Abstract
Purpose
Chronic Lymphocytic Leukemia (CLL) is incurable with current chemotherapy treatments. Curcumin (diferuloylmethane), an active ingredient in the spice turmeric, inhibits tumor metastasis, invasion, and angiogenesis in tumor cell lines. We evaluated the effects of curcumin on the viability of primary CLL B-cells and its ability to overcome stromal mediated protection.
Experimental Design
The in vitro effect of curcumin on primary CLL B-cells was evaluated using FACS analysis and Western blotting. For some experiments, CLL B-cells were co-cultured with human stromal cells to evaluate the effects of curcumin on leukemia cells cultured in their micro-environment. Finally, the effect of curcumin in combination with the green tea extract EGCG was evaluated.
Results
Curcumin induced apoptosis in CLL B-cells in a dose-dependent (5–20 μM) manner and inhibited constitutively active pro-survival pathways including STAT3, AKT and NF-κB. Moreover, curcumin suppressed expression of the anti-apoptotic proteins Mcl-1 and XIAP, and up-regulated the pro-apoptotic protein BIM. Co-culture of CLL B-cells with stromal cells resulted in elevated levels of STAT3, increased expression of Mcl-1, XIAP and decreased sensitivity to curcumin. When curcumin was administered simultaneously with EGCG, antagonism was observed for most patient samples. In contrast, sequential administration of these agents led to substantial increases in CLL B-cell death and could overcome stromal protection.
Conclusions
Curcumin treatment was able to overcome stromal protection of CLL B-cells on in vitro testing and to synergize with EGCG when administered in a sequential fashion. Additional evaluation of curcumin as a potential therapeutic agent for treatment of CLL appears warranted.
Introduction
B-cell chronic lymphocytic leukemia (CLL) is the most common leukemia in North America and, with the possible exception of allogeneic stem cell transplant, is incurable with current treatments (1). CLL B-cells are largely non-cycling (2), and their accumulation is primarily secondary to decreased apoptosis rather than increased proliferation (3). Notably, the apoptotic resistance of CLL B-cells to chemotherapeutic agents and monoclonal antibodies is, in part, related to increased levels of anti-apoptotic proteins Mcl-1, Bcl-2, and X-linked inhibitor of apoptosis protein (XIAP) (1, 3–7). CLL B-cells have also been found to have a constitutively active STAT3 pathway (8, 9), possess an autocrine VEGF signaling loop (10), and to express constitutively activated NF-κB (11) and PI3K. Importantly, selective inhibition of PI3K induces apoptosis of CLL B-cells (12, 13).
Previous work by our laboratory and others suggests that autocrine survival signals and interactions between the CLL B-cell and its microenvironment influence apoptotic resistance and sensitivity to chemotherapeutic agents (8–10, 14). In the marrow, physical contact between stromal elements and leukemic cells promotes CLL B-cell survival (15–18), an effect mediated in part through integrins (e.g., VLA-4(18)) on the surface of CLL B-cells and their interaction with various ligands (e.g., VCAM1; fibronectin; iC3b) expressed on marrow stromal cells(19–22). To add to the complexity of environmental/stromal protection several other interactions (mediated by both contact and soluble factors) between CLL B-cells and their microenvironment have been shown to promote survival, proliferation, and upregulation of anti-apoptotic proteins (20, 22–25).
There is now great interest in identifying pharmacologic agents that are able to modulate these interactions that impact survival pathways in the hope of identifying potential novel therapies for treatment of CLL. Naturally occurring compounds are a potential source of agents that could modulate these survival signals and interrupt stromal nurturing. We have previously shown that the green tea extract, epigallocatechin-3 gallate (EGCG), inhibits VEGF receptor activation and induces apoptosis in primary CLL B-cells (10). This agent has now entered clinical testing in patients with early-stage CLL (26, 27).
Another natural compound, curcumin (diferuloylmethane), one of the active ingredients in the spice turmeric, has emerged as an attractive therapeutic agent that combines clinical tolerability with intriguing pharmacologic properties, including anti-tumor, anti-inflammatory and anti-oxidant properties (28–30). Curcumin has recently been shown to inhibit tumor metastasis, invasion and angiogenesis (29–32). Curcumin induces apoptosis in a variety of cancer cell lines and down-regulates expression of cell-proliferation (cyclooxygenase-2, cyclin D1 and c-myc) as well as anti-apoptotic (IAP1, IAP2, XIAP, Bcl-2, Bcl-xL, Bfl-1/A1, TNF-receptor-associated factor-1, cellular FLIP) and metastatic gene products (VEGF, matrix metalloproteinase-9, ICAM-1) through suppression of IκBα kinase and Akt activation (29). Importantly, Everett et al., recently reported that curcumin can induce apoptosis in CLL B-cells and suggested this effect may relate to inhibition of constitutively activated NF-κB (33).
In the present study, therefore, we have further assessed the impact of curcumin on CLL B-cell viability and dissected the mechanism of curcumin mediated cytotoxic effects on CLL B-cells. Specifically, we evaluated the effect of curcumin on pro-survival pathways constitutively activated in CLL B-cells including NF-κB, STAT3 and AKT. We have also examined the cytotoxic effect of curcumin on CLL B-cells in the presence of stromal cells. Finally, we have evaluated the effect of curcumin in combination with EGCG and the ability of combination therapy to overcome stromal protection.
Materials and Methods
Patient selection and CLL sample processing
Blood was obtained from CLL patients who had provided written informed consent under a protocol approved by the Mayo Clinic Institutional Review Board according to the regulations of the Declaration of Helsinki or from healthy volunteers. All CLL patients had a confirmed diagnosis using the National Cancer Institute (NCI) Working Group definition (34). Patients in this cohort were from all Rai stages and had not been treated prior to blood processing for this study within the last 2 years. CLL cells were isolated from heparinized venous blood by density gradient centrifugation. When assessed by flow cytometry (FACScan, Becton Dickinson, Sunnyvale, CA), the isolated cells were predominantly CLL B-cells (>90% CD5+/CD19+). Lymphocytes from healthy volunteers (n=5) were separated by density gradient centrifugation. Freshly isolated CLL B-cells or PBMC from normal individuals were cultured in serum-free AIM-V medium at 37°C in an atmosphere containing 95% air and 5% CO2.
Reagents
Immunological reagents that recognize the following antigens were purchased from the indicated suppliers: mouse monoclonal antibodies to Bcl-2, Bcl-xL, XIAP, BAD, BAX and rabbit polyclonal antibody to BID from BD Pharmingen/Transduction Laboratories (San Diego, CA); antibodies to actin and survivin from Novus Biologicals (Littleton, CO); antibody to Mcl-1 from Chemicon (Billerica, MA); antibody to poly (adenosine diphosphate-ribose) polymerase (PARP) from BIOMOL (Plymouth Meeting, PA); antibodies to caspase-3, -9, phospho-BAD (Ser 136), phospho-AKT (Ser 473), phospho-IκBα, phospho-STAT3 (Ser 727), AKT and STAT3 from Cell Signaling Technology (Beverly, MA); and antibodies to IκBα and BIM from Santa Cruz (Santa Cruz, CA). The following reagents were purchased from the indicated suppliers: curcumin (>94% purified, Sigma, St. Louis, MO); fluorescein isothiocyanate (FITC)-conjugated annexin V (Invitrogen, Carlsbad, CA); propidium iodide (PI) (Becton Dickinson, San Diego, CA); pan-caspase inhibitor Z-VAD-fmk (BD Pharmingen); and AIM-V medium (Gibco, Grand Island, NY). Epigallocatechin (EGCG) was a gift from Dr. Y. Hara (Mitsui Norin, Japan).
Apoptosis assay
Primary CLL B-cells (1.0 × 106 cells/ml) were treated with either vehicle (DMSO) or curcumin for 24–48 hours at increasing doses (5–20 μM) in serum-free AIM-V medium. Cells were washed with phosphate-buffered saline (PBS), stained with annexin V-FITC and PI, and analyzed for apoptosis by flow cytometry (FACScan). Similarly, freshly isolated normal PBMC (1.0 × 106/ml) were treated with curcumin at various doses (5–25 μM) for 24–48 hours, and we then analyzed cell death by staining with CD19-APC, annexin-FITC and PI (Sigma, St. Louis, MO) on a FACSCalibur Instrument (Becton Dickinson) using Cell Quest software. Cells staining with annexin V-FITC and/or PI were considered positive for cell death.
Treatment of CLL B-cells with curcumin in presence of stromal cells
For stromal experiments, the human bone marrow stromal cell line HS-5 (35) was grown and maintained in DMEM (Biosource, Camarillo, CA) containing 10% fetal bovine serum (FBS) (Biosource) as described previously (14). HS-5 cells were cultured in 12-well tissue-culture plates at a cell density of 1.0 × 105/well overnight, washed twice with serum-free AIM V medium and then incubated with primary CLL B-cells at a cell density of 1 × 106 cells/well. CLL B-cells were cultured by either direct contact with HS-5 cells or indirectly exposed to HS-5 cells via transwells (pore size 0.45 μ) with serum-free AIM V medium for 24 hours prior to then being cultured with increasing doses of curcumin (10, 15 and 20 μM) or DMSO. For comparison, CLL B-cells cultured without stromal cells were treated similarly with curcumin or DMSO. After 24 hours, CLL B-cells were harvested, washed in PBS and stained with APC-conjugated antibody to CD19, annexin V-FITC and PI. Apoptosis in CD19 positive lymphocytes was then analyzed on a FACSCalibur Instrument.
Assessment of combination treatment with curcumin and EGCG on CLL B-cell death
CLL B-cells were treated with various doses of curcumin (2.5–15 μM) or EGCG (25–150 μM) individually or in combination using a constant ratio (1:10) for 24 hours. Cells were harvested, stained with annexin/PI and viability analyzed by flow cytometry. After concentration-effect curves were generated for each agent, data were analyzed using the CalcuSyn software program (Biosoft, Cambridge, UK), which uses the method of Chou and Talalay (36), to determine whether combination treatment yields greater effects than expected from summation alone. A combination index (CI) of 1 indicates an additive effect, a CI above 1 indicates an antagonistic effect and a CI below 1 indicates a synergistic effect (37). In other experiments CLL B-cells were treated with DMSO, curcumin (10 μM), EGCG (100 μM) or sequentially with both drugs. For sequential treatment experiments, 1.0 × 106 CLL B-cells/ml were treated with DMSO, curcumin (10 μM) alone, EGCG (100 μM) alone, or both agents for 24 hours, washed and cultured for another 24 hours in media alone or with the addition of the second agent (EGCG or curcumin) as indicated. Cells were then harvested and stained with annexin-FITC/PI to analyze cell death on FACScan flow cytometer (Becton Dickinson). Similarly, sequential treatment of the primary CLL B-cells was also performed in co-culture with the stromal cells in experiments to determine if combination therapy could reduce the survival impact of stromal cells.
Immunoblotting
For immunoblot experiments, primary CLL B-cells were treated with DMSO or curcumin (20 μM) for 36–48 hours and then lysed in lysis buffer containing 50 mM Tris HCl, pH 7.5, 150 mM NaCl, 2 mM EGTA, 1% NP-40, 10 mM NaF, 1mM Na3V04 and a cocktail of protease inhibitors. Following incubation on ice for 20 min, cell lysates were passed through 27 G needle and sonicated briefly (30–40 seconds). The whole cell extract was collected after a centrifugation at 16,000 × g for 15 minutes. Protein content was determined and equal amounts of proteins were loaded on SDS-polyacrylamide gels after digesting in Laemmli SDS-sample buffer. Separated proteins were transferred onto 0.45 μ nitrocellulose papers (BioRad) and immunostained with specific antibodies. Protein bands were detected using an enhanced chemiluminescence detection kit (Pierce).
Statistical Analysis
The percent kill and percentage viable cells were evaluated across CLL patients and were summarized both graphically and quantitatively. These percentages were analyzed graphically for each dose level independently as well as across dose levels graphically. Means and standard errors were calculated using Excel (Microsoft Corporation). Graphical analyses were done using Sigma plot software. See the previous section on combination treatment for discussion, mathematical analysis of additive, synergistic or antagonistic effects of combination therapy using the CalcuSyn software program.
Results
Curcumin induces apoptosis in primary CLL B-cells in vitro
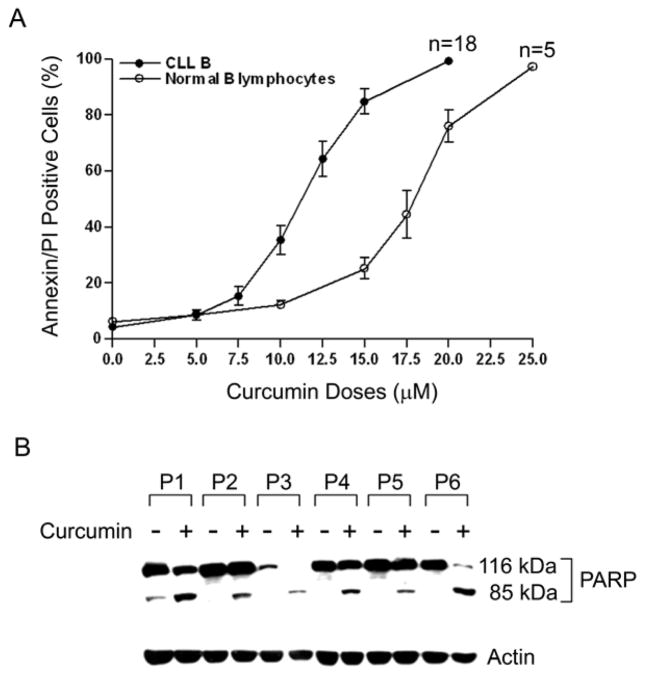
Primary CLL B-cells in serum-free AIM-V medium were treated with increasing doses of curcumin for 24 hours. At the indicated time, cells were harvested, stained with annexin V/PI and analyzed by flow cytometry for viability. Curcumin treatment induced apoptosis in CLL B-cells in a dose-dependent manner (Fig. 1A). The mean IC50 dose of curcumin at 24 hours was between 10 and 12.5 μM. Under the same experimental conditions, the average IC50 dose for the normal CD19+ B-lymphocytes was higher at between 17.5 and 20 μM. The sensitivity of CLL B-cells to curcumin did not correlate with the Rai stage using the IC50 data and appeared to be independent of prognostic parameters (Table 1).
Fig. 1. Curcumin induces apoptosis in primary CLL B-cells in a dose-dependent manner.
(A) Primary PBMC (≥90% CD5+/CD19+ lymphocytes) isolated from CLL patients (n=18) were treated with increasing doses of curcumin for 24 hours. Cells were harvested, stained with annexin/PI and analyzed by flow cytometry for the induction of cell death. Similarly, PBMC from normal individuals (n=5) were treated with curcumin and induction of cell death in CD19+ B-lymphocytes was analyzed by flow cytometry (annexin/PI positivity). Mean values were plotted with standard error bars. (B) Curcumin induced cell death involves PARP cleavage. Lysates from CLL B-cells (n=6, P=patient) treated with curcumin or DMSO were analyzed for PARP cleavage by Western blot. Curcumin-treated cells displayed cleavage of the native PARP (116 kDa) into its signature 85 kDa polypeptide fragment.
Table 1.
Assessment of In Vitro Curcumin Sensitivity in Leukemic Cells from B-CLL Patients
| Patient No. | Rai Stage | WBC/ALC (×109/L) | CD38* | ZAP-70** | VH Status | FISH | Curcumin IC50 (μM) Range |
|---|---|---|---|---|---|---|---|
| 1 | IV | 163.9/144.15 | Negative | Negative | UM | 13q- | 12.5 |
| 2 | IV | 30/16.91 | Positive | ND | UM | ND | 10.0 |
| 3 | I | 25.5/14.53 | Negative | Negative | ND | 11q- | 10–12.5 |
| 4 | I | 55.4/51.69 | Negative | Negative | M | 13q- | 10–12.5 |
| 5 | I | 32.5/26.32 | Positive | Positive | UM | 13q- | 12.5–15.0 |
| 6 | IV | 79.1/66.44 | Negative | Positive | ND | 17p- | 15.0 |
| 7 | II | 100.8/97.77 | Negative | Positive | UM | 11q- | 15–20 |
| 8 | I | 61.4/58.33 | Negative | Negative | M | Normal | 12.5–15.0 |
| 9 | II | 45.9/44.06 | Positive | Positive | UM | Trisomy 12 | 10–12.5 |
| 10 | I | 29.8/27.11 | ND | Positive | UM | 13q- | 12.5 |
| 11 | IV | 56.2/50.58 | Negative | Negative | ND | Trisomy 12 | 10–12.5 |
| 12 | I | 40.5/38.47 | Positive | Positive | UM | 6–39.0 | 10–12.5 |
| 13 | I | 42.8/33.81 | Negative | Negative | M | 13q- | 12.5–15.0 |
| 14 | II | 23.9/12.66 | Positive | Negative | ND | Trisomy 12 | 10–12.5 |
| 15 | I | 43.1/32.32 | Positive | ND | UM | Trisomy 12 | 5–7.5 |
| 16 | 0 | 46.2/42.04 | ND | Positive | UM | Normal | 10–12.5 |
| 17 | III | 19.4/17.04 | Positive | Positive | UM | 13q- | 7.5–10.0 |
| 18 | I | 31.1/26.74 | Negative | Negative | ND | Normal | 7.5–10.0 |
ND: Not Done
Cut off value ≥30%
Cut off value ≥20%
To examine whether curcumin-induced apoptosis involved PARP cleavage, we analyzed lysates obtained from curcumin-treated CLL B-cells by Western blot. PARP was cleaved from the native form (116 kD) into its 85 kD signature polypeptide after treatment with curcumin (Fig. 1B). Activation of upstream effector or initiator caspases (e.g., caspases 3 and 9) was not observed with curcumin treatment (data not shown), suggesting that curcumin-induced apoptosis is not dependent on the caspase pathway. To confirm curcumin induced apoptosis was caspase-independent; CLL B-cells were treated with curcumin in the presence or absence of the pan-caspase inhibitor zVAD-fmk. Treatment of CLL B-cells with zVAD-fmk (100 μM) failed to protect them from curcumin-induced apoptosis (data not shown).
Curcumin inhibits pro-survival pathways in CLL B-cells
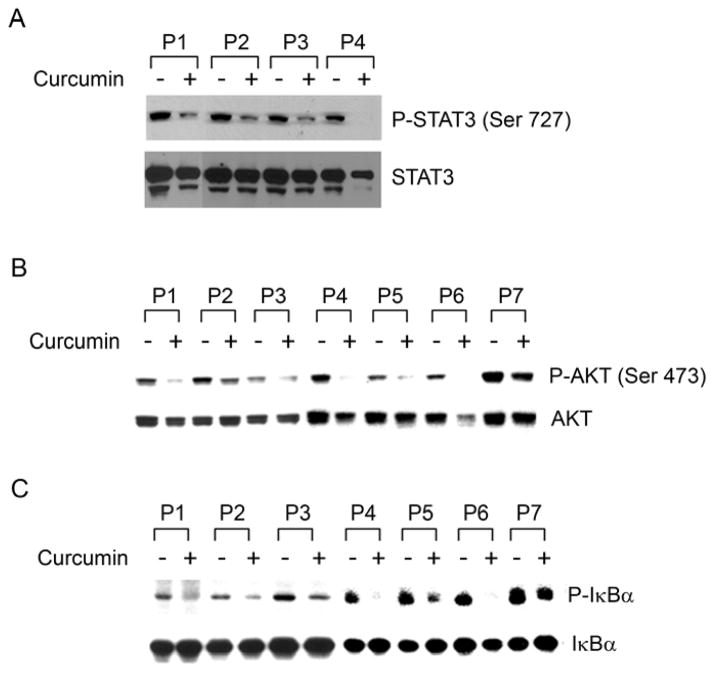
A number of pro-survival signaling pathways are known to be constitutively activated in CLL B-cells including STAT3, PI3-kinase and NF-κB [reviewed in(30) and (13)]. Therefore, to examine the effect of curcumin on these survival pathways, we analyzed lysates from primary CLL B-cells following treatment with curcumin by Western blot (Fig. 2). Curcumin treatment decreased phosphorylation of both STAT3 (Fig. 2A) and AKT (Fig. 2B), a downstream target of activated PI3-kinase. In most cell types, NF-κB is present constitutively in the cytosol in a latent, inactive form where it is retained through its interaction with inhibitory IκB (inhibitor of NF-κB) proteins, masking its nuclear localization sequence. A variety of stimuli induce phosphorylation of IκB at N-terminal serine residues by the IκB kinase complex, followed by ubiquitination and degradation of IκB by the proteosome (38). Its degradation leads to activation of NF-κB complex with subsequent translocation to the nucleus, where it can induce transcription of its target genes. However, CLL B-cells express constitutively activated NF-κB (11) and consistent with the previous report (33), we also found curcumin inhibited phosphorylation of IκBα in CLL B-cells (Fig. 2C) suggesting NF-κB inhibition.
Fig. 2. Curcumin inhibits pro-survival signaling pathways active in CLL B-cells.
Curcumin-treated CLL B-cells were analyzed by Western blot using phospho-specific antibodies to assess the phosphorylation profile of the pro-survival signaling molecules known to be constitutively elevated in CLL including: (A) STAT3 (n=4), (B) AKT (n=7) and (C) IκBα (n =7). Curcumin treatment decreased phosphorylation levels of STAT3, AKT, and IκBα in primary CLL B-cells. Total STAT3, AKT and IκBα were used as loading controls for the respective experiments. Representative figures of, at least, ten CLL B patients’ samples.
Curcumin modulates expression of anti- and pro-apoptotic proteins in CLL B-cells
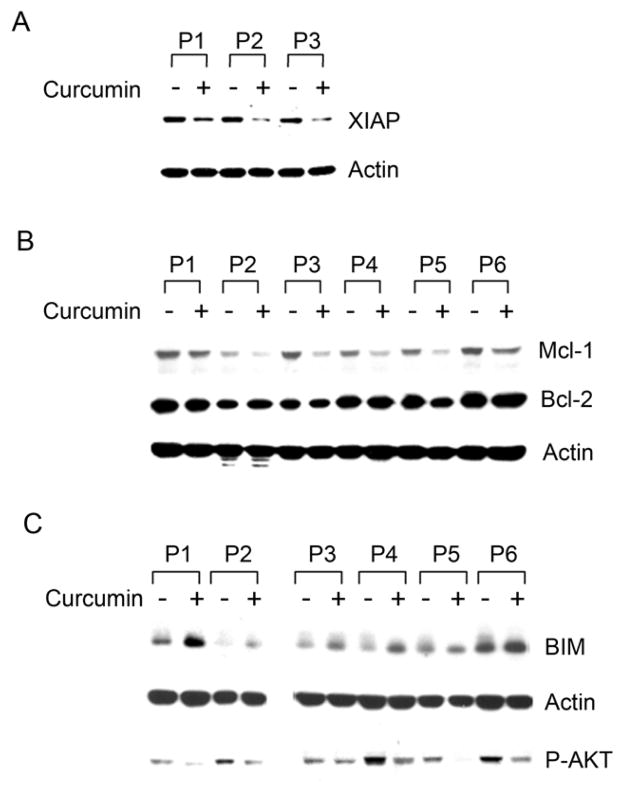
Several anti-apoptotic proteins including Mcl-1, XIAP and Bcl-2 are elevated in CLL B-cells and contribute to apoptotic resistance. Since Mcl-1 is the downstream target of STAT3 and NF-κB regulates the expression of XIAP, survivin and Bcl-2 (39, 40), we analyzed the lysates of CLL B-cells treated with curcumin for the expression of XIAP (Fig. 3A), Mcl-1 and Bcl-2 (Fig. 3B) by Western blot. We found that curcumin did decrease the expression of both XIAP and Mcl-1 in CLL B-cells while Bcl-2 and survivin (data not shown) expression levels remained unaltered. Together, these results suggest that curcumin treatment reduces XIAP and Mcl-1 levels in CLL B-cells possibly through the inhibition of the upstream pro-survival signaling pathways STAT3 and NF-κB.
Fig. 3. Curcumin modulates the expression of certain pro- and anti-apoptotic proteins in CLL B-cells.
Lysates of CLL B-cells isolated from various patients as indicated treated with curcumin were analyzed to assess the effect of curcumin on the anti-apoptotic proteins XIAP (n=3), Mcl-1 (n=6), and Bcl-2 (n=6) as well as the pro-apoptotic protein BIM (n=6) using specific antibodies. Actin was used as the loading control. Curcumin treatment of CLL B-cells suppressed the expression of XIAP (A) and Mcl-1 (B, top row) but, not Bcl-2 (B, middle row). Curcumin treatment of CLL B-cells also resulted in up-regulation of the pro-apoptotic protein BIM expression (C, top row). AKT is the upstream negative regulator of BIM expression. Inhibition of AKT-phosphorylation by curcumin (C, bottom row) is also shown.
One major way by which AKT mediates cell survival is through the phosphorylation and inactivation of Forkhead box class O (FOXO) proteins, a family of transcription factors regulating cell proliferation, survival, and stress responses (41). Recently, it has been found that FOXO3a is constitutively phosphorylated on its AKT target site Thr32 in B-CLL cells (42), suggesting constitutive inactivation of FOXO3a. One key FOXO target gene is BIM, a BH3 domain protein that is capable of inducing apoptosis (43, 44). Given the inhibitory effects of curcumin on AKT phosphorylation, we then examined BIM expression in CLL B-cells after treatment with curcumin. Curcumin treatment increased expression of BIM in CLL B-cells, a finding which correlated with AKT inhibition (Fig. 3C). However, we did not observe up-regulation of other pro-apoptotic proteins of the Bcl-2 family including Bid, Bad or Bax in CLL B-cells following curcumin treatment (data not shown). Together, these data suggest that curcumin inhibits the expression of constitutively elevated anti-apoptotic proteins (XIAP and Mcl-1) and specifically up-regulates the pro-apoptotic protein BIM in CLL B-cells.
Curcumin overcomes stromal protection of CLL B-cells
Previously, we have demonstrated that stromal cells protect CLL B-cells from spontaneous and drug-induced apoptosis through both soluble and contact mediated interactions (14). To begin to dissect the mechanism of stromal cell mediated protection of CLL B-cells, we analyzed pro-survival signaling pathways and anti-apoptotic protein levels in CLL B-cells after co-culture of CLL B-cells with the human stromal cell line HS-5 (separated by transwells). After a 48-hour co-culture with HS-5 in transwells, CLL B-cells were lysed and analyzed for the activation of STAT3, AKT, and NF-κB by Western blot. We found that co-culture with stromal cells increased phosphorylation of STAT3 (serine-727) (Fig. 4A), but not NF-κB or AKT (data not shown) in CLL B-cells. Interestingly, this increase in STAT3-phosphorylation appeared to be due to an increase in the level of total STAT3 protein rather than simply an effect on phosphorylation. Co-culture of CLL B-cells with stromal cells also increased expression of Mcl-1 (Fig. 4B) and XIAP (Fig. 4C, top row), although Bcl-2 levels remained unaltered (Fig. 4C, middle row). Similar results were found when CLL B-cells were co-cultured in direct physical contact with the stromal cells (data not shown). Together, these results suggest that soluble factors secreted in the co-culture system of CLL B and HS-5 human stromal cells as well as direct contact between the latter two cell types induce up-regulation and activation of STAT3 and increased levels of XIAP and Mcl-1 in CLL B-cells which are likely responsible, at least in part, for stromal cell mediated protection.
Fig. 4. Coculture of CLL B-cells with HS-5 modulates STAT3 activation and apoptosis-regulatory proteins.
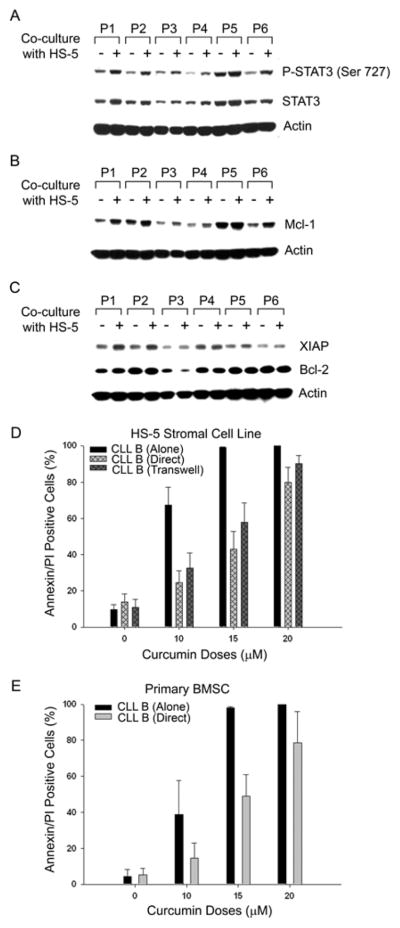
(A) Primary CLL B-cells cocultured with HS-5 human stromal cells in transwells for 48 hours were harvested and the cell lysates (n=6) were analyzed for enhancement of STAT3 activation by western blot using a specific antibody to phosphorylated STAT3 (Ser 727). Total STAT3 level was also analyzed by stripping the membrane using a specific antibody. Actin was used as the loading control. Up-regulation of total STAT3 and enhancement of STAT3 activation from the basal level was observed when CLL B-cells were cocultured with stromal cells. (B & C) CLL B-cell lysates described in (A) were also analyzed for the expression of Mcl-1, XIAP and Bcl-2 by Western blot. Co-culture with stromal cells resulted in increased expression of Mcl-1 (B) and XIAP (C top row) in CLL B-cells. Bcl-2 expression remained unaltered (C middle row). (D) Modulation of curcumin induced apoptosis in CLL B cells when cocultured with stromal cells. Primary CLL B-cells (n=9) were cultured alone or together with HS-5 human stromal cells in either transwells or direct cell contact for 24 hours. Cells were then treated with the indicated doses of curcumin for 24 hours. Cells were harvested, stained with CD19-APC and annexin-FITC/PI by flow cytometric analysis. Viability of CD19+ lymphocytes was assessed and is represented by mean values with standard error bars. Higher dose curcumin (20 μM) appeared to overcome the effects of stromal protection.
We next examined whether curcumin was able to overcome this stromal protection of CLL B-cells. For this, CLL B-cells were cultured either alone or with human stromal cells [HS-5 or primary bone marrow stroma (14)] in either direct contact or in a transwell system. After 24 hours of co-culture, curcumin was added at various increasing concentrations (0, 10, 15 and 20 μM) and cells were cultured for an additional 24 hours. CLL B-cells were then harvested and analyzed for apoptosis by staining with annexin/PI. Co-culture with HS-5 stromal cells provided substantial protection of CLL B-cells against apoptosis at lower doses of curcumin (10–15 μM); however, a higher dose (20 μM) of curcumin was able to overcome stromal protection (Fig. 4D). We also observed similar results when primary human bone marrow cells (14) were used as the source of stromal cells for these experiments (data not shown).
Combination treatment with curcumin and the dietary polyphenol EGCG increases death in CLL B-cells
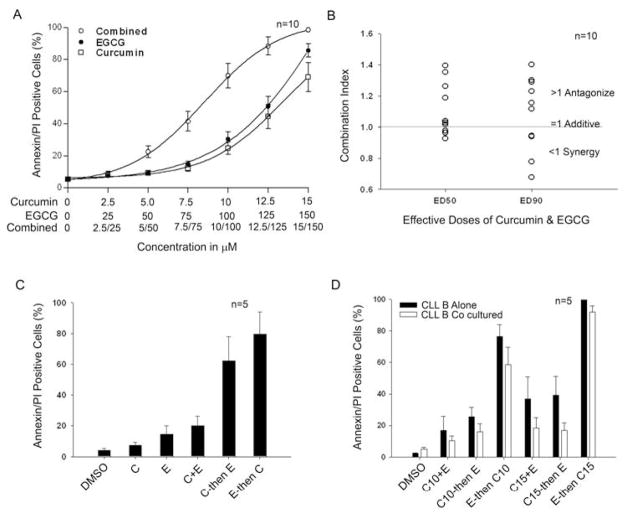
We previously demonstrated that the dietary polyphenol EGCG induces apoptosis in CLL B-cells in vitro (10), and this agent is currently in phase II testing as a treatment for CLL (27). To evaluate the combined effect of these two dietary products with favorable toxicity profiles in initial human (45) testing, we treated primary CLL B-cells (n=10) with either curcumin (2.5–15 μM) or EGCG (25–150 μM) alone or in combination at a constant ratio (1:10). Following 24-hour treatment, cells were harvested and induction of cell death was assessed using annexin/PI staining. On average, the combination of curcumin and EGCG appeared to increase apoptosis more than treatment with the individual drug alone (Fig. 5A).
Fig. 5. Effect of combination treatment with curcumin and EGCG on primary CLL B-cell survival.
(A) Primary CLL B-cells (n=10) were treated with increasing doses of curcumin or EGCG alone or in combination using a constant ratio (1:10). After 24 hours of treatment, viability was assessed using annexin/PI staining. The mean value at each dose level is represented in the figure along with the standard error. (B) Combination index values at effective dose (ED) 50 (50% cell death) and ED90 (90% cell death) for curcumin and EGCG (constant ratio 1:10) for CLL B-cells from 10 patients were calculated using Calcusyn software. Values less than 1 imply synergy; values equal to 1 imply an additive effect; and values greater than 1 imply antagonism. As shown, the simultaneous administration of curcumin and EGCG led to antagonism in the majority of patients tested. (C) Sequential treatment is superior to concurrent therapy. Based on the results in Panel B suggesting that although both agents have single-agent activity they are antagonistic in the majority of patients when administered simultaneously, we next evaluated the effect of sequential administration. CLL B-cells were cultured (n=5) with sub-lethal doses of curcumin (C, 10 μM), EGCG (E, 100 μM) or both drugs together (C+E) for 24 hours. Cells were then washed and cultured for another 24 hours in either media alone or with the second agent (C then E; E then C) for an additional 24 hours using the same doses. Cells were harvested and apoptosis assessed using annexin/PI staining as analyzed by flow cytometry. The results show sequential administration (C then E; E then C) was dramatically superior to simultaneous administration (C+E) and that the E then C sequence appeared superior to the reverse. (D) Sequential treatment of CLL B cells with curcumin/EGCG overcomes stromal protection. CLL B-cells (n=5) were treated with curcumin (C), at 10 and 15 μM), EGCG (E) at 100 μM or together (C10+E and C15+E) either cocultured in direct contact with HS-5 stromal cells or cultured alone for 24 hours. Cells were then washed and cultured for another 24 hours in either media alone or with the second agent (C10 then E; C15 then E; E then C10 and E then C15) for an additional 24 hours. Cells were harvested and induction of apoptosis was assessed using annexin/PI staining as analyzed by flow cytometry. The results show sequential administration (E then C) was dramatically superior to simultaneous administration (C+E) or the reverse sequence and that this approach can overcome stromal protection. Results are presented as mean values with standard error bars.
To more accurately determine the effects (additive, synergistic, or antagonistic) of combination therapy on the individual sample, these results were evaluated mathematically using the method of Chou and Toulay (36). Although synergy (i.e., combination index <1.0) was observed in 4 of 10 samples, combination therapy was actually antagonistic (i.e., combination index >1.0) in the remaining 6 cases (Fig. 5B). Therefore, based on these results suggesting both the agents had single-agent activity but that they are antagonistic in the majority of patients when administered simultaneously, we evaluated the effect of sequential administration on induction of CLL B-cell death. For these experiments, CLL B-cells were cultured with sub-lethal doses of either curcumin (10 μM), EGCG (100 μM) or both drugs together for 24 hours. Cells were then washed and immediately cultured for another 24 hours in either media alone or with the second agent (EGCG or curcumin) for an additional 24 hours. Cells were harvested and induction of apoptosis was assessed using annexin/PI staining as analyzed by flow cytometry. Consistent with the suggestion of antagonism indicated by the method of Chou and Talalay (Fig. 5B), simultaneous culture had a less than additive effect. In contrast, sequential exposure to EGCG and curcumin appeared to have more than an additive effect (Fig. 5C). Remarkably, the sequence of exposure also appeared to impact leukemic cell death, where exposure to EGCG followed by curcumin induced more apoptosis than the reverse sequence. This observation was consistent in samples from all 5 patients studied. Thus, these results suggest that pre-treatment with either drug can sensitize the CLL B-cells to the second drug (EGCG or curcumin) when administered in a sequential fashion and that sequential administration led to dramatically more leukemic cell death than simultaneous administration.
Sequential treatment with EGCG and Curcumin overcomes stromal protection of CLL B-cells
Building on these experiments which demonstrated sequential administration was superior to concurrent treatment when cells were cultured in media alone, we next evaluated the ability of sequential treatment to overcome stromal mediated protection. For these experiments freshly isolated CLL B-cells were treated with the EGCG and curcumin concurrently or sequentially (as described above) in either media alone or in direct contact with HS-5 human stromal cells. These experiments demonstrated that sequential therapy was superior to concurrent therapy and that the EGCG then curcumin sequence was superior to the reverse sequence (Fig. 5D) in for overcoming stromal mediated survival of CLL B-cells.
Discussion
In this study, we demonstrate that curcumin is a potent cytotoxic agent for primary CLL B-cells that inhibits specific pro-survival pathways known to be relevant to CLL B-cell biology. Importantly, our data demonstrate that curcumin differs from most other therapeutic agents currently under study in lymphoid malignancies. First, curcumin-induced apoptosis in CLL B-cells is not dependent on caspase activation. Second, curcumin specifically down-regulated Mcl-1 and XIAP without discernible effects on levels of Bcl-2 or survivin, a finding that is relatively uncommon among most therapeutics used to treat lymphoid malignancy (46). Third, the biologic effects of curcumin on CLL B-cells seems to be explained, at least in part, by inhibition of the pro-survival pathways known to be constitutively active in CLL B-cells including STAT3, AKT, and NF-κB. Although co-culture of CLL B-cells with stromal cells selectively up-regulates STAT3 protein and its phosphorylation status, curcumin was still able to overcome the stromal-induced protection at higher doses or when administered with EGCG in a sequential fashion. Finally, it is known that curcumin is well tolerated and showed minimal toxicity at up to 8 g/day (approximately, 115 mg/kg/day) in a phase I clinical trial (47). Collectively, this data indicates that the use of curcumin in the treatment of CLL may be worthy of study.
Curcumin has been shown to inhibit neoplastic initiation, promotion, and progression in several cancers where numerous mechanisms have been proposed to account for the ability of curcumin to induce apoptosis in malignant cells. We have examined the potential relevance of those mechanisms that are more specific to CLL leukemic B biology: inhibition of STAT3, AKT and NF-κB signaling pathways (13, 48). We confirm the findings of Everett and colleagues (33) that curcumin reduces the constitutive phosphorylation level of IκBα, suggesting an inhibition of NF-κB active in CLL B-cells. Our analysis demonstrates curcumin induces a significant decrease in XIAP expression, an anti-apoptotic protein elevated in CLL and also a downstream target of NF-κB pathway. Curcumin treatment inhibited STAT3 activity and decreased the expression of Mcl-1, a downstream target of activated STAT3, in the majority of CLL B-cells. Curcumin treatment of CLL B-cells also reduced the phosphorylation level of AKT. The serine/threonine kinase AKT has been considered an attractive target for cancer therapy and prevention (49). AKT kinase plays critical roles in mammalian cell survival and is constitutively active in various cancers including CLL (48). Since curcumin inhibited AKT, we evaluated its effects on the pro-apoptotic protein BIM, a downstream target of AKT. Indeed, we found that curcumin treatment of primary CLL B-cells resulted in increased expression of BIM which correlated well with inhibition of AKT (Fig. 3C). The precise mechanism by which curcumin inhibited these constitutively active pro-survival pathways in CLL B-cells remains unclear.
While numerous compounds have been shown to induce apoptosis when CLL B-cells are cultured in isolation, in their tissue microenvironment these cells experience a variety of nurturing signals through interactions with stromal cells. Modulators of CLL B-cell survival are complex and include both soluble factors and signals derived from direct cell-cell contact (20). Extensive work has indicated that stromal cells play an important role in progression of cancers and protect the malignant cells from apoptosis induced by various chemotherapeutic agents (20). For maximal efficacy, therapeutic strategies able to overcome stromal mediated protection are needed. To design and develop such strategies, it is necessary to understand precisely what signaling pathways are modulated through leukemic cell stromal cell interactions. Our work here further demonstrates that soluble factors produced on co-culture of marrow stroma and primary CLL B-cells increase expression and enhance the phosphorylation status of STAT3. Moreover, soluble factors induced enhanced expression of the anti-apoptotic proteins Mcl-1 and XIAP, which could explain increased resistance of CLL B-cells to apoptosis in presence of stromal cells.
We performed additional experiments to evaluate the effect of curcumin on CLL B-cells when leukemic cells were allowed to interact with human stromal cells. Similar to other agents (20), we found that co-culture with bone marrow stromal cells protect CLL B-cells from curcumin-induced apoptosis. Although higher dose (20 μM) of curcumin was able to overcome the protection of CLL B-cells by marrow stroma, it is not known if these levels can be achieved in vivo.
We believe it is unlikely that a single-agent, no matter how active, will be able to cure this disease. Therefore, to evaluate the effect of curcumin in combination with other agents, we explored the combination of curcumin with the green tea extract, EGCG (10). This combination was selected since both are dietary products with favorable toxicity profiles in phase I trials (14, 27, 47) that may be an attractive combination for clinical testing in patients with early-stage disease. Although on average we found that the combination of curcumin and EGCG increased the apoptotic cell death of CLL B-cells compared to either drug alone, mathematical modeling of the combined effects at the level of the individual patient demonstrated antagonism in the majority of patients (6 of 10 samples). This observation prompted us to examine the effect of sequential administration of curcumin and EGCG. Sequential exposure to these agents was superior to simultaneous treatment with a 3- to 4-fold increase in cell death compared to simultaneous administration. The sequence of administration also appeared to impact efficacy where EGCG followed by curcumin induced more cell death than the reverse sequence. Sequential administration of these two agents was also able to overcome stromal mediated protection of CLL B-cells at lower curcumin doses. These preclinical results strongly encourage us to use a sequential approach in designing subsequent clinical trials with these agents. In addition, these studies indicate how in vitro experiments can inform the design of clinical trials beyond simply a summary curve such as that in Fig. 5A. Subsequent assessment of association with clinical outcome will be of great interest.
In conclusion, the present study indicates that curcumin is cytotoxic to primary CLL B-cells. This cytotoxic effect of curcumin is complex and is associated with the inhibition of pro-survival pathways, down-regulation of anti-apoptotic proteins Mcl-1 and XIAP which are characteristics associated with leukemic cell resistance to chemotherapeutic agents (1, 3, 4, 6) and increased expression of the pro-apoptotic protein BIM. Importantly, curcumin treatment was able to overcome protection of CLL B-cells by marrow stroma on in vitro testing and to synergize with EGCG when the agents were administered in a sequential fashion. Additional evaluation of curcumin as a potential therapeutic agent for the treatment of CLL appears warranted.
Statement of Clinical Relevance.
The authors evaluate the effects of the spice curcumin on primary chronic lymphocytic leukemia cells in vitro. These experiments confirm that curcumin induces cell death in these leukemic cells and begins to dissect both the mechanisms of these effects and how they are modulated by leukemia cell-stromal cell interactions. Given the tolerability of curcumin in initial human testing, the results of these studies provide insight for the design of potential clinical testing of this agent in chronic lymphocytic leukemia.
Acknowledgments
Support through grants from the National Cancer Institute (NCI CA113408; NCI CA95241) CLL Global Research Foundation, CLL Topics, and Polyphenon E International are gratefully acknowledged.
Footnotes
Contribution: A.K.G., T.D.S. and N.E.K. designed the research; A.K.G. and C.R.S. performed the experiments; A.K.G. and T.D.S. analyzed the data and wrote the paper; A.K.G., T.D.S. and N.E.K. approved the final manuscript.
Conflict of Interest: T.D.S. and N.E.K. receive research grant support from Polyphenon E International.
References
- 1.Keating MJ. Chronic lymphocytic leukemia [Review] [65 refs] Seminars in Oncology. 1999;26:107–14. [PubMed] [Google Scholar]
- 2.Messmer BT, Messmer D, Allen SL, et al. In vivo measurements document the dynamic cellular kinetics of chronic lymphocytic leukemia B cells. J Clin Invest. 2005;115:755–64. doi: 10.1172/JCI23409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reed JC. Molecular biology of chronic lymphocytic leukemia. Semin Oncol. 1998;25:11–8. [PubMed] [Google Scholar]
- 4.Thomas A, El Rouby S, Reed JC, et al. Drug-induced apoptosis in B-cell chronic lymphocytic leukemia: relationship between p53 gene mutation and bcl-2/bax proteins in drug resistance. Oncogene. 1996;12:1055–62. [PubMed] [Google Scholar]
- 5.McConkey DJ, Chandra J, Wright S, et al. Apoptosis sensitivity in chronic lymphocytic leukemia is determined by endogenous endonuclease content and relative expression of BCL-2 and BAX. J Immunol. 1996;156:2624–30. [PubMed] [Google Scholar]
- 6.Saxena A, Viswanathan S, Moshynska O, Tandon P, Sankaran K, Sheridan D. Mcl-1 and Bcl-2/Bax ratio are associated with treatment response but not with Rai stage in B-cell chronic lymphocytic leukeia. American journal of hematology. 2004;75:22–33. doi: 10.1002/ajh.10453. [DOI] [PubMed] [Google Scholar]
- 7.Bernal A, Pastore RD, Asgary Z, et al. Survival of leukemic B cells promoted by engagement of the antigen receptor. Blood. 2001;98:3050–7. doi: 10.1182/blood.v98.10.3050. [DOI] [PubMed] [Google Scholar]
- 8.Frank DA, Mahajan S, Ritz J. B lymphocytes from patients with chronic lymphocytic leukemia contain signal transducer and activator of transcription (STAT) 1 and STAT3 constitutively phosphorylated on serine residues. J Clin Invest. 1997;100:3140–8. doi: 10.1172/JCI119869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee YK, Shanafelt TD, Bone ND, Strege AK, Jelinek DF, Kay NE. VEGF receptors on chronic lymphocytic leukemia (CLL) B cells interact with STAT 1 and 3: implication for apoptosis resistance. Leukemia. 2005;19:513–23. doi: 10.1038/sj.leu.2403667. [DOI] [PubMed] [Google Scholar]
- 10.Lee YK, Bone ND, Strege AK, Shanafelt TD, Jelinek DF, Kay NE. VEGF receptor phosphorylation status and apoptosis is modulated by a green tea component, epigallocatechin-3-gallate (EGCG), in B-cell chronic lymphocytic leukemia. Blood. 2004;104:788–94. doi: 10.1182/blood-2003-08-2763. [DOI] [PubMed] [Google Scholar]
- 11.Furman RR, Asgary Z, Mascarenhas JO, Liou HC, Schattner EJ. Modulation of NF-kappa B activity and apoptosis in chronic lymphocytic leukemia B cells. J Immunol. 2000;164:2200–6. doi: 10.4049/jimmunol.164.4.2200. [DOI] [PubMed] [Google Scholar]
- 12.Barragan M, Bellosillo B, Campas C, Colomer D, Pons G, Gil J. Involvement of protein kinase C and phosphatidylinositol 3-kinase pathways in the survival of B-cell chronic lymphocytic leukemia cells. Blood. 2002;99:2969–76. doi: 10.1182/blood.v99.8.2969. [DOI] [PubMed] [Google Scholar]
- 13.Ringshausen I, Schneller F, Bogner C, et al. Constitutively activated phosphatidylinositol-3 kinase (PI-3K) is involved in the defect of apoptosis in B-CLL: association with protein kinase Cdelta. Blood. 2002;100:3741–8. doi: 10.1182/blood-2002-02-0539. [DOI] [PubMed] [Google Scholar]
- 14.Kay NE, Shanafelt TD, Strege AK, Lee YK, Bone ND, Raza A. Bone biopsy derived marrow stromal elements rescue chronic lymphocytic leukemia B-cells from spontaneous and drug induced cell death and facilitates an “angiogenic switch”. Leuk Res. 2007;31:899–906. doi: 10.1016/j.leukres.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burger JA, Burger M, Kipps TJ. Chronic lymphocytic leukemia B cells express functional CXCR4 chemokine receptors that mediate spontaneous migration beneath bone marrow stromal cells. Blood. 1999;94:3658–67. [PubMed] [Google Scholar]
- 16.Burger JA, Tsukada N, Burger M, Zvaifler NJ, Dell’Aquila M, Kipps TJ. Blood-derived nurse-like cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through stromal cell-derived factor-1. Blood. 2000;96:2655–63. [PubMed] [Google Scholar]
- 17.Panayiotidis P, Jones D, Ganeshaguru K, Foroni L, Hoffbrand A. Human bone marrow stromal cells prevent apoptosis and support the survival of chronic lymphocytic leukaemia cells in vitro. British journal of haematology. 1996;92:97–103. doi: 10.1046/j.1365-2141.1996.00305.x. [DOI] [PubMed] [Google Scholar]
- 18.Lagneaux L, Delforge A, Bron D, De Bruyn C, Stryckmans P. Chronic lymphocytic leukemic B cells but not normal B cells are rescued from apoptosis by contact with normal bone marrow stromal cells. Blood. 1998;91:2387–96. [PubMed] [Google Scholar]
- 19.Burger JA, Zvaifler NJ, Tsukada N, Firestein GS, Kipps TJ. Fibroblast-like synoviocytes support B-cell pseudoemperipolesis via a stromal cell-derived factor-1- and CD106 (VCAM-1)-dependent mechanism. J Clin Invest. 2001;107:305–15. doi: 10.1172/JCI11092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pedersen IM, Kitada S, Leoni LM, et al. Protection of CLL B cells by a follicular dendritic cell line is dependent on induction of Mcl-1. Blood. 2002;100:1795–801. [PubMed] [Google Scholar]
- 21.Plate JM, Long BW, Kelkar SB. Role of beta2 integrins in the prevention of apoptosis induction in chronic lymphocytic leukemia B cells. Leukemia. 2000;14:34–9. doi: 10.1038/sj.leu.2401621. [DOI] [PubMed] [Google Scholar]
- 22.de la Fuente MT, Casanova B, Garcia-Gila M, Silva A, Garcia-Pardo A. Fibronectin interaction with alpha4beta1 integrin prevents apoptosis in B cell chronic lymphocytic leukemia: correlation with Bcl-2 and Bax. Leukemia. 1999;13:266–74. doi: 10.1038/sj.leu.2401275. [DOI] [PubMed] [Google Scholar]
- 23.Buske C, Gogowski G, Schreiber K, Rave-Frank M, Hiddemann W, Wormann B. Stimulation of B-chronic lymphocytic leukemia cells by murine fibroblasts, IL-4, anti-CD40 antibodies, and the soluble CD40 ligand. Exp Hematol. 1997;25:329–37. [PubMed] [Google Scholar]
- 24.Kitada S, Zapata JM, Andreeff M, Reed JC. Bryostatin and CD40-ligand enhance apoptosis resistance and induce expression of cell survival genes in B-cell chronic lymphocytic leukaemia. British journal of haematology. 1999;106:995–1004. doi: 10.1046/j.1365-2141.1999.01642.x. [DOI] [PubMed] [Google Scholar]
- 25.Granziero L, Ghia P, Circosta P, et al. Survivin is expressed on CD40 stimulation and interfaces proliferation and apoptosis in B-cell chronic lymphocytic leukemia. Blood. 2001;97:2777–83. doi: 10.1182/blood.v97.9.2777. [DOI] [PubMed] [Google Scholar]
- 26.Shanafelt TD, Lee YK, Call TG, et al. Clinical effects of oral green tea extracts in four patients with low grade B-cell malignancies. Leuk Res. 2006;30:707–12. doi: 10.1016/j.leukres.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 27.Shanafelt TD, Kaufmann SH, Call TG, et al. A Phase I Trial of Daily Oral Green Tea Extract in Asymptomatic, Rai Stage 0-II Patients with Chronic Lymphocytic Leukemia. Blood - American Society of Hematology - 49th Annual Meeting. 2007;110:610a. (abst #2047) [Google Scholar]
- 28.Aggarwal BB, Sundaram C, Malani N, Ichikawa H. Curcumin: the Indian solid gold. Adv Exp Med Biol. 2007;595:1–75. doi: 10.1007/978-0-387-46401-5_1. [DOI] [PubMed] [Google Scholar]
- 29.Aggarwal S, Ichikawa H, Takada Y, Sandur SK, Shishodia S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of IkappaBalpha kinase and Akt activation. Mol Pharmacol. 2006;69:195–206. doi: 10.1124/mol.105.017400. [DOI] [PubMed] [Google Scholar]
- 30.Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as “Curecumin”: from kitchen to clinic. Biochemical pharmacology. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 31.Bae MK, Kim SH, Jeong JW, et al. Curcumin inhibits hypoxia-induced angiogenesis via down-regulation of HIF-1. Oncol Rep. 2006;15:1557–62. [PubMed] [Google Scholar]
- 32.Lin YG, Kunnumakkara AB, Nair A, et al. Curcumin inhibits tumor growth and angiogenesis in ovarian carcinoma by targeting the nuclear factor-kappaB pathway. Clin Cancer Res. 2007;13:3423–30. doi: 10.1158/1078-0432.CCR-06-3072. [DOI] [PubMed] [Google Scholar]
- 33.Everett PC, Meyers JA, Makkinje A, Rabbi M, Lerner A. Preclinical assessment of curcumin as a potential therapy for B-CLL. American journal of hematology. 2007;82:23–30. doi: 10.1002/ajh.20757. [DOI] [PubMed] [Google Scholar]
- 34.Cheson BD, Bennett JM, Grever M, et al. National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood. 1996;87:4990–7. [PubMed] [Google Scholar]
- 35.Roecklein BA, Torok-Storb B. Functionally distinct human marrow stromal cell lines immortalized by transduction with the human papilloma virus E6/E7 genes. Blood. 1995;85:997–1005. [PubMed] [Google Scholar]
- 36.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Advances in Enzyme Regulation. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 37.Chang T, Chou T. Rational approach to the clinical protochol design for drug combinations: a review. Acta Paediatr Taiwan. 2000;41:294–302. [PubMed] [Google Scholar]
- 38.Gilmore TD. The Rel/NF-kappaB signal transduction pathway: introduction. Oncogene. 1999;18:6842–4. doi: 10.1038/sj.onc.1203237. [DOI] [PubMed] [Google Scholar]
- 39.Cuni S, Perez-Aciego P, Perez-Chacon G, et al. A sustained activation of PI3K/NF-kappaB pathway is critical for the survival of chronic lymphocytic leukemia B cells. Leukemia. 2004;18:1391–400. doi: 10.1038/sj.leu.2403398. [DOI] [PubMed] [Google Scholar]
- 40.Kawakami H, Tomita M, Matsuda T, et al. Transcriptional activation of survivin through the NF-kappaB pathway by human T-cell leukemia virus type I tax. International journal of cancer. 2005;115:967–74. doi: 10.1002/ijc.20954. [DOI] [PubMed] [Google Scholar]
- 41.Burgering BM, Medema RH. Decisions on life and death: FOXO Forkhead transcription factors are in command when PKB/Akt is off duty. J Leukoc Biol. 2003;73:689–701. doi: 10.1189/jlb.1202629. [DOI] [PubMed] [Google Scholar]
- 42.Ticchioni M, Essafi M, Jeandel PY, et al. Homeostatic chemokines increase survival of B-chronic lymphocytic leukemia cells through inactivation of transcription factor FOXO3a. Oncogene. 2007;26:7081–91. doi: 10.1038/sj.onc.1210519. [DOI] [PubMed] [Google Scholar]
- 43.Dijkers PF, Medema RH, Lammers JW, Koenderman L, Coffer PJ. Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-L1. Curr Biol. 2000;10:1201–4. doi: 10.1016/s0960-9822(00)00728-4. [DOI] [PubMed] [Google Scholar]
- 44.Gilley J, Coffer PJ, Ham J. FOXO transcription factors directly activate bim gene expression and promote apoptosis in sympathetic neurons. J Cell Biol. 2003;162:613–22. doi: 10.1083/jcb.200303026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chow HH, Cai Y, Hakim IA, et al. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin Cancer Res. 2003;9:3312–9. [PubMed] [Google Scholar]
- 46.Liu Q, Zhao X, Frissora F, et al. FTY720 demonstrates promising preclinical activity for chronic lymphocytic leukemia and lymphoblastic leukemia/lymphoma. Blood. 2008;111:275–84. doi: 10.1182/blood-2006-10-053884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheng AL, Hsu CH, Lin JK, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–900. [PubMed] [Google Scholar]
- 48.Shankar S, Chen Q, Sarva K, Siddiqui I, Srivastava RK. Curcumin enhances the apoptosis-inducing potential of TRAIL in prostate cancer cells: molecular mechanisms of apoptosis, migration and angiogenesis. J Mol Signal. 2007;2:10. doi: 10.1186/1750-2187-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–27. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]