Abstract
Ehrlichioses are emerging tick-borne bacterial diseases of humans and animals for which no vaccines are available. The diseases are caused by obligately intracellular bacteria belonging to the genus Ehrlichia. Several immunoreactive proteins of ehrlichiae have been identified based on their reactivity with immune sera from human patients and animals. These include the major outer membrane proteins, ankyrin repeat proteins and tandem repeat proteins (TRP). Polyclonal antibodies directed against the tandem repeats (TRs) of Ehrlichia chaffeensis TRP32, TRP47 and TRP120 have been shown to provide protection in mice. In the present study, we evaluated E. muris P29, which is the ortholog of E. chaffeensis TRP47 and E. canis TRP36, as a subunit vaccine in a mouse model of ehrlichiosis. Our study indicated that unlike E. chaffeensis TRP47 and E. canis TRP36, orthologs of E. muris (P29) and E. muris-like agent (EMLA) do not contain tandem repeats. Immunization of mice with recombinant E. muris P29 induced significant protection against a challenge infection. The protection induced by E. muris P29 was associated with induction of strong antibody responses. In contrast to development of P29-specific IgG antibodies following immunization, development of P29-specific IgG antibodies, but not IgM antibodies, was impaired during persistent E. muris infection. Furthermore, our study indicated that CD4+ T cells target P29 during E. muris infection and differentiate into IFN-γ-producing Th1 effector/memory cells. In conclusion, our study indicated that orthologs of E. muris P29 showed considerable variation in the central tandem repeat region among different species, induction of P29-specific IgG antibody response was impaired during persistent E. muris infection, and rP29 induced protective immune responses.
Keywords: Ehrlichia, intracellular bacteria, vaccine, protective immunity, antigen, antibody
Introduction
Ehrlichioses are emerging tick-transmitted human zoonoses caused by obligately intracellular bacteria belonging to the genus Ehrlichia. Human ehrlichioses are caused by E. chaffeensis, the etiologic agent of human monocytotropic ehrlichiosis (HME), E. ewingii, which causes E. ewingii ehrlichiosis, and the newly discovered E. muris-like agent (EMLA) from the upper midwestern USA [1–3]. Animal pathogens include E. canis and E. ruminantium, which cause canine monocytic ehrlichiosis and heartwater in ruminants, respectively [4]. E. chaffeensis and E. ewingii also infect dogs. Currently human or veterinary vaccines are not commercially available for ehrlichiosis [5;6].
Several antigens of Ehrlichia spp. have been identified based on their reactivity with immune sera from infected hosts that include the major outer membrane proteins (OMP-1/P28) encoded by a multi-gene family, ferric ion-binding protein (Fbp), disulfide bond formation (Dsb) protein, ankyrin repeat proteins, and tandem repeat proteins (TRP) [7–12]. Ortholog tandem repeat proteins of E. chaffeensis and E. canis, TRP120/TRP140, TRP75/TRP95, TRP47/TRP36, and TRP32/TRP19, contain major antibody epitopes in the tandem repeat regions [13–16]. Ehrlichial TRPs are secreted, serine/threonine-rich, and acidic, which results in higher electrophoretic mobility than their predicted molecular masses [17]. The TRPs contain varying numbers of TRs in different ehrlichial species and strains [18;19]. The TRPs interact with a diverse group of host proteins, suggesting functional importance in establishment of a productive infection [20–22]. The protective role of antibodies directed against the E. chaffeensis P28-19 was demonstrated in SCID mice [23]. Recently, we demonstrated the protective roles of Ehrlichia heat-shock protein 60 and the OMPs: P28-9, P28-12, and P28-19 in the E. muris-C57BL/6 mouse model [24;25]. Furthermore, antibodies directed against the major epitopes in the TR regions of E. chaffeensis TRP120, TRP47 and TRP32 inhibit ehrlichial replication in vitro and reduce the bacterial burden in vivo. [26].
Neither E. chaffeensis nor E. ewingii causes disease in immunocompetent mice; thus, surrogate ehrlichial pathogens that infect mice have been used in animal models [24;27–30]. In the present study, we evaluated the recombinant E. muris P29, which is an ortholog of E. chaffeensis TRP47 and E. canis TRP36, as a subunit vaccine candidate in the E.muris-C57BL/6 mouse model. Our study indicated that, unlike E. chaffeensis TRP47 and E. canis TRP36, their orthologs in E. muris (P29) and EMLA do not contain tandem repeats. Immunization with recombinant E. muris P29 conferred significant protection against challenge infection.
Materials and Methods
Mice
Six to eight-week old female C57BL/6 mice used in the study were purchased from the Jackson Laboratory (Bar Harbor, ME) and housed and cared for in the Animal Research Center at the University of Texas Medical Branch. All experiments were carried out in accordance with the protocol (No. 95-09-066) approved by the Institutional Animal Care and Use Committee.
Bacteria
E. muris AS145 strain was cultured in the canine macrophage-like cell line DH82. For infection of mice, ehrlichial stocks were prepared from the spleens of syngeneic mice inoculated by the intraperitoneal (i.p.) route with E. muris grown in DH82 cells as described previously [31].
PCR amplification, cloning and expression of recombinant Ehrlichia proteins
We amplified the E. muris p29 gene by PCR using primers P29F1 – CACCAATATTCATAGTGGGGACAGG and P29R1S – CTAAGCAGCTATTTGTTCACG, which covered the entire ORF except for 17 codons on the 5’ end and 9 codons on the 3’ end. The amplified PCR product was cloned into the pET151/D-TOPO vector (Invitrogen, CA) and expressed as a recombinant protein with an N-terminal tag containing the V5 epitope and a 6xHis-tag. The recombinant histidine-tagged protein was purified by immobilized metal ion affinity chromatography using HisTrap HP columns packed with Ni sepharose (GE Heathcare Life Sciences, NJ). The purified protein was dialyzed against PBS to remove detergents and salts. The N-terminal fusion tag was removed from the recombinant E. muris P29 (rP29) using the Tobacco Etch Virus protease (Invitrogen, CA). The recombinant protein purity was tested by SDS-PAGE, and concentration was determined by the Bradford method.
Bioinformatic analysis
Multiple protein sequences were aligned by the ClustalW method, and similarity index was calculated following pairwise alignment of protein sequences by the Lipman-Pearson method (MegAlign program; DNASTAR Inc.,WI). We used the Tandem Repeats Finder program to identify tandem repeats in the sequences [32].
Animal immunizations and challenge infections
Mice were immunized with recombinant proteins (50 µg per mouse) in complete Freund’s adjuvant (CFA) by the i.p. route, followed by a booster immunization in incomplete Freund’s adjuvant (IFA) 30 days after primary immunization. Mice immunized with recombinant Chlamydia pneumoniae MOMP and mice previously infected with E. muris (E. muris-immune mice) served as controls. All immunized and control mice were challenged with the splenic ehrlichial stock containing ~ 2 × 103 bacteria (low dose) or 1 × 104 E. muris (high dose) by the i.p. route 60 days after the booster immunization.
Determination of ehrlichial copy numbers in splenic stocks and quantification of ehrlichial load in organs
Ehrlichial copy numbers in stocks and organs were determined by a quantitative real-time PCR as described previously [33].
Splenocyte cultures and in vitro assay of CD4+ T cell responses
Frequencies of Ehrlichia-specific IFN-γ-producing CD4+ T cells in the splenocyte population from separate groups of mice infected with E. muris were determined by flow cytometry as described previously [25;34].
Indirect antibody ELISA
Indirect antibody ELISA was performed using a Protein Detector ELISA Kit (KPL, Inc. Gaithersburg, MD). ELISA plates were coated with rP29 (without the N-terminal fusion tag) or E. muris lysate antigen at a concentration of 10 µg/ml (100 µl per well). The plates were incubated with serum samples diluted 1:300. Wells without coated antigen that were reacted with primary and secondary antibodies were used as blanks.
Western immunoblot analysis
Western blot analysis was carried out as described previously [35]. Blots were incubated with serum samples diluted 1:1000 (Figure 2B) or 1:300 (Figure 2C). All gel and western blot images were analyzed using the MyImageAnalysis software version 1.0 (Thermo Fisher Scientific Inc., Rockford, IL).
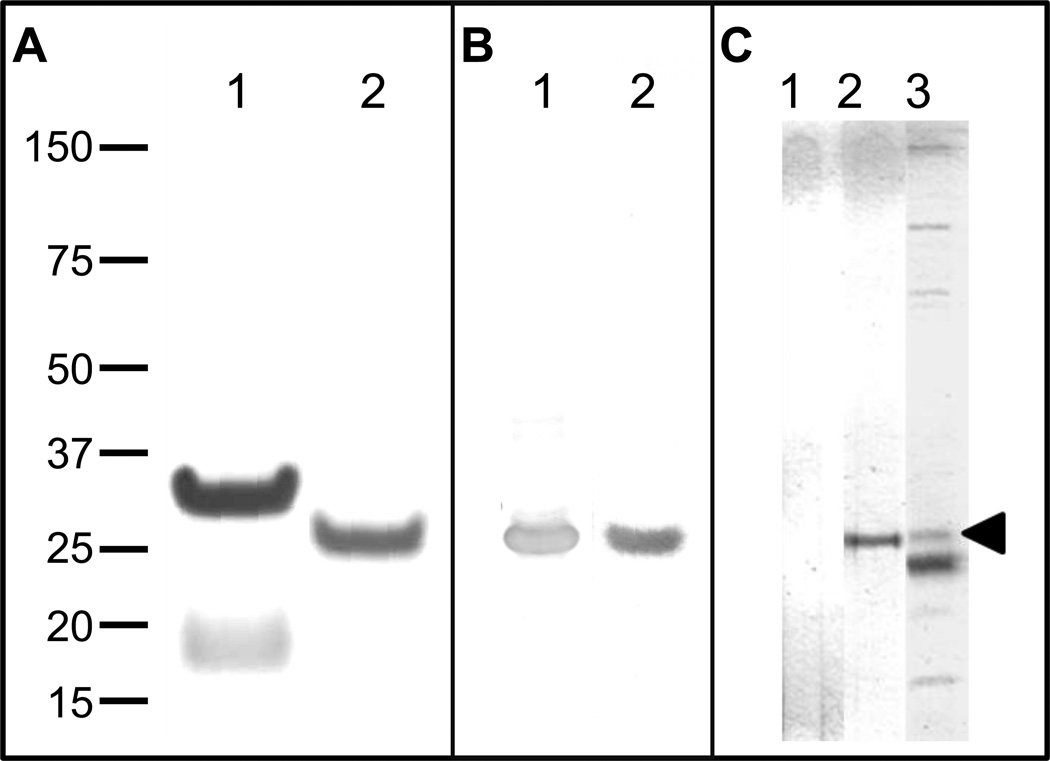
Figure 2. Analysis of recombinant E. muris P29 protein, and identification of native P29 protein.
(A) Purified recombinant E. muris P29 protein with N-terminal fusion tag (lane 1) or without N-terminal fusion tag (lane 2) was analyzed by SDS-PAGE and stained with Coomassie Brilliant Blue G-250. (B) Western-blot analysis of rP29 protein. An anti-rP29 immune serum (lane 1) and an immune-serum from E. muris-infected mice containing detectable concentration of anti-rP29 IgG antibodies by ELISA (lane 2) reacted specifically with rP29. (C) Identification of native E. muris P29 protein. E. muris lysate antigen was separated on a NuPAGE Zoom gel containing an IPG well (Invitrogen) by SDS-PAGE and developed with naïve serum (lane 1), anti-rP29-serum (lane 2) or E. muris-immune serum containing detectable concentration of anti-rP29 IgG antibodies by ELISA (lane 3) using a Mini-PROTEAN II multiscreen apparatus (Bio-Rad laboratories). The anti-rP29-serum recognized a 29 kDa native E. muris protein (lane 2). The arrow head indicates the native P29 protein recognized by the E. muris-immune serum (lane 3). All images were analyzed using the MyImageAnalysis software (Thermo Scientific).
Statistical analysis
The data were square root transformed and analyzed by one way ANOVA with Bonferroni post test for comparison of multiple groups using the GraphPad Prism software. Statistical significance was determined at 95 % (P < 0.05). Data presented are expressed as means plus standard deviations and are representative of two to three independent experiments.
Results
Comparison of orthologs of E. muris P29 protein
We identified the open reading frame (ORF) of E. muris p29 gene from a draft genome of E. muris based on homology to E. chaffeensis trp47 and E. canis trp36 and the synteny. E. muris p29 gene is flanked by the tRNA(Pro)GGG gene on the 5’ end and the 5-amino-6-(5-phosphoribosylamino) uracil reductase (ribD) gene on the 3’ end on the same strand. The E. muris p29 gene is 687 bp long and is predicted to encode a 228 amino acid protein with a predicted molecular mass of 25.17 kDa.
We compared the E. muris P29 with the orthologs in the other ehrlichial genomes (Table 1). E. muris P29 orthologs show high variation in size, which corresponds to differences in the repeat region. Both E. muris P29 and its ortholog in EMLA do not contain tandem repeats compared to the presence of tandem repeats of varying length and number in the orthologs (Table 1). Determination of the similarity index (SI) indicated that E. muris P29 has greatest sequence similarity to EMLA P29 protein followed by E. chaffeensis TRP47 and E. canis TRP36. The calculated isoelectric point (pI) of E. muris P29 was low (4.22) indicative of its acidic nature, which is similar to orthologs in other Ehrlichia (Table 1). It was reported that the tandem repeat region of E. chaffeensis TRP47 is more acidic than the flanking N-terminal and C-terminal non-repeat regions [17]. The predicted pI of the central region of E. muris P29 was 3.71 compared to the predicted pI of 4.76 for the combined N-terminal and C-terminal regions, suggesting the more acidic nature of the central region than the flanking N-terminal and C-terminal regions (data not shown).
Table 1.
Comparison of orthologs of Ehrlichia muris P29 protein
| Organism /strain | Length (aa) | Similarity Index (%)a |
Repeatsb | Percent of protein in repeats (%)d |
pIe | GenBank Accession Number |
|
|---|---|---|---|---|---|---|---|
| No | Length (aa) | ||||||
| E. muris P29 | 228 | - | None | - | 0 | 4.22 | KC595883 |
| E. muris-like agent P29 | 252 | 62.4 | None | - | 0 | 4.26 | K523728 |
| E. chaffeensis TRP47 | |||||||
| Arkansas | 316 | 53.5 | 7 | 19 | 42.08 | 4.03 | DQ085430.1 |
| Sapulpa | 328 | 56.7 | 4.5 | 33 | 40.24 | 4.04 | DQ085431.1 |
| E. canis TRP36 | |||||||
| Jake | 279 | 41.7 | 12.2 | 9 | 38.70 | 3.93 | DQ085427.1 |
| Oklahoma | 218 | 39.3 | 5.2 | 9 | 20.64 | 4.26 | DQ085428.1 |
| E. ruminantium mucin-like protein | |||||||
| Gardel | 530 | 29.9 | 16.9c | 22 | 66.41 | 3.75 | CR925677 |
| Welgevonden | 625 | 29 | 51.8c | 9 | 73.44 | 4.19 | CR925678.1 |
Similarity index was calculated following pairwise alignment of E.muris P29 protein sequences with or thologs in other ehlichial genomes by the Lipman-Pearson method using the MegAlign program (DNASTAR Inc., Madison, WI)
Tandem Repeats Finder program was used to identify the tandem repeats in the sequences
Short four-amino acid tandem repeats of less than three numbers present in these sequences are not included in the analysis
Proportion of protein in repeats expressed in percent
Calculated isoelectric point (pI)
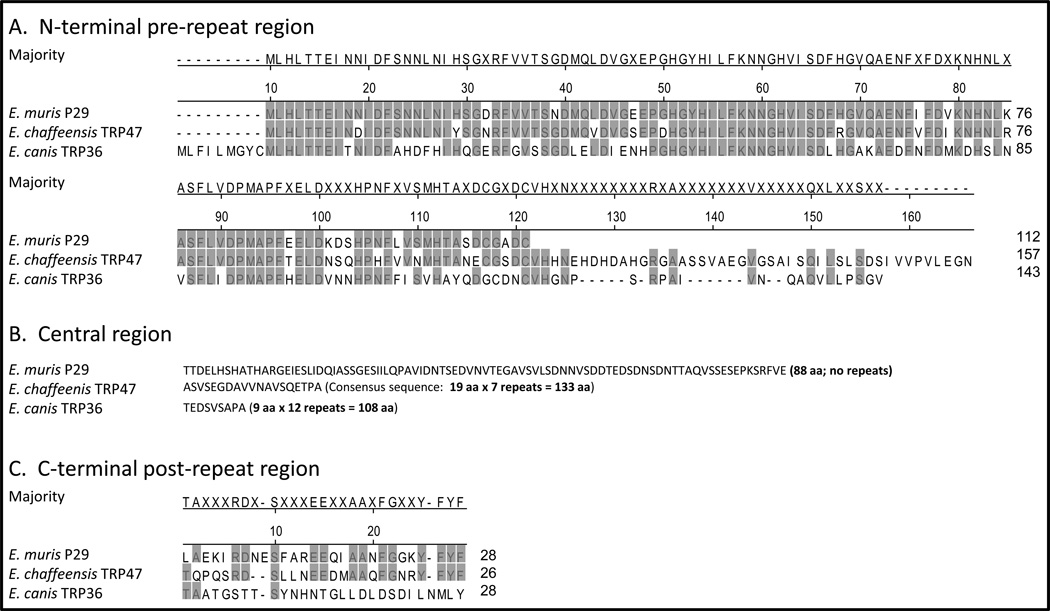
Multiple sequence alignment by ClustalW method indicated that the N-terminal region (first 112 aa) of E. muris P29 has high amino acid sequence homology to the pre-repeat regions of E. chaffeensis TRP47 and E. canis TRP36 (Figure 1A). The central region (88 aa) of E. muris P29 showed little homology to repeat regions of orthologous E. chaffeensis TRP47 and E. canis TRP36 (Figure 1B). The post-repeat region of E. chaffeensis TRP47 showed some degree of homology to the C-terminal region (28 aa) of E. muris P29 (Figure 1C). Conservation of amino acid usage was noted in the repeat regions of E. chaffeensis TRP47, E. canis TRP36 and orthologous E. ruminantium mucin-like proteins [7]. The repeats in these proteins utilized a total of 10 amino acids with predominant use of serine, threonine, alanine, proline, valine and glutamic acid [7]. Examination of the amino acid composition of E. muris P29 indicated that the central region of E. muris P29 utilized a total of 16 amino acids with occurrence of higher frequencies of serine, threonine, valine and glutamate residues in the central region compared to the combined N-terminal and C-terminal regions.
Figure 1. Orthologs of Ehrlichia muris P29 show high variation in the central region.
The N-terminal regions (A) and C-terminal regions (C) of E. muris P29 and its orthologs of E. chaffeensis Arkansas strain (TRP47) and E. canis Jake strain (TRP36) were aligned by ClustalW method. The central region of E. muris P29 and the repeat regions of orthologous E. chaffeensis TRP47 and E. canis TRP36 (B). The amino acid residues that match the consensus sequence are highlighted in grey.
Analysis of recombinant E. muris P29 and identification of native P29
SDS-PAGE analysis indicated that rP29 without the N-terminal tag exhibited migration mobility of an approximate molecular mass of 29 kDa compared to the predicted molecular mass of 22.71 kDa, suggestive of abnormal electrophoretic mobility similar to orthologs in other Ehrlichia due to acidic nature of these proteins (Figure 2A; lane 2)[17]. Western blot analysis indicated that anti-rP29 immune sera and an E. muris-immune serum containing a detectable concentration of anti-rP29 IgG antibodies by ELISA recognized the rP29 without the N-terminal tag (Figure 2B). Western blot analysis of E. muris-lysate antigen using anti-rP29 immune serum identified a 29 kDa native E. muris protein (Figure 2C; lane 2). The predicted molecular mass of native E. muris P29 is 25.17 kDa compared to observed electrophoretic mobility of 29 kDa. An E. muris-immune serum containing detectable concentration of anti-rP29 IgG antibodies by ELISA also recognized the native P29 on Western blot (Figure 2C; lane 3).
Mice immunized with recombinant E. muris P29 developed protective immune responses against challenge infection
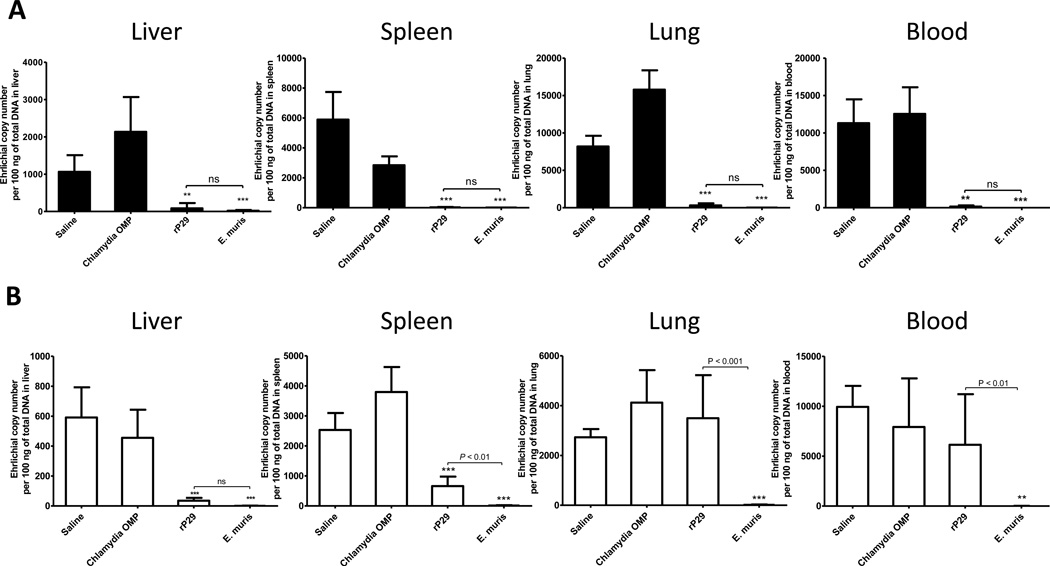
We assessed the development of protective immune responses in mice immunized with rP29 by challenge with a low dose (2 × 103 bacteria) or a high dose (1 × 104 bacteria) of E. muris (Figure 3). E. muris does not cause lethal disease in C57BL/6 mice, and our preliminary studies indicated the bacteremia peaked around day 10 post-infection. Therefore, we used reduction in bacterial burden on day 10 after challenge infection as an indicator of protective immunity. Mice immunized with rP29 or E. muris-immune mice had significantly reduced bacterial loads in the liver, spleen, lung and blood after the low dose E. muris challenge infection compared to naïve unimmunized control mice or mice immunized with Chlamydia pneumoniae MOMP (Figure 3A). However, immunization with rP29 significantly reduced bacterial burdens in the liver and spleen, but not in the lung and blood, following high dose challenge infection compared to significant reductions in bacterial burdens in all the organs examined in E. muris-immune mice (Figure 3B). The reduced bacterial burdens in the liver after the high dose challenge infection were comparable in mice immunized with rP29 and E. muris-immune mice (Figure 3B).
Figure 3. Immunization with recombinant E. muris P29 induces significant protection against challenge infection.
C57BL/6 mice were immunized with two doses of rP29 and challenged with a low dose (A; 2 × 103 bacteria) or a high dose (B; 1 × 104 bacteria) of E. muris by the i.p. route 60 days later. Mice were sacrificed on day 10 post-challenge, and the bacterial burdens in the liver, spleen, lung, and blood were determined by quantitative real-time PCR. Unimmunized mice (saline), mice immunized with recombinant Chlamydia pneumoniae MOMP, and E. muris-immune mice served as controls. Ehrlichial copy numbers were normalized to the total DNA. Each group contained three to four mice, and data were square-root transformed and analyzed by one way ANOVA with Bonferroni post-test for comparison of multiple groups. The error bars represent the standard deviation. **, P < 0.01 and ***, P < 0.001 compared to the saline control group. ns – not significant
Protection induced by rP29 is associated with induction of antibody responses
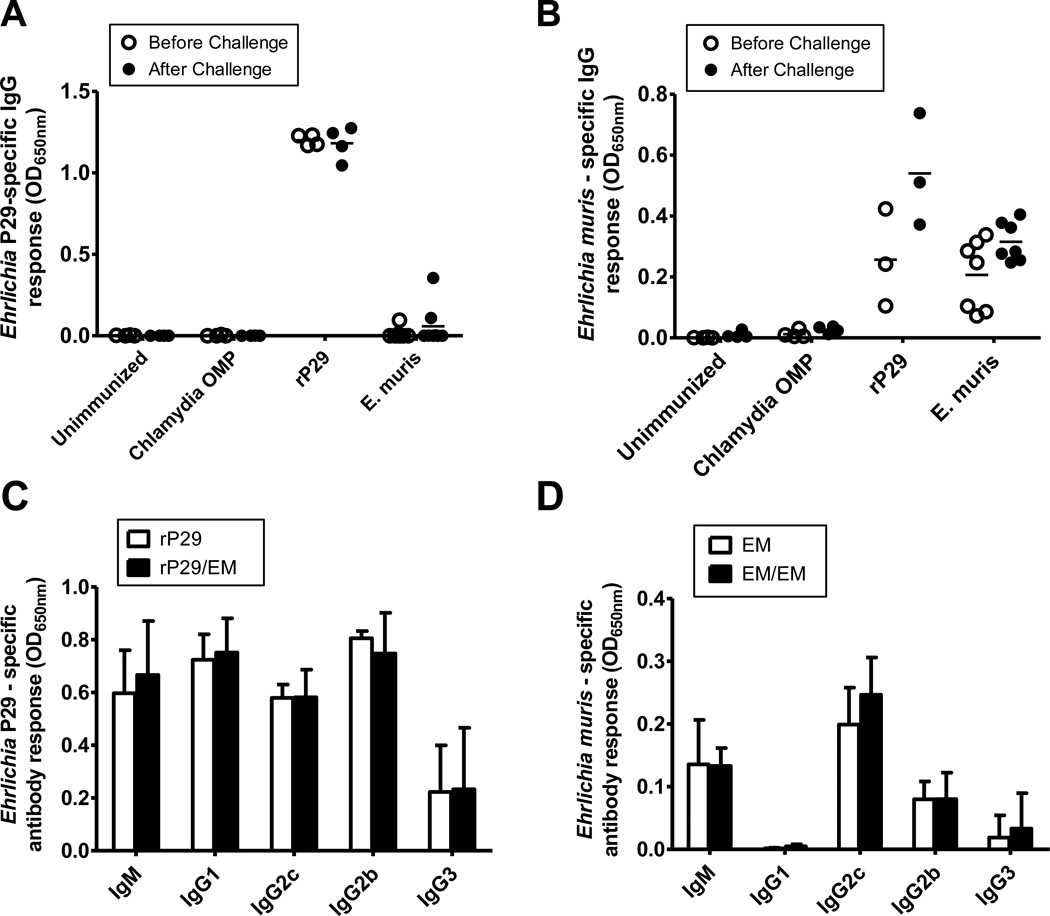
Antibodies have been demonstrated to play a significant role in protection against Ehrlichia [36– 38]. Mice immunized with the rP29 had high levels of rP29-specific IgG antibodies in sera before and after E. muris challenge (Figure 4A). However, immune sera from only one out of ten mice after primary E. muris infection and only two out of eight mice after secondary E. muris infection had detectable concentration of rP29-specific IgG antibodies (Figure 4A). Immune sera from mice immunized with rP29, and E. muris-immune mice showed strong reactivity with the E. muris lysate antigen (Figure 4B). Examination of immunoglobulin isotypes indicated that mice immunized with rP29 developed high concentrations of anti-rP29 serum IgG1, IgG2c and IgG2b, and low concentrations of IgG3 antibodies (Figure 4C). In contrast, immune sera collected from mice after primary or secondary E. muris infection had high concentrations of IgG2c and lower concentration of IgG2b and IgG3 with no detectable concentration of IgG1 directed against the E. muris-lysate antigen (Figure 4D). All groups had antigen-specific IgM antibodies.
Figure 4. Protection induced by recombinant E. muris P29 is associated with induction of a broad IgG isotype responses.
Serum IgG antibody responses specific to rP29 (A) and E. muris lysate antigen (B) in experimental groups before and on day 10 after E. muris challenge were determined by ELISA. (C) Serum immunoglobulin isotype responses specific to rP29 in mice immunized with rP29 before (rP29) and on day 10 after E. muris challenge (rP29/EM). (D) Serum immunoglobulin isotype responses specific to E. muris-lysate antigen in mice after primary (EM) and on day 10 after secondary E. muris infection (EM/EM). Data are representative of two independent experiments. (A & B) Each data point represents an individual immunized animal before E. muris challenge infection (open circles) or on day 10 after E. muris challenge (closed circles) and the horizontal solid bars represent the means. (C & D) Each bar represents the average of four immunized mice before E. muris challenge infection (open bars) or on day 10 after E. muris challenge (solid bars) and the error bars represent the standard deviation.
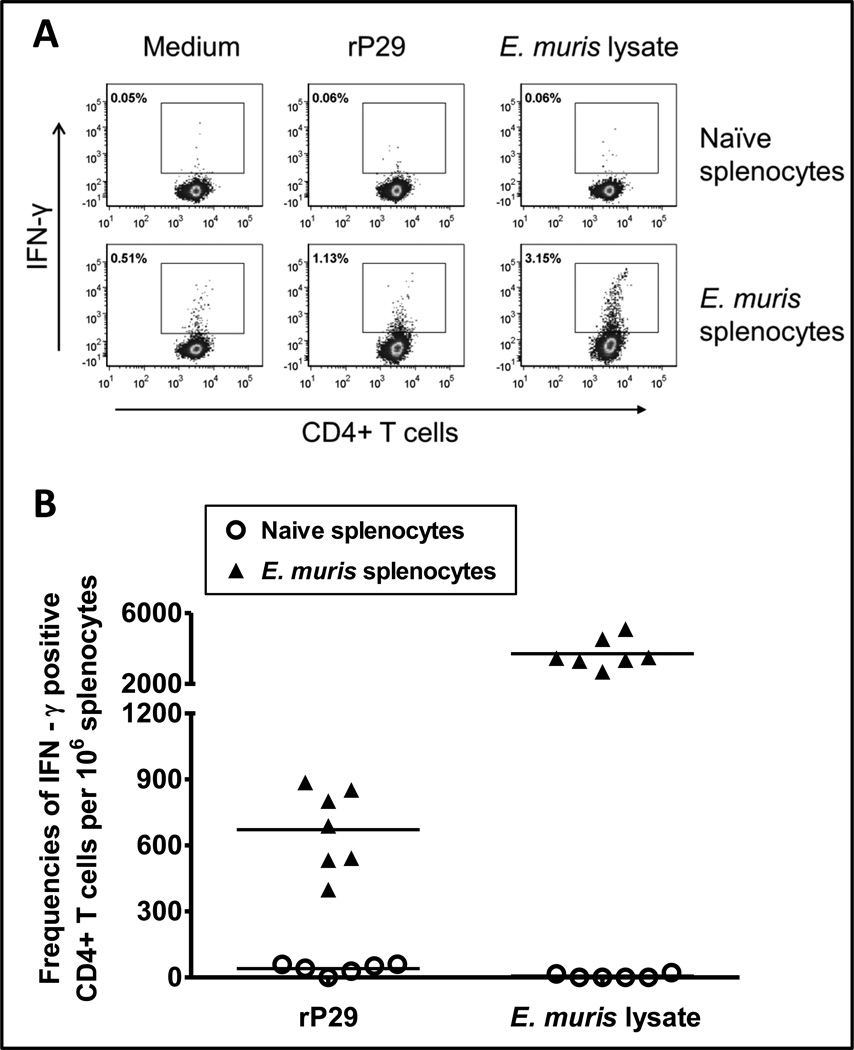
P29-specific effector/memory CD4+ Th1 responses are induced during E. muris infection
We examined by flow cytometry whether CD4+ Th1 cells target P29 during E. muris infection. Splenocytes from mice infected with E. muris at day 30 post-infection were stimulated in vitro with rP29 or E. muris lysate antigen for 24h. Flow cytometric analysis indicated that E. muris-infected mice had significantly higher frequencies of P29-specific CD4+ Th1 cells in the spleen compared to naïve uninfected mice (Figure 5).
Figure 5. E. muris P29 induces effector/memory Th1 CD4+ T cells in infected mice.
Frequencies of antigen-specific IFN-γ-producing CD4+ T cells in the spleens from separate groups of mice infected with E. muris were determined by flow cytometry. (A) Dot plots showing the percentages of P29-specific IFN-γ-producing CD4+ T cells in the spleens on day 30 after E. muris infection. (B) Frequencies of P29-specific IFN-γ-producing CD4+ T cells per million splenocytes in mice infected with E. muris on day 30 after infection. Each data point represents an individual animal, and data from two independent experiments are combined. Splenocytes from uninfected naïve mice stimulated with rP29 or E. muris lysate antigen served as controls. Background values from wells containing unstimulated splenocytes (medium only controls) were subtracted from antigen-stimulated wells for each mouse. Frequencies of IFN-γ-producing CD4+ T cells responding to E. muris lysate antigen from the same mice are presented for comparison.
Discussion
Orthologs of Ehrlichia tandem repeat proteins show considerable genetic variation in the repeat region, presumably due to host immune pressure or host adaptation, and some lack tandem repeats. The present study revealed that E. muris p29 gene and its ortholog in EMLA lack tandem repeats and show considerable genetic variation in the central region from the corresponding repeat regions of orthologous E. chaffeensis trp47 and E. canis trp36 genes, which is consistent with the divergence of repeat regions of TRP47 and TRP36 [7]. Analysis of the amino acid composition and predicted isoelectric point indicated that the central region of E. muris P29 has similar properties to that of the repeat regions of orthologs in related Ehrlichia.
Our study indicated that recombinant E. muris P29 induced significant protection, which was comparable to protection observed in E. muris-immune mice, against low dose challenge infection in the E. muris-C57BL/6 mouse model. However, immunization with rP29 reduced the bacterial burden in only some of the organs examined following high dose challenge infection compared to E. muris-immune mice. The reasons for the observed differences in protection against the high dose challenge infection in different organs of mice immunized with rP29 are not known, and these could be due to differences in the bacterial burdens or due to differences in the number and composition of the cells of the immune system and their functional heterogeneity in different organs [39–41]. The data suggested that immunity generated by natural E. muris infection is superior to protection induced by rP29. Thus, a multicomponent vaccine mimicking infection-induced immunity may provide better protection against high dose challenge. Our results indicate that P29 could be an important subunit vaccine component against ehrlichiosis.
Immunization with rP29 induced strong IgG antibody responses in all mice examined. In contrast, E. muris infection generated inconsistent P29-specfic IgG antibody responses in C57BL/6 mice, indicating that the infection does not effectively stimulate the development of IgG antibody responses to P29. However, all mice infected with E. muris developed P29-specific IgM responses (Supplementary Figure S1). In addition, all mice infected with E. muris developed IgG responses to E. muris-lysate antigen and to E. muris P13 protein (Supplementary Figure S1). E. muris P13 is an ortholog of E. chaffeensis TRP32 and E. canis TRP19 with a predicted molecular mass of 13 kDa. A previous study reported the lack of development of specific IgM antibodies in BALB/c mice and IgG antibodies in C57BL/6 mice to Trypanosoma cruzi proline racemase (TcPRAC) during experimental T. cruzi infection in contrast to development of IgG antibodies to other T. cruzi antigens [42]. In addition, genetic immunization with recombinant TcPRAC DNA resulted in generation of high-titer recombinant TcPRAC-specific IgG antibodies [42]. The mechanisms that contribute to impaired IgG responses to E. muris P29 during persistent E. muris infection or its biological significance are not clear.
In contrast to impaired IgG responses to E. muris P29 in mice, previous studies indicated that all 15 dogs experimentally infected with E. canis developed IgG antibodies to TRP36, and 24 out of 31 serum samples (77.4%) from HME patients with detectable antibodies to E. chaffeensis by IFA had IgG antibodies reactive with TRP47 [7;8;14]. In addition, C57BL/6 and AKR mice infected with E. muris developed a high titer of anti-E. muris IgG antibodies in contrast to the development of poor antibody responses in BALB/c mice infected with E. muris [29;43]. These studies indicate the potential host differences in antibody responses to Ehrlichia.
The protection induced by the rP29 was associated with induction of a broad IgG isotype response (Figure 4C). As reported previously, our study indicated that immunization with antigens, which generally involves use of adjuvants, induces a broader isotype response than antibody responses induced by Ehrlichia infection [24;27]. A previous study indicated that antibodies directed against the tandem repeat regions of E. chaffeensis TRP120, TRP47 and TRP32, which mainly consisted of IgG1 isotype, were effective in reducing bacterial burdens and splenomegaly in C57BL/6-scid and C57BL/6 mice infected with E. chaffeensis [26]. It has been suggested that antibodies directed against E. chaffeensis TRP47 or its orthologs could potentially interfere with their interactions with the host cell proteins and thereby prevent the establishment of infection [26].
In addition to antibody responses, T cells play an important role in protection against Ehrlichia [36;44;45]. Our data indicated that CD4+ T cells target P29 during E. muris infection and differentiate into IFN-γ-producing Th1 effector/memory cells. CD4+ Th1 cells mediate immune responses against intracellular pathogens, and IFN-γ produced by CD4+ Th1 cells activates macrophages and enhances their microbicidal activity [46]. Taken together, these studies suggest that orthologs of E. muris P29, although exhibit considerable variation in the central tandem repeat regions, are targeted by the host immune responses and could serve as potential vaccine candidates.
In conclusion, our study indicated that (i) orthologs of E. muris P29 showed considerable variation in the central tandem repeat region among different species, (ii) induction of P29-specific IgG antibody response was impaired during persistent E. muris infection, and (iii) rP29 induced protective immune responses.
Supplementary Material
Highlights.
Immunization with recombinant E. muris P29 induced protective immune responses
Protection induced by rP29 was associated with development of IgG antibodies
P29-specific effector/memory CD4+ Th1 responses induced during E. muris infection
Acknowledgements
We thank Tahereh Dadfarnia and Beau DiCicco for their help with cloning and expression of the recombinant protein. A part of this study was supported by grant AI31431 from the National Institute of Allergy and Infectious Diseases to DHW. NRT is supported by an NIAID Research Scholar Development Award (5K22AI089973). The excellent secretarial assistance by Rachel Stella is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interests
The authors have no financial conflicts of interest
References
- 1.Pritt BS, Sloan LM, Johnson DK, Munderloh UG, Paskewitz SM, McElroy KM, et al. Emergence of a new pathogenic Ehrlichia species, Wisconsin and Minnesota 2009. N Engl J Med. 2011;365(5):422–429. doi: 10.1056/NEJMoa1010493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker DH, Ismail N, Olano JP, McBride JW, Yu XJ, Feng HM. Ehrlichia chaffeensis : a prevalent, life-threatening, emerging pathogen. Trans Am Clin Climatol Assoc. 2004;115:375–382. [PMC free article] [PubMed] [Google Scholar]
- 3.Buller RS, Arens M, Hmiel SP, Paddock CD, Sumner JW, Rikhisa Y, et al. Ehrlichia ewingii, a newly recognized agent of human ehrlichiosis. N Engl J Med. 1999;341(3):148–155. doi: 10.1056/NEJM199907153410303. [DOI] [PubMed] [Google Scholar]
- 4.McBride JW, Walker DH. Molecular and cellular pathobiology of Ehrlichia infection: targets for new therapeutics and immunomodulation strategies. Expert Rev Mol Med. 2011;13:e3. doi: 10.1017/S1462399410001730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McBride JW, Walker DH. Progress and obstacles in vaccine development for the ehrlichioses. Expert Rev Vaccines. 2010;9(9):1071–1082. doi: 10.1586/erv.10.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rudoler N, Baneth G, Eyal O, van SM, Harrus S. Evaluation of an attenuated strain of Ehrlichia canis as a vaccine for canine monocytic ehrlichiosis. Vaccine. 2012;31(1):226–233. doi: 10.1016/j.vaccine.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Doyle CK, Nethery KA, Popov VL, McBride JW. Differentially expressed and secreted major immunoreactive protein orthologs of Ehrlichia canis and E. chaffeensis elicit early antibody responses to epitopes on glycosylated tandem repeats. Infect Immun. 2006;74(1):711–720. doi: 10.1128/IAI.74.1.711-720.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McBride JW, Corstvet RE, Gaunt SD, Boudreaux C, Guedry T, Walker DH. Kinetics of antibody response to Ehrlichia canis immunoreactive proteins. Infect Immun. 2003;71(5):2516–2524. doi: 10.1128/IAI.71.5.2516-2524.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nethery KA, Doyle CK, Zhang X, McBride JW. Ehrlichia canis gp200 contains dominant species-specific antibody epitopes in terminal acidic domains. Infect Immun. 2007;75(10):4900–4908. doi: 10.1128/IAI.00041-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohashi N, Unver A, Zhi N, Rikihisa Y. Cloning and characterization of multigenes encoding the immunodominant 30-kilodalton major outer membrane proteins of Ehrlichia canis and application of the recombinant protein for serodiagnosis. J Clin Microbiol. 1998;36(9):2671–2680. doi: 10.1128/jcm.36.9.2671-2680.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doyle CK, Zhang X, Popov VL, McBride JW. An immunoreactive 38-kilodalton protein of Ehrlichia canis shares structural homology and iron-binding capacity with the ferric ion-binding protein family. Infect Immun. 2005;73(1):62–69. doi: 10.1128/IAI.73.1.62-69.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McBride JW, Ndip LM, Popov VL, Walker DH. Identification and functional analysis of an immunoreactive DsbA-like thio-disulfide oxidoreductase of Ehrlichia spp. Infect Immun. 2002;70(5):2700–2703. doi: 10.1128/IAI.70.5.2700-2703.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo T, Zhang X, Wakeel A, Popov VL, McBride JW. A variable-length PCR target protein of Ehrlichia chaffeensis contains major species-specific antibody epitopes in acidic serine-rich tandem repeats. Infect Immun. 2008;76(4):1572–1580. doi: 10.1128/IAI.01466-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo T, Zhang X, Nicholson WL, Zhu B, McBride JW. Molecular characterization of antibody epitopes of Ehrlichia chaffeensis ankyrin protein 200 and tandem repeat protein 47 and evaluation of synthetic immunodeterminants for serodiagnosis of human monocytotropic ehrlichiosis. Clin Vaccine Immunol. 2010;17(1):87–97. doi: 10.1128/CVI.00331-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McBride JW, Zhang X, Wakeel A, Kuriakose JA. Tyrosine-phosphorylated Ehrlichia chaffeensis and Ehrlichia canis tandem repeat orthologs contain a major continuous cross-reactive antibody epitope in lysine-rich repeats. Infect Immun. 2011;79(8):3178–3187. doi: 10.1128/IAI.01347-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo T, Zhang X, McBride JW. Major species-specific antibody epitopes of the Ehrlichia chaffeensis p120 and E. canis p140 orthologs in surface-exposed tandem repeat regions. Clin Vaccine Immunol. 2009;16(7):982–990. doi: 10.1128/CVI.00048-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wakeel A, Zhang X, McBride JW. Mass spectrometric analysis of Ehrlichia chaffeensis tandem repeat proteins reveals evidence of phosphorylation and absence of glycosylation. PLoS One. 2010;5(3):e9552. doi: 10.1371/journal.pone.0009552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McBride JW, Doyle CK, Zhang X, Cardenas AM, Popov VL, Nethery KA, et al. Identification of a glycosylated Ehrlichia canis 19-kilodalton major immunoreactive protein with a species-specific serine-rich glycopeptide epitope. Infect Immun. 2007;75(1):74–82. doi: 10.1128/IAI.01494-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sumner JW, Childs JE, Paddock CD. Molecular cloning and characterization of the Ehrlichia chaffeensis variable-length PCR target: an antigen-expressing gene that exhibits interstrain variation. J Clin Microbiol. 1999;37(5):1447–1453. doi: 10.1128/jcm.37.5.1447-1453.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo T, Kuriakose JA, Zhu B, Wakeel A, McBride JW. Ehrlichia chaffeensis TRP120 interacts with a diverse array of eukaryotic proteins involved in transcription, signaling, and cytoskeleton organization. Infect Immun. 2011;79(11):4382–4391. doi: 10.1128/IAI.05608-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo T, McBride JW. Ehrlichia chaffeensis TRP32 interacts with host cell targets that influence intracellular survival. Infect Immun. 2012;80(7):2297–2306. doi: 10.1128/IAI.00154-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wakeel A, Kuriakose JA, McBride JW. An Ehrlichia chaffeensis tandem repeat protein interacts with multiple host targets involved in cell signaling, transcriptional regulation, and vesicle trafficking. Infect Immun. 2009;77(5):1734–1745. doi: 10.1128/IAI.00027-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li JS, Yager E, Reilly M, Freeman C, Reddy GR, Reilly AA, et al. Outer membrane protein-specific monoclonal antibodies protect SCID mice from fatal infection by the obligate intracellular bacterial pathogen Ehrlichia chaffeensis. J Immunol. 2001;166(3):1855–1862. doi: 10.4049/jimmunol.166.3.1855. [DOI] [PubMed] [Google Scholar]
- 24.Crocquet-Valdes PA, Thirumalapura NR, Ismail N, Yu X, Saito TB, Stevenson HL, et al. Immunization with Ehrlichia P28 outer membrane proteins confers protection in a mouse model of ehrlichiosis. Clin Vaccine Immunol. 2011;18(12):2018–2025. doi: 10.1128/CVI.05292-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas S, Thirumalapura NR, Crocquet-Valdes PA, Luxon BA, Walker DH. Structure-based vaccines provide protection in a mouse model of ehrlichiosis. PLoS One. 2011;6(11):e27981. doi: 10.1371/journal.pone.0027981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuriakose JA, Zhang X, Luo T, McBride JW. Molecular basis of antibody mediated immunity against Ehrlichia chaffeensis involves species-specific linear epitopes in tandem repeat proteins. Microbes Infect. 2012;14(12):1054–1063. doi: 10.1016/j.micinf.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nandi B, Hogle K, Vitko N, Winslow GM. CD4 T-cell epitopes associated with protective immunity induced following vaccination of mice with an ehrlichial variable outer membrane protein. Infect Immun. 2007;75(11):5453–5459. doi: 10.1128/IAI.00713-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winslow GM, Yager E, Shilo K, Collins DN, Chu FK. Infection of the laboratory mouse with the intracellular pathogen Ehrlichia chaffeensis. Infect Immun. 1998;66(8):3892–3899. doi: 10.1128/iai.66.8.3892-3899.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olano JP, Wen G, Feng HM, McBride JW, Walker DH. Histologic, serologic, and molecular analysis of persistent ehrlichiosis in a murine model. Am J Pathol. 2004;165(3):997–1006. doi: 10.1016/S0002-9440(10)63361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sotomayor EA, Popov VL, Feng HM, Walker DH, Olano JP. Animal model of fatal human monocytotropic ehrlichiosis. Am J Pathol. 2001;158(2):757–769. doi: 10.1016/S0002-9440(10)64018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ismail N, Soong L, McBride JW, Valbuena G, Olano JP, Feng HM, et al. Overproduction of TNF-alpha by CD8+ type 1 cells and down-regulation of IFN-gamma production by CD4+ Th1 cells contribute to toxic shock-like syndrome in an animal model of fatal monocytotropic ehrlichiosis. J Immunol. 2004;172(3):1786–1800. doi: 10.4049/jimmunol.172.3.1786. [DOI] [PubMed] [Google Scholar]
- 32.Benson G. Tandem repeats finder: a program to analyze DNA sequences. Nucleic Acids Res. 1999;27(2):573–580. doi: 10.1093/nar/27.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stevenson HL, Jordan JM, Peerwani Z, Wang HQ, Walker DH, Ismail N. An intradermal environment promotes a protective type-1 response against lethal systemic monocytotropic ehrlichial infection. Infect Immun. 2006;74(8):4856–4864. doi: 10.1128/IAI.00246-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thirumalapura NR, Crossley EC, Walker DH, Ismail N. Persistent infection contributes to heterologous protective immunity against fatal ehrlichiosis. Infect Immun. 2009;77(12):5682–5689. doi: 10.1128/IAI.00720-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thirumalapura NR, Stevenson HL, Walker DH, Ismail N. Protective heterologous immunity against fatal ehrlichiosis and lack of protection following homologous challenge. Infect Immun. 2008;76(5):1920–1930. doi: 10.1128/IAI.01293-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feng HM, Walker DH. Mechanisms of immunity to Ehrlichia muris : a model of monocytotropic ehrlichiosis. Infect Immun. 2004;72(2):966–971. doi: 10.1128/IAI.72.2.966-971.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li JS, Chu F, Reilly A, Winslow GM. Antibodies highly effective in SCID mice during infection by the intracellular bacterium Ehrlichia chaffeensis are of picomolar affinity and exhibit preferential epitope and isotype utilization. J Immunol. 2002;169(3):1419–1425. doi: 10.4049/jimmunol.169.3.1419. [DOI] [PubMed] [Google Scholar]
- 38.Winslow GM, Yager E, Shilo K, Volk E, Reilly A, Chu FK. Antibody-mediated elimination of the obligate intracellular bacterial pathogen Ehrlichia chaffeensis during active infection. Infect Immun. 2000;68(4):2187–2195. doi: 10.1128/iai.68.4.2187-2195.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gebhardt T, Mueller SN, Heath WR, Carbone FR. Peripheral tissue surveillance and residency by memory T cells. Trends Immunol. 2013;34(1):27–32. doi: 10.1016/j.it.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 40.Gordon S, Pluddemann A. Tissue macrophage heterogeneity: issues and prospects. Semin Immunopathol. 2013;35(5):533–540. doi: 10.1007/s00281-013-0386-4. [DOI] [PubMed] [Google Scholar]
- 41.Michel T, Poli A, Domingues O, Mauffray M, Thérésine M, Brons NH, et al. Mouse lung and spleen natural killer cells have phenotypic and functional differences, in part influenced by macrophages. PLoS One. 2012;7(12):e51230. doi: 10.1371/journal.pone.0051230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bryan MA, Norris KA. Genetic immunization converts the Trypanosoma cruzi B-Cell mitogen proline racemase to an effective immunogen. Infect Immun. 2010;78(2):810–822. doi: 10.1128/IAI.00926-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kawahara M, Suto C, Shibata S, Futohashi M, Rikihisa Y. Impaired antigen specific responses and enhanced polyclonal stimulation in mice infected with Ehrlichia muris. Microbiol Immunol. 1996;40(8):575–581. doi: 10.1111/j.1348-0421.1996.tb01111.x. [DOI] [PubMed] [Google Scholar]
- 44.Bitsaktsis C, Huntington J, Winslow G. Production o f IFN-gamma by CD4 T cells is essential for resolving Ehrlichia infection. J Immunol. 2004;172(11):6894–6901. doi: 10.4049/jimmunol.172.11.6894. [DOI] [PubMed] [Google Scholar]
- 45.Ganta RR, Wilkerson MJ, Cheng C, Rokey AM, Chapes SK. Persistent Ehrlichia chaffeensis infection occurs in the absence of functional major histocompatibility complex class II genes. Infect Immun. 2002;70(1):380–388. doi: 10.1128/IAI.70.1.380-388.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112(5):1557–1569. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.