Abstract
Concanavalin A, a phytohemagglutinin isolated from the jack bean, crystallizes at pH 6.8 in the orthorhombic space group 1222 with a = 89.9, b = 87.2, and c = 63.1 Å. We have analyzed x-ray diffraction intensity data to 4 Å resolution on native concanavalin A and five heavy-metal derivatives: lead, mersalyl, chloroplatinate, uranyl, and o-mercuri-p-nitrophenol. Heavy-atom positions, occupancies, and isotropic thermal parameters have been refined by least-squares methods.
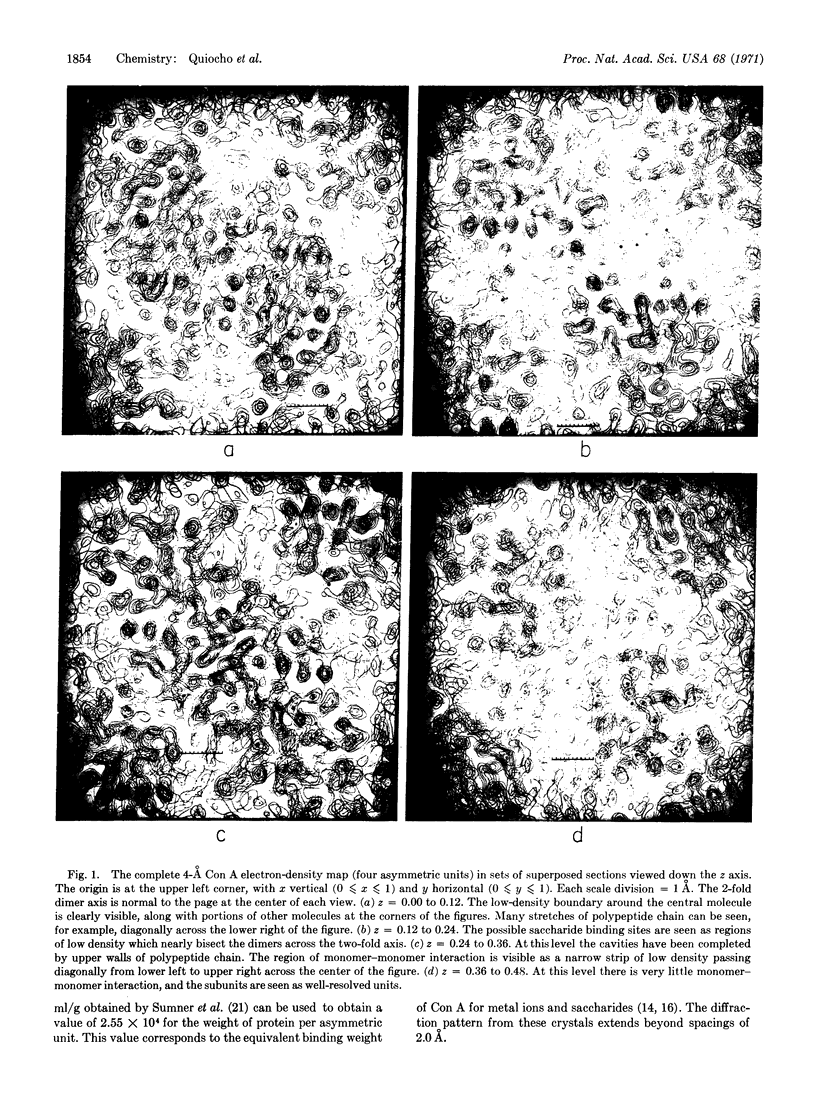
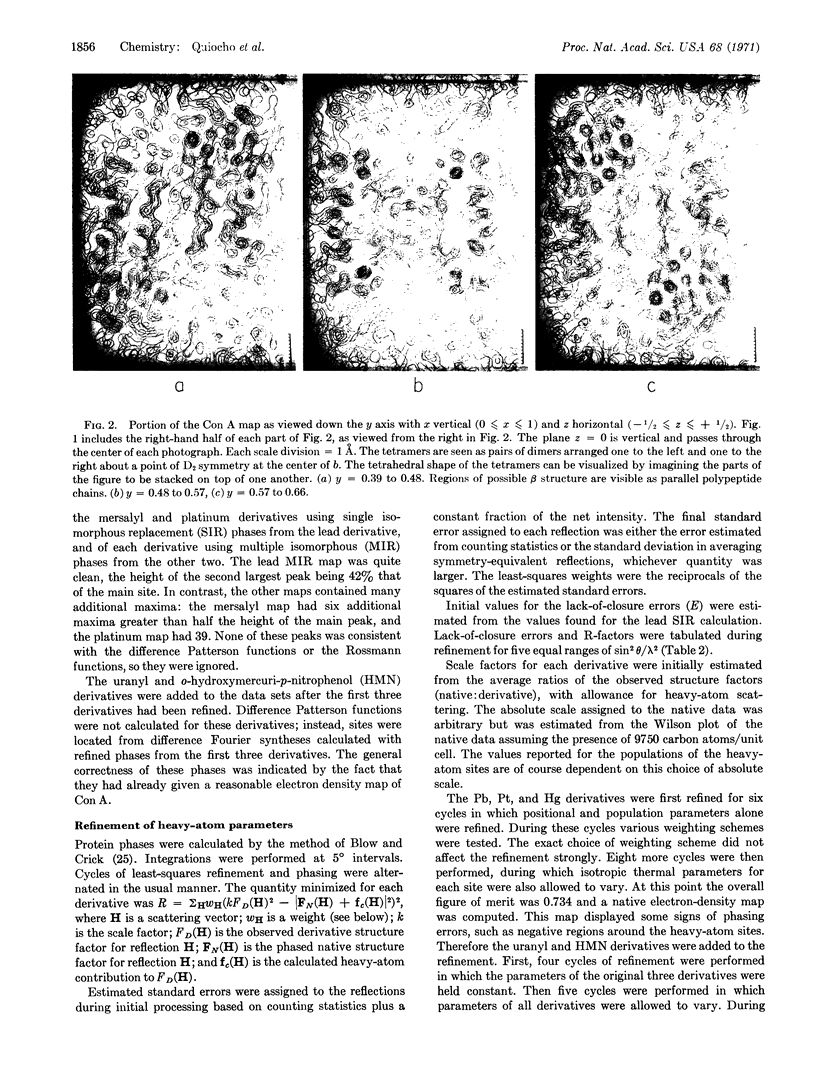
The electron density maps clearly show the molecular shape and the packing of the concanavalin A molecules. The asymmetric unit (mol wt 27,000) forms an elliptical dome or “gumdrop” with a base of approximately 46 × 26 Å and a height of 42 Å. The subunits are paired across 2-fold axes parallel to the c-axis to form dimers. The dimers are in turn paired across points of D2 symmetry to form tetramers of roughly tetrahedral shape. Each unit has a depression located on the surface which could be the site of saccharide binding. In many regions we have been able to trace the course of the polypeptide chain.
Keywords: x-ray diffraction, heavy-atom derivatives, lectins
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYD W. C. The lectins: their present status. Vox Sang. 1963 Jan-Feb;8:1–32. doi: 10.1111/j.1423-0410.1963.tb04146.x. [DOI] [PubMed] [Google Scholar]
- Burger M. M., Noonan K. D. Restoration of normal growth by covering of agglutinin sites on tumour cell surface. Nature. 1970 Nov 7;228(5271):512–515. doi: 10.1038/228512a0. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN I. J., HOLLERMAN C. E., MERRICK J. M. PROTEIN-CARBOHYDRATE INTERACTION. I. THE INTERACTION OF POLYSACCHARIDES WITH CONCANAVALIN A. Biochim Biophys Acta. 1965 Jan 4;97:68–76. doi: 10.1016/0304-4165(65)90270-9. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN I. J., HOLLERMAN C. E., SMITH E. E. PROTEIN-CARBOHYDRATE INTERACTION. II. INHIBITION STUDIES ON THE INTERACTION OF CONCANAVALIN A WITH POLYSACCHARIDES. Biochemistry. 1965 May;4:876–883. doi: 10.1021/bi00881a013. [DOI] [PubMed] [Google Scholar]
- Goldstein I. J., So L. L. Protein-carbonhydrate interaction. 3. Agar gel-diffusion studies on the interaction of Concanavalin A, a lectin isolated from jack bean, with polysaccharides. Arch Biochem Biophys. 1965 Aug;111(2):407–414. doi: 10.1016/0003-9861(65)90203-1. [DOI] [PubMed] [Google Scholar]
- Greer J., Kaufman H. W., Kalb A. J. An x-ray crystallographic study of concanavalin A. J Mol Biol. 1970 Mar 14;48(2):365–366. doi: 10.1016/0022-2836(70)90169-5. [DOI] [PubMed] [Google Scholar]
- Inbar M., Sachs L. Interaction of the carbohydrate-binding protein concanavalin A with normal and transformed cells. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1418–1425. doi: 10.1073/pnas.63.4.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inbar M., Sachs L. Structural difference in sites on the surface membrane of normal and transformed cells. Nature. 1969 Aug 16;223(5207):710–712. doi: 10.1038/223710a0. [DOI] [PubMed] [Google Scholar]
- Kalb A. J., Levitzki A. Metal-binding sites of concanavalin A and their role in the binding of alpha-methyl d-glucopyranoside. Biochem J. 1968 Oct;109(4):669–672. doi: 10.1042/bj1090669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalb A. J., Lustig A. The molecular weight of concanavalin A. Biochim Biophys Acta. 1968 Oct 21;168(2):366–367. doi: 10.1016/0005-2795(68)90161-x. [DOI] [PubMed] [Google Scholar]
- Nakamura S., Suzuno R. Crystallization of concanavalins A and B and canavalin from Japanese jack beans. Arch Biochem Biophys. 1965 Sep;111(3):499–505. doi: 10.1016/0003-9861(65)90228-6. [DOI] [PubMed] [Google Scholar]
- Shoham J., Inbar M., Sachs L. Differential toxicity on normal and transformed cells in vitro and inhibition of tumour development in vivo by concanavalin A. Nature. 1970 Sep 19;227(5264):1244–1246. doi: 10.1038/2271244a0. [DOI] [PubMed] [Google Scholar]
- So L. L., Goldstein I. J. Protein-carbohydrate interaction. IV. Application of the quantitative precipitin method to polysaccharide-concanavalin A interaction. J Biol Chem. 1967 Apr 10;242(7):1617–1622. [PubMed] [Google Scholar]
- So L. L., Goldstein I. J. Protein-carbohydrate interaction. IX. Application of the quantitative hapten inhibition technique to polysaccharide-concanavalin A interaction. Some comments on the forces involved n concanavalin A-polysaccharide interaction. J Immunol. 1967 Jul;99(1):158–163. [PubMed] [Google Scholar]
- Sumner J. B., Howell S. F. Identification of Hemagglutinin of Jack Bean with Concanavalin A. J Bacteriol. 1936 Aug;32(2):227–237. doi: 10.1128/jb.32.2.227-237.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyckoff H. W., Hardman K. D., Allewell N. M., Inagami T., Tsernoglou D., Johnson L. N., Richards F. M. The structure of ribonuclease-S at 6 A resolution. J Biol Chem. 1967 Aug 25;242(16):3749–3753. [PubMed] [Google Scholar]
- Yariv J., Kalb A. J., Levitzki A. The interaction of concanavalin A with methyl alpha-D-glucopyranoside. Biochim Biophys Acta. 1968 Sep 3;165(2):303–305. doi: 10.1016/0304-4165(68)90063-9. [DOI] [PubMed] [Google Scholar]





