Abstract
Alzheimer’s disease (AD) is a global public health threat that continues to rise as the proportion of the population over the age of 60 rapidly increases. Aging and dementia are both associated with cognitive decline and share some features in terms of structural and functional alterations in neural circuitry. In this review, we attempt to highlight the network perturbations that occur in “typical” aging and emphasize how they may differ from those that manifest in dementia. We focus in particular on neuroimaging studies of the medial temporal lobe (MTL) network, which is involved in episodic memory and is known to change both with age and with AD pathology. We propose a temporal model of structural and functional alterations in the MTL along the aging-dementia continuum. The earliest changes are synaptic in nature and are detectable in particularly vulnerable white matter pathways such as the perforant path. These are followed by structural degradation in the transentorhinal region and subsequently neurodegeneration of the hippocampus as a result of accumulating pathology as well as deafferentation from entorhinal input. We believe that testing this model explicitly is an important direction for future research, particularly in the context of biomarker discovery and clinical trial design.
Keywords: aging, mild cognitive impairment, Alzheimer’s disease, magnetic resonance imaging, diffusion tensor imaging, medial temporal lobe
1. Introduction
By 2050, almost a third of the world’s population will be 60 years or older. The population over 65 is projected to increase to 86.7 million by 2050 (U.S. Census Bureau, Population Estimates and Projections, 2004). In addition, the risk for developing Alzheimer’s disease (AD) increases with age, with people 85 and older facing the highest risk (Brookmeyer et al., 1998). An estimated 5.4 million Americans are living with AD today (Alzheimer’s Association, 2012). If there is no prevention or treatment discovered, the number of individuals with AD in 2050 could range between 11 and 16 million. Since age is the single most striking risk factor for AD, the rapid increase in the aging population significantly complicates this public health problem and places a strain on the nation’s health care system. Indeed, the costs of care for AD in 2012 alone are estimated at $200 billion (Alzheimer’s Association, 2012).
Although much of the research conducted on memory loss focuses on AD, aging in the absence of Alzheimer’s pathology is itself associated with neurocognitive decline. Differentiating the mechanisms for neural changes in successful and pathological aging is critical to accurate diagnosis and effective intervention. In order for this discrimination process to be relevant in the therapeutic arena, it must take place early enough before AD pathology has initiated irreversible neurodegenerative processes (i.e. frank cell loss). There is widespread agreement that intervention at this stage is too late, thus providing the impetus for early diagnosis based on biomarkers during the preclinical / asymptomatic stage (Sperling et al., 2011). During this stage, molecular and synaptic changes take place (thus giving rise to potential biomarker signatures that can be detected using physiological measures), however cognition remains largely intact.
Here, we review evidence from brain imaging studies that may be used to differentiate aging from AD and its prodromal phase, mild cognitive impairment (MCI). In particular, we focus on neural circuitry that is essential for the formation of new declarative memories, as memory is one of the first domains to show change in the course of AD and memory complaints with aging are fairly common. Although we restrict our review to studies of neural circuits in humans using brain imaging techniques such as structural magnetic resonance imaging (MRI), functional MRI (fMRI), and diffusion tensor imaging (DTI), it is worth noting that studies in aged animals and genetic models of AD have contributed much to our understanding of neural circuits underlying age and pathology-related changes in cognition (e.g., see recent reviews by Gallagher and Koh, 2011; Kitazawa et al., 2012).
2. Neuroimaging studies in cognitive aging
It is commonly known that many forms of memory decline with age. Older adults report memory complaints more frequently than problems with any other cognitive skill (Newson and Kemps, 2006). The medial temporal lobes (MTL), which include the hippocampus and surrounding cortices, play an essential role in declarative (fact and event) memory processing (Milner et al., 1998). Changes in this system with normal aging have been documented in animal studies (see Burke and Barnes, 2010 and Wilson et al., 2006 for reviews) and human brain imaging studies (Chee et al., 2006; Murty et al., 2008; Raz et al., 2005; Sperling, 2007). At a cognitive level, aging is associated with a decline in the ability to form new episodic memories (Craik and Simon, 1980; Hedden and Gabrieli, 2004; Hedden and Gabrieli, 2005; Small et al., 1999) spatial memory and navigation (Newman and Kaszniak, 2000), and contextual source memory (Henkel et al., 1998), all functions thought to be subserved by the MTL and the prefrontal cortex.
2.1. Structural Imaging
Structural neuroimaging methods measured across the lifespan have revealed many age-related changes in the brain. There is a decrease in total brain volume resultant from cortical thinning and gyral atrophy (Uylings and de Brabander, 2002). Specifically, the prefrontal cortex and the hippocampal formation display volume loss in advanced aging that significantly accelerates from normal aging to MCI to AD (Jack et al., 2000; Raz et al., 2005). Some MRI studies have also shown that the extent of hippocampal and entorhinal volume decline with increasing age predicted performance on memory tasks (Rodrigue and Raz, 2004; Rosen et al., 2003). Despite these studies, it is not clear whether any of these changes are actually the result of frank cell loss with age, or perhaps are secondary to synaptic and dendritic loss. Studies in aged rodents and non-human primates have reliably demonstrated the absence of frank cell loss in the hippocampus with age (Rasmussen et al., 1996; Rapp and Gallagher, 1996; Rapp et al., 2002) but regions in the prefrontal cortex are found to undergo cell loss (Peters et al., 1994; Smith et al., 2004; Stranahan et al., 2012).
2.2. Functional Imaging
Early positron emission tomography (PET) studies of the MTL demonstrated differential activation patterns between young and older adults on memory tasks. For example, Grady et al. (1995) and subsequently Cabeza et al. (1997) found that left prefrontal activity during memory encoding was decreased in older adults. Cabeza and colleagues additionally found a bilaterality in older adults (equivalent levels of activity in the right and left prefrontal cortex) that was not present in young adults (conceptualized in the Hemispheric Asymmetry Reduction in Older Adults - HAROLD model). Grady and colleagues also found that the correlation between hippocampal and prefrontal activity found in the young (.94) was remarkably diminished in the old (.02), suggesting that there is a decrease in connectivity between these regions with age.
Morcom et al. (2003) reported that both older and younger individuals showed more hippocampal activity for remembered items than forgotten items in a subsequent memory paradigm1, with younger adults showing more activity in the left inferior temporal cortex than older adults. Several other fMRI studies confirmed that activity in the hippocampus was similar in young and old individuals across a range of memory tasks (Duverne et al., 2008; Rand-Giovannetti et al., 2006; Sperling, 2007). This is in contrast to findings reported by Daselaar et al. (2003) who used a similar task to Morcom and colleagues, although remembered items were compared to baseline instead of forgotten items due to an inadequate number of stimuli. The authors found that high-performing older adults showed similar levels of hippocampal activity to young adults, whereas low-performing older adults showed less medial temporal lobe activation for remembered items. Another study by Gutchess et al. (2005) used an incidental picture-encoding task and observed that older adults had lower parahippocampal activation and higher left frontal activation for remembered items. Other studies have also suggested that there are reductions in activity in the hippocampus with aging (Cabeza et al., 2004; Daselaar et al., 2006; Dennis et al., 2008; Murty et al., 2008), coupled with an increase in compensatory activity in the left frontal cortex. The lack of consensus across fMRI studies could be due to the wide variability in tasks and statistical contrasts used to assess hippocampal function. It is also possible that age difference could derive from a reduced ability to use the correct strategy to resolve the memory task instead of a failure of neural circuitry (Dennis et al., 2007; Logan et al., 2002; Morcom et al., 2003; Reuter-Lorenz and Lustig, 2005). Rodent studies have shown increased variability in learning index in aged compared to young rodents (Gallagher et al., 2006), suggesting that the variability seen in fMRI studies of MTL function could be reflecting this variability in memory performance with age. The differential distribution of fMRI variability across young and aged groups further complicates fMRI group analyses and must be taken into consideration especially with small samples.
Functional MRI studies of the in older adults have some additional noteworthy limitations. First, aging is associated with changes in basal state factors such as cerebrovascular coupling and metabolic rate (Small et al., 2004; Ances et al., 2009; Fleischer et al., 2008) which can severely affect group comparisons and may even lead to misleading results. This issue is magnified when baseline tasks such as rest or fixation are used, as they involve hippocampal activity on par with mnemonic tasks (Stark and Squire, 2001). A potential solution to these problems is to compare groups on within-participant contrasts only. Let us assume for example that groups A and B were both administered two active conditions X and Y. The comparison of (XA – YA) vs. (XB –YB) should be relatively immune from basal effects unless one assumes a complex interaction between condition and basal state.
Yassa et al. (2010a) recently used this approach in a study of hippocampal memory in young and older adults. The investigators reported hyperactivity in the hippocampus, but it was limited to the dentate gyrus and CA3 subregions, and only in a specific memory contrast, which accounted for baseline metabolic factors. The extent of hyperactivity predicted the degree to which participants generated false alarms (judged items previously not seen as familiar), suggesting that hyperactivity is not compensatory but rather may be an index of network dysfunction. These results are also consistent with findings from Miller et al. (2008a) who demonstrated that low-performing older adults have greater hippocampal activation on a face-name paired associate learning task that did not manifest in high-performing older adults.
Overall, the results from functional MRI studies of the aging brain, and in particular as they pertain to episodic memory and the medial temporal lobe, have been mixed and studies have been plagued with many experimental and methodological confounds (for example, baseline metabolic factors, strategy use, the lack of behavioral effects, using blocked designs, and using whole-brain alignment techniques that miss hippocampal activity at a group level). The most recent work discussed above takes into consideration all of these issues and suggests a new perspective on age-related hippocampal alterations. The idea is that particular subregions of the hippocampus are vulnerable to age-related loss of inhibitory input, which has been shown in rodent studies (see Wilson et al. 2006 for review), and as a result show hyperactivation, the extent of which predicts memory deficits. This hyperactivation is even more dramatic in the context of mild cognitive impairment (MCI), which will be discussed later in the review. Hyperactivation in the dentate and CA3 regions of the hippocampus can be viewed as an index of network dysfunction that may predict later decline to MCI, although this hypothesis has not yet been directly tested.
In addition to task-activated fMRI, correlational approaches have also been applied to studies of the aging brain. These resting-state functional connectivity magnetic resonance imaging (rs-fcMRI) studies examine connectivity by analyzing spontaneous fluctuations in brain networks (Biswal et al., 1995; Fox and Raichle, 2007). Most analyses focus on the integrity of brain circuitry that is active during rest, otherwise known as the “default mode network (DMN)” (Gusnard and Raichle, 2001; Raichle et al., 2001) - a network that involves the medial prefrontal cortex (mPFC), posterior cingulate cortex (PCC), precuneus, anterior cingulate cortex (ACC), parietal cortex, and the medial temporal lobe, including the hippocampus (Greicius and Menon, 2004; Buckner et al., 2008). These regions are active during rest and are typically inactivated during task performance (Raichle et al., 2001).
The DMN is hypothesized to mediate task-independent “internal” thought rather than extrinsic stimulus processing. Individuals perform better on tasks involving extrinsic stimulus processing when more of the DMN regions are being suppressed. In the healthy brain, greater suppression of the DMN is associated with better memory formation (Daselaar et al., 2004; Daselaar et al., 2009). As task difficulty increases, DMN suppression increases (Singh and Fawcett, 2008; McKiernan et al., 2003), suggesting that attentional resources are diverted away from internal processing and toward more difficult task-related processing. In addition to DMN activation being associated with attention and memory processing task performance, many studies have attributed this network to self-referential processing (D'Argembeau et al., 2005; Gusnard et al., 2001; Kelley et al., 2002; Northoff and Bermpohl, 2004; Northoff et al., 2006; Whitfield-Gabrieli et al., 2011) and with remembering the past, planning one’s future, and forming beliefs (Buckner et al., 2008; Raichle and Snyder, 2007).
Studies of the DMN in older adults have generally shown a decrease in connectivity between the DMN regions compared to young adults. Specifically, the PCC, superior and middle frontal gyrus, and the superior parietal regions show reduced connectivity compared to young adults (Andrews-Hanna et al., 2007; Damoiseaux et al., 2008). A negative association between age and DMN connectivity has also been demonstrated (Biswal et al., 2010). The reduction in connectivity within the DMN may reflect a reduction in the ability to suspend DMN activity when more complex cognitive processes are required. There may be some difficulty in switching from a “default mode” to a task-related mode of brain function (Grady et al., 2006; 2010). Some studies in healthy older subjects have shown increased activity at rest, mainly in anterior brain areas such as the anterior cingulate (Davis et al., 2008; Grady et al., 2006; van den Heuvel and Hulshoff Pol, 2010), medial prefrontal, and superior frontal cortices (Lustig et al., 2003). This increased activity at rest in frontal DMN regions in elderly adults has been viewed to reflect a compensatory mechanism that may be compensating for the decrease of resting-state activity in posterior DMN areas (Mevel et al., 2011).
In addition to investigations of the DMN, some recent work has focused on the medial temporal lobe network using high-resolution functional MRI. One recent study (Yassa et al., 2011b) showed an age-related decrease in connectivity between the entorhinal cortex and the dentate and CA3 regions of the hippocampus, which are implicated in the hippocampus’ ability to support pattern separation (storing similar experiences as distinct memories using orthogonalized neural codes). This local connection can be thought of as a functional assay of the perforant path, a major input pathway to the hippocampus from the entorhinal cortex. The age-related decline was tightly coupled to levels of activity in the dentate and CA3 regions, behavioral performance on a task sensitive to pattern separation deficits, as well as structural integrity of the perforant path measured using diffusion tensor imaging (this method is discussed in detail in the next section). Future studies investigating both DMN circuitry and more fine-grained details of intra-network connectivity such as the MTL network will be required to further understand age-related brain dynamics. This step will require high-resolution sequences that are capable of querying individual subfields of the hippocampus as well as the entire brain, which is quite elusive given our current scanning capabilities.
To summarize, resting state fMRI studies, unlike task-activated fMRI studies, have generally provided a more consistent picture with most studies observing decreases in posterior DMN connectivity, increases in anterior DMN connectivity, overall loss of coherence in the DMN, and loss of intra-network connectivity in the medial temporal lobe, a critical component of the DMN, all of which have been associated with age-related memory deficits.
2.3. Diffusion Imaging
Diffusion tensor imaging (DTI) has been used to investigate the microstructural features of white matter (Taylor et al., 2004). A number of DTI studies have shown white matter loss with aging (see review by Chua et al., 2008), most likely due to thin myelinated fiber degeneration (Marner et al., 2003; Meier-Ruge et al., 1992; Sandell and Peters, 2003; Tang et al., 1997). The majority of DTI studies assess white matter integrity using voxel-wise values such as fractional anisotropy (FA). FA is a scalar quantity that measures the anisotropy (i.e. directionality) of the diffusion signal in any given voxel and is taken as an indirect proxy to white matter integrity. There are many factors that affect FA including axonal degeneration, demyelination, disorganization, packing density, and other microstructural features. Although the neural basis of FA is still not completely understood, it has been used as an index of white matter integrity in thousands of studies of the human and animal brain. Typically the higher the FA value, the more intact a fiber pathway is thought to be.
Stebbins et al. (2002) examined frontal lobe FA in selected regions-of-interest (corrected for atrophy) in ten younger and ten older right-handed healthy participants and found that frontal FA was significantly reduced in older compared to younger participants. Age-related FA reductions also seem to have a temporal pattern where they start in more anterior brain regions then proceed gradually to more posterior regions (Davis et al., 2008). In addition to white matter FA decline in aging, mean diffusivity (MD) increases (Chen et al., 2001; Engelter et al., 2000; Head et al., 2004; Helenius et al., 2002; Naganawa et al., 2003; Ota et al., 2006; Pfefferbaum et al., 2005; Pfefferbaum and Sullivan, 2003). MD is another scalar measure of overall diffusivity within any given voxel and tends to increase in regions where white matter is compromised.
A novel high-resolution DTI sequence capable of resolving fibers with sub-millimeter resolution was recently used to quantify the perforant path in young and older adults, with results demonstrating a significant decline in perforant path integrity with aging (Yassa et al., 2010a). Furthermore, the degree of such decline was negatively correlated with scores on tests of hippocampal function as well as functional activity in the dentate and CA3 regions of the hippocampus in the same individuals (Yassa et al., 2011a).
To summarize, diffusion imaging studies of the aging brain have generally noted a decrease in white matter integrity throughout the entire brain starting with more anterior (prefrontal) regions, as well as more specific degradations in pathways critical for episodic memory, such as the perforant path. Diffusion imaging, compared to other imaging modalities, however is still in its infancy and will likely make more significant contributions in the future as our techniques for high-resolution scanning and tractography improve.
2.4. Summary
Structural, functional, and diffusion imaging techniques have all been applied to the study of the aging human brain. Structurally the entire brain undergoes gray matter volume loss, with some regions such as the medial temporal lobe (particularly the entorhinal cortex and the hippocampus) and the prefrontal cortex being the most vulnerable. White matter structural loss (using DTI) has also been reported in the prefrontal cortex and in a specific hippocampal pathway, the perforant path, which conveys input from the entire brain to the hippocampus and is critical for episodic memory processing.
Functionally, there is a shift in brain dynamics where the following age-related features have been reported: (1) reduced laterality in the prefrontal cortex in episodic memory tasks, (2) increased activity in the dentate and CA3 regions of the hippocampus in low-performing older adults in episodic memory tasks sensitive to hippocampal pattern separation, (3) decreased entorhinal – hippocampal connectivity in low-performing older adults during rest, and (4) decreased DMN connectivity particularly in the posterior regions, e.g. posterior cingulate and precuneus. Many of these features have been linked to episodic memory deficits, thus they may be used to track individuals longitudinally to determine which of them may predict later decline. Since there is no way to exclude AD pathology in the aged brain (amyloid imaging is a step in the right direction, but is in no way diagnostic), some of these features may be specific to the aging brain and some may be precursors for Alzheimer’s disease. Longitudinal follow-up studies are essential to determine the specificity of these neurobiological alterations.
3. Neuroimaging studies in MCI and early AD
Episodic memory deficits have been documented as important and early-detected risk factors for AD (DeCarli et al., 2004; Grober et al., 2008; Grober et al., 2000; Kawas et al., 2003; Welsh et al., 1992). The hippocampus and entorhinal cortex are most affected in AD, with increasing atrophy in these structures from normal aging to MCI to AD (Du et al., 2001; Welsh-Bohmer et al., 2012). Frank neuronal loss in the hippocampus (CA1 field) and layer II of the entorhinal cortex has been observed in histological studies of Alzheimer’s disease (Gomez-Isla et al., 1996; Nestor et al., 2004; Price et al., 2001; West et al., 1994; 2004). Further, such work has even been able to show that cellular loss is correlated with the onset of disease symptoms (Price et al., 2001; West et al., 2004). In discussing neuroimaging findings, we will once again focus on the medial temporal lobe, since histological studies have all pointed to this network as the one where AD pathology first manifests. Also, we will specifically focus on the earliest stages of Alzheimer’s disease (i.e., mild cognitive impairment), which is where much of the diagnosis and intervention effort is focused.
3.1. Structural Imaging
Although dramatic neuronal loss is not observed in preclinical AD or MCI, several studies have shown mild hippocampal atrophy during these stages. Hippocampal atrophy has been linked to cognitive impairment suggestive of AD (Convit et al., 1993; 1995; Killiany et al., 1993). Several human structural MRI studies have used very-high-dimension transformation techniques to observe changes in the shape of the hippocampus associated with AD. Consistent with the histological data, changes in the area of the CA1 fields in the hippocampus have been reported (Csernansky et al., 2000; Csernansky et al., 2005; Wang et al., 2006). Notably, in one of these studies, the same region of CA1 identified as differing in shape between non-demented and mildly demented patients also varied in the non-demented patients as a function of whether or not they later converted to a CDR (Clinical Dementia Rating) of 0.5. More recent work by the same group suggests that surface deflections across all hippocampal subfields (CA1 lateral zone, dentate gyrus/CA2-4 superior zone, and subiculum inferior medial zone) differentiate non-demented controls from early AD patients (Qiu and Miller, 2008).
Recent high-resolution structural imaging studies in MCI patients where subfields of the hippocampus were manually segmented have suggested that specific subfields are more vulnerable than others. Yassa et al. (2010b) found that the CA1 and CA3/dentate gyrus regions both show volumetric loss, with left-lateralized changes in both subregions. The subiculum and other medial temporal regions were no different in MCI patients and controls. Similar techniques showed that the subiculum, CA1, and entorhinal cortex are further affected in AD (Mueller et al., 2010; Mueller and Weiner, 2009). Mueller and Weiner (2009) also found that ApoE4 status was associated with volumetric decline in the CA3/dentate subregions, suggesting that early risk for AD may selectively affect this region, consistent with the loss of synaptic input reported in animal studies.
Several reports based on the Alzheimer’s Disease Neuroimaging Initiative (ADNI) have strongly suggested that entorhinal cortical thickness declines early in MCI and continues to do so linearly as the disease progresses to AD. For example, a recent study by Desikan et al. (2010) found that entorhinal cortical thinning predicted later hippocampal decline in MCI and AD and that this MRI feature was linked to p-tau elevations found in cerebrospinal fluid (CSF) of the same sample, consistent with histopathological data. Another recent study by Ewers et al. (2012) suggested that the entorhinal cortex was one of the best predictors of MCI conversion to AD, even surpassing multimarker models.
Thus, results from structural MRI studies have generally shown that both the entorhinal cortex and the hippocampus show robust volumetric declines in MCI and AD (with the entorhinal change occurring earlier) and may be used as an early diagnostic feature, although functional changes may well precede structural changes in this network.
3.2. Functional Imaging
Several studies have used fMRI to examine functional changes in MCI and early AD. In one example (Dickerson et al., 2004), MCI patients were scanned during memory encoding. Consistent with studies of young, healthy individuals, the amount of activity in the medial temporal lobe during this encoding phase correlated with participants’ subsequent memory performance. Critically, the amount of activity in these regions also varied as a function of the patients’ CDR-SB (Clinical Dementia Rating – Sum of Boxes) scores. Further, the amount of activation in the parahippocampal gyrus varied as a function of whether the patients’ CDR-SB scores remained constant or declined over several years. Studies of novel encoding in mild AD patients have consistently found decreased fMRI activation in hippocampal and parahippocampal regions compared to healthy older adults (Rombouts et al., 2000; Small et al., 1999; Sperling et al., 2003). Several studies have also found evidence of increased activation in some neocortical regions in AD patients, which may be secondary or even compensatory to MTL network failure (Dickerson et al., 2005; Pariente et al., 2005).
Dickerson and colleagues (2005) found increased hippocampal activity during learning in individuals with MCI compared to normal controls and individuals with AD. Another study by the same group (Celone et al., 2006) using an independent component analysis found that less impaired MCI patients showed this increase, while more impaired MCI patients showed a decrease in activity similar to mild AD cases (Dickerson et al., 2005; Kato et al., 2001; Rombouts et al., 2000; Small et al., 1999; Sperling, 2007). Additional data from Miller et al. (2008b) shows that hippocampal activation at baseline predicts cognitive decline as measured by the CDR-SB scores over 4 years after scanning. This paradoxical “hyperactivity” seen in some MCI patients has also been noted in cognitively intact ApoE 4 carriers (Bookheimer et al., 2000) and in asymptomatic offspring of autopsy-confirmed AD patients (Bassett et al., 2006).
Recent work has shown that this hippocampal hyperactivity in MCI, similar to that present in low-performing older adults, is specific to the dentate and CA3 subregions of the hippocampus (Yassa et al., 2010b). This is consistent with studies in aged rodents, where the CA3 region demonstrates maladaptive hyperexcitability (see Wilson et al., 2006 for review). This elevation in activity can be targeted with inhibitory pharmacological manipulations (Koh et al., 2010) reversing memory deficits in treated animals. The remarkable consistency across animal and human studies here was used recently to leverage translational work in a clinical trial with a low-dose anti-epileptic (levetiracetam) in individuals with MCI. The trial reported positive results where levetiracetam successfully reduced hippocampal hyperactivity and reversed deficits on a test of hippocampal pattern separation (Bakker et al., 2012).
Thus, fMRI studies have generally shown hippocampal hyperactivation in MCI, likely secondary to loss of inhibitory tone as well as loss of perforant path input (see Wilson et al., 2006). As the disease progresses and hippocampal atrophy takes hold, studies have reported declines in hippocampal activity, which one would expect given the neurodegenerative process.
Resting-state functional connectivity between the hippocampus and the posterior cingulate cortex (a major part the default mode network) is disrupted with AD (Greicius and Menon, 2004). This finding is in agreement with PET studies of AD which report hypometabolism in the posterior cingulate (see review by Mosconi et al., 2005). Recently, amyloid imaging methods able to measure AD pathology have revealed another link to the default network: pathology preferentially accumulates in the DMN (regions typically forming well-connected and highly active hubs) even before symptoms emerge (Buckner et al., 2008). DMN abnormalities are typically found to increase with disease progression (Petrella et al., 2011; Sanz-Arigita et al., 2010; Supekar et al., 2008; Zhang et al., 2010; Zhou et al., 2010; Agosta et al., 2011). Brier et al. (2012) recently showed that AD is associated with widespread loss of both intra-network and inter-network correlations. A recent study of functional connectivity within the medial temporal lobe reported a relative disconnection of the MTL from other neocortical structures, but increased connectivity locally within the MTL in MCI patients (Das et al., 2012). This suggests that altered activity in components of the DMN may act as an early marker for AD pathology, although much work has yet to be done to understand the individual roles of each of the DMN sub-networks in disease pathophysiology.
3.3. Diffusion Imaging
DTI has recently been applied to the study of AD (see review by Chua et al., 2008). DTI studies of MCI and AD show widespread declines in white matter integrity throughout the brain with the most reliable changes reported in the temporal lobes (Bozzali et al., 2002; Chua et al., 2008; Huang and Auchus, 2007; Huang et al., 2007; Naggara et al., 2006; Xie et al., 2006). Investigations of white matter connectivity changes in aging and AD have focused on the fornix and the cingulum, as they are the major links between the limbic system and the rest of the brain. The fornix is the largest input/output fiber bundle of the hippocampus and connects it to the hypothalamus, while the cingulum connects the cingulate and the parahippocampal gyri to the septal cortex (Haines and Lancon, 2003). Damage to the fornix in animal studies has been found to reproduce learning and memory deficits resulting from hippocampal lesions (McDonald and White, 1993; Sutherland et al., 1982). DTI fiber tracking studies show reduced fractional anisotropy in the fornix in AD (Teipel et al., 2007). Several studies have found white matter changes in the cingulum in MCI and mild AD cases (Choo et al., 2010; Firbank et al., 2007; Villain et al., 2008). At least two studies have suggested a reduction in fractional anisotropy in the MTL in ApoE4 carriers (Nierenberg et al., 2005; Persson et al., 2006), suggesting that microscopic changes precede the onset of dementia and can be detected in the absence of any cognitive symptoms.
One critical pathway for episodic memory is the perforant path, discussed earlier in the sections on aging. A recent study by Kalus et al. (2006) found reductions in intervoxel coherence in the perforant path zone in MCI patients compared to controls, possibly indicating synaptic loss in the region. However, since there are many crossing fibers in the region, it was not possible to uniquely attribute these signal losses to the perforant path itself. In order to measure the integrity of the perforant path, a high-resolution sequence similar to the one used by Yassa et al. (2010a; 2011b) in older adults may be applied. Preliminary results from this method suggest that perforant path decline is much more dramatic in MCI and AD (Yassa and Stark, unpublished observations) compared to the loss exhibited in normal aging.
3.4. Summary
Structural, functional and diffusion imaging studies in MCI and early AD have shown volume changes and thinning in the entorhinal cortex and the hippocampus and demonstrated a link between these changes and disease status and progression. Functional MRI studies of episodic memory have generally shown hippocampal hyperactivity in the early stages followed by hypoactivity in the later stages of the disease. Resting state studies of the DMN suggest general disconnection among its components in MCI and furthermore in AD. DTI studies have reliably demonstrated loss of integrity in the fornix, the cingulum, and the perforant path, with the latter being one feature that is directly linked to hippocampal function and episodic memory performance.
4. Synthesis and Hypothetical Biomarker Model
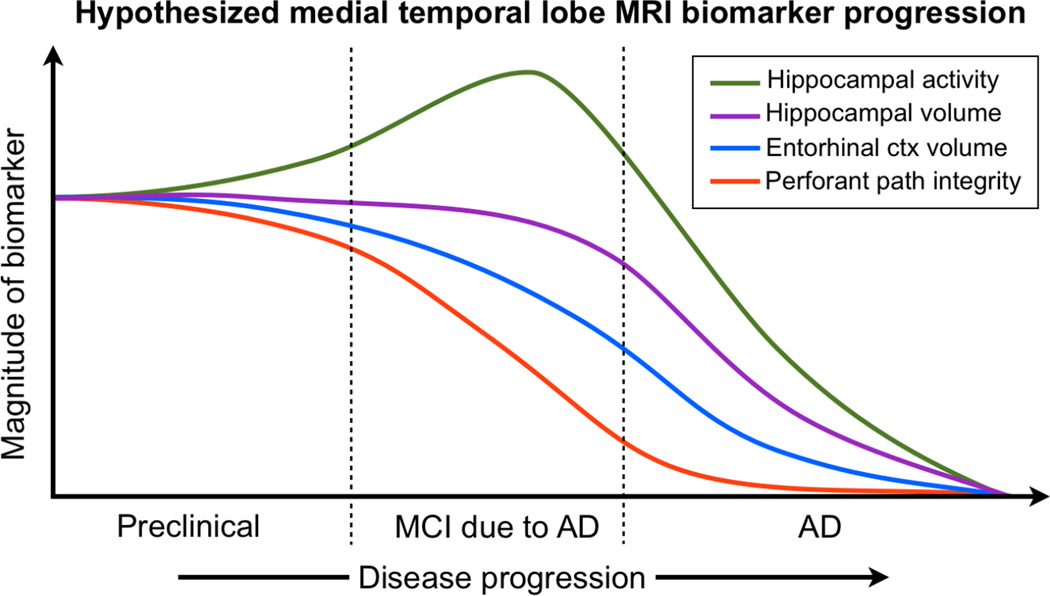
Based on a survey of the neuroimaging literature in aging, MCI, and AD, we propose a preliminary model to understanding medial temporal lobe structural and functional changes along the continuum from aging to MCI to AD. Many of the features in this model are on a quantitative, rather than a qualitative scale, suggesting that there are key features that change very early in the course of the disease and which can be targeted for diagnosis and intervention. We choose to focus on the medial temporal lobe here, as the picture in this region is far clearer than anywhere else in the brain, although much still remains to be accomplished. The hypothetical model is shown in Figure 1, and extends several models recently hypothesized in the literature (e.g., Sperling et al., 2011; Ewers et al., 2011) with more specific emphasis on neurobiological features in the MTL network. Our model specifically adds two features with their hypothesized temporal parameters, namely entorhinal cortical thinning and perforant path degeneration.
Figure 1.
Summary model for hypothesized changes in the MTL in the preclinical phase (asymptomatic), MCI, and AD. Functional activity in the hippocampus increases during the preclinical stage and continues to do so during the MCI stage, then declines towards the end of the MCI stage and more so in AD. The earliest structural decline occurs in the perforant path (measured using DTI), followed by entorhinal cortical thinning, followed by hippocampal volume loss, with each of these features independently predicting episodic memory deficits and disease decline. See text for discussion.
We suggest that during the preclinical stage, the biomarker signals undergoing the most change are hippocampal hyperactivity and perforant path loss, with the structural signals in the entorhinal cortex and the hippocampus being much more difficult to detect as frank neurodegeneration has not yet occurred. During the MCI stage, hippocampal hyperactivity reaches its peak, coupled with a considerable decline in the perforant path. Decline in this pathway is hypothesized to have retrograde effects (loss of entorhinal cortical neurons leading to cortical thinning) and anterograde effects (loss of neurons in the hippocampus leading to volume decline). Given converging reports from autopsy and MRI, we further suggest that the retrograde effect on the entorhinal cortex precedes and predicts the later change to the hippocampus. Since AD pathology tends to accumulate first in the transentorhinal region (Braak stage I), we suggest that the impact of synaptic loss in the perforant path may manifest first in the entorhinal cortex by interacting with hyperphosphorylated tau pathology in this region.
In MCI, the most sensitive biomarkers (those exhibiting the largest change) will vary as a function of how far along individuals are in the disease process. If individuals are earlier in that spectrum, then hippocampal hyperactivity and perforant path loss will be the most salient (with detectable but smaller changes in entorhinal cortical thickness and hippocampal volume). If individuals are farther along in that spectrum, hippocampal hyperactivity will have diminished, thus making this biomarker less useful. Using structural features such as perforant path integrity, entorhinal cortical thickness, and hippocampal volume as longitudinal markers of decline may be more robust against individual variations in progression. In AD, the perforant path is completely deteriorated, thus biomarker change here may not be salient; however, structural assays of neurodegeneration, i.e. hippocampal volume and entorhinal cortical thickness, will likely be the most salient. It is worthy of note that although hippocampal activity may also be a salient feature here (lower magnitude in AD), functional markers are most helpful in the absence of structural change (i.e. in the preclinical and early MCI phase).
Figure 1 represents our current understanding of how different regions of the medial temporal lobe change from healthy aging to MCI to AD, but it is important to note that it is a work in progress and much still remains to be understood. For example, the individual contributions of each of the hippocampal subfields is not entirely understood, although we suggest that the dentate and CA3 are especially vulnerable earlier on, with the vulnerability extending to the CA1 and subiculum later on in the disease.
Furthermore, other subregions of the medial temporal lobe such as the perirhinal cortex and the parahippocampal cortex have not received as much attention in the literature on neurocognitive aging and the pathophysiology of AD, although they are undoubtedly implicated by virtue of pathology accumulation and network connectivity. Finally, the relationship between these biomarkers and other indices of brain dysfunction is not well understood. Future studies focused on investigating connectivity changes within and across brain networks with focus on the medial temporal lobe and posterior parietal networks will significantly inform this understanding.
5. Conclusion
We conclude that although most of the perturbations in medial temporal network dynamics found in aging, MCI, and AD are a continuous spectrum and can be construed as varying quantitatively rather than qualitatively, pathology tends to accelerate the decline of certain features thus making them more detectable and perhaps diagnostic of the disease process. Understanding the nature of these pathological changes and how they affect network and circuit dynamics is critical to understanding the aging brain, differentiating healthy aging from dementia, and improving diagnostic and therapeutic tools for dementia.
Highlights.
-
-
Structural and functional changes in the medial temporal lobe occur in aging, mild cognitive impairment and Alzheimer’s disease.
-
-
Synaptic dysfunction precedes frank neurodegeneration and can be used as a sentinel to predict decline.
-
-
Changes in the medial temporal lobe follow a distinct temporal biomarker pattern, potentially informing clinical intervention trials.
Acknowledgements
M.Y. is supported by grants from the NIA P50 AG05146 and R01 AG034613. S.L. is supported by the Robert S. and Dorothy L. Waldrop Research Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The Subsequent Memory paradigm is a commonly used fMRI task where performance during retrieval is used to sort encoding trials into subsequently remembered (S-R) and subsequently forgotten (S-F). Much research using this task has shown hippocampal activity during the S-R trials compared to the S-F trials.
References
- Alzheimer's Association. Alzheimer's disease facts and figures. Alzheimer's and Dementia. 2012;8:131–168. doi: 10.1016/j.jalz.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Ances BM, Liang CL, Leontiev O, Perthen JE, Fleisher AS, Lansing AE, Buxton RB. Effects of aging on cerebral blood flow, oxygen metabolism, and blood oxygenation level dependent responses to visual stimulation. Human brain mapping. 2009;30:1120–1132. doi: 10.1002/hbm.20574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker A, Krauss GL, Albert MS, Speck CL, Jones LR, Stark CE, Yassa MA, Bassett SS, Shelton AL, Gallagher M. Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron. 2012;74:467–474. doi: 10.1016/j.neuron.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett SS, Yousem DM, Cristinzio C, Kusevic I, Yassa MA, Caffo BS, Zeger SL. Familial risk for Alzheimer's disease alters fMRI activation patterns. Brain. 2006;129:1229–1239. doi: 10.1093/brain/awl089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic resonance in medicine. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, Dogonowski AM, Ernst M, Fair D, Hampson M, Hoptman MJ, Hyde JS, Kiviniemi VJ, Kotter R, Li SJ, Lin CP, Lowe MJ, Mackay C, Madden DJ, Madsen KH, Margulies DS, Mayberg HS, McMahon K, Monk CS, Mostofsky SH, Nagel BJ, Pekar JJ, Peltier SJ, Petersen SE, Riedl V, Rombouts SA, Rypma B, Schlaggar BL, Schmidt S, Seidler RD, Siegle GJ, Sorg C, Teng GJ, Veijola J, Villringer A, Walter M, Wang L, Weng XC, Whitfield-Gabrieli S, Williamson P, Windischberger C, Zang YF, Zhang HY, Castellanos FX, Milham MP. Toward discovery science of human brain function. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, Small GW. Patterns of brain activation in people at risk for Alzheimer's disease. The New England journal of medicine. 2000;343:450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozzali M, Falini A, Franceschi M, Cercignani M, Zuffi M, Scotti G, Comi G, Filippi M. White matter damage in Alzheimer's disease assessed in vivo using diffusion tensor magnetic resonance imaging. Journal of neurology, neurosurgery, and psychiatry. 2002;72:742–746. doi: 10.1136/jnnp.72.6.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brier MR, Thomas JB, Snyder AZ, Benzinger TL, Zhang D, Raichle ME, Holtzman DM, Morris JC, Ances BM. Loss of intranetwork and internetwork resting state functional connections with Alzheimer's disease progression. The Journal of neuroscience. 2012;32:8890–8899. doi: 10.1523/JNEUROSCI.5698-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset. American journal of public health. 1998;88:1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Burke SN, Barnes CA. Senescent synapses and hippocampal circuit dynamics. Trends in neurosciences. 2010;33:153–161. doi: 10.1016/j.tins.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Grady CL, Nyberg L, McIntosh AR, Tulving E, Kapur S, Jennings JM, Houle S, Craik FI. Age-related differences in neural activity during memory encoding and retrieval: a positron emission tomography study. The Journal of neuroscience. 1997;17:391–400. doi: 10.1523/JNEUROSCI.17-01-00391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Prince SE, Daselaar SM, Greenberg DL, Budde M, Dolcos F, LaBar KS, Rubin DC. Brain activity during episodic retrieval of autobiographical and laboratory events: an fMRI study using a novel photo paradigm. Journal of cognitive neuroscience. 2004;16:1583–1594. doi: 10.1162/0898929042568578. [DOI] [PubMed] [Google Scholar]
- Celone KA, Calhoun VD, Dickerson BC, Atri A, Chua EF, Miller SL, DePeau K, Rentz DM, Selkoe DJ, Blacker D, Albert MS, Sperling RA. Alterations in memory networks in mild cognitive impairment and Alzheimer's disease: an independent component analysis. The Journal of neuroscience. 2006;26:10222–10231. doi: 10.1523/JNEUROSCI.2250-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee MW, Goh JO, Venkatraman V, Tan JC, Gutchess A, Sutton B, Hebrank A, Leshikar E, Park D. Age-related changes in object processing and contextual binding revealed using fMR adaptation. Journal of cognitive neuroscience. 2006;18:495–507. doi: 10.1162/jocn.2006.18.4.495. [DOI] [PubMed] [Google Scholar]
- Chen ZG, Li TQ, Hindmarsh T. Diffusion tensor trace mapping in normal adult brain using single-shot EPI technique. A methodological study of the aging brain. Acta radiologica. 2001;42:447–458. doi: 10.1080/028418501127347160. [DOI] [PubMed] [Google Scholar]
- Choo IH, Lee DY, Oh JS, Lee JS, Lee DS, Song IC, Youn JC, Kim SG, Kim KW, Jhoo JH, Woo JI. Posterior cingulate cortex atrophy and regional cingulum disruption in mild cognitive impairment and Alzheimer's disease. Neurobiology of aging. 2010;31:772–779. doi: 10.1016/j.neurobiolaging.2008.06.015. [DOI] [PubMed] [Google Scholar]
- Chua TC, Wen W, Slavin MJ, Sachdev PS. Diffusion tensor imaging in mild cognitive impairment and Alzheimer's disease: a review. Current opinion in neurology. 2008;21:83–92. doi: 10.1097/WCO.0b013e3282f4594b. [DOI] [PubMed] [Google Scholar]
- Convit A, de Leon MJ, Golomb J, George AE, Tarshish CY, Bobinski M, Tsui W, De Santi S, Wegiel J, Wisniewski H. Hippocampal atrophy in early Alzheimer's disease: anatomic specificity and validation. The Psychiatric quarterly. 1993;64:371–387. doi: 10.1007/BF01064929. [DOI] [PubMed] [Google Scholar]
- Convit A, de Leon MJ, Tarshish C, De Santi S, Kluger A, Rusinek H, George AE. Hippocampal volume losses in minimally impaired elderly. Lancet. 1995;345:266. doi: 10.1016/s0140-6736(95)90265-1. [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Wang L, Joshi S, Miller JP, Gado M, Kido D, McKeel D, Morris JC, Miller MI. Early DAT is distinguished from aging by high-dimensional mapping of the hippocampus. Neurology. 2000;55:1636–1643. doi: 10.1212/wnl.55.11.1636. [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Wang L, Swank J, Miller JP, Gado M, McKeel D, Miller MI, Morris JC. Preclinical detection of Alzheimer's disease: hippocampal shape and volume predict dementia onset in the elderly. NeuroImage. 2005;25:783–792. doi: 10.1016/j.neuroimage.2004.12.036. [DOI] [PubMed] [Google Scholar]
- D'Argembeau A, Collette F, Van der Linden M, Laureys S, Del Fiore G, Degueldre C, Luxen A, Salmon E. Self-referential reflective activity and its relationship with rest: a PET study. NeuroImage. 2005;25:616–624. doi: 10.1016/j.neuroimage.2004.11.048. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita EJ, Barkhof F, Scheltens P, Stam CJ, Smith SM, Rombouts SA. Reduced resting-state brain activity in the"default network" in normal aging. Cerebral cortex. 2008;18:1856–1864. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- Das SR, Pluta J, Mancuso L, Kliot D, Orozco S, Dickerson BC, Yushkevich PA, Wolk DA. Increased functional connectivity within medial temporal lobe in mild cognitive impairment. Hippocampus. 2012 doi: 10.1002/hipo.22051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Dobbins IG, Madden DJ, Cabeza R. Effects of healthy aging on hippocampal and rhinal memory functions: an event-related fMRI study. Cerebral cortex. 2006;16:1771–1782. doi: 10.1093/cercor/bhj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM, Prince SE, Cabeza R. When less means more: deactivations during encoding that predict subsequent memory. NeuroImage. 2004;23:921–927. doi: 10.1016/j.neuroimage.2004.07.031. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Prince SE, Dennis NA, Hayes SM, Kim H, Cabeza R. Posterior midline and ventral parietal activity is associated with retrieval success and encoding failure. Frontiers in human neuroscience. 2009;3:13. doi: 10.3389/neuro.09.013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM, Rombouts SA, Veltman DJ, Raaijmakers JG, Jonker C. Similar network activated by young and old adults during the acquisition of a motor sequence. Neurobiology of aging. 2003;24:1013–1019. doi: 10.1016/s0197-4580(03)00030-7. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA? The posterior-anterior shift in aging. Cerebral cortex. 2008;18:1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarli C, Mungas D, Harvey D, Reed B, Weiner M, Chui H, Jagust W. Memory impairment, but not cerebrovascular disease, predicts progression of MCI to dementia. Neurology. 2004;63:220–227. doi: 10.1212/01.wnl.0000130531.90205.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis NA, Kim H, Cabeza R. Effects of aging on true and false memory formation: an fMRI study. Neuropsychologia. 2007;45:3157–3166. doi: 10.1016/j.neuropsychologia.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Dennis NA, Kim H, Cabeza R. Age-related differences in brain activity during true and false memory retrieval. Journal of cognitive neuroscience. 2008;20:1390–1402. doi: 10.1162/jocn.2008.20096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Sabuncu MR, Schmansky NJ, Reuter M, Cabral HJ, Hess CP, Weiner MW, Biffi A, Anderson CD, Rosand J, Salat DH, Kemper TL, Dale AM, Sperling RA, Fischl B, Alzheimer's Disease Neuroimaging I. Selective disruption of the cerebral neocortex in Alzheimer's disease. PloS one. 2010;5:e12853. doi: 10.1371/journal.pone.0012853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Bates JF, Atiya M, Killiany RJ, Greve DN, Dale AM, Stern CE, Blacker D, Albert MS, Sperling RA. Medial temporal lobe function and structure in mild cognitive impairment. Annals of neurology. 2004;56:27–35. doi: 10.1002/ana.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Greve DN, Chua EF, Rand-Giovannetti E, Rentz DM, Bertram L, Mullin K, Tanzi RE, Blacker D, Albert MS, Sperling RA. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005;65:404–411. doi: 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duverne S, Habibi A, Rugg MD. Regional specificity of age effects on the neural correlates of episodic retrieval. Neurobiology of aging. 2008;29:1902–1916. doi: 10.1016/j.neurobiolaging.2007.04.022. [DOI] [PubMed] [Google Scholar]
- Engelter ST, Provenzale JM, Petrella JR, DeLong DM, MacFall JR. The effect of aging on the apparent diffusion coefficient of normal-appearing white matter. American journal of roentgenology. 2000;175:425–430. doi: 10.2214/ajr.175.2.1750425. [DOI] [PubMed] [Google Scholar]
- Ewers M, Sperling RA, Klunk WE, Weiner MW, Hampel H. Neuroimaging markers for the prediction and early diagnosis of Alzheimer's disease dementia. Trends Neurosci. 2011;34:430–442. doi: 10.1016/j.tins.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firbank MJ, Blamire AM, Krishnan MS, Teodorczuk A, English P, Gholkar A, Harrison R, O'Brien JT. Atrophy is associated with posterior cingulate white matter disruption in dementia with Lewy bodies and Alzheimer's disease. NeuroImage. 2007;36:1–7. doi: 10.1016/j.neuroimage.2007.02.027. [DOI] [PubMed] [Google Scholar]
- Fleisher AS, Podraza KM, Bangen KJ, Taylor C, Sherzai A, Sidhar K, Liu TT, Dale AM, Buxton RB. Cerebral perfusion and oxygenation differences in Alzheimer's disease risk. Neurobiology of aging. 2009;30:1737–1748. doi: 10.1016/j.neurobiolaging.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature reviews Neuroscience. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Colantuoni C, Eichenbaum H, Haberman RP, Rapp PR, Tanila H, Wilson IA. Individual differences in neurocognitive aging of the medial temporal lobe. Age. 2006;28:221–233. doi: 10.1007/s11357-006-9017-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, Koh MT. Episodic memory on the path to Alzheimer's disease. Current opinion in neurobiology. 2011;21:929–934. doi: 10.1016/j.conb.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Isla T, Price JL, McKeel DW, Jr, Morris JC, Growdon JH, Hyman BT. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer's disease. The Journal of neuroscience. 1996;16:4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Horwitz B, Maisog JM, Ungerleider LG, Mentis MJ, Pietrini P, Schapiro MB, Haxby JV. Age-related reductions in human recognition memory due to impaired encoding. Science. 1995;269:218–221. doi: 10.1126/science.7618082. [DOI] [PubMed] [Google Scholar]
- Grady CL, Protzner AB, Kovacevic N, Strother SC, Afshin-Pour B, Wojtowicz M, Anderson JA, Churchill N, McIntosh AR. A multivariate analysis of age-related differences in default mode and task-positive networks across multiple cognitive domains. Cerebral cortex. 2010;20:1432–1447. doi: 10.1093/cercor/bhp207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Springer MV, Hongwanishkul D, McIntosh AR, Winocur G. Age-related changes in brain activity across the adult lifespan. Journal of cognitive neuroscience. 2006;18:227–241. doi: 10.1162/089892906775783705. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Menon V. Default-mode activity during a passive sensory task: uncoupled from deactivation but impacting activation. Journal of cognitive neuroscience. 2004;16:1484–1492. doi: 10.1162/0898929042568532. [DOI] [PubMed] [Google Scholar]
- Grober E, Hall CB, Lipton RB, Zonderman AB, Resnick SM, Kawas C. Memory impairment, executive dysfunction, and intellectual decline in preclinical Alzheimer's disease. Journal of the International Neuropsychological Society. 2008;14:266–278. doi: 10.1017/S1355617708080302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grober E, Lipton RB, Hall C, Crystal H. Memory impairment on free and cued selective reminding predicts dementia. Neurology. 2000;54:827–832. doi: 10.1212/wnl.54.4.827. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nature reviews Neuroscience. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Welsh RC, Hedden T, Bangert A, Minear M, Liu LL, Park DC. Aging and the neural correlates of successful picture encoding: frontal activations compensate for decreased medial-temporal activity. Journal of cognitive neuroscience. 2005;17:84–96. doi: 10.1162/0898929052880048. [DOI] [PubMed] [Google Scholar]
- Head D, Buckner RL, Shimony JS, Williams LE, Akbudak E, Conturo TE, McAvoy M, Morris JC, Snyder AZ. Differential vulnerability of anterior white matter in nondemented aging with minimal acceleration in dementia of the Alzheimer type: evidence from diffusion tensor imaging. Cerebral cortex. 2004;14:410–423. doi: 10.1093/cercor/bhh003. [DOI] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JD. Insights into the ageing mind: a view from cognitive neuroscience. Nature reviews Neuroscience. 2004;5:87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JD. Healthy and pathological processes in adult development: new evidence from neuroimaging of the aging brain. Current opinion in neurology. 2005;18:740–747. doi: 10.1097/01.wco.0000189875.29852.48. [DOI] [PubMed] [Google Scholar]
- Helenius J, Soinne L, Perkio J, Salonen O, Kangasmaki A, Kaste M, Carano RA, Aronen HJ, Tatlisumak T. Diffusion-weighted MR imaging in normal human brains in various age groups. American journal of neuroradiology. 2002;23:194–199. [PMC free article] [PubMed] [Google Scholar]
- Henkel LA, Johnson MK, De Leonardis DM. Aging and source monitoring: cognitive processes and neuropsychological correlates. Journal of experimental psychology. General. 1998;127:251–268. doi: 10.1037//0096-3445.127.3.251. [DOI] [PubMed] [Google Scholar]
- Huang J, Auchus AP. Diffusion tensor imaging of normal appearing white matter and its correlation with cognitive functioning in mild cognitive impairment and Alzheimer's disease. Annals of the New York Academy of Sciences. 2007;1097:259–264. doi: 10.1196/annals.1379.021. [DOI] [PubMed] [Google Scholar]
- Huang J, Friedland RP, Auchus AP. Diffusion tensor imaging of normal-appearing white matter in mild cognitive impairment and early Alzheimer disease: preliminary evidence of axonal degeneration in the temporal lobe. American journal of neuroradiology. 2007;28:1943–1948. doi: 10.3174/ajnr.A0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Petersen RC, Xu Y, O'Brien PC, Smith GE, Ivnik RJ, Boeve BF, Tangalos EG, Kokmen E. Rates of hippocampal atrophy correlate with change in clinical status in aging and AD. Neurology. 2000;55:484–489. doi: 10.1212/wnl.55.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalus P, Slotboom J, Gallinat J, Mahlberg R, Cattapan-Ludewig K, Wiest R, Nyffeler T, Buri C, Federspiel A, Kunz D, Schroth G, Kiefer C. Examining the gateway to the limbic system with diffusion tensor imaging: the perforant pathway in dementia. NeuroImage. 2006;30:713–720. doi: 10.1016/j.neuroimage.2005.10.035. [DOI] [PubMed] [Google Scholar]
- Kato T, Knopman D, Liu H. Dissociation of regional activation in mild AD during visual encoding: a functional MRI study. Neurology. 2001;57:812–816. doi: 10.1212/wnl.57.5.812. [DOI] [PubMed] [Google Scholar]
- Kawas CH, Corrada MM, Brookmeyer R, Morrison A, Resnick SM, Zonderman AB, Arenberg D. Visual memory predicts Alzheimer's disease more than a decade before diagnosis. Neurology. 2003;60:1089–1093. doi: 10.1212/01.wnl.0000055813.36504.bf. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. Journal of cognitive neuroscience. 2002;14:785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Killiany RJ, Moss MB, Albert MS, Sandor T, Tieman J, Jolesz F. Temporal lobe regions on magnetic resonance imaging identify patients with early Alzheimer's disease. Archives of neurology. 1993;50:949–954. doi: 10.1001/archneur.1993.00540090052010. [DOI] [PubMed] [Google Scholar]
- Kitazawa M, Medeiros R, Laferla FM. Transgenic mouse models of Alzheimer disease: developing a better model as a tool for therapeutic interventions. Current pharmaceutical design. 2012;18:1131–1147. doi: 10.2174/138161212799315786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh MT, Haberman RP, Foti S, McCown TJ, Gallagher M. Treatment strategies targeting excess hippocampal activity benefit aged rats with cognitive impairment. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:1016–1025. doi: 10.1038/npp.2009.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL. Under-recruitment and nonselective recruitment: dissociable neural mechanisms associated with aging. Neuron. 2002;33:827–840. doi: 10.1016/s0896-6273(02)00612-8. [DOI] [PubMed] [Google Scholar]
- Lustig C, Snyder AZ, Bhakta M, O'Brien KC, McAvoy M, Raichle ME, Morris JC, Buckner RL. Functional deactivations: change with age and dementia of the Alzheimer type. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:14504–14509. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marner L, Nyengaard JR, Tang Y, Pakkenberg B. Marked loss of myelinated nerve fibers in the human brain with age. The Journal of comparative neurology. 2003;462:144–152. doi: 10.1002/cne.10714. [DOI] [PubMed] [Google Scholar]
- McDonald RJ, White NM. A triple dissociation of memory systems: hippocampus, amygdala, and dorsal striatum. Behavioral neuroscience. 1993;107:3–22. doi: 10.1037//0735-7044.107.1.3. [DOI] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. Journal of cognitive neuroscience. 2003;15:394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Meier-Ruge W, Ulrich J, Bruhlmann M, Meier E. Age-related white matter atrophy in the human brain. Annals of the New York Academy of Sciences. 1992;673:260–269. doi: 10.1111/j.1749-6632.1992.tb27462.x. [DOI] [PubMed] [Google Scholar]
- Mevel K, Chetelat G, Eustache F, Desgranges B. The default mode network in healthy aging and Alzheimer's disease. International journal of Alzheimer's disease. 2011;2011:535816. doi: 10.4061/2011/535816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SL, Celone K, DePeau K, Diamond E, Dickerson BC, Rentz D, Pihlajamaki M, Sperling RA. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proceedings of the National Academy of Sciences of the United States of America. 2008a;105:2181–2186. doi: 10.1073/pnas.0706818105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SL, Fenstermacher E, Bates J, Blacker D, Sperling RA, Dickerson BC. Hippocampal activation in adults with mild cognitive impairment predicts subsequent cognitive decline. J Neurol Neurosurg Psychiatry. 2008b;79:630–635. doi: 10.1136/jnnp.2007.124149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner B, Squire LR, Kandel ER. Cognitive neuroscience and the study of memory. Neuron. 1998;20:445–468. doi: 10.1016/s0896-6273(00)80987-3. [DOI] [PubMed] [Google Scholar]
- Morcom AM, Good CD, Frackowiak RS, Rugg MD. Age effects on the neural correlates of successful memory encoding. Brain. 2003;126:213–229. doi: 10.1093/brain/awg020. [DOI] [PubMed] [Google Scholar]
- Mosconi L, Tsui WH, De Santi S, Li J, Rusinek H, Convit A, Li Y, Boppana M, de Leon MJ. Reduced hippocampal metabolism in MCI and AD: automated FDG-PET image analysis. Neurology. 2005;64:1860–1867. doi: 10.1212/01.WNL.0000163856.13524.08. [DOI] [PubMed] [Google Scholar]
- Mueller SG, Schuff N, Yaffe K, Madison C, Miller B, Weiner MW. Hippocampal atrophy patterns in mild cognitive impairment and Alzheimer's disease. Human brain mapping. 2010;31:1339–1347. doi: 10.1002/hbm.20934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SG, Weiner MW. Selective effect of age Apo e4, and Alzheimer's disease on hippocampal subfields. Hippocampus. 2009;19:558–564. doi: 10.1002/hipo.20614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murty VP, Sambataro F, Das S, Tan HY, Callicott JH, Goldberg TE, Meyer-Lindenberg A, Weinberger DR, Mattay VS. Age-related alterations in simple declarative memory and the effect of negative stimulus valence. Journal of cognitive neuroscience. 2009;21:1920–1933. doi: 10.1162/jocn.2009.21130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naganawa S, Sato K, Katagiri T, Mimura T, Ishigaki T. Regional ADC values of the normal brain: differences due to age gender, and laterality. European radiology. 2003;13:6–11. doi: 10.1007/s00330-002-1549-1. [DOI] [PubMed] [Google Scholar]
- Naggara O, Oppenheim C, Rieu D, Raoux N, Rodrigo S, Dalla Barba G, Meder JF. Diffusion tensor imaging in early Alzheimer's disease. Psychiatry research. 2006;146:243–249. doi: 10.1016/j.pscychresns.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Nestor PJ, Scheltens P, Hodges JR. Advances in the early detection of Alzheimer's disease. Nature medicine. 2004;10:S34–S41. doi: 10.1038/nrn1433. [DOI] [PubMed] [Google Scholar]
- Newman MC, Kaszniak AW. Spatial memory and aging: Performance on a human analog of the Morris water maze. Aging Neuropsychol C. 2000;7:86–93. [Google Scholar]
- Newson RS, Kemps EB. The nature of subjective cognitive complaints of older adults. International journal of aging & human development. 2006;63:139–151. doi: 10.2190/1EAP-FE20-PDWY-M6P1. [DOI] [PubMed] [Google Scholar]
- Nierenberg J, Pomara N, Hoptman MJ, Sidtis JJ, Ardekani BA, Lim KO. Abnormal white matter integrity in healthy apolipoprotein E epsilon4 carriers. Neuroreport. 2005;16:1369–1372. doi: 10.1097/01.wnr.0000174058.49521.16. [DOI] [PubMed] [Google Scholar]
- Northoff G, Bermpohl F. Cortical midline structures and the self. Trends in cognitive sciences. 2004;8:102–107. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain--a meta-analysis of imaging studies on the self. NeuroImage. 2006;31:440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Ota M, Obata T, Akine Y, Ito H, Ikehira H, Asada T, Suhara T. Age-related degeneration of corpus callosum measured with diffusion tensor imaging. NeuroImage. 2006;31:1445–1452. doi: 10.1016/j.neuroimage.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Pariente J, Cole S, Henson R, Clare L, Kennedy A, Rossor M, Cipoloti L, Puel M, Demonet JF, Chollet F, Frackowiak RS. Alzheimer's patients engage an alternative network during a memory task. Annals of neurology. 2005;58:870–879. doi: 10.1002/ana.20653. [DOI] [PubMed] [Google Scholar]
- Persson J, Lind J, Larsson A, Ingvar M, Cruts M, Van Broeckhoven C, Adolfsson R, Nilsson LG, Nyberg L. Altered brain white matter integrity In healthy carriers of the APOE epsilon4 allele: a risk for AD? Neurology. 2006;66:1029–1033. doi: 10.1212/01.wnl.0000204180.25361.48. [DOI] [PubMed] [Google Scholar]
- Peters A, Leahu D, Moss MB, McNally KJ. The effects of aging on area 46 of the frontal cortex of the rhesus monkey. Cerebral cortex. 1994;4:621–635. doi: 10.1093/cercor/4.6.621. [DOI] [PubMed] [Google Scholar]
- Petrella JR, Sheldon FC, Prince SE, Calhoun VD, Doraiswamy PM. Default mode network connectivity in stable vs progressive mild cognitive impairment. Neurology. 2011;76:511–517. doi: 10.1212/WNL.0b013e31820af94e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Frontal circuitry degradation marks healthy adult aging: Evidence from diffusion tensor imaging. NeuroImage. 2005;26:891–899. doi: 10.1016/j.neuroimage.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV. Increased brain white matter diffusivity in normal adult aging: relationship to anisotropy and partial voluming. Magnetic resonance in medicine. 2003;49:953–961. doi: 10.1002/mrm.10452. [DOI] [PubMed] [Google Scholar]
- Price JL, Ko AI, Wade MJ, Tsou SK, McKeel DW, Morris JC. Neuron number in the entorhinal cortex and CA1 in preclinical Alzheimer disease. Archives of neurology. 2001;58:1395–1402. doi: 10.1001/archneur.58.9.1395. [DOI] [PubMed] [Google Scholar]
- Qiu A, Miller MI. Multi-structure network shape analysis via normal surface momentum maps. NeuroImage. 2008;42:1430–1438. doi: 10.1016/j.neuroimage.2008.04.257. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. NeuroImage. 2007;37:1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- Rand-Giovannetti E, Chua EF, Driscoll AE, Schacter DL, Albert MS, Sperling RA. Hippocampal and neocortical activation during repetitive encoding in older persons. Neurobiology of aging. 2006;27:173–182. doi: 10.1016/j.neurobiolaging.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Deroche PS, Mao Y, Burwell RD. Neuron number in the parahippocampal region is preserved in aged rats with spatial learning deficits. Cerebral cortex. 2002;12:1171–1179. doi: 10.1093/cercor/12.11.1171. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Gallagher M. Preserved neuron number in the hippocampus of aged rats with spatial learning deficits. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:9926–9930. doi: 10.1073/pnas.93.18.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen T, Schliemann T, Sorensen JC, Zimmer J, West MJ. Memory impaired aged rats: no loss of principal hippocampal and subicular neurons. Neurobiology of aging. 1996;17:143–147. doi: 10.1016/0197-4580(95)02032-2. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cerebral cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Lustig C. Brain aging: reorganizing discoveries about the aging mind. Current opinion in neurobiology. 2005;15:245–251. doi: 10.1016/j.conb.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Rodrigue KM, Raz N. Shrinkage of the entorhinal cortex over five years predicts memory performance in healthy adults. The Journal of neuroscience. 2004;24:956–963. doi: 10.1523/JNEUROSCI.4166-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombouts SA, Barkhof F, Veltman DJ, Machielsen WC, Witter MP, Bierlaagh MA, Lazeron RH, Valk J, Scheltens P. Functional MR imaging in Alzheimer's disease during memory encoding. AJNR. American journal of neuroradiology. 2000;21:1869–1875. [PMC free article] [PubMed] [Google Scholar]
- Rosen AC, Prull MW, Gabrieli JD, Stoub T, O'Hara R, Friedman L, Yesavage JA, deToledo-Morrell L. Differential associations between entorhinal and hippocampal volumes and memory performance in older adults. Behavioral neuroscience. 2003;117:1150–1160. doi: 10.1037/0735-7044.117.6.1150. [DOI] [PubMed] [Google Scholar]
- Sandell JH, Peters A. Disrupted myelin and axon loss in the anterior commissure of the aged rhesus monkey. The Journal of comparative neurology. 2003;466:14–30. doi: 10.1002/cne.10859. [DOI] [PubMed] [Google Scholar]
- Sanz-Arigita EJ, Schoonheim MM, Damoiseaux JS, Rombouts SA, Maris E, Barkhof F, Scheltens P, Stam CJ. Loss of 'small-world' networks in Alzheimer's disease: graph analysis of FMRI resting-state functional connectivity. PloS one. 2010;5:e13788. doi: 10.1371/journal.pone.0013788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh KD, Fawcett IP. Transient and linearly graded deactivation of the human default-mode network by a visual detection task. NeuroImage. 2008;41:100–112. doi: 10.1016/j.neuroimage.2008.01.051. [DOI] [PubMed] [Google Scholar]
- Small SA, Chawla MK, Buonocore M, Rapp PR, Barnes CA. Imaging correlates of brain function in monkeys and rats isolates a hippocampal subregion differentially vulnerable to aging. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:7181–7186. doi: 10.1073/pnas.0400285101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small SA, Stern Y, Tang M, Mayeux R. Selective decline in memory function among healthy elderly. Neurology. 1999;52:1392–1396. doi: 10.1212/wnl.52.7.1392. [DOI] [PubMed] [Google Scholar]
- Smith DE, Rapp PR, McKay HM, Roberts JA, Tuszynski MH. Memory impairment in aged primates is associated with focal death of cortical neurons and atrophy of subcortical neurons. The Journal of neuroscience. 2004;24:4373–4381. doi: 10.1523/JNEUROSCI.4289-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R. Functional MRI studies of associative encoding in normal aging, mild cognitive impairment, and Alzheimer's disease. Annals of the New York Academy of Sciences. 2007;1097:146–155. doi: 10.1196/annals.1379.009. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Jr, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & dementia. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Bates JF, Chua EF, Cocchiarella AJ, Rentz DM, Rosen BR, Schacter DL, Albert MS. fMRI studies of associative encoding in young and elderly controls and mild Alzheimer's disease. Journal of neurology, neurosurgery, and psychiatry. 2003;74:44–50. doi: 10.1136/jnnp.74.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark CE, Squire LR. When zero is not zero: the problem of ambiguous baseline conditions in fMRI. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:12760–12766. doi: 10.1073/pnas.221462998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Jiam NT, Spiegel AM, Gallagher M. Aging reduces total neuron number in the dorsal component of the rodent prefrontal cortex. The Journal of comparative neurology. 2012;520:1318–1326. doi: 10.1002/cne.22790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, Menon V, Rubin D, Musen M, Greicius MD. Network analysis of intrinsic functional brain connectivity in Alzheimer's disease. PLoS computational biology. 2008;4:e1000100. doi: 10.1371/journal.pcbi.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland RJ, Kolb B, Whishaw IQ. Spatial mapping: definitive disruption by hippocampal or medial frontal cortical damage in the rat. Neuroscience letters. 1982;31:271–276. doi: 10.1016/0304-3940(82)90032-5. [DOI] [PubMed] [Google Scholar]
- Tang Y, Nyengaard JR, Pakkenberg B, Gundersen HJ. Age-induced white matter changes in the human brain: a stereological investigation. Neurobiology of aging. 1997;18:609–615. doi: 10.1016/s0197-4580(97)00155-3. [DOI] [PubMed] [Google Scholar]
- Taylor WD, Hsu E, Krishnan KR, MacFall JR. Diffusion tensor imaging: background, potential, and utility in psychiatric research. Biological psychiatry. 2004;55:201–207. doi: 10.1016/j.biopsych.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Teipel SJ, Stahl R, Dietrich O, Schoenberg SO, Perneczky R, Bokde AL, Reiser MF, Moller HJ, Hampel H. Multivariate network analysis of fiber tract integrity in Alzheimer's disease. NeuroImage. 2007;34:985–995. doi: 10.1016/j.neuroimage.2006.07.047. [DOI] [PubMed] [Google Scholar]
- Uylings HB, de Brabander JM. Neuronal changes in normal human aging and Alzheimer's disease. Brain and cognition. 2002;49:268–276. doi: 10.1006/brcg.2001.1500. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: a review on resting-state fMRI functional connectivity. European neuropsychopharmacology. 2010;20:519–534. doi: 10.1016/j.euroneuro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Villain N, Desgranges B, Viader F, de la Sayette V, Mezenge F, Landeau B, Baron JC, Eustache F, Chetelat G. Relationships between hippocampal atrophy, white matter disruption, and gray matter hypometabolism in Alzheimer's disease. The Journal of neuroscience. 2008;28:6174–6181. doi: 10.1523/JNEUROSCI.1392-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Miller JP, Gado MH, McKeel DW, Rothermich M, Miller MI, Morris JC, Csernansky JG. Abnormalities of hippocampal surface structure in very mild dementia of the Alzheimer type. NeuroImage. 2006;30:52–60. doi: 10.1016/j.neuroimage.2005.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh KA, Butters N, Hughes JP, Mohs RC, Heyman A. Detection and staging of dementia in Alzheimer's disease. Use of the neuropsychological measures developed for the Consortium to Establish a Registry for Alzheimer's Disease. Archives of neurology. 1992;49:448–452. doi: 10.1001/archneur.1992.00530290030008. [DOI] [PubMed] [Google Scholar]
- West MJ, Coleman PD, Flood DG, Troncoso JC. Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer's disease. Lancet. 1994;344:769–772. doi: 10.1016/s0140-6736(94)92338-8. [DOI] [PubMed] [Google Scholar]
- West MJ, Kawas CH, Stewart WF, Rudow GL, Troncoso JC. Hippocampal neurons in pre-clinical Alzheimer's disease. Neurobiology of aging. 2004;25:1205–1212. doi: 10.1016/j.neurobiolaging.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Moran JM, Nieto-Castanon A, Triantafyllou C, Saxe R, Gabrieli JD. Associations and dissociations between default and self-reference networks in the human brain. NeuroImage. 2011;55:225–232. doi: 10.1016/j.neuroimage.2010.11.048. [DOI] [PubMed] [Google Scholar]
- Wilson IA, Gallagher M, Eichenbaum H, Tanila H. Neurocognitive aging: prior memories hinder new hippocampal encoding. Trends in neurosciences. 2006;29:662–670. doi: 10.1016/j.tins.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie S, Xiao JX, Gong GL, Zang YF, Wang YH, Wu HK, Jiang XX. Voxel-based detection of white matter abnormalities in mild Alzheimer disease. Neurology. 2006;66:1845–1849. doi: 10.1212/01.wnl.0000219625.77625.aa. [DOI] [PubMed] [Google Scholar]
- Yassa MA, Lacy JW, Stark SM, Albert MS, Gallagher M, Stark CE. Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus. 2011a;21:968–979. doi: 10.1002/hipo.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Mattfeld AT, Stark SM, Stark CE. Age-related memory deficits linked to circuit-specific disruptions in the hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2011b;108:8873–8878. doi: 10.1073/pnas.1101567108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Muftuler LT, Stark CE. Ultrahigh-resolution microstructural diffusion tensor imaging reveals perforant path degradation in aged humans in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2010a;107:12687–12691. doi: 10.1073/pnas.1002113107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark SM, Bakker A, Albert MS, Gallagher M, Stark CE. High-resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic Mild Cognitive Impairment. NeuroImage. 2010b;51:1242–1252. doi: 10.1016/j.neuroimage.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Lu G, Zhong Y, Tan Q, Liao W, Wang Z, Wang Z, Li K, Chen H, Liu Y. Altered spontaneous neuronal activity of the default-mode network in mesial temporal lobe epilepsy. Brain research. 2010;1323:152–160. doi: 10.1016/j.brainres.2010.01.042. [DOI] [PubMed] [Google Scholar]