networks (Fuster, 2001). Therefore, the frontal lobes may play a key role in supporting memory for the temporal order of items in a sequence (Shimamura, 1995).
People with frontal lobe damage have impaired temporal order memory (Daum and Mayes, 2000; Milner et al, 1985; Shimamura et al, 1990). Functional neuroimaging studies have recorded frontal lobe activation in healthy adults performing tasks that involve temporally sequenced stimuli (Cabeza et al, 2000; Hayes et al, 2004; Knutson et al, 2004; Rowe and Passingham, 2001). The temporal lobes also may be involved in temporal order memory. People with damage to the temporal lobes, including the hippocampus, show impairments on tasks requiring memory for the temporal order of stimuli (Downes et al, 2002; Hopkins et al, 1995; Mayes et al, 2001; Spiers et al, 2001). Neuroimaging studies have shown increased medial temporal lobe activity during tasks requiring temporal order retrieval (Ekstrom and Bookheimer, 2007; Lehn et al, 2009). Therefore, there is evidence that both the frontal and temporal lobes are essential for temporal order memory (Devito and Eichenbaum, 2011; Ekstrom et al, 2011).
As discussed by Tolentino et al (2012), age-related changes in both gray and white matter have been documented in many brain regions, including the medial temporal and frontal lobes (Cardenas et al, 2011; Davis et al, 2009; Kramer et al, 2007; Murray et al, 2010; Raz et al, 2010; Ziegler et al, 2010). Given such structural changes, it is not surprising that studies have reported significant age-related impairments in the ability to encode and retrieve the sequential order of stimuli and events (Kessels et al, 2007; Newman et al, 2001; Old and Naveh-Benjamin, 2008; Ulbrich et al, 2009).
Tolentino et al’s 2012 study of nondemented older adults demonstrated impaired temporal order memory for fixed and random sequences, particularly when temporal interference (presumed increased overlap among memory representations) was high, ie, when items appeared close together in a sequence. However, the participants’ temporal order memory for fixed sequences improved to the level of young adults when temporal interference was minimized (ie, when items appeared farther apart in a sequence). The results suggest that temporal order memory in older adults is less efficient and more susceptible to interference.
People perform temporal order memory tasks more efficiently when items are presented further apart in time than when they are closer (Kesner and Hopkins, 2006). This is referred to as the temporal separation effect. This phenomenon is thought to occur because there is more interference among temporally proximal than temporally distant events (Gilbert et al, 2001; Tolentino et al 2012). For example, people experience numerous events throughout a typical day. If one were asked whether an event that he or she experienced in the morning had occurred earlier in the day than an event experienced in the evening, one could easily recollect which event had happened first. However, one would find it much more difficult to make a similar temporal judgment between 2 events that had occurred minutes apart, presumably because temporally proximal events may share a common temporal context that produces more interference between the events.
One subpopulation of the HIV epidemic for whom temporal order memory may be especially relevant is older adults, who represent an ever-increasing proportion of the persons living with HIV infection in the cART era (Centers for Disease Control and Prevention, 2009). Older HIV-infected individuals experience faster disease progression (Centers for Disease Control and Prevention, 2009) and worse functional outcomes (Morgan et al, 2012; Thames et al, 2011). HIV and aging appear to have largely additive effects on brain structure (Ances et al, 2012) and function (Valcour et al, 2004), even in studies that control for variables such as treatment, disease severity, and psychiatric confounds (Valcour et al, 2004). These additive effects may be particularly evident in the FSTC pathways (Ances et al, 2012) and associated neurocognitive functions (Iudicello et al, 2012). For example, older age and HIV infection have additive adverse effects on those aspects of prospective memory that make stronger strategic or executive demands (Weber et al, 2011; Woods et al, 2010).
In light of these findings, it is reasonable to hypothesize that temporal order memory may be disrupted in older persons living with HIV infection. Functional magnetic resonance imaging studies measuring semantic event sequencing in middle-aged people with HIV have shown compromised temporal perception, associated with hypoactivation of the caudate and prefrontal cortex (Melrose et al, 2008). We are unaware, however, of any study to date that has specifically investigated the effect of temporal order interference on memory in HIV-infected older adults.
Our purpose in this study was to compare temporal order memory in older adults with HIV infection (HIV+) and matched seronegative (HIV−) adults. We used a computerized task to investigate the effects of varying levels of interference on temporal order memory for sequences of visuospatial stimuli.
METHODS
Participants
We tested 50 HIV+ participants aged 50 years or older from the HIV Neurobehavioral Research Program at the University of California San Diego. These people had originally been recruited via local print publications and in HIV clinical settings. We excluded candidates who had clinical evidence of neurologic disease, severe psychiatric illness, current substance dependence, or a urine toxicology screen that was positive for illicit drugs other than marijuana on the day of assessment.
The Institutional Review Board at the University of California San Diego approved all study procedures, and all of the HIV+ participants provided signed consent.
As a normal comparison group, we recruited 50 age- and sex-matched HIV− participants from the San Diego community. We screened candidate controls for dementia with the Dementia Rating Scale (Mattis, 1976). We excluded candidates who had a history of a neurologic condition (eg, seizures, head injury), a neurodegenerative disease, a history of alcohol or other substance abuse, or clinical evidence of a psychiatric illness within the past 6 months.
Written informed consent was received from all HIV− participants according to the Declaration of Helsinki, and all procedures were approved by the University of California San Diego and San Diego State University.
The participants’ demographic variables are shown in Table 1. A 1-way analysis of variance did not detect significant age differences between the HIV+ and HIV− groups, F(1,98) = 0.41, P = 0.53. Chi-square analyses revealed no significant differences between the 2 groups in sex, χ2 (1, N = 100) = 1.77, P = 0.18, or in ethnicity, χ2 (1, N = 100) = 1.87, P = 0.17. However, the HIV− group had significantly more years of education than the HIV+ group, F(1,98) = 8.66, P < 0.01. As noted below in the Results, the primary between-group findings did not change when education or ethnicity was considered as a covariate.
For disease staging, the HIV+ participants underwent a standardized neuromedical evaluation that included a blood draw. They also completed the Composite International Diagnostic Interview (World Health Organization, 1998); this structured, computerized evaluation, conducted by a research associate, elicits lifetime diagnoses of mood, anxiety, and substance use disorders. Table 2 lists the HIV+ group’s clinical data.
Temporal Order Memory Task
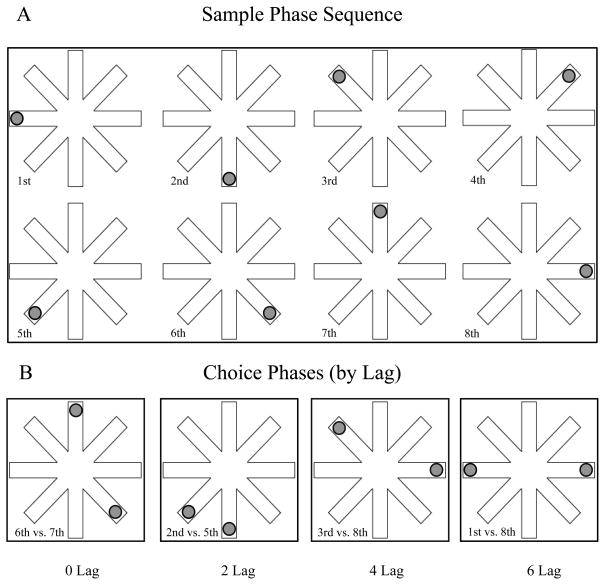
We used a task that we have described in previously published experiments (Nicoll et al, 2013; Pirogovsky et al, 2009; Tolentino et al, 2012) to assess the effects of interference on temporal order memory for random sequences of visuospatial stimuli. Participants were seated in front of a computer monitor. At the beginning of each trial, they were asked to focus on the screen, where we presented a radial 8-arm maze (Figure 1). They were told that a circle would appear at the end of each arm, one at a time, and they should remember the sequence in which the circles had appeared.
FIGURE 1.
Schematic of our visuospatial temporal order memory task. Panel A: Sample phase: Participants are shown 1 circle at a time at the end of an arm of an 8-arm radial maze, and are asked to remember the sequence in which the circles appeared. This panel shows a random sequence of the presentation of circles from the 1st through the 8th arms. Panel B: Choice phase: Participants are shown 2 circles at once and are asked which of the 2 circles had appeared earlier than the other during the sample phase. This panel shows examples of 0-, 2-, 4-, and 6-lag temporal separations. A lag is defined as the number of circles presented between the 2 circles shown. (Adapted from Tolentino et al, 2012. Used by permission of Cold Spring Harbor Laboratory Press.)
Each trial consisted of a sample phase followed by a choice phase. In the sample phase (Figure 1A), a 3-cm diameter gray circle appeared at the end of a randomly selected arm for 2 seconds. Then the display went gray for 2 seconds to eliminate after-image effects. When the display reappeared, the first circle was gone and a new circle appeared at the end of a different randomly selected arm for 2 seconds, again followed by a 2-second gray mask over the display. This process continued until a circle had appeared once at the end of each of the 8 arms. The sequence of presentation was randomized, varying in each trial.
In the choice phase (Figure 1B), we again showed the participants gray circles at the ends of the maze arms, but this time we presented 2 gray circles simultaneously for 5 seconds, and we asked the participants to say which of the 2 circles had appeared earlier than the other 1 during the sample phase.
We randomly selected temporal separations of 0, 2, 4, and 6 lags for each choice phase. We defined a lag as the number of circles presented during the sample-phase sequence between the 2 circles presented simultaneously during the choice phase (Figure 1B). For example, a 6-lag separation trial would have 2 choice-phase circles that, during the sample-phase sequence, had been presented with 6 circles between them, ie, they were the first and eighth circles shown.
We presented a total of 16 sample phase sequences, with each sequence followed by 3 choice phases. Each of the 3 choice phases presented a different lag: 0, 2, 4, or 6 circles. All 4 possible lags were counterbalanced across sequences. Thus, we gave a total of 12 choice-phase trials for each of the 4 lags. Participants had a 15-second interval after each trial.
As noted, evidence suggests that items appearing farther apart in a temporal sequence are easier to remember than items that are temporally adjacent. Therefore, we hypothesized that participants would have more difficulty in separating the temporally proximal circles in 0- and 2-lag trials than the temporally distant circles in 4- and 6-lag trials because of greater temporal interference.
Neuropsychological Assessment
Our HIV+ participants (but not the controls) took a neuropsychological test battery before they completed the temporal order task. The battery included the Wechsler Test of Adult Reading to estimate their verbal intelligence quotient (Psychological Corporation, 2001), and measures designed to assess 7 neurocognitive domains relevant to HAND, in accordance with Frascati research criteria (Antinori et al, 2007) (Table 3).
We converted the raw scores from the individual neuropsychological tests to demographically adjusted T-scores that we then used to derive global and domain-specific clinical ratings of neuropsychological function (Woods et al, 2004). Higher ratings indicated worse neuropsychological performance. Ratings ranged from 1 (above average, T-score ≥ 55) to 9 (severely impaired, T-score < 20).
We had several important reasons to use clinical ratings of neurocognitive impairment rather than raw scores or simple T-scores. Clinical ratings are considered the gold standard of assessment in HAND, according to the recommendations of the US National Institutes of Health working group’s 2007 research diagnostic scheme (Antinori et al, 2007). Clinical ratings have strong evidence of reliability and validity (see Woods et al, 2004) in neuroAIDS. These ratings also provide a robust assay of the neurocognitive domain of interest in that they include at least 2 test scores per ability area. This redundancy also helps to minimize the risk of type I error.
To reduce our risk of type I error even further in this study, we confined the neurocognitive domains that we assessed to those of greatest relevance to temporal order memory: delayed memory, attention, executive function, and processing speed. We also tested motor skills to provide a measure of conceptual divergence (ie, a noncognitive construct for which we did not expect to see an association with temporal order memory). Finally, we limited type I error by analyzing a summary measure of temporal order memory in a multiple regression with the 5 neurocognitive domains of interest. There was low risk of multicollinearity given that the Spearman’s rho intercorrelations between the clinical rating domains ranged from −0.04 to 0.38.
RESULTS
To analyze the data from the temporal order task, we used a 2 × 4 analysis of variance with serostatus group (HIV+, HIV−) as a between-group factor and with temporal separation lag (0, 2, 4, 6) as a within-group factor. The analysis revealed a significant main effect of serostatus group, F (1,98) = 29.08, P < 0.001, eta squared (η2)= 0.094, in which the HIV− participants scored significantly higher on the task than the HIV+ participants (Figure 2). The analysis of variance also revealed a significant main effect of lag, F(3, 294) = 63.89, P < 0.001, η2 = 0.231, with participants in both groups performing better when the temporal separation lags were larger.
FIGURE 2.
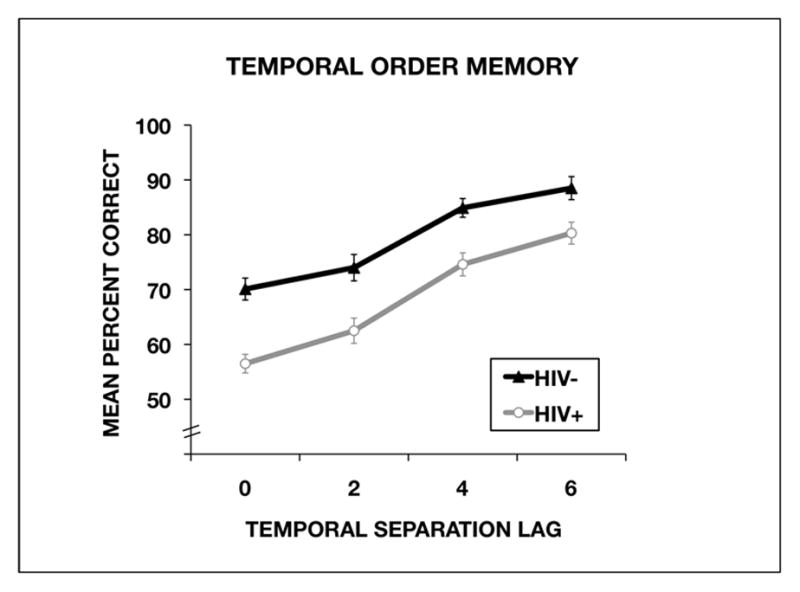
Performance by the HIV-seropositive (HIV+) group and the seronegative (HIV−) control group on the temporal order memory task as a function of temporal separation lag. The percentages of correct answers are shown as mean ± standard error.
Planned polynomial contrasts revealed a significant linear effect of temporal separation lag, F(1, 98) = 182.93, P < 0.001, η2 = 0.654, indicating that performance improved linearly as a function of temporal separation. Analysis of temporal separation lag by serostatus group did not reach statistical significance, F (3, 294) = 0.84, P = 0.47, η2 = 0.003.
We conducted planned analyses with Cohen’s d to examine the effect size for group differences at each temporal separation. The effect size was large for the 0-lag separation (d = 1.022), 2-lag separation (d = 0.689), and 4-lag separation (d = 0.752). The effect size for the 6-lag separation was medium (d = 0.535).
Because the HIV+ group had significantly less education (mean = 13.74 years) than the HIV− group (mean = 15.21 years), we conducted a follow-up analysis of covariance with years of education entered as a covariate. The analysis revealed that the main effect of group still reached significance with education in the model, F (1,98) = 22.21, P < 0.001, η2 = 0.094.
Ethnicity (% white) did not differ by serostatus and was not uniquely associated with temporal order memory in the HIV+ group (P > 0.10). Moreover, when we included ethnicity as a covariate in the statistical model examining the association between HIV and temporal order memory, there were no main effects of ethnicity and no interaction between ethnicity and HIV (P > 0.10 for both). As with education, the main effect of serostatus remained significant with ethnicity included in the model, F (1,96) = 15.8, P < 0.001.
We conducted a multiple linear regression to predict the temporal order summary score from the clinical ratings for the domains of delayed memory, executive function, attention, processing speed, and motor skills. Given the lack of statistical interaction between HIV serostatus and temporal order lag, we used a single measure of temporal order task performance for these analyses (ie, the average accuracy across all test lag conditions) to minimize our risk of type I error. The results showed a significant overall model (adjusted correlation coefficient R2 = 0.18, P = 0.012), which was driven by unique contributions of memory (standardized coefficient B = −0.34, P = 0.02) and executive function (B = −0.31, P = 0.03). None of the other cognitive domains were significantly associated with temporal order memory in this model (all P values > 0.20).
We performed a post hoc analysis to determine if specific aspects of delayed memory might be driving these effects. In an effort to minimize type I error, we used a critical alpha of 0.01 and limited the analyses to 2 variables selected on theoretical grounds. We chose semantic clustering because it is a strategically driven aspect of list learning and recall, and subjective clustering because it offers a comparison standard for purposes of specificity. We found a significant correlation between temporal order memory and the semantic clustering score on the long delayed free recall trial of the California Verbal Learning Test-Second Edition (Spearman rank correlation coefficient [rho] = 0.38, P = 0.008), but none with subjective clustering (rho = 0.10, P = 0.51).
Among the 50 members of the HIV+ group, the temporal order summary variable was not significantly associated with current or nadir CD4 count, plasma HIV ribonucleic acid, estimated duration of infection, or cART status (all P values > 0.05). As for the possible influence of medical and psychiatric co-factors in the HIV+ group, the temporal order summary score was not significantly associated with co-infection with hepatitis C, current affective distress as measured by the Profile of Mood States (McNair et al, 1981), or lifetime diagnoses of major depressive, generalized anxiety, or substance use disorders (all P values > 0.05). Moreover, the sum total of comorbidities in the HIV+ group (hepatitis C, mood disorder, alcohol disorder, and other substance use disorders, range = 0 to 4) was not significantly correlated with temporal order memory (P > 0.20).
Finally, we found that 12 (24%) of the HIV+ participants had positive urine toxicology screens for marijuana, reflecting cannabis use up to 30 days before assessment or possibly certain commonly prescribed antiretroviral drugs. Importantly, however, we found no significant differences in temporal order memory performance between the HIV+ participants with positive and negative urine toxicology screens (all P values > 0.05).
DISCUSSION
Our aim in this study was to examine how HIV infection in older adults affected their temporal order memory for visuospatial sequences with varying levels of temporal interference. We hypothesized that as temporal separation lag decreased (ie, the circles shown in the choice phases had been presented closer together in the sequence during the sample phase), interference was likely to increase, resulting in poorer temporal order memory. Indeed, both our HIV+ and HIV− participants did better on the temporal order task when the temporal separation lag increased and the temporal interference decreased. However, the HIV-group outperformed the HIV+ group across temporal separations with high, moderate, and low temporal interference.
These findings suggest that temporal order memory is generally less efficient in older adults with HIV infection. Cohen’s d effect size analyses revealed that the group differences were largest on trials involving the highest level of temporal interference (0 lag trials, d = 1.022); the HIV+ group’s performance improved as a function of decreased interference, as evidenced by smaller group differences at the largest temporal separation (6 lag trials, d = 0.535). In other words, despite the lack of significant statistical interaction between HIV serostatus and temporal order lag, the magnitude of the HIV effect on temporal order memory in the highest temporal interference condition was nearly double that in the lowest interference condition. Our findings agree with an earlier study in which we found that HIV-associated prospective memory deficits in adults are exacerbated by increasing cognitive load (Woods et al, 2010), an effect posited to stem from an executive burden on episodic memory systems.
To recap several points from the Introduction: Impaired temporal order memory for sequences of stimuli or events may adversely affect goal-directed behavior in numerous cognitive domains; these include executive function and episodic memory, processes critical to the execution of daily living skills. The frontal lobes may play a critical role in memory for sequences of stimuli and events (Shimamura, 1995). Our study offers support for the idea of an impairment of temporal order memory associated with HIV infection. HIV is neurovirulent, with an affinity for the pre-FSTC circuits that support executive control of episodic memory. Our prior studies found that patients with manifest Huntington disease (Nicoll et al, 2013) and premanifest gene carriers for Huntington disease (Pirogovsky et al, 2009) also showed significant deficits on our temporal order memory task because of presumed frontal-striatal circuit disruption.
Given that memory for temporally sequenced events, stimuli, or actions may be critical for many aspects of executive function (eg, strategic planning, prospective memory, problem solving), impaired temporal order memory may contribute to executive dysfunction in individuals infected with HIV. In fact, in this study we observed moderate associations between temporal order memory and 2 clinical tests of executive function that measured visual planning as well as complex attention and sequencing. While such cognitive correlations are nonspecific and inferential, they provide some indirect support for the possibility of frontal system involvement. A 2008 functional neuroimaging study had implicated the caudate and prefrontal cortex in HIV-associated impairment of semantic event sequencing (Melrose et al, 2008).
Besides the relationship between temporal order memory and executive function, our analyses also detected a unique association between temporal order memory and delayed memory. Temporal order memory for sequences may be critical for the formation and subsequent retrieval of episodic memories (Tolentino et al, 2012). Episodic memory is the ability to remember a personally experienced event and to place that event in space and time (Tulving, 2002). The encoding of contextual information may be critical to the formation of episodic memories because of the need to form associations among stimuli, actions, and places that make up an event (Eichenbaum, 2004).
Although episodic memory relies on the functioning of the temporal and frontal lobes (Peters and Daum, 2009; Squire et al, 2004; Turnock and Becker, 2008), the functional contributions of each cortical region can be dissociated (Ekstrom et al, 2011; Kramer et al, 2005). The hippocampus may support specific mnemonic processes that help the encoding and retrieval of episodic memories to improve memory accuracy, as has been shown directly in clinicopathologic (Moore et al, 2006) and neuroimaging (Maki et al, 2009) studies in HIV. The frontal lobes may be more important for decision-making and strategic aspects of episodic memory (Kramer et al, 2005), suggesting that HIV-associated deficits in temporal order memory may relate to strategic aspects of “working with memory” (Moscovitch, 1992). “Working with memory” is the idea that FSTC-driven executive processes work in concert with the temporal lobes to organize information to be remembered. However, studies are needed to investigate further how temporal order memory relates to episodic memory and working memory in the HIV+ population. In particular, it would be of interest to incorporate a delayed memory condition into a temporal order task to better dissociate medial temporal and frontal lobe contributions to task performance.
Our study had some limitations. First, our interpretations of the neural substrates of the HIV-associated temporal order memory deficit are inferential and await confirmation with neuroimaging and biomarkers (eg, tau, Aβ42, leptin). For example, Woods et al (2006) showed a significant, unique association between tau and HIV-associated prospective memory impairment.
A second limitation is the lack of a factorial design by which to determine the combined effects of HIV and aging on temporal order memory. Without such a design, we cannot interpret our findings beyond a simple serostatus effect among “older” adults.
Third, not having comparable neuropsychological data between the HIV+ and HIV− groups limits our ability to generate inferences about brain-behavior relationships in the HIV− group.
A notable final limitation is that, consistent with other studies of the HIV epidemic in the US (eg, Rodriguez-Penney et al, 2013), our HIV+ cohort had a high rate of comorbid conditions (eg, hepatitis C co-infection, substance use disorders) not found in the HIV− control group. This might raise concern that the temporal order effects that we observed in our HIV+ group are just an artifact of their comorbid conditions, especially considering the cross-sectional nature of our study. As explained in the Results, our HIV+ group’s temporal order performance was not directly associated with their nadir CD4 or any of the other HIV disease parameters that we measured. We did not see any associations between these comorbidities and the group’s temporal order performance, lending some support to the possibility that these clinical factors do not exclusively explain our findings.
To rule out the possibility that comorbidities accounted for the HIV+ group’s task performance, we further analyzed the effects of their comorbidities on their temporal order memory summary score. We did not find temporal order to be associated with hepatitis C co-infection, current affective distress, mood disorder, history of substance use disorder, or a positive urine toxicology for marijuana. The absence of mood effects on our HIV+ group’s cognitive functioning is consistent with a large body of literature showing that major depressive disorder does not confer a risk for HIV-associated neurocognitive disorders (eg, Cysique et al, 2007).
None of our HIV+ participants met current diagnostic criteria for any substance use disorder. This is important because while recent substance abuse (eg, alcohol, stimulants) can have additive effects on neurocognitive impairment in HIV (eg, Grauzas et al, 2013; Rippeth et al, 2004), abstinence brings considerable neurocognitive recovery. Indeed, 2011 data from a large national cohort show that HIV-infected persons with a remote history of substance use disorders are not at greater-than-average risk for neurocognitive impairment (Byrd et al, 2011). Moreover, numerous studies show significant HIV serostatus effects on high-level memory tasks even when the study groups have well-matched substance use histories (eg, Martin et al, 2007). Therefore, although we could not match our control group to the comorbidities of our HIV+ group, we can state with some confidence that our findings in the HIV+ group were not solely an artifact of their comorbidities.
In conclusion, our results show that HIV infection is associated with poorer temporal order memory, particularly when temporal interference is high. Our findings suggest that temporal order memory is less efficient and more susceptible to interference in HIV+ individuals. Future systematic investigations are warranted to explore further the construct of temporal order memory (eg, divergent validity, convergent validity, and predictive validity) and to determine whether temporal order memory is selectively impaired in individuals with HIV infection. Considering that HIV-associated memory deficits affect a wide range of functional outcomes, future research may be warranted to study the possible unique contribution of temporal order memory impairment to HIV-related disability. In particular, deficits in temporal order memory might affect daily living activities that involve sequencing and timing, such as automobile driving, self-management of health care (eg, medication adherence) (Woods et al, 2009), finances, and such household chores as shopping and cooking. Future studies are also needed to determine the incremental value of temporal order memory in predicting functional and clinical outcomes in HIV, above and beyond other cognitive measures
Although prior studies have shown HIV minimally affecting the ability to sequence daily tasks (Scott et al, 2011b), sequencing errors in HIV are moderately related to executive dysfunction (Scott et al, 2011a) and to less effective multitasking during daily activities (Scott et al, 2011a). If our predicted associations between temporal order memory and HIV-associated disability are borne out in future research, behavioral interventions that minimize temporal interference and structure daily living tasks into fixed, repetitive sequences may improve HIV+ individuals’ memory and perhaps increase their functional independence.
Our results may have further clinical implications in light of a 2012 report that impaired temporal order memory may be a selective behavioral marker of Alzheimer disease (Bellassen et al, 2012). In coming years, these combined findings could have particular meaning for the growing number of people with HIV infection who are living into their 60’s and beyond (High et al, 2012).
Supplementary Material
Acknowledgments
Supported by National Institute of Mental Health grants R01-AG034202 (P.E.G.), R01-MH73419 (S.P.W.), and P30-MH62512 (I.G.). The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, or the US Government.
The authors thank P. Katie Riggs for her help with study management.
Glossary
- cART
combination antiretroviral therapy
- FSTC
fronto-striato-thalamo-cortical
- HAND
HIV-associated neurocognitive disorders
- HIV
human immunodeficiency virus
- HIV+
HIV-seropositive
- HIV−
HIV-seronegative
Footnotes
The authors declare no conflicts of interest.
References
- Ances BM, Ortega M, Vaida F, et al. Independent effects of HIV, aging, and HAART on brain volumetric measures. J Acquir Immune Defic Syndr. 2012;59:469–477. doi: 10.1097/QAI.0b013e318249db17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellassen V, Iglói K, de Souza LC, et al. Temporal order memory assessed during spatiotemporal navigation as a behavioral cognitive marker for differential Alzheimer’s disease diagnosis. J Neurosci. 2012;32:1942–1952. doi: 10.1523/JNEUROSCI.4556-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd DA, Fellows RP, Morgello S, et al. Neurocognitive impact of substance use in HIV infection. J Acquir Immune Defic Syndr. 2011;58:154–162. doi: 10.1097/QAI.0b013e318229ba41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Houle S, et al. Age-related differences in neural activity during item and temporal-order memory retrieval: a positron emission tomography study. J Cogn Neurosci. 2000;12:197–206. doi: 10.1162/089892900561832. [DOI] [PubMed] [Google Scholar]
- Cardenas VA, Chao LL, Studholme C, et al. Brain atrophy associated with baseline and longitudinal measures of cognition. Neurobiol Aging. 2011;32:572–580. doi: 10.1016/j.neurobiolaging.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Gonzalez R, et al. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol. 2004;26:307–319. doi: 10.1080/13803390490510031. [DOI] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Rippeth JD, et al. Prospective memory in HIV-1 infection. J Clin Exp Neuropsychol. 2006;28:536–548. doi: 10.1080/13803390590949494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattie JE, Woods SP, Arce M, et al. Construct validity of the item-specific deficit approach to the California Verbal Learning Test (2nd Ed) in HIV infection. Clin Neuropsychol. 2012;26:288–304. doi: 10.1080/13854046.2011.653404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. HIV/AIDS Surveillance Report, 2007. Atlanta, GA: US Department of Health and Human Services, CDC; 2009. [Google Scholar]
- Chana G, Everall IP, Crews L, et al. Cognitive deficits and degeneration of interneurons in HIV+ methamphetamine users. Neurology. 2006;67:1486–1489. doi: 10.1212/01.wnl.0000240066.02404.e6. [DOI] [PubMed] [Google Scholar]
- Culbertson WC, Zillmer EA. The Tower of London DX (TOLDX) Manual. North Tonawanda, NY: Multi-Health Systems; 2001. [Google Scholar]
- Cysique LA, Deutsch R, Atkinson JH, et al. Incident major depression does not affect neuropsychological functioning in HIV-infected men. J Int Neuropsychol Soc. 2007;13:1–11. doi: 10.1017/S1355617707070026. [DOI] [PubMed] [Google Scholar]
- Daum I, Mayes AR. Memory and executive function impairments after frontal or posterior cortex lesions. Behav Neurol. 2000;12:161–173. doi: 10.1155/2000/327304. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Buchler NG, et al. Assessing the effects of age on long white matter tracts using diffusion tensor tractography. Neuroimage. 2009;46:530–541. doi: 10.1016/j.neuroimage.2009.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, et al. California Verbal Learning Test. 3. San Antonio, TX: Psychological Corporation; 2000. (CVLT-II) [Google Scholar]
- Delis DC, Peavy G, Heaton R, et al. Do patients with HIV-associated minor cognitive/motor disorder exhibit a “subcortical” memory profile? evidence using the California Verbal Learning Test. Assessment. 1995;2:151–165. [Google Scholar]
- Devito LM, Eichenbaum H. Memory for the order of events in specific sequences: contributions of the hippocampus and medial prefrontal cortex. J Neurosci. 2011;31:3169–3175. doi: 10.1523/JNEUROSCI.4202-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes JJ, Mayes AR, MacDonald C, et al. Temporal order memory in patients with Korsakoff’s syndrome and medial temporal amnesia. Neuropsychologia. 2002;40:853–861. doi: 10.1016/s0028-3932(01)00172-5. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron. 2004;44:109–120. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Ekstrom AD, Bookheimer SY. Spatial and temporal episodic memory retrieval recruit dissociable functional networks in the human brain. Learn Mem. 2007;14:645–654. doi: 10.1101/lm.575107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom AD, Copara MS, Isham EA, et al. Dissociable networks involved in spatial and temporal order source retrieval. Neuroimage. 2011;56:1803–1813. doi: 10.1016/j.neuroimage.2011.02.033. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Deutsch R, Heaton RK, et al. Neurocognitive impairment is an independent risk factor for death in HIV infection. Arch Neurol. 1997;54:416–424. doi: 10.1001/archneur.1997.00550160054016. [DOI] [PubMed] [Google Scholar]
- Everall I, Vaida F, Khanlou N, et al. Cliniconeuropathologic correlates of human immunodeficiency virus in the era of antiretroviral therapy. J Neurovirol. 2009;15:360–370. doi: 10.3109/13550280903131915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex--an update: time is of the essence. Neuron. 2001;30:319–333. doi: 10.1016/s0896-6273(01)00285-9. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP, Lee I. Dissociating hippocampal subregions: double dissociation between dentate gyrus and CA1. Hippocampus. 2001;11:626–636. doi: 10.1002/hipo.1077. [DOI] [PubMed] [Google Scholar]
- Gongvatana A, Woods SP, Taylor MJ, et al. Semantic clustering inefficiency in HIV-associated dementia. J Neuropsychiatry Clin Neurosci. 2007;19:36–42. doi: 10.1176/jnp.2007.19.1.36. [DOI] [PubMed] [Google Scholar]
- Grauzas V, Rubin LH, Martin E, et al. HIV and recent illicit drug use interact to affect verbal memory in women. J Acquir Immune Defic Syndr. 2013;63:67–76. doi: 10.1097/QAI.0b013e318289565c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SM, Ryan L, Schnyer DM, et al. An fMRI study of episodic memory: retrieval of object, spatial, and temporal information. Behav Neurosci. 2004;118:885–896. doi: 10.1037/0735-7044.118.5.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Miller SW, Taylor MJ, et al. Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults. Lutz, FL: Psychological Assessment Resources; 2004. [Google Scholar]
- High KP, Brennan-Ing M, Clifford DB, et al. HIV and aging: state of knowledge and areas of critical need for research. A report to the NIH Office of AIDS Research by the HIV and Aging Working Group. J Acquir Immune Defic Syndr. 2012;60(Suppl 1):S1–S18. doi: 10.1097/QAI.0b013e31825a3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins RO, Kesner RP, Goldstein M. Item and order recognition memory in subjects with hypoxic brain injury. Brain Cogn. 1995;27:180–201. doi: 10.1006/brcg.1995.1016. [DOI] [PubMed] [Google Scholar]
- Iudicello JE, Woods SP, Deutsch R, et al. Combined effects of aging and HIV infection on semantic verbal fluency: a view of the cortical hypothesis through the lens of clustering and switching. J Clin Exp Neuropsychol. 2012;34:476–488. doi: 10.1080/13803395.2011.651103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP, Hopkins RO. Mnemonic functions of the hippocampus: a comparison between animals and humans. Biol Psychol. 2006;73:3–18. doi: 10.1016/j.biopsycho.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Kessels RP, Hobbel D, Postma A. Aging, context memory and binding: a comparison of “what, where and when” in young and older adults. Int J Neurosci. 2007;117:795–810. doi: 10.1080/00207450600910218. [DOI] [PubMed] [Google Scholar]
- Kløve H. Grooved Pegboard. Lafayette, IN: Lafayette Instruments; 1963. [Google Scholar]
- Knutson K, Wood JN, Grafman J. Brain activation in processing temporal sequence: an fMRI study. Neuroimage. 2004;23:1299–1307. doi: 10.1016/j.neuroimage.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Mungas D, Reed BR, et al. Longitudinal MRI and cognitive change in healthy elderly. Neuropsychology. 2007;21:412–418. doi: 10.1037/0894-4105.21.4.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer JH, Rosen HJ, Du AT, et al. Dissociations in hippocampal and frontal contributions to episodic memory performance. Neuropsychology. 2005;19:799–805. doi: 10.1037/0894-4105.19.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehn H, Steffenach HA, van Strien NM, et al. A specific role of the human hippocampus in recall of temporal sequences. J Neurosci. 2009;29:3475–3484. doi: 10.1523/JNEUROSCI.5370-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, Cohen MH, Weber K, et al. Impairments in memory and hippocampal function in HIV-positive vs HIV-negative women: a preliminary study. Neurology. 2009;72:1661–1668. doi: 10.1212/WNL.0b013e3181a55f65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin EM, Nixon H, Pitrak DL, et al. Characteristics of prospective memory deficits in HIV-seropositive substance-dependent individuals: preliminary observations. J Clin Exp Neuropsychol. 2007;29:496–504. doi: 10.1080/13803390600800970. [DOI] [PubMed] [Google Scholar]
- Mattis S. Mental status examination for organic mental syndrome in the elderly patient. In: Bellack L, Katsau TB, editors. Geriatric Psychiatry: A Handbook for Psychiatrists and Primary Care Physicians. New York: Grune and Stratton; 1976. pp. 77–121. [Google Scholar]
- Mayes AR, Isaac CL, Holdstock JS, et al. Memory for single items, word pairs, and temporal order of different kinds in a patient with selective hippocampal lesions. Cogn Neuropsychol. 2001;18:97–123. doi: 10.1080/02643290125897. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppelman LF. Manual for the Profile of Mood States. San Diego, CA: Educational and Industrial Testing Service; 1981. [Google Scholar]
- Melrose RJ, Tinaz S, Castelo JM, et al. Compromised fronto-striatal functioning in HIV: an fMRI investigation of semantic event sequencing. Behav Brain Res. 2008;188:337–447. doi: 10.1016/j.bbr.2007.11.021. [DOI] [PubMed] [Google Scholar]
- Milner B, Petrides M, Smith ML. Frontal lobes and the temporal organization of memory. Hum Neurobiol. 1985;4:137–142. [PubMed] [Google Scholar]
- Moore DJ, Masliah E, Rippeth JD, et al. Cortical and subcortical neurodegeneration is associated with HIV neurocognitive impairment. AIDS. 2006;20:879–887. doi: 10.1097/01.aids.0000218552.69834.00. [DOI] [PubMed] [Google Scholar]
- Morgan EE, Woods SP, Smith C, et al. Lower cognitive reserve among individuals with syndromic HIV-associated neurocognitive disorders (HAND) AIDS Behav. 2012;16:2279–2285. doi: 10.1007/s10461-012-0229-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan EE, Woods SP, Weber E, et al. HIV-associated episodic memory impairment: evidence of a possible differential deficit in source memory for complex visual stimuli. J Neuropsychiatry Clin Neurosci. 2009;21:189–198. doi: 10.1176/appi.neuropsych.21.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovitch M. Memory and working-with-memory: a component process model based on modules and central systems. J Cogn Neurosci. 1992;4:257–267. doi: 10.1162/jocn.1992.4.3.257. [DOI] [PubMed] [Google Scholar]
- Murray ME, Senjem ML, Petersen RC, et al. Functional impact of white matter hyperintensities in cognitively normal elderly subjects. Arch Neurol. 2010;67:1379–1385. doi: 10.1001/archneurol.2010.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman MC, Allen JB, Kaszniak AW. Tasks for assessing memory for temporal order versus memory for items in aging. Aging Neuropsychol Cogn. 2001;8:72–78. [Google Scholar]
- Nicoll D, Pirogovsky E, Collazo A, et al. The effect of interference on temporal order memory in premanifest and manifest Huntington’s disease. J Huntingtons Dis. 2013;2:177–184. doi: 10.3233/JHD-130064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old SR, Naveh-Benjamin M. Memory for people and their actions: further evidence for an age-related associative deficit. Psychol Aging. 2008;23:467–472. doi: 10.1037/0882-7974.23.2.467. [DOI] [PubMed] [Google Scholar]
- Peavy G, Jacobs D, Salmon DP, et al. Verbal memory performance of patients with human immunodeficiency virus infection: evidence of subcortical dysfunction. J Clin Exp Neuropsychol. 1994;16:508–523. doi: 10.1080/01688639408402662. [DOI] [PubMed] [Google Scholar]
- Peters J, Daum I. Frontal but not parietal positivity during source recollection is sensitive to episodic content. Neurosci Lett. 2009;454:182–186. doi: 10.1016/j.neulet.2009.03.019. [DOI] [PubMed] [Google Scholar]
- Pirogovsky E, Goldstein J, Peavy G, et al. Temporal order memory deficits prior to clinical diagnosis in Huntington’s disease. J Int Neuropsychol Soc. 2009;15:662–670. doi: 10.1017/S1355617709990427. [DOI] [PubMed] [Google Scholar]
- Power C, Selnes OA, Grim JA, et al. HIV Dementia Scale: a rapid screening test. J AIDS. 1995;8:273–278. doi: 10.1097/00042560-199503010-00008. [DOI] [PubMed] [Google Scholar]
- Psychological Corporation. WAIS-III and WMS-III Technical Manual. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- Psychological Corporation. Manual for the Wechsler Test of Adult Reading (WTAR) San Antonio, TX: Psychological Corporation; 2001. [Google Scholar]
- Raz N, Ghisletta P, Rodrigue KM, et al. Trajectories of brain aging in middle-aged and older adults: regional and individual differences. Neuroimage. 2010;51:501–511. doi: 10.1016/j.neuroimage.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippeth JD, Heaton RK, Carey CL, et al. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. J Int Neuropsychol Soc. 2004;10:1–14. doi: 10.1017/S1355617704101021. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Penney AT, Iudicello JE, Riggs PK, et al. Co-morbidities in persons infected with HIV: increased burden with older age and negative effects on health-related quality of life. AIDS Patient Care STDS. 2013;27:5–16. doi: 10.1089/apc.2012.0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JB, Passingham RE. Working memory for location and time: activity in prefrontal area 46 relates to selection rather than maintenance in memory. Neuroimage. 2001;14:77–86. doi: 10.1006/nimg.2001.0784. [DOI] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Patterson KA, et al. Recency effects in HIV-associated dementia are characterized by deficient encoding. Neuropsychologia. 2006;44:1336–1343. doi: 10.1016/j.neuropsychologia.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Vigil O, et al. A neuropsychological investigation of multitasking in HIV infection: implications for everyday functioning. Neuropsychology. 2011a;25:511–519. doi: 10.1037/a0022491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Vigil O, et al. Script generation of activities of daily living in HIV-associated neurocognitive disorders. J Int Neuropsychol Soc. 2011b;17:740–745. doi: 10.1017/S135561771100052X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura AP. Memory and the prefrontal cortex. Ann N Y Acad Sci. 1995;769:151–159. doi: 10.1111/j.1749-6632.1995.tb38136.x. [DOI] [PubMed] [Google Scholar]
- Shimamura AP, Janowsky JS, Squire LS. Memory for the temporal order of events in patients with frontal lobe lesions and amnesic patients. Neuropsychologia. 1990;28:803–813. doi: 10.1016/0028-3932(90)90004-8. [DOI] [PubMed] [Google Scholar]
- Spiers HJ, Burgess N, Maguire EA, et al. Unilateral temporal lobectomy patients show lateralized topographical and episodic memory deficits in a virtual town. Brain. 2001;124:2476–2489. doi: 10.1093/brain/124.12.2476. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Thames AD, Kim MS, Becker BW, et al. Medication and finance management among HIV-infected adults: the impact of age and cognition. J Clin Exp Neuropsychol. 2011;33:200–209. doi: 10.1080/13803395.2010.499357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolentino JC, Pirogovsky E, Luu T, et al. The effect of interference on temporal order memory for random and fixed sequences in nondemented older adults. Learn Mem. 2012;19:251–255. doi: 10.1101/lm.026062.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. Episodic memory: from mind to brain. Annu Rev Psychol. 2002;53:1–25. doi: 10.1146/annurev.psych.53.100901.135114. [DOI] [PubMed] [Google Scholar]
- Turnock M, Becker S. A neural network model of hippocampal-striatal-prefrontal interactions in contextual conditioning. Brain Res. 2008;1202:87–98. doi: 10.1016/j.brainres.2007.06.078. [DOI] [PubMed] [Google Scholar]
- Ulbrich P, Churan J, Fink M, et al. Perception of temporal order: the effects of age, sex, and cognitive factors. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2009;16:183–202. doi: 10.1080/13825580802411758. [DOI] [PubMed] [Google Scholar]
- Valcour V, Shikuma C, Shiramizu B, et al. Higher frequency of dementia in older HIV-1 individuals: the Hawaii Aging with HIV-1 Cohort. Neurology. 2004;63:822–827. doi: 10.1212/01.wnl.0000134665.58343.8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- War Department Adjutant General’s Office. Army Individual Test Battery: Manual of Directions and Scoring. Washington, DC: War Department Adjutant General’s Office; 1944. [Google Scholar]
- Weber E, Woods SP, Delano-Wood L, et al. An examination of the age-prospective memory paradox in HIV-infected adults. J Clin Exp Neuropsychol. 2011;33:1108–1118. doi: 10.1080/13803395.2011.604027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Composite International Diagnostic Interview (CIDI, version 2.1) Geneva, Switzerland: 1998. [Google Scholar]
- Woods SP, Dawson MS, Weber E, et al. Timing is everything: antiretroviral nonadherence is associated with impairment in time-based prospective memory. J Int Neuropsychol Soc. 2009;15:42–52. doi: 10.1017/S1355617708090012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Dawson MS, Weber E, et al. The semantic relatedness of cue-intention pairings influences event-based prospective memory failures in older adults with HIV infection. J Clin Exp Neuropsychol. 2010;32:398–407. doi: 10.1080/13803390903130737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Morgan EE, Marquie-Beck J, et al. Markers of macrophage activation and axonal injury are associated with prospective memory in HIV-1 disease. Cogn Behav Neurol. 2006;19:217–221. doi: 10.1097/01.wnn.0000213916.10514.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Rippeth JD, Frol AB, et al. Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. J Clin Exp Neuropsychol. 2004;26:759–778. doi: 10.1080/13803390490509565. [DOI] [PubMed] [Google Scholar]
- Woods SP, Scott JC, Sires DA, et al. Action (verb) fluency: test-retest reliability, normative standards, and construct validity. J Int Neuropsychol Soc. 2005;11:408–415. [PubMed] [Google Scholar]
- Ziegler DA, Piguet O, Salat DH, et al. Cognition in healthy aging is related to regional white matter integrity, but not cortical thickness. Neurobiol Aging. 2010;31:1912–1926. doi: 10.1016/j.neurobiolaging.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.