Abstract
Rat fetal spinal cord (FSC) tissue, naturally enriched with interneuronal progenitors, was introduced into high cervical, hemi-resection (Hx) lesions. Electrophysiological analyses were conducted to determine if such grafts exhibit physiologically-patterned neuronal activity and if stimuli which increase respiratory motor output also alter donor neuron bursting. Three months following transplantation, the bursting activity of FSC neurons and the contralateral phrenic nerve were recorded in anesthetized rats during a normoxic baseline period and brief respiratory challenges. Spontaneous neuronal activity was detected in 80 % of the FSC transplants, and autocorrelation of action potential spikes revealed distinct correlogram peaks in 87% of neurons. At baseline, the average discharge frequency of graft neurons was 13.0 ± 1.7 Hz, and discharge frequency increased during a hypoxic respiratory challenge (p < 0.001). Parallel studies in unanesthetized rats showed that FSC tissue recipients had larger inspiratory tidal volumes during brief hypoxic exposures (p < 0.05 vs. C2Hx rats). Anatomical connectivity was explored in additional graft recipients by injecting a transynaptic retrograde viral tracer (pseudorabies virus, PRV) directly into matured transplants. Neuronal labeling occurred throughout graft tissues and also in the host spinal cord and brainstem nuclei, including those associated with respiratory control. These results underscore the neuroplastic potential of host-graft interactions and training approaches to enhance functional integration within targeted spinal circuitry.
Keywords: fetal spinal cord, transplantation, spinal cord repair, cervical spinal cord injury, hypoxia, respiratory recovery
Introduction
A longstanding focus of spinal cord repair has centered on cell-based approaches to promote axonal growth and restored neural communication across sites of injury (Reier, 2004). However, neuroanatomical and electrophysiological findings have indicated that intraspinal transplantation of neuronal progenitors also can promote functional changes (Mitsui et al., 2005) such as by serving as novel neuronal relays (Jakeman and Reier, 1991; Reier, 1985; Reier et al., 1986; White et al., 2010). Neuronal progenitor-enriched tissue repair strategies also have the potential to reconstruct disrupted spinal circuitries (i.e., “gray matter repair”) (Bonner et al., 2010; Ketschek et al., 2012; Reier et al., 2002) either alone or in combination with other treatments (Hooshmand et al., 2009; Kim et al., 2006; Nikulina et al., 2004).
The efficacy of a neuronal progenitor-based therapeutic approach is dependent on many variables (Bonner et al., 2011; Reier et al., 2002). Whether specific neuronal precursors will be required or if donor neuronal activity can be entrained within a defined spinal circuit remains unclear. Being able to effectively entrain donor neuron activity and possibly evoke activity-dependent plasticity within donor cells could be advantageous for achieving optimal graft-mediated recoveries.
While there are many examples of activity induced plasticity within naïve or injured spinal circuitries (Baker-Herman et al., 2004; Fuller et al., 2001; Golder and Mitchell, 2005; Trumbower et al., 2012), to date there has been no attempt to determine if studies of activity-dependent plasticity would be feasible in neuronally-enriched intraspinal grafts. In the present study, transplants of FSC tissue were made into lesions immediately rostral to the phrenic motor nucleus (i.e., at C2). Since many cells in that circuit fire rhythmically in phase with the respiratory cycle (Lee and Fuller, 2011), we thus assessed whether matured, neuronally-enriched grafts acquired site-specific, physiologically-patterned bursting activity. To determine if studies of activity-dependent plasticity would be feasible in this model, the response of donor neurons to elevated CO2 (i.e., hypercapnia) or reductions in O2 (i.e., hypoxia) were evaluated. Both stimuli are known to increase respiratory-related output of the host phrenic motor system (Fuller et al., 2003; Lee et al., 2009; Vinit et al., 2009).
Materials and Methods
Animals
All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Florida. Adult (N = 27) Sprague-Dawley rats purchased from Harlan Inc. (Indianapolis, IN, USA) were used to investigate the bursting patterns of neurons in mature FSC grafts under baseline and respiratory challenge conditions, and also the impact of the FSC grafts on the pattern of breathing in unanesthetized rats. The experimental animals were divided into three groups: control, uninjured (N = 7), C2 hemisection (Hx) (N = 9), or C2Hx followed by immediate transplantation of embryonic day 14/15 FSC tissue (C2Hx+FSC, N = 11). An additional group of C2Hx+FSC rats was used exclusively for anatomical tracing studies (N = 6).
Spinal cord injury and FSC transplantation
Rats were injured at age 3 month as previously described (Lane et al., 2008; Lee et al., 2010). Briefly, the animals were anesthetized by injection of xylazine (10 mg/kg, s.c.) and ketamine (140 mg/kg, i.p., Fort Dodge Animal Health, IA, USA). A dorsal cervical incision was made from base of the skull to the C3 segment followed by C2 laminectomy and left C2 dura section. A left C2Hx was performed by microscalpel incision and aspiration. In a subset of rats FSC was transplanted into the lesion cavity as previously described by our group (Jakeman and Reier, 1991; White et al., 2010). In brief, pregnant Sprague-Dawley rats at 14 days of gestation (E13.5–E14) were anesthetized by xylazine (10 mg/kg, s.c.) and ketamine (140 mg/kg, i.p.). Following a laparotomy, embryos were removed from the placenta and placed in Hank’s balanced salt solution (Mediatech, Inc., Manassas, VA, USA). FSC tissue was then removed and cut into ~1–2 mm tissue blocks) which were introduced into the lesion cavity until filling was achieved. The dura and overlying muscles were sutured with 9-0 (Ethicon, NJ, USA) and 4-0 polyglycolic acid sutures (Webster Veterinary, MA, USA), respectively. The skin was closed with stainless steel wound clips (Stoelting, IL, USA), and animals received an injection of yohimbine (1.2 mg/kg, s.c., Lloyd, IA, USA) to reverse the effect of xylazine. Following surgery, animals were given an analgesic (buprenorphine, 0.03 mg/kg, s.c., Hospira, IL, USA) and sterile lactated Ringers solution (5 ml s.c.). The post-surgical care protocol included injection of lactated Ringers solution (5 ml/day, s.c.) and oral supply of Nutrical supplements (1–3 ml, Webster Veterinary, MA, USA), given until adequate volitional drinking and eating resumed.
Plethysmography
Tidal volume, minute ventilation and breathing frequency were quantified in unanesthetized animals at both 5 and 10 weeks post-injury using whole body plethysmography (Buxco Inc., Wilmington, NC, USA). The plethysmograph system was calibrated using standard procedures as previously described (Fuller et al., 2008). Animals were placed in Plexiglas chambers for a 60–90 min baseline period while the chamber was flushed with a normoxic gas mixture (21% O2, balance N2). The animals were then exposed to a 5 min hypoxic challenge during which the chamber was flushed with 10% O2 (balance N2).
Neurophysiology
Between 11–13 weeks post-injury, rats were anesthetized with urethane (1.6 g/kg, i.p.) and prepared for terminal neurophysiological recordings, as described previously (Lee and Fuller, 2010; Lee et al., 2009; Lee et al., 2010; Sandhu et al., 2010). Intubation was via an endotracheal tube below the larynx. Cannulae were inserted into the femoral artery and vein for blood pressure measurement and drug administration, respectively. The rectal temperature was monitored by an electrical thermometer and maintained at 37.5 ± 1 °C by a servo-controlled heating pad (model TC-1000, CWE Inc., Ardmore, PA, USA). The animal was then placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA, USA) to stabilize the spinal cord, paralyzed with pancuronium bromide (2.5 mg/kg, i.v., Hospira, Inc., Lake Forest, IL, USA) and mechanically ventilated (Model 683; Harvard Apparatus, Inc, South Natick, MA, USA) with an oxygen/nitrogen mixture throughout the experimental procedure (50–60 % O2, balance N2, volume = 7 ml/kg; frequency = 60–65/min). The partial pressure of end-tidal CO2 (PETCO2) was analyzed with a Capnogard neonatal CO2 monitor placed on the expiratory line of the ventilator circuit (Novametrix Medical Systems, Wallingford, CT, USA) and maintained at 45–50 mmHg by adjusting inspired CO2. This value was selected to ensure a robust inspiratory signal in the phrenic nerve recording.
The right phrenic nerve (i.e. contralateral to the C2Hx lesion) was exposed using a dorsal approach. The contralateral nerve was selected for recording since it provides a very robust host respiratory pattern after C2Hx (Doperalski and Fuller, 2006; Lee et al., 2010). The isolated nerve was sectioned distally and placed over monopolar hook silver electrodes. Recordings were amplified (1000x, Model 1700, A–M Systems, Carlsborg, WA, USA), band-pass filtered (0.3 – 10 kHz) and integrated (time constant 100 ms; model MA-1000; CWE Inc., Ardmore, PA, USA). The raw and integrated neural signals were digitized by the CED Power 1401 data acquisition interface and recorded on a PC using Spike2 software (Cambridge Electronic Design Limited, Cambridge, England).
For FSC graft recordings, the C1–C3 vertebrate bone and dura were removed to re-expose the transplant site. Single (0.4–0.8 MΩ, carbostar-3, Kation Scientific, MN, USA)(N=9) or array electrode (20 kΩ, Tucker Davis Technologies, FL, USA)(N=2) with sixteen 50 μm tungsten electrodes with 250 × 500 μm column spacing were mounted on the microelectrode holder (David Kopf Instruments, Tujunga, CA, USA) and inserted into the graft. Neural signals from the single electrode were amplified (10,000x, ExAmp Extracellular amplifier, Kation Scientific) and digitized by the CED Power 1401 and recorded on a PC as well as phrenic nerve signals. Neural signals recorded from multi-electrode array were amplified (5,000x), band-pass filtered (0.5–6 kHz), and recorded with hardware (sampled at 24414.06 Hz) and software from Tucker Davis Technologies (Alachua, FL, USA). After stable recording of graft neurons during the baseline condition (50–60% O2, balance N2; PETCO2 = 45–50 mmHg), animals were exposed to 3 min period of hypoxic (13–15% O2, balance N2) and/or hypercapnic (PETCO2 = 70–80 mmHg) gas.
Histological Evaluation
At the end of experiments, each animal was intracardially perfused with paraformaldehyde [4% w/v in 0.1M phosphate buffered saline (PBS), pH 7.4]. The spinal cord was then removed from the vertebral column and stored in paraformaldehyde (2% w/v in PBS) for histological processing. Cervical spinal cord tissue was processed for vibratome (40 μm) sectioning (described in detail in Lane et al., 2008). Serotonin immunohistochemistry was used to examine the distribution of serotonergic fibers. Longitudinal sections of cervical spinal cord (40 μm) were incubated overnight with primary antibody solution (anti-serotonin, 1:20,000), 0.75% triton X-100 and 1% normal goat serum). Sections were then incubated with a secondary antibody (goat-anti-rabbit, 1:100) and then reacted with DAB prior to mounting on glass slides and counterstained with cresyl violet.
To obtain an index of host-graft connectivity, a subset of animals (N = 6) received intratransplant injections of the transneuronal tracer pseudorabies virus (PRV152, titer ~2.0 × 108). Once surgical plane of anesthesia was achieved (anesthetized as described above in Spinal cord injury and FSC transplantation), the site of injury and transplant was surgically re-exposed, and a small (~1 mm) incision was made in the overlying dura. PRV was then injected into the transplant tissue (~1 microliter via a 35 gauge needle and Hamilton syringe) at a depth of ~0.8 mm. The dura and overlaying muscles and skin closed as described above. Animals were left to recover for 72 hours before being terminally anesthetized with Beuthanasia® (9:1, sodium pentobarbitone to phenytoin solution) and intracardially perfused with paraformaldehyde (4 % w/v in 0.1M PBS, pH 7.4) The spinal cord was then removed from the vertebral column and stored in paraformaldehyde (2% w/v in PBS) for histological processing. Brainstem and cervical spinal cord tissue was processed for vibratome (40 μm) sectioning (described in detail in Lane et al., 2008). Briefly, coronal or longitudinal vibratome sections, through the caudal medulla and cervical spinal cord respectively, were immunolabeled with antibodies against PRV (rabbit anti-PRV (Rb134), provided by Dr. Lynn Enquist, Princeton University, as a service of the National Center for Experimental Neuroanatomy with Neurotropic Viruses: NCRR P40 RRO118604) to examine distribution of PRV labeled neurons in the host spinal cord. Optimal dilution of all antibodies has been previously determined in our laboratory. Prior to incubation with the primary antibody, sections were washed in PBS (0.1 M, pH = 7.4, 3 × 5 min), blocked against endogenous peroxidase activity (30 % methanol, 0.6 % hydrogen peroxide in 0.1 M PBS, incubated for 1 hour), re-washed in PBS, and blocked against non-specific protein labeling (10 % serum in 0.1 M PBS with 0.03 % Triton-X, incubated for 1 hour). Sections were incubated overnight at 4 °C in Rb134 (1:10,000 - raised against whole, purified PRV particles that were acetone inactivated). The following day, tissue was washed in PBS (0.1 M, 3 × 5 min), incubated for 2 hours at room temperature in a biotinylated secondary antibody (donkey anti-rabbit, Jackson Immunocytochemicals, 1:200), re-washed in PBS (3 × 5 min) and incubated for another 2 hours in an avidin-biotin complex (ABC, Elite Vectastain Kit, Vector Labs). Sections were then given a third series of washes in PBS and antigen was visualized with diaminobenzidine (DAB, Sigma). Immunoprecipitation and SDS-page has previously demonstrated that these PRV antibodies are specific for the major capsid, tegument and viral membrane proteins of PRV (Card et al., 1990; Card et al., 1991). Immunolabeled sections were then slide mounted and cover-slipped. Transverse sections through the medulla were counterstained with cresyl violet to identify morphological landmarks. Sections were examined using a Zeiss Axiophot or Axioplan microscope and photographs taken using a digital camera (Axiocam HRc) linked to a computer for image analysis.
Data analyses
All neurophysiological data were analyzed using Spike 2 software. Standard spike sorting methods were used to identify action potentials from individual neurons. In addition, spikes were examined using auto-correlogram analysis (200 ms with a resolution of 0.5 ms bin during 3–5 min baseline condition) (Isokawa-Akesson et al., 1987). To investigate relationship between graft neurons and contralateral phrenic bursting, the raw phrenic signals were converted into events by setting a horizontal threshold above the phrenic neurogram noise level as described previously (Sandhu et al., 2009). The graft spikes were then cross-correlated with the phrenic bursting events (200 ms with a resolution of 0.5 ms bin during 3–5 min baseline condition).
The discharge frequency of graft neurons was examined in relation to the respiratory cycle (i.e., inspiratory and expiratory periods as determined from the phrenic nerve recordings) as previously described (Lee et al., 2009). Data were averaged over 30 sec intervals during baseline as well as during respiratory challenges induced by hypoxia or hypercapnia. Data obtained during hypoxia and hypercapnia were expressed as absolute value (Hz) but also were normalized as percentage of baseline bursting (% baseline).
Plethysmography data (tidal volume, respiratory frequency and minute ventilation) were analyzed during 10 s intervals. Data were averaged over a 10 min baseline recording and during the final 2 min of the hypoxic challenge. Tidal volume and minute ventilation data were represented as both absolute value and normalized to body weight (Fuller et al., 2008).
A paired t-test was used to compare discharge frequency of graft neurons during baseline vs. respiratory challenge. The relationship between baseline graft neuronal discharge during baseline and challenge conditions was also analyzed by a linear regression analysis. Ventilation and body weight were compared using a two way repeated measures analysis of variance [factor one: animal group (control, C2Hx and C2Hx + FSC); factor two: condition (baseline and hypoxia) or time point (5 weeks and 10 weeks post-injury)] followed by the Student-Newman-Keuls post hoc test. For the present analyses, p < 0.05 was considered statistically significant.
Results
Histology
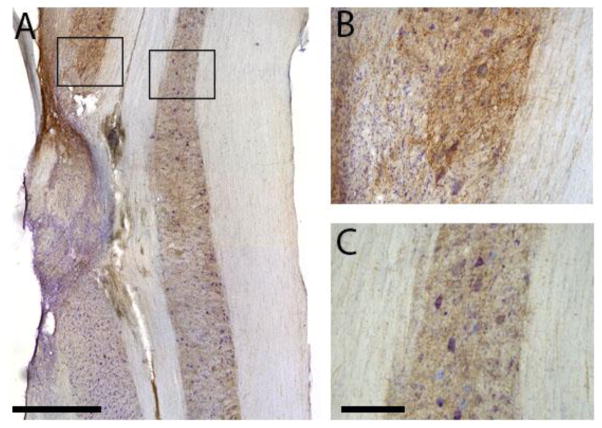
Histological characterization of intraspinal FSC transplants has been extensively described elsewhere (Houle and Reier, 1988; Jakeman and Reier, 1991; Jakeman et al., 1989; White et al., 2010), and the distinction between host and graft tissues was readily identifiable consistent with these prior publications. The grafts typically filled nearly the entire lesion cavity with consistent graft approximation being seen most often with host gray matter along the rostral, medial, and caudal borders (Figs. 1–2). Despite general host-graft approximation, tissue confluence was patchy due to frequent partitioning by small, densely packed cells morphologically typical of glia especially at the rostral-caudal white matter interfaces. Immunostaining for serotonin revealed modest axonal projections into the grafts which even occurred at points of donor tissue approximation to host white matter. Fig. 1A shows images which are representative of the histology obtained from each animal. Within neighboring regions of the host spinal cord, serotonergic immunoreactivity was robust ipsilateral and immediately rostral to the graft (Fig 1B). Serotonin-positive fibers were densely packed around spinal neurons in the intermediate gray matter and at the rostral host-graft interface, while immunoreactivity was considerably less on the contralateral side of the spinal cord (Fig. 1C), and both ipsi- and contralateral sides of the cord caudal to injury/transplant.
Fig. 1. Longitudinal sections through the cervical spinal cord at the site of the the C2 hemilesion and transplant.
Tissues were immunolabeled for the presence serotonin and counterstained with cresyl violet. The grafts typically filled nearly the entire C2Hx lesion cavity (A). Serotonergic immunoreactivity was robust immediately rostral to the graft (B) but staining intensity was considerably less on the contralateral side of the spinal cord (C). Scale bars indicate 1 mm (A) and 200 microns (B, C).
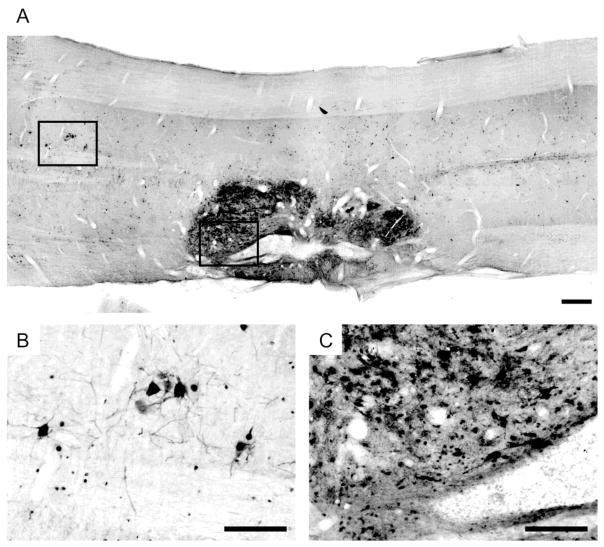
Fig. 2. Longitudinal sections through the cervical spinal cord immunolabeled for the presence of PRV.
Sixty four hours following PRV injection into donor tissue, there was extensive PRV labeling observed throughout the transplant. Note that the donor tissue in this animal appears to be in two large pieces, separated at the center of the transplant. Labeled neurons were observed bilaterally in the cervical host spinal cord, both rostral and caudal to the transplant (A). Labeled host neurons have dendrites extending rostro-caudally and laterally (B). In some cases dendrites appear to be crossing the spinal midline. Neurons labeled within the donor tissue (C) appear highly interconnected. Smaller labeled structures may represent with transverse section through a dendritic process or labeling in small glia. Scale bars indicate 500 (A) and 200 microns (B, C).
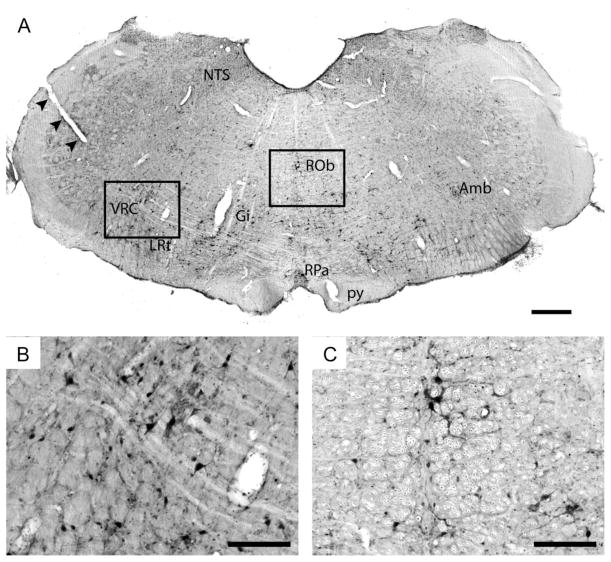
The transynaptic retrograde tracer PRV was used to confirm synaptic connectivity within intraspinal grafts, and also between graft neurons and the surrounding host tissue. At 64 hours following direct injection of PRV into the graft, PRV-infected neurons could be observed throughout the graft tissue (Fig. 2). Spread of the virus at this and earlier post-PRV survival times was, however, confined to the donor tissue. In addition, host-graft connectivity was indicated by the presence of PRV-positive neurons throughout the host cervical spinal cord, including areas both rostral and caudal to the graft. Specifically, PRV labeled interneurons were noted in close proximity to the host-graft interface, but also in contralateral gray matter (Fig. 2B). In some cases the dendritic profiles of PRV positive cervical interneurons in spinal laminae X appeared to project cross the spinal midline. Neuronal PRV labeling was also noted in medullary regions which are known to be important to respiratory control including the gigantocellular and lateral reticular nuclei, the midline raphé nuclei including obscurus and pallidus, the nucleus ambiguous, the nucleus of the solitary tract, and finally the ventral respiratory column (Fig. 3).
Fig. 3. Transverse section through the caudal medulla immunolabeled using antibodies against PRV (brown), and counterstained with cresyl violet (blue).
A small incision (arrowheads) has been made on the left of the medulla to indicate the side ipsilateral to injury and transplant. Sixty four hours following labeling, there was a substantial number of PRV labeled neurons through the medulla. This representative section demonstrates labeling in the medial and lateral reticular nuclei (gigantocellular (Gi) and lateral reticular (LRt)), raphé nuclei (obscures (ROb) and pallidus (RPa)), nucleus of the solitary tract (NTS), nucleus ambiguous (Amb) and the ventral respiratory column (VRC). The pyramidal tract is indicated by ‘py’. Scale bar represents 500 (A) and 200 microns (B, C).
Spontaneous neuronal activity within spinal cord grafts
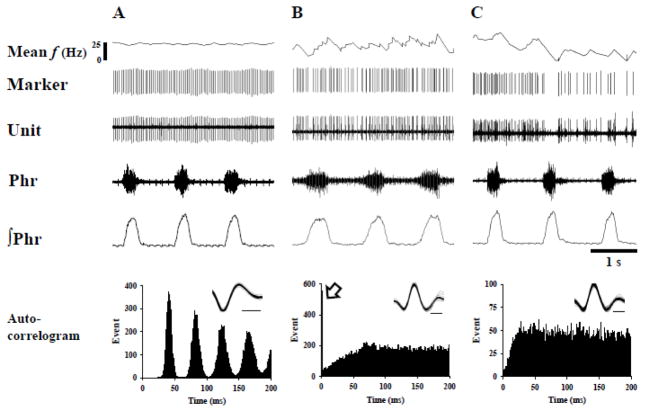
Spontaneous electrical discharges were readily detectable in 8 of 10 grafts (see Figs. 4–6). Overall, a total of 23 neurons were recorded and the pattern of neuronal spikes was examined using both auto- and cross-correlation methods (Duffin, 2000; Sandhu et al., 2009). The majority of auto-correlograms contained readily discernible peaks (e.g., Fig. 4) – a feature that is consistent with non-random neuronal burst patterns (Duffin, 2000). Three types of auto-correlogram profiles were generated, as follows. Seventy percent of the recorded neurons were characterized by an initial trough followed by several periodic peaks (Fig. 4A). Another 17% of graft neurons showed a single peak in the auto-correlogram profile (Fig. 4B). The remaining 13% had no discernible peaks (Fig. 4C). No apparent differences in burst frequency were noted when neurons were grouped according to their correlogram profile (periodic peaks: 11.7 ± 1.9 Hz, single peak: 18.1 ± 2.5 Hz; no peaks: 10.7 ± 4.6 Hz) (p = 0.275).
Fig. 4. Spontaneous firing patterns of FSC graft neurons and the associated auto-correlogram profiles.
Graft neuron activity (Unit) was simultaneously recorded with the phrenic nerve originating on the uninjured side of the spinal cord. “Marker” indicates individual neural spikes sorted using Spike 2 software. The mean discharge frequency (f) of graft neurons was calculated using 200 ms bins. Auto-correlation analysis revealed three main spontaneous firing patterns of graft neurons. The neuron in panel A has a regular interspike interval and displays a periodic firing in the auto-correlogram. Panel B shows a graft neuron with an initial peak (arrow) in the auto-correlogram. Panel C depicts a graft neuron with an irregular interspike interval. The inlet in auto-correlogram represents the superimposition of individual graft neuron spikes (time scale bar: 0.2 ms). Phr and ∫Phr represents raw and integrated phrenic signals, respectively.
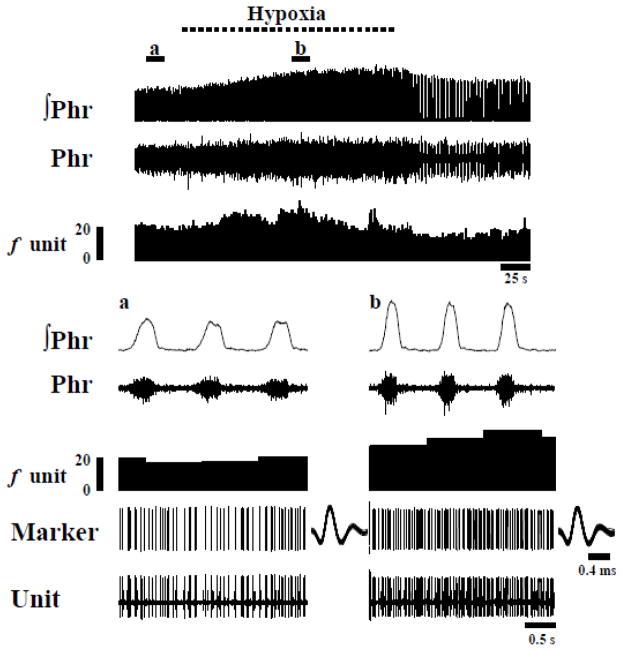
Fig. 6. Representative example of the phrenic nerve response to hypoxia along with discharge of single graft neuron.
Note that hypoxia induces a progressive increase in the phrenic motor output along with an increase in the discharge frequency (Hz) of the graft neuron. The areas marked a and b in the top panel are shown at an expanded time scale trace in the lower panels. The trace labeled as “Marker” shows individual neuronal spikes. The spikes are shown superimposed to the right of each trace.
Cross-correlation (Sandhu et al., 2009; Shen et al., 2003) of graft neuronal activity and the host phrenic signal was performed for all recorded neurons. These analyses revealed no indications of synchronous activity between the graft and the host’s respiratory activity. That is, the correlogram profiles showed no peaks that could be discerned relative to baseline noise (data not shown) (Kirkwood, 1979; Vaughan and Kirkwood, 1997).
Differential Effects of Hypoxia and Hypercapnia on Donor Neuron Activity
In view of our neuroanatomical data, the question arose whether altered respiratory drive would change intrinsic donor neuronal discharge patterns via host spinal afferent projections to the grafts. We therefore examined the impact of two physiological stimuli which are known to robustly increase phrenic motoneuron excitability. During baseline conditions, the burst frequency of graft neurons was 13.0 ± 1.7 Hz, and a majority (80%) of neurons increased discharge frequency upon exposure to hypoxia (Figs. 5–7). Representative examples of the impact of hypoxia on graft neuronal activity are provided in Figs. 5 and 6. On average, hypoxia triggered a 67 ± 24 % increase in frequency to 16.0 ± 1.5 Hz (p < 0.01, Fig. 7). However, Fig. 7 illustrates the relatively wide range of responses that were recorded, with burst frequency ranging from 5.2–28.9 Hz during hypoxia. Regression analyses indicated that cells with low baseline burst frequency tended to respond with the most robust increase in bursting during hypoxia (Fig. 8). No tendencies in hypoxic sensitivity (i.e., high vs. low responders) were noted based on the auto-correlogram profile groupings.
Fig. 5. Representative examples of graft neuron activity during baseline conditions and hypoxic challenge.
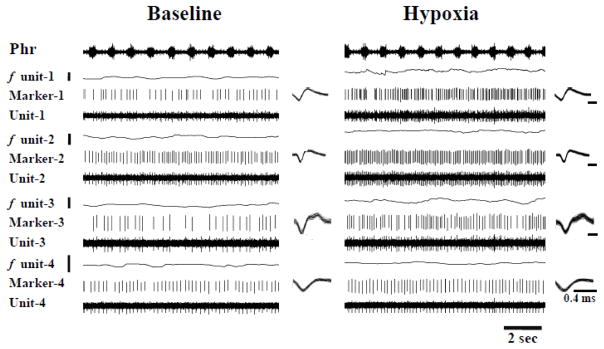
Four graft neurons were simultaneously recorded by the array electrode. Note that discharge frequency increased in each neuron during hypoxia. The trace labeled as “Marker” shows individual neuronal spikes. The spikes are superimposed to the right of each trace. The superimposition waveform is identical during baseline and hypoxia suggesting spikes are from the same neuron under both conditions. The scale bar near the discharge rate (f unit) represents 0–10 Hz.
Fig. 7. The impact of hypoxia (A) and hypercapnia (B) on the average discharge rate of graft neurons.
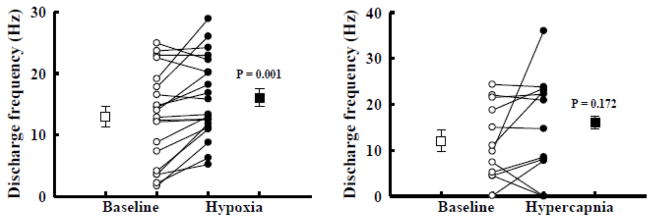
The mean (□/■) and individual (○/●) discharge frequency of graft neurons is shown during baseline as well as hypoxic (A) or hypercapnic (B) challenge. The mean discharge frequency of graft neurons significantly increased during hypoxia (p=0.001) but not hypercapnia (p=0.172).
Fig. 8. Linear regression analysis of the discharge frequency of graft neurons during baseline (abscissa) and respiratory challenge (ordinate).
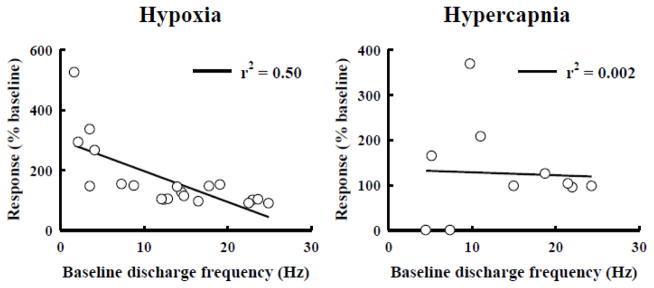
Graft neurons with a lower baseline discharge frequency tended to increase bursting during hypoxia (A) but not hypercapnia (B).
Graft neuron responses to hypercapnia were more variable than what was observed during hypoxia, Thus, hypercapnia did not, on average, have a statistically significant impact on graft neuronal discharge (p = 0.172). Overall, 50% of graft neurons increased burst frequency during hypercapnia with changes ranging from 0–23.8 Hz. In contrast to the hypoxic data, there was no detectable correlation between the baseline discharge frequency and the ability of a particular neuron to increase burst frequency during hypercapnia (Fig. 8). Both hypoxia and hypercapnia caused robust increases in phrenic nerve output (Table 1) that were quantitatively similar to previous reports from rats with C2Hx injury (Fuller et al., 2008).
Table 1.
Cardiorespiratory responses of grafted animals during hypoxia and hypercapnia.
| Baseline | Hypoxia | Baseline | Hypercapnia | |
|---|---|---|---|---|
| ∫ Phr (% baseline) | 100 ± 0 | 125 ± 6** | 100 ± 0 | 144 ± 10* |
| MAP (mmHg) | 80 ± 9 | 63 ± 9** | 86 ± 12 | 99 ± 11** |
∫Phr: integrated phrenic signal amplitude; MAP: mean arterial blood pressure.
P < 0.05;
P < 0.01 compared with the value during baseline condition.
Impact of FSC Grafts on Ventilation
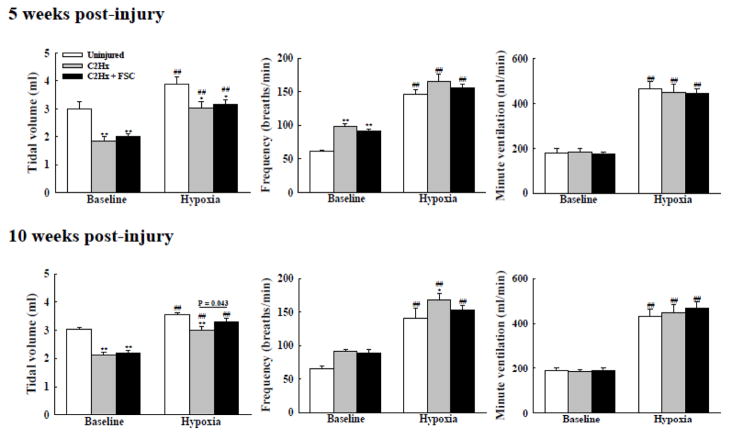
Body weight was similar between uninjured, injury-only, and C2Hx rats with FSC transplant (Table 2), and respiratory volume data are presented as both absolute values (ml/breath; Fig. 9) and relative to body mass (ml/breath/100g; Table 3) (Fuller et al., 2008). During baseline, normoxic conditions, the injury-only and FSC recipients had similar rapid, shallow breathing patterns as compared to the uninjured rats. Baseline breathing did not differ between 5 and 10 weeks post-injury (Fig. 9). Exposure to hypoxia triggered an increase in ventilation in all rats, as expected (Powell et al., 1998). More importantly, there was evidence for a time-dependent impact of the FSC grafts on the ventilatory response to hypoxia. At 5 weeks post-injury, the injury-only and transplant recipient groups had similar inspiratory tidal volume during the hypoxic challenge (p < 0.05 vs. uninjured group, Fig. 9). However, at 10 weeks post-injury, the FSC transplant group showed a significant increase in tidal volume during hypoxia when compared to the injury-only group (p = 0.043, Fig. 9).
Table 2.
The body weight (g) of uninjured control, C2Hx and C2Hx + FSC animals at 5 and 10 weeks post-injury.
| 5 weeks | 10 weeks | |
|---|---|---|
| Control (N=7) | 365 ± 9 | 405 ± 6** |
| C2Hx (N=9) | 381 ± 7 | 391 ± 12 |
| C2Hx + FSC (N=11) | 378 ± 8 | 402 ± 13 |
Values are mean ± SE.
P < 0.05 compared with the value at 5 weeks post-injury
Fig. 9. The ventilatory behavior of uninjured control, C2Hx and C2Hx+FSC animals at 5 and 10 weeks post-injury.
Both C2Hx and C2Hx+FSC animals had similar rapid shallow breathing pattern during baseline conditions at 5 and 10 weeks post-injury. The tidal volume (left panel) of both the C2Hx and C2Hx+FSC animals was similarly blunted compared to control animals during hypoxia at 5 weeks post-injury. However, the tidal volume of FSC grafted animals was significantly larger than the C2Hx only group at 10 weeks post-injury. At the 10 week time point, the respiratory frequency (breaths/min; middle panel) of FSC grafted animals during the hypoxic challenge was lower when compared to the C2Hx only group. Minute ventilation (right panel) was similar across all groups during all conditions. **: p < 0.01 compared with control animals. ##: p < 0.01 compared with the value during the baseline.
Table 3.
The tidal volume and minute ventilation of uninjured control, C2Hx and C2Hx + FSC animals at 5week and 10 week post-injury.
| Tidal volume (ml/100g)
| ||||
|---|---|---|---|---|
| 5 weeks
|
10 weeks
|
|||
| Baseline | Hypoxia | Baseline | Hypoxia | |
| Control | 0.82±0.06 | 1.05±0.06## | 0.75±0.03 | 0.88±0.03## |
| C2Hx | 0.50±0.04** | 0.80±0.06**,## | 0.55±0.03** | 0.78±0.05## |
| C2Hx + FSC | 0.53±0.03** | 0.84±0.04**,## | 0.55±0.03** | 0.85±0.04## |
| Minute ventilation (ml/min/100g)
| ||||
|---|---|---|---|---|
| 5 weeks
|
10 weeks
|
|||
| Baseline | Hypoxia | Baseline | Hypoxia | |
| Control | 49.0±4.9 | 127.2±5.7## | 47.0±3.2 | 106.5±7.9## |
| C2Hx | 48.2±4.0 | 118.1±9.6## | 47.8±3.1 | 116.3±10.4## |
| C2Hx + FSC | 46.9±2.2 | 117.7±4.9## | 47.7±3.6 | 118.2±8.1## |
Values are mean ± SE.
P < 0.05;
P < 0.01 compared with control animals.
P < 0.01 compared with the value during the baseline condition.
Discussion
Our findings demonstrate that grafts consisting of neuronal progenitors introduced in proximity to the phrenic nucleus can become neuroanatomically integrated with host spinal interneurons and are capable of responding to a physiologic stimulus (hypoxia) that also increases host respiratory output (Mitchell et al., 2001). This shift in firing patterns may translate to some beneficial changes in ventilatory function since whole-body plethysmography indicated increased inspiratory tidal volume during hypoxia in rats with spinal grafts. The response of the graft neurons to the hypoxic stimulus further suggests the potential for enhancing communication between host and graft tissue by “training the transplant” (Reier, 2004). In turn, this also raises a fundamental question: is stringent donor cell selection necessary to achieve a desired functional effect, or can rehabilitation paradigms be used to reprogram naïve donor neurons?
Electrophysiological Signatures of Fetal CNS Grafts
Previous studies of the intrinsic electrophysiological activity of fetal CNS grafts have shown that neuronal activity patterns can differ relative to the region from which graft tissue was derived (Bragin and Vinogradova, 1983). Nevertheless, random, spontaneous activity has been generally observed in graft recordings including studies of intraocular and intracerebral grafts of embryonic hippocampal (Kichigina et al., 1984) and raphe (Segal and Azmitia, 1986) tissue. Likewise, previous extracellular recordings of intraocular FSC transplants showed spontaneously active cells with some having high amplitudes and discharge rates (Henschen et al., 1985).
Our neurophysiology data are consistent with the hypothesis that donor neuronal bursting patterns within cervical FSC grafts are driven, at least in part, by network activity as opposed to purely random discharge. This hypothesis is based on interpretation of the autocorrelogram results (e.g. Fig. 4) in the context of prior mathematical modeling studies (Bar-Gad et al., 2001; Duffin, 2000). This prior work indicates that autocorrelograms without peaks (i.e., a “flat” appearance, see Fig. 4c) reflect neurons with random bursting patterns, whereas oscillatory functions are associated with non-random changes in the firing probability. For instance, a modeling study by Duffin showed that neurons with no synaptic inputs had flat autocorrelograms, but providing a tonic excitatory input to a neuron (with an appropriate refractory period) resulted in autocorrelograms with multiple peaks appearing in an oscillating manner. Indeed, the modeling study produced results that were remarkably similar to the autocorrelograms derived from graft neurons in the present study (e.g. compare Fig. 2 in (Duffin, 2000) with Fig. 4a in the present paper). The salient point is that oscillations in the autocorrellogram appear when neurons are receiving a non-random synaptic input, and this is consistent with the hypothesis that a functional neuronal network is present within most FSC grafts. This hypothesis is further supported by neuroanatomical evidence for extensive intrinsic graft connectivity (Jakeman and Reier, 1991) (also see Fig. 2).
With rare exception (Clowry and Vrbova, 1992), FSC grafts represent transplants of lineage-restricted precursors [e.g., interneurons (Jakeman et al., 1989)], as well as residual populations of stem cells (Chow et al., 2000; Lepore et al., 2005; Lepore and Fischer, 2005; Shihabuddin et al., 2000; Theele et al., 1996). FSC grafts are therefore a close prototype to lineage-restricted progenitor cell populations (Ketschek et al., 2012). In theory, interneuronally-enriched grafts have the potential of serving as novel neuronal relays (Bonner et al., 2011; Reier, 2004; Reier et al., 2002) which, if appropriately entrained, could enhance motoneuron output below sites of spinal cord injury. The fact we did not observe respiratory patterned discharge in the FSC graft neural recordings underscores the need for combinatorial approaches such as chondroitinase (Jefferson et al., 2011), growth factors (Bonner et al., 2011), or rehabilitation paradigms (see below) to establish greater functional connectivity between donor and host respiratory neurons.
Training a transplant?
Some studies have shown that graft-mediated functional outcomes can be influenced by the physical activity levels and surrounding environment of the recipient (Dupont-Versteegden et al., 2000; Eaton et al., 2008; Kubasak et al., 2008; Peterson et al., 2000). However, whether this involves changes in synaptic connectivity is unclear, and to our knowledge, no previous studies have examined whether targeted rehabilitation can be used to modify the activity of donor neurons themselves. In that regard, our results suggest that hypoxia could have value as a rehabilitative training tool to potentially enhance host-graft integration. Hypoxic episodes will activate phrenic motoneurons (Baker-Herman et al., 2004) and interneurons (Lane et al., 2009) in the immediate vicinity of the graft, and the current data show that hypoxia will also activate graft neurons (Figs. 5–7). It is presently unknown if and to what extent host respiratory interneurons projected to the grafts made in this study. Nevertheless, hypoxia provides a tool to introduce (or increase) appropriately patterned bursting around the graft, and possibly within the graft. In turn, this could lead to activity-dependent plasticity that strengthens host-graft and/or inter-graft synaptic connections possibly via Hebbian mechanisms.
The increased graft bursting during hypoxia probably did not reflect non-specific increases in respiratory motor drive since hypercapnic stimulation did not produce the same effect (i.e., the response was specific to the hypoxic stimulus). However, it is possible that setting the baseline PETCO2 well above the predicted CO2 threshold for phrenic bursting could have blunted the subsequent response of graft neurons to hypercapnia. Arguing against this possibility is the lack of relationship between baseline graft activity and the hypercapnic response (Fig. 8). In regards to the hypoxia response, there are several possibilities as to how reductions in O2 could stimulate graft neurons. For example, graft neurons could have an intrinsic sensitivity to O2, or bursting could be stimulated secondary to altered blood flow during hypoxia. We currently favor the latter hypothesis since the hypoxic sensitivity of graft neurons was similar across the three bursting profiles (as established via the autocorrelation results). That is, cells that had some neurophysiologic evidence of connectivity (i.e., oscillations in the autocorrelogram, Fig. 4a) and those that did not (i.e., flat profile, Fig. 4c) all responded similarly to hypoxia. Alternatively, it is possible that host neurons which respond to hypoxia are providing synaptic input to the graft, as seen in other neural graft models (Auerbach et al., 2000; Itoh et al., 1996; Zhou et al., 1998). This suggestion is further supported by transneuronal tracing findings (White et al., 2010) showing host-graft polysynaptic connectivity in the C2Hx injury-transplant model. In addition, Raphe neurons can respond vigorously to hypoxic stimulation (Morris et al., 2001), and modest serotonergic innervation of the graft was observed. Accordingly serotonin release during hypoxia (Kinkead et al., 2001) could have played a role in the change in graft bursting.
The acute response of the graft neurons to hypoxia raises the possibility of using repeated exposures as a method to “train the transplant”. A body of literature supports the use intermittent hypoxia (IH) as a rehabilitative tool [reviewed in (Feldman et al., 2003; Mitchell and Johnson, 2003; Vinit et al., 2009)]. Both acute (i.e. min-hours) and chronic (i.e. days-wks) IH is associated with spinal neuroplasticity and persistently enhanced phrenic output in SCI (Fuller et al., 2003; Vinit et al., 2009) and spinal intact rats (Baker-Herman et al., 2004; Ling et al., 2001). Increased phrenic output after IH (i.e. long-term facilitation) is also associated with synthesis of brain-derived neurotrophic factor (BDNF) in or around phrenic motoneurons (Baker-Herman et al., 2004), and IH may also induce secretion of BDNF from microvascular cells (Wang et al., 2006). Thus, in addition to synaptic plasticity, IH has the potential to enhance neuronal sprouting via BDNF-dependent mechanisms (Cohen-Cory and Fraser, 1995). IH may also have potential for clinical use since preliminary work shows improved limb motor output in SCI patients after IH treatment (Rymer, 2007; Trumbower, 2009).
Significance
The goals of tissue transplantation after SCI include replacing lost cells (e.g. motoneurons, interneurons, oligodendrocytes), filling and/or “bridging” cyst cavities, and creating a favorable growth environment (Horner and Gage, 2000; Reier, 2004). While varying degrees of success in each of these areas has been reported [reviewed in (Reier, 2004)], effective neuronal replacement remains elusive. In this regard, a detailed understanding of if and how donor neurons connect to the host spinal cord is a prerequisite for the design of rationale rehabilitation interventions after transplantation. Overall, the current results indicate that grafted FSC tissues develop into an interneuronal network which is capable of altering host motor output, at least under certain conditions. Future studies should focus on enhancing the synaptic connectivity between FSC graft neurons and the host phrenic motor circuit (Jakeman and Reier, 1991; White et al., 2010).
Highlights.
Rats received intraspinal transplantation of neuronal precursors
Anatomical connectivity was demonstrated between host and graft neurons
Spontaneous neuronal activity was detected in the mature grafts
Exposure to hypoxia resulted in increased burst frequency of graft neurons
Compared to shams, transplant recipients had larger inspiratory tidal volumes during hypoxia
Acknowledgments
Support for this work was provided by grants from the National Institutes of Health (NIH): R21 HL104294-01 (DDF) and P40RR018604 to Dr. Card who supplied the PRV. Support was also provided by the Oscar and Anne Lackner Chair in Medicine (PJR) the Paralyzed Veterans of America (MAL & KZL), and the Brain and Spinal Cord Injury Research Trust Fund, University of Florida.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Auerbach JM, Eiden MV, McKay RD. Transplanted CNS stem cells form functional synapses in vivo. Eur J Neurosci. 2000;12:1696–1704. doi: 10.1046/j.1460-9568.2000.00067.x. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci. 2004;7:48–55. doi: 10.1038/nn1166. [DOI] [PubMed] [Google Scholar]
- Bar-Gad I, Ritov Y, Bergman H. The neuronal refractory period causes a short-term peak in the autocorrelation function. Journal of neuroscience methods. 2001;104:155–163. doi: 10.1016/s0165-0270(00)00335-6. [DOI] [PubMed] [Google Scholar]
- Bonner JF, Blesch A, Neuhuber B, Fischer I. Promoting directional axon growth from neural progenitors grafted into the injured spinal cord. J Neurosci Res. 2010;88:1182–1192. doi: 10.1002/jnr.22288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner JF, Connors TM, Silverman WF, Kowalski DP, Lemay MA, Fischer I. Grafted neural progenitors integrate and restore synaptic connectivity across the injured spinal cord. J Neurosci. 2011;31:4675–4686. doi: 10.1523/JNEUROSCI.4130-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin AG, Vinogradova OS. Comparison of neuronal activity in septal and hippocampal grafts developing in the anterior eye chamber of the rat. Brain research. 1983;312:279–286. doi: 10.1016/0165-3806(83)90144-x. [DOI] [PubMed] [Google Scholar]
- Card JP, Rinaman L, Schwaber JS, Miselis RR, Whealy ME, Robbins AK, Enquist LW. Neurotropic properties of pseudorabies virus: uptake and transneuronal passage in the rat central nervous system. J Neurosci. 1990;10:1974–1994. doi: 10.1523/JNEUROSCI.10-06-01974.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card JP, Whealy ME, Robbins AK, Moore RY, Enquist LW. Two alpha-herpesvirus strains are transported differentially in the rodent visual system. Neuron. 1991;6:957–969. doi: 10.1016/0896-6273(91)90236-s. [DOI] [PubMed] [Google Scholar]
- Chow SY, Moul J, Tobias CA, Himes BT, Liu Y, Obrocka M, Hodge L, Tessler A, Fischer I. Characterization and intraspinal grafting of EGF/bFGF-dependent neurospheres derived from embryonic rat spinal cord. Brain Res. 2000;874:87–106. doi: 10.1016/s0006-8993(00)02443-4. [DOI] [PubMed] [Google Scholar]
- Clowry GJ, Vrbova G. Observations on the development of transplanted embryonic ventral horn neurones grafted into adult rat spinal cord and connected to skeletal muscle implants via a peripheral nerve. Exp Brain Res. 1992;91:249–258. doi: 10.1007/BF00231658. [DOI] [PubMed] [Google Scholar]
- Cohen-Cory S, Fraser SE. Effects of brain-derived neurotrophic factor on optic axon branching and remodelling in vivo. Nature. 1995;378:192–196. doi: 10.1038/378192a0. [DOI] [PubMed] [Google Scholar]
- Doperalski NJ, Fuller DD. Long-term facilitation of ipsilateral but not contralateral phrenic output after cervical spinal cord hemisection. Exp Neurol. 2006;200:74–81. doi: 10.1016/j.expneurol.2006.01.035. [DOI] [PubMed] [Google Scholar]
- Duffin J. Simulation of cross-correlograms resulting from synaptic connections between neurons. Journal of neuroscience methods. 2000;99:65–70. doi: 10.1016/s0165-0270(00)00213-2. [DOI] [PubMed] [Google Scholar]
- Dupont-Versteegden EE, Murphy RJ, Houle JD, Gurley CM, Peterson CA. Mechanisms leading to restoration of muscle size with exercise and transplantation after spinal cord injury. Am J Physiol Cell Physiol. 2000;279:C1677–1684. doi: 10.1152/ajpcell.2000.279.6.C1677. [DOI] [PubMed] [Google Scholar]
- Eaton MJ, Pearse DD, McBroom JS, Berrocal YA. The combination of human neuronal serotonergic cell implants and environmental enrichment after contusive SCI improves motor recovery over each individual strategy. Behav Brain Res. 2008;194:236–241. doi: 10.1016/j.bbr.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Doperalski NJ, Dougherty BJ, Sandhu MS, Bolser DC, Reier PJ. Modest spontaneous recovery of ventilation following chronic high cervical hemisection in rats. Exp Neurol. 2008;211:97–106. doi: 10.1016/j.expneurol.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Johnson SM, Olson EB, Jr, Mitchell GS. Synaptic pathways to phrenic motoneurons are enhanced by chronic intermittent hypoxia after cervical spinal cord injury. J Neurosci. 2003;23:2993–3000. doi: 10.1523/JNEUROSCI.23-07-02993.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Zabka AG, Baker TL, Mitchell GS. Phrenic long-term facilitation requires 5-HT receptor activation during but not following episodic hypoxia. J Appl Physiol. 2001;90:2001–2006. doi: 10.1152/jappl.2001.90.5.2001. discussion 2000. [DOI] [PubMed] [Google Scholar]
- Golder FJ, Mitchell GS. Spinal synaptic enhancement with acute intermittent hypoxia improves respiratory function after chronic cervical spinal cord injury. J Neurosci. 2005;25:2925–2932. doi: 10.1523/JNEUROSCI.0148-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henschen A, Hoffer B, Olson L. Spinal cord grafts in oculo: survival, growth, histological organization and electrophysiological characteristics. Exp Brain Res. 1985;60:38–47. doi: 10.1007/BF00237016. [DOI] [PubMed] [Google Scholar]
- Hooshmand MJ, Sontag CJ, Uchida N, Tamaki S, Anderson AJ, Cummings BJ. Analysis of host-mediated repair mechanisms after human CNS-stem cell transplantation for spinal cord injury: correlation of engraftment with recovery. PLoS One. 2009;4:e5871. doi: 10.1371/journal.pone.0005871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner PJ, Gage FH. Regenerating the damaged central nervous system. Nature. 2000;407:963–970. doi: 10.1038/35039559. [DOI] [PubMed] [Google Scholar]
- Houle JD, Reier PJ. Transplantation of fetal spinal cord tissue into the chronically injured adult rat spinal cord. J Comp Neurol. 1988;269:535–547. doi: 10.1002/cne.902690406. [DOI] [PubMed] [Google Scholar]
- Isokawa-Akesson M, Wilson CL, Babb TL. Diversity in periodic pattern of firing in human hippocampal neurons. Exp Neurol. 1987;98:137–151. doi: 10.1016/0014-4886(87)90079-3. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Waldeck RF, Tessler A, Pinter MJ. Regenerated dorsal root fibers form functional synapses in embryonic spinal cord transplants. J Neurophysiol. 1996;76:1236–1245. doi: 10.1152/jn.1996.76.2.1236. [DOI] [PubMed] [Google Scholar]
- Jakeman LB, Reier PJ. Axonal projections between fetal spinal cord transplants and the adult rat spinal cord: a neuroanatomical tracing study of local interactions. J Comp Neurol. 1991;307:311–334. doi: 10.1002/cne.903070211. [DOI] [PubMed] [Google Scholar]
- Jakeman LB, Reier PJ, Bregman BS, Wade EB, Dailey M, Kastner RJ, Himes BT, Tessler A. Differentiation of substantia gelatinosa-like regions in intraspinal and intracerebral transplants of embryonic spinal cord tissue in the rat. Exp Neurol. 1989;103:17–33. doi: 10.1016/0014-4886(89)90181-7. [DOI] [PubMed] [Google Scholar]
- Ketschek AR, Haas C, Gallo G, Fischer I. The roles of neuronal and glial precursors in overcoming chondroitin sulfate proteoglycan inhibition. Exp Neurol. 2012;235:627–637. doi: 10.1016/j.expneurol.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kichigina VF, Bragin AG, Vinogradova OS. Functional integration of the nervous tissue of the rat following xenotransplantation into the brain of a rabbit. Zhurnal vysshei nervnoi deiatelnosti imeni I P Pavlova. 1984;34:932–940. [PubMed] [Google Scholar]
- Kim BG, Dai HN, Lynskey JV, McAtee M, Bregman BS. Degradation of chondroitin sulfate proteoglycans potentiates transplant-mediated axonal remodeling and functional recovery after spinal cord injury in adult rats. J Comp Neurol. 2006;497:182–198. doi: 10.1002/cne.20980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinkead R, Bach KB, Johnson SM, Hodgeman BA, Mitchell GS. Plasticity in respiratory motor control: intermittent hypoxia and hypercapnia activate opposing serotonergic and noradrenergic modulatory systems. Comp Biochem Physiol A Mol Integr Physiol. 2001;130:207–218. doi: 10.1016/s1095-6433(01)00393-2. [DOI] [PubMed] [Google Scholar]
- Kirkwood PA. On the use and interpretation of cross-correlations measurements in the mammalian central nervous system. Journal of neuroscience methods. 1979;1:107–132. doi: 10.1016/0165-0270(79)90009-8. [DOI] [PubMed] [Google Scholar]
- Kubasak MD, Jindrich DL, Zhong H, Takeoka A, McFarland KC, Munoz-Quiles C, Roy RR, Edgerton VR, Ramon-Cueto A, Phelps PE. OEG implantation and step training enhance hindlimb-stepping ability in adult spinal transected rats. Brain. 2008;131:264–276. doi: 10.1093/brain/awm267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MA, Lee KZ, Fuller DD, Reier PJ. Spinal circuitry and respiratory recovery following spinal cord injury. Respir Physiol Neurobiol. 2009;169:123–132. doi: 10.1016/j.resp.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MA, White TE, Coutts MA, Jones AL, Sandhu MS, Bloom DC, Bolser DC, Yates BJ, Fuller DD, Reier PJ. Cervical prephrenic interneurons in the normal and lesioned spinal cord of the adult rat. J Comp Neurol. 2008;511:692–709. doi: 10.1002/cne.21864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KZ, Fuller DD. Preinspiratory and inspiratory hypoglossal motor output during hypoxia-induced plasticity in the rat. J Appl Physiol. 2010;108:1187–1198. doi: 10.1152/japplphysiol.01285.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KZ, Fuller DD. Neural control of phrenic motoneuron discharge. Respir Physiol Neurobiol. 2011;179:71–79. doi: 10.1016/j.resp.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KZ, Reier PJ, Fuller DD. Phrenic motoneuron discharge patterns during hypoxia-induced short-term potentiation in rats. Journal of neurophysiology. 2009;102:2184–2193. doi: 10.1152/jn.00399.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KZ, Sandhu MS, Dougherty BJ, Reier PJ, Fuller DD. Influence of vagal afferents on supraspinal and spinal respiratory activity following cervical spinal cord injury in rats. J Appl Physiol. 2010;109:377–387. doi: 10.1152/japplphysiol.01429.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepore AC, Bakshi A, Swanger SA, Rao MS, Fischer I. Neural precursor cells can be delivered into the injured cervical spinal cord by intrathecal injection at the lumbar cord. Brain Res. 2005;1045:206–216. doi: 10.1016/j.brainres.2005.03.050. [DOI] [PubMed] [Google Scholar]
- Lepore AC, Fischer I. Lineage-restricted neural precursors survive, migrate, and differentiate following transplantation into the injured adult spinal cord. Exp Neurol. 2005;194:230–242. doi: 10.1016/j.expneurol.2005.02.020. [DOI] [PubMed] [Google Scholar]
- Ling L, Fuller DD, Bach KB, Kinkead R, Olson EB, Jr, Mitchell GS. Chronic intermittent hypoxia elicits serotonin-dependent plasticity in the central neural control of breathing. J Neurosci. 2001;21:5381–5388. doi: 10.1523/JNEUROSCI.21-14-05381.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB., Jr Invited review: Intermittent hypoxia and respiratory plasticity. J Appl Physiol. 2001;90:2466–2475. doi: 10.1152/jappl.2001.90.6.2466. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Johnson SM. Neuroplasticity in respiratory motor control. J Appl Physiol. 2003;94:358–374. doi: 10.1152/japplphysiol.00523.2002. [DOI] [PubMed] [Google Scholar]
- Mitsui T, Shumsky JS, Lepore AC, Murray M, Fischer I. Transplantation of neuronal and glial restricted precursors into contused spinal cord improves bladder and motor functions, decreases thermal hypersensitivity, and modifies intraspinal circuitry. J Neurosci. 2005;25:9624–9636. doi: 10.1523/JNEUROSCI.2175-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris KF, Shannon R, Lindsey BG. Changes in cat medullary neurone firing rates and synchrony following induction of respiratory long-term facilitation. J Physiol. 2001;532:483–497. doi: 10.1111/j.1469-7793.2001.0483f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikulina E, Tidwell JL, Dai HN, Bregman BS, Filbin MT. The phosphodiesterase inhibitor rolipram delivered after a spinal cord lesion promotes axonal regeneration and functional recovery. Proc Natl Acad Sci U S A. 2004;101:8786–8790. doi: 10.1073/pnas.0402595101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson CA, Murphy RJ, Dupont-Versteegden EE, Houle JD. Cycling exercise and fetal spinal cord transplantation act synergistically on atrophied muscle following chronic spinal cord injury in rats. Neurorehabil Neural Repair. 2000;14:85–91. doi: 10.1177/154596830001400201. [DOI] [PubMed] [Google Scholar]
- Powell FL, Milsom WK, Mitchell GS. Time domains of the hypoxic ventilatory response. Respiration physiology. 1998;112:123–134. doi: 10.1016/s0034-5687(98)00026-7. [DOI] [PubMed] [Google Scholar]
- Reier PJ. Neural tissue grafts and repair of the injured spinal cord. Neuropathol Appl Neurobiol. 1985;11:81–104. doi: 10.1111/j.1365-2990.1985.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Reier PJ. Cellular transplantation strategies for spinal cord injury and translational neurobiology. NeuroRx. 2004;1:424–451. doi: 10.1602/neurorx.1.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reier PJ, Bregman BS, Wujek JR. Intraspinal transplantation of embryonic spinal cord tissue in neonatal and adult rats. J Comp Neurol. 1986;247:275–296. doi: 10.1002/cne.902470302. [DOI] [PubMed] [Google Scholar]
- Reier PJ, Golder FJ, Bolser DC, Hubscher C, Johnson R, Schrimsher GW, Velardo MJ. Gray matter repair in the cervical spinal cord. Prog Brain Res. 2002;137:49–70. doi: 10.1016/s0079-6123(02)37007-9. [DOI] [PubMed] [Google Scholar]
- Rymer WZ, Hornby T, Mitchell GS, Schmit BD, Trumbower RD. Effects of intermittent hypoxia on motor function in persons with incomplete SCI. Abstract; Society for Neuroscience Annual Meeting; San Diego California. Nov. 3, 2007.2007. [Google Scholar]
- Sandhu MS, Dougherty BJ, Lane MA, Bolser DC, Kirkwood PA, Reier PJ, Fuller DD. Respiratory recovery following high cervical hemisection. Respir Physiol Neurobiol. 2009;169:94–101. doi: 10.1016/j.resp.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu MS, Lee KZ, Fregosi RF, Fuller DD. Phrenicotomy alters phrenic long-term facilitation following intermittent hypoxia in anesthetized rats. J Appl Physiol. 2010;109:279–287. doi: 10.1152/japplphysiol.01422.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M, Azmitia EC. Fetal raphe neurons grafted into the hippocampus develop normal adult physiological properties. Brain research. 1986;364:162–166. doi: 10.1016/0006-8993(86)90997-2. [DOI] [PubMed] [Google Scholar]
- Shen L, Li YM, Duffin J. Inhibitory connections among rostral medullary expiratory neurones detected with cross-correlation in the decerebrate rat. Pflugers Arch. 2003;446:365–372. doi: 10.1007/s00424-003-1024-0. [DOI] [PubMed] [Google Scholar]
- Shihabuddin LS, Horner PJ, Ray J, Gage FH. Adult spinal cord stem cells generate neurons after transplantation in the adult dentate gyrus. J Neurosci. 2000;20:8727–8735. doi: 10.1523/JNEUROSCI.20-23-08727.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theele DP, Schrimsher GW, Reier PJ. Comparison of the growth and fate of fetal spinal iso- and allografts in the adult rat injured spinal cord. Exp Neurol. 1996;142:128–143. doi: 10.1006/exnr.1996.0184. [DOI] [PubMed] [Google Scholar]
- Trumbower RD, Jayaraman A, Mitchell GS, Rymer WZ. Exposure to acute intermittent hypoxia augments somatic motor function in humans with incomplete spinal cord injury. Neurorehabil Neural Repair. 2012;26:163–172. doi: 10.1177/1545968311412055. [DOI] [PubMed] [Google Scholar]
- Trumbower RD, Jayaraman A, Schmit BD, Hornby TG, Mitchell GS, Rymer WZ. Effects of repetitive acute intermittent hypoxia on lumbosacral motor function in human SCI. 2009 Neuroscience Meeting Planner; Chicago, IL: Society for Neuroscience; 2009. 2009 Online. [Google Scholar]
- Vaughan CW, Kirkwood PA. Evidence from motoneurone synchronization for disynaptic pathways in the control of inspiratory motoneurones in the cat. J Physiol. 1997;503 (Pt 3):673–689. doi: 10.1111/j.1469-7793.1997.673bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinit S, Lovett-Barr MR, Mitchell GS. Intermittent hypoxia induces functional recovery following cervical spinal injury. Respir Physiol Neurobiol. 2009;169:210–217. doi: 10.1016/j.resp.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Ward N, Boswell M, Katz DM. Secretion of brain-derived neurotrophic factor from brain microvascular endothelial cells. Eur J Neurosci. 2006;23:1665–1670. doi: 10.1111/j.1460-9568.2006.04682.x. [DOI] [PubMed] [Google Scholar]
- White TE, Lane MA, Sandhu MS, O’Steen BE, Fuller DD, Reier PJ. Neuronal progenitor transplantation and respiratory outcomes following upper cervical spinal cord injury in adult rats. Exp Neurol. 2010;225:231–236. doi: 10.1016/j.expneurol.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Raisman G, Zhou C. Transplanted embryonic entorhinal neurons make functional synapses in adult host hippocampus. Brain Res. 1998;788:202–206. doi: 10.1016/s0006-8993(97)01539-4. [DOI] [PubMed] [Google Scholar]