Abstract
Coral reefs are facing a biodiversity crisis due to increasing human impacts, consequently, one third of reef-building corals have an elevated risk of extinction. Logistic challenges prevent broad-scale species-level monitoring of hard corals; hence it has become critical that effective proxy indicators of species richness are established. This study tests how accurately three potential proxy indicators (generic richness on belt transects, generic richness on point-intercept transects and percent live hard coral cover on point-intercept transects) predict coral species richness at three different locations and two analytical scales. Generic richness (measured on a belt transect) was found to be the most effective predictor variable, with significant positive linear relationships across locations and scales. Percent live hard coral cover consistently performed poorly as an indicator of coral species richness. This study advances the practical framework for optimizing coral reef monitoring programs and empirically demonstrates that generic richness offers an effective way to predict coral species richness with a moderate level of precision. While the accuracy of species richness estimates will decrease in communities dominated by species-rich genera (e.g. Acropora), generic richness provides a useful measure of phylogenetic diversity and incorporating this metric into monitoring programs will increase the likelihood that changes in coral species diversity can be detected.
Introduction
A critical challenge facing conservation scientists and ecosystem managers is curtailing the loss of biodiversity in the face of rapid global change [1], [2]. Coral reefs support more than 35% of all known marine biodiversity, hence there is strong impetus to forecast, detect and mitigate losses in this ecosystem [3]–[5]. Coral reef biodiversity is however, at risk due to the recent destruction of 20% of the world's coral reefs and a further 50% of reefs in decline [6]. Consequently, there has been a dramatic increase in the threatened status of reef-building corals, with 33% of species now listed in elevated categories of threat by the IUCN [7]. The urgent need to conserve corals is emphasized further by the thousands of other marine species that rely on these habitat-forming organisms and the millions of people dependent on reefs for food security [8].
The task of protecting coral biodiversity is immense and exacerbated not only by the logistic challenges of conducting species-level surveys on SCUBA and the high level of taxonomic expertise needed to identify corals; but by the fact that coral communities are characterized by a large proportion of rare species [9]. Recent studies have shown that rare species disproportionately increase the potential breadth of functions provided by ecosystems across spatial scales [10], [11]. Thus, protecting rare species, and moreover, the full complement of species richness insures against future uncertainty arising from environmental change [11]. Despite species richness influencing ecosystem functioning, resilience and resistance to environmental change [12], for most coral communities, there is a critical shortage of rigorous species-level baseline data and that presents a major challenge for the conservation of diversity [13]–[16].
In the absence of species-level data, conservation decisions relevant to protecting coral biodiversity are based on subsets of data relating to indicator species [17], cross-taxon surrogates [18] or broad habitat-based proxy metrics [19]. Developing indicator, surrogate or proxy metrics that accurately represent trends in biodiversity is an important and pragmatic conservation objective [20]. While proxies reduce the time and cost required for data collection [21], [22], their effectiveness varies considerably and all have limitations [23], [24]. Hence, numerous studies have questioned the ability for proxy metrics to effectively represent biodiversity [18], [20]–[25] especially if their performance is not evaluated with empirical data [26].
On coral reefs, ‘reefscape proxies’ are commonly used to quantify the condition of coral reef habitat, with percent live hard coral cover being the most widely used metric in monitoring studies [27]–[29]. Despite its popularity, hard coral cover is not a robust indicator of coral biodiversity [30]. Subsequently, protecting reefs with a high level of coral cover may not have the expected flow-on biodiversity benefits. As such, there is a need to optimize the data collected in coral reef monitoring programs and find an effective way to make better predictions about the status of coral biodiversity.
In terrestrial systems, a high level of congruence has been documented between species and higher-taxon richness [31]–[38]. A similar significant positive linear relationship has been proposed to exist between species and generic richness in coral communities [30], [39]. Before generic richness can be broadly applied as a proxy indicator of species richness in coral communities, it is necessary for the relationship between these variables to be further characterized. To build on the initial findings of a companion study [30], here we examine if the linear relationship between generic and species richness is scale-dependent and how it is impacted by diversity.
The overall objective of this study was to examine the relationship between coral species richness and three potential proxy indicators: generic richness (measured on belt transects); generic richness (measured on point-intercept transects); and percent live hard coral cover (measured on point-intercept transects). Specifically, this study aims to: 1. Determine which proxy is the best predictor of species richness; 2. Determine if the relationships between predictor variables and species richness are affected by the spatial scale in which the data is analyzed and; 3. Determine whether the relationships between predictor variables and coral species richness vary between locations of varying diversity.
Materials and Methods
Ethics Statement
All necessary permits were obtained for the described field studies. Permits were obtained from the Department of Environment, Water and Heritage, Australia; the Marshall Island Marine Resource Authority; and the Kosrae Conservation and Safety Organization.
Study Sites
From 2009 to 2012 underwater visual surveys of hermatypic scleractinian (hard) corals and percent coral cover were conducted at 14 sites at Majuro Atoll in the Republic of the Marshall Islands, Pacific Ocean (7°4′N 171°16′E); 22 sites at Kosrae, Micronesia, Pacific Ocean (5°19′N 162°59′E); and at eight sites within the Ashmore and Cartier National Marine Reserves, Timor Sea, NW Australia (Ashmore: 12°17′S 123°02′E; Cartier:12°32′S 123°33′E).
Generic and Species Richness
In this study we examine observed species richness (the total number of species observed in a sample i.e. alpha-diversity) rather than estimated species richness which may be obtained through the application of statistical methods to correct for undetected species (e.g. Chao and jackknife estimators). Generic and species richness of reef building hard corals was documented on six replicate 50 m long ×2 m wide belt transects (i.e. 100 m per transect, 600 m per site). Belt transects were surveyed at two depths (three replicates at 3–5 m depth and three at 8–10 m depth). Within the belt transects, every coral colony (over 5 cm diameter) was identified to species level. Colonies under 5 cm were considered juveniles and not able to be identified in-situ because of the lack of skeletal development. In the case of large stands of coral (e.g. branching Acropora, large colonies of Porites), every 1 m2 was counted as a separate colony.
Hard Coral Cover
The percent cover of hard corals was documented to generic level on point-intercept transects (PIT). This methodology involved resurveying the same transect used to document species richness (thus referred to here as ‘paired transects’). On the second pass of the transect tape, all benthos occurring directly below 100 uniformly distributed points (50 cm apart) per were recorded (see [30] for an illustration of the survey method).
Data Analysis
The relationship between species richness (response variable) and 3 predictor variables {generic richness from belt transects (GRb), generic richness from point-intercept transects (GRp), and percent live hard coral cover (HCC)} were examined at two scales, site (mean across transects) and transect (total of all transects). Linear regression analyses were performed using the R statistical program version 2.15.3 [40]. The coefficient of determination (R2) was used as the measure of fit and standard errors indicate the accuracy of the prediction.
For strong (R2>0.7) and significant (p<0.0005) relationships, the regression equation is reported (ŷ = a+bx) (where ŷ represents mean species richness and x represents the predictor variable, a = intercept coefficient (±SE) and b represents x variable coefficient (±SE). Application of this equation enables species richness to be estimated. A series of one-way analyses-of-variance (ANOVA) were conducted on untransformed data to test the null hypothesis that there is no significant difference in the performance of predictor variables across scales of analysis (site versus transect).
Results
A total of 193 ‘paired’ replicate transects were conducted at 3 locations (Table 1). In total, 19,300 m2 of coral reef habitat was surveyed and 29,406 colonies were identified. The highest species richness was at Ashmore Reef (191 species), followed by Kosrae (154 species) and Majuro (135 species). There was no significant relationship between species richness and the area surveyed (F = 121.06, df = 1, p = 0.058).
Table 1. The study locations, total number of colonies, total species and generic richness, total number of sites surveyed, transects per site and total number of paired transects per location.
| Location | Number of colonies | Species richness | Generic richness | Number of sites | Number of transects per site | Total number of paired transects |
| Kosrae | 10884 | 154 | 49 | 22 | 3 | 66 |
| Majuro Atoll | 9125 | 135 | 44 | 14 | 6 at 12 sites plus 3 at 1 site and 4 at 1site | 79 |
| Ashmore and Cartier Reefs | 9397 | 191 | 51 | 8 | 6 | 48 |
The composition of the coral communities differed between the three locations. Ashmore Reef was dominated by colonies of Acropora, Seriatopora, Porites and Montipora; whilst Majuro was dominated by Acropora, Porites and Pocillopora, and Kosrae was dominated by Porites, Acropora and Galaxea (Figure S1a). Notable differences in the composition of communities at the three locations include the high abundance of Porites and Galaxea colonies at Kosrae; and there being twice as many Montipora spp. at Ashmore Reef than at the other locations. While Acropora colonies were commonly encountered at all three locations, there was far greater species-level diversity at Ashmore Reef and Majuro Atoll than observed at Kosrae (Figure S1b).
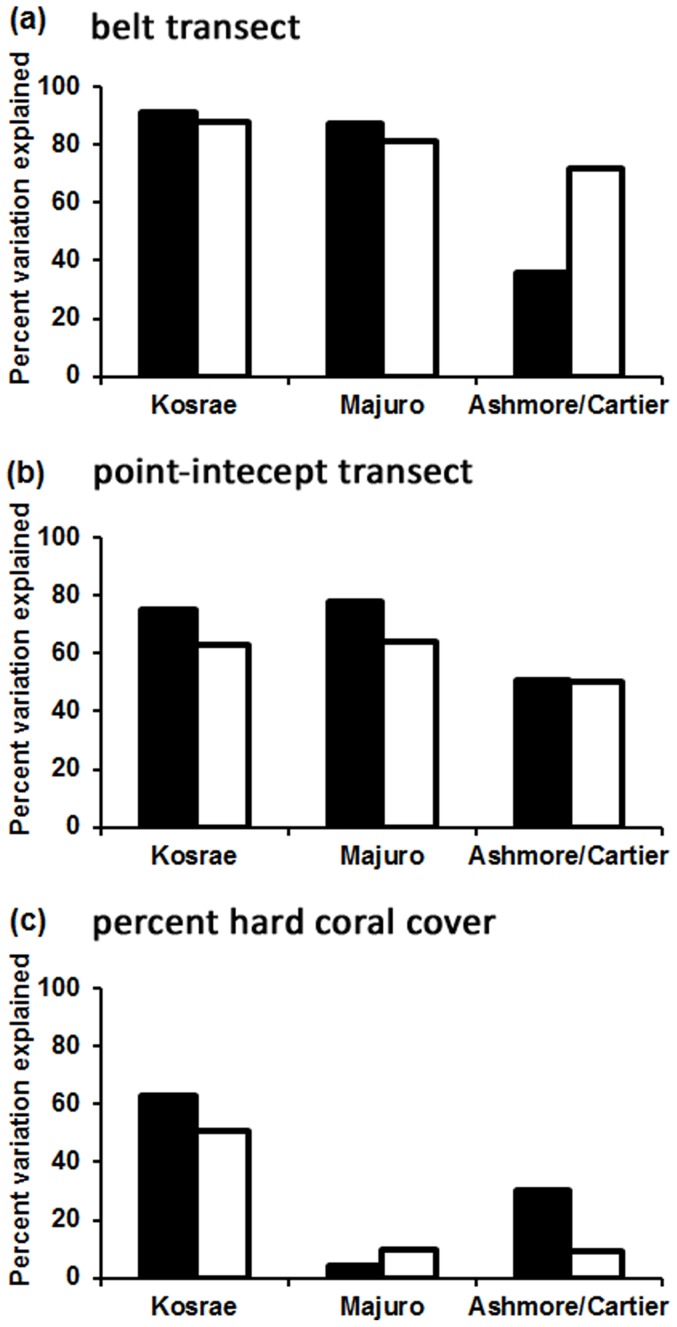
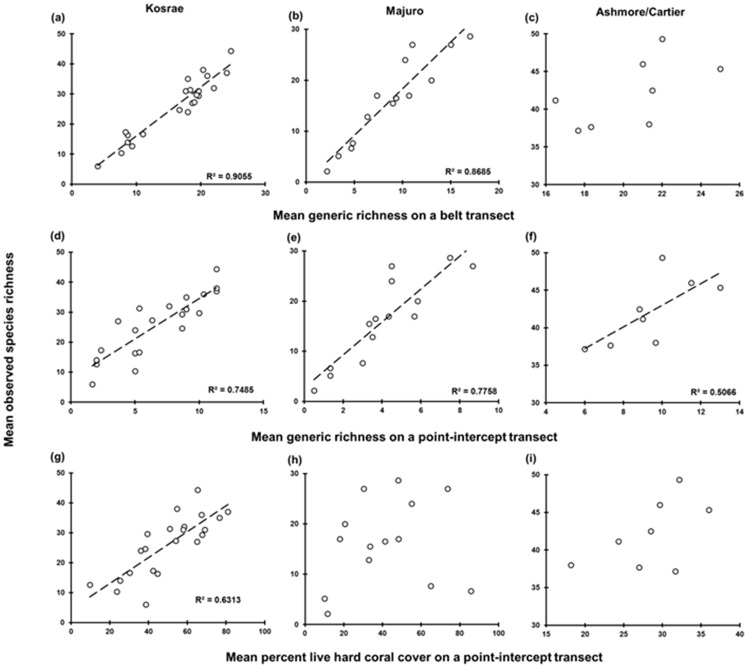
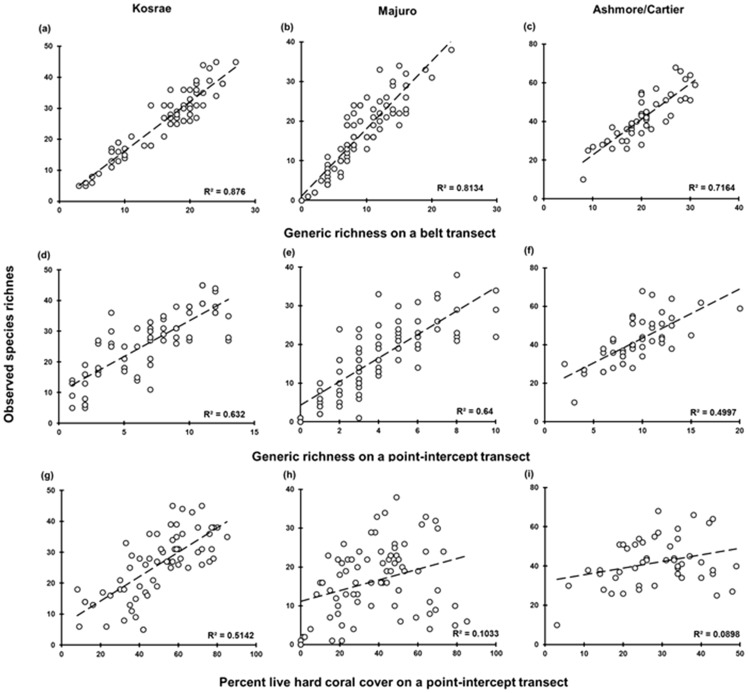
The ability for input variables (HCC, GRp, GRb) to predict species richness varied considerably, ranging from 4–91% variation explained depending on location and scale examined. Overall, GRb provided the most accurate predictions of species richness as evidenced by the high R2 values (i.e. >70% variation explained in 5/6 analyses, Figure 1a; Figure 2a–c; Figure 3a–c), and highly significant linear relationships (p<0.001; Table 2). GRb is a particularly strong explanatory variable species richness at Kosrae where 91% of variation was explained based on site data (Table 2). Based upon this result, the regression equation from Kosrae site data is ŷ = 1.624 (±0.117)x−0.234 (±2.011) and from this equation species richness can be predicted (see Table 3).
Figure 1. Histogram depicting the percent of variation in species richness that is explained by 3 predictor variables at 2 scales (black bars: site means; white bars: transect).
(a) generic richness measured on a belt transect, (b) generic richness measured on a point-intercept transect, (c) percent live hard coral cover measured on a point-intercept transect.
Figure 2. Site-level linear regressions of species richness on predictor variables (in rows) at the three locations (in columns).
The number of sites for each location is shown in Table 1. R2 and the 1∶1 relationship are shown for significant associations.
Figure 3. Transect-level linear regressions of species richness on predictor variables (in rows) at the three locations (in columns).
The number of sites for each location is shown in Table 1. R2 and the 1∶1 relationship are shown for significant associations.
Table 2. Regression statistics showing the strength and significance of linear relationships between species richness and a) Generic richness measured on a belt transect; b) Generic richness measured on a point intercept transect; and c) Percent live hard coral cover.
| Location | Predictor Variable | Scale of Analysis | R2 | Adj R2 | SE | df | F-value | P-value | Sig. |
| KOSRAE | GRb | Site | 0.905 | 0.901 | 3.189 | 21 | 191.576 | 1.050E-11 | *** |
| KOSRAE | GRb | Transect | 0.876 | 0.874 | 3.718 | 65 | 452.115 | 1.035E-30 | *** |
| KOSRAE | GRp | Site | 0.749 | 0.736 | 5.202 | 21 | 59.532 | 2.029E-07 | *** |
| KOSRAE | GRp | Transect | 0.632 | 0.626 | 6.404 | 65 | 109.917 | 1.584E-15 | *** |
| KOSRAE | HCC | Site | 0.631 | 0.613 | 6.299 | 21 | 34.251 | 1.003E-05 | *** |
| KOSRAE | HCC | Transect | 0.514 | 0.507 | 7.358 | 65 | 67.750 | 1.263E-11 | *** |
| MAJURO | GRb | Site | 0.868 | 0.858 | 3.243 | 13 | 79.240 | 1.240E-06 | *** |
| MAJURO | GRb | Transect | 0.813 | 0.811 | 3.999 | 78 | 335.585 | 8.580E-30 | *** |
| MAJURO | GRp | Site | 0.776 | 0.757 | 4.234 | 13 | 41.522 | 3.189E-05 | *** |
| MAJURO | GRp | Transect | 0.636 | 0.631 | 5.588 | 78 | 134.299 | 1.493E-18 | *** |
| MAJURO | HCC | Site | 0.038 | −0.043 | 8.772 | 13 | 0.470 | 5.061E-01 | ns |
| MAJURO | HCC | Transect | 0.103 | 0.092 | 8.766 | 78 | 8.873 | 3.869E-03 | ** |
| ASHMORE | GRb | Site | 0.360 | 0.253 | 3.863 | 7 | 3.371 | 1.160E-01 | ns |
| ASHMORE | GRb | Transect | 0.716 | 0.710 | 6.502 | 47 | 116.174 | 3.556E-14 | *** |
| ASHMORE | GRp | Site | 0.507 | 0.424 | 3.391 | 7 | 6.161 | 4.767E-02 | * |
| ASHMORE | GRp | Transect | 0.500 | 0.489 | 8.635 | 47 | 45.953 | 1.957E-08 | *** |
| ASHMORE | HCC | Site | 0.296 | 0.178 | 4.052 | 7 | 2.521 | 1.634E-01 | ns |
| ASHMORE | HCC | Transect | 0.090 | 0.070 | 11.647 | 47 | 4.538 | 3.853E-02 | * |
= 0.05;
= 0.005;
= 0.0005.
Strong (>0.70) and highly significant (p<0.0005) linear relationships are shaded.
Table 3. Regression equations for predicting species richness (y) based on generic richness (GR) on belt transects where R2>0.7.
| Regression equation | Range in y for x = 20 | Range in y for x = 30 | Range in y for x = 40 | |
| Kosrae | ||||
| Site data | ŷ = 1.624 (±0.117)x−0.234 (±2.011) | 28–33 | 43–54 | 58–71 |
| Transect data | y = 1.590 (±0.748)x+0.319 (±1.290) | 16–48 | 24–72 | 33–95 |
| Majuro Atoll | ||||
| Site data | ŷ = 1.829 (±0.206)x+0.033 (±2.015) | 30–43 | 47–63 | 63–83 |
| Transect data | y = 1.706 (±0.093)x+1.038 (±0.951) | 32–38 | 48–56 | 65–74 |
| Ashmore-Cartier | ||||
| Site data | R2 too low to formulate a reliable equation | |||
| Transect data | y = 1.846 (±0.171)x+4.171 (±3.620) | 34–48 | 51–68 | 68–88 |
The scale upon which generic richness data was collected impacted upon the ability for species richness to be predicted. When data were collected on a point-intercept transect, generic richness provided modest to strong (R2 = 0.50–0.78) and significant (p<0.05) positive linear relationships with coral species richness across all locations (Figure 2d–f; Figure 3d–f, Table 2). GRp provided a strong explanatory variable for species richness at Majuro Atoll with 78% of variation explained as a positive linear function based on the site data. At all other locations and scales of analysis however, R2 values were considered too low (i.e. below 0.7) and/or SE values were too high to provide meaningful or precise estimates of species richness.
Percent live hard coral cover was the least reliable predictor of coral species richness (4–63% variation explained, Figure 1c, Table 2). For HCC, both the strength and significance of the relationship with coral species richness varied. At Kosrae, a significant (p<0.001) linear relationship exists between HCC and coral species richness (R2 = 0.51–0.63); while at Majuro and Ashmore/Cartier, the relationship between HCC and coral species richness was weak (R2<0.30) and of low, or no statistical significance (Figure 2g–i, Figure 3g–i, Table 2). Overall R2 values were considered too low (i.e. below 0.7) to provide meaningful estimates of coral species richness.
There was no significant difference (p<0.001) in the performance of GRb, GRp or HCC across the two scales of measurement (Table S1). Hence, analyzing the data at a finer spatial scale (i.e. transect level) did not necessarily increase predictive ability. There is one important exception however, at Ashmore Reef, the location with the highest number of species (see Table 1) the linear regression provided a better fit when analyzed at the transect level (R2 = 0.72 for transect versus 0.36 for site, Figure 1, Table 2).
Discussion
Evidence is mounting that there is limited redundancy in complex marine ecosystems like coral reefs [41]–[43]; and individual species can be surprisingly important to ecosystem resilience [44], [45]. Thus, even small changes in species diversity can have significant impacts on ecosystem function [46], [47]. Protecting species-level diversity is therefore a key component of maintaining the functional diversity of coral reefs. However, given that the task of species-level monitoring and management is immense, coral reef management authorities necessarily adopt ecosystem-based monitoring approaches. If managers of sensitive coral reefs are only informed at this level, the future of coral biodiversity could be jeopardized. Thus, striking a balance between ecosystem and species-level monitoring is an acute challenge to conservation science. This study confronts this challenge by examining the potential for 3 proxy metrics to represent species diversity.
It is standard practice for the status of coral reefs to be assessed based upon the level of hard coral cover [6], [27], [29], [48]–[51]. Under this model, a reef with high HCC is considered ‘healthy’ and a reef with low HCC is not. This study has confirmed however, that HCC is a poor linear predictor of coral species richness, thus a reef with high coral cover does not necessarily have high coral species diversity. Moreover, in apparent accordance with predictions of the intermediate disturbance hypothesis [52] at the locations examined here (see also [30]), species richness tends to peak at intermediate levels of coral cover rather than having a positive linear trend. Thus, the relationship between HCC and species richness is complex and this precludes its usefulness as a proxy indicator. Nevertheless, HCC remains an important habitat monitoring target because it has been correlated with other variables such as coral disease prevalence [53], and the abundance and composition of reef fishes [54] and overall reef condition [6], [27].
This dataset suggests that generic richness provides a more reliable indicator of species richness based upon the strong and significant linear relationships between these two variables. Since Veron [39] highlighted the association between generic and species richness in coral communities, there have been few empirical examinations of the nature of the relationship (but see [30]). Our study confirms that GRb provides a strong explanatory variable for the observed patterns of species richness at three Indo-Pacific locations of varying diversity. Thus, we conclude that GRb provides not only a tangible measure of phylogenetic diversity (which is in itself important for insuring against losses of evolutionary history [55], [56]); but a reliable proxy indicator of species richness.
In other taxa, the strength of the relationship between species and generic richness is influenced by scale, location; ecosystem complexity and community age [57]–[60]. Similarly, our results suggest the relationship between coral species and generic richness is sensitive to scale, most notably at locations where there is a high level of within-genus diversity. For example, at Kosrae and Majuro Atoll, GRb explained 81%–91% of the variation in species richness regardless of the scale of analysis. Whereas at Ashmore Reef GRb performed less reliably, explaining only 36% of the variation at a site level (and 72% at a transect level). The variable performance of GRb at Ashmore Reef in comparison to the other localities relates to the differences in diversity and community structure at this location. Ashmore Reef has the highest level of species richness among the locations examined, and the community is dominated by species from diverse genera such as Acropora and Montipora (Figure S1). Conversely, the Kosrae and Majuro communities are dominated by less diverse genera including Porites and Pocillopora. Therefore, if species-rich genera dominate the community, the strength of the generic-species richness relationship diminishes.
Moreover, for a community comprised of mono-specific genera there is an exact relationship between generic and species richness. As the number of species in each genus increases, the strength of the relationship with species richness decreases. Therefore, a limitation of using generic richness as a proxy indicator of species richness is that the accuracy of estimates will decrease in communities dominated by species-rich genera. Conducting the regression analysis at a finer spatial scale (e.g. transect level) can help improve the predictive capability of GRb; however the equal weighting of genera regardless of number of species within, is an important limitation of the GRb proxy approach which detracts from its usefulness, especially in diverse tropical communities that are dominated by species-rich genera.
Considering species-level data collection is not feasible for corals within the scope of most coral reef monitoring programs, we advocate that generic richness data should be collected for numerous reasons. Firstly, it provides a measure of phylogenetic diversity. Secondly, if applied with caution, GRb data can be used to obtain a robust estimate of species richness; and thirdly, generic richness is easily quantified. Although it takes longer to document generic richness on a belt transect than to collect generic richness or benthic cover data on a point-intercept transect, it is possible for a suitably trained diver to complete up to six 50×2 m belt transects per 60 minute dive. While identifying corals to genus is far easier than identifying to species, para-taxonomic training is still necessary because there are 86 genera of scleractinian corals in the Indo-Pacific. However, genus-level identification guides are available and it is realistic for field guides to be taken underwater to help confirm identifications.
Despite its potential as a proxy indicator, caution must be applied when using GRb data to predict species richness because it is still a relatively coarse measure and the level of precision obtained is contingent on numerous conditions. For example, at Kosrae, if a mean of 20 genera were recorded across a site, we would predict between 28–33 species were present. With application of the regression equation based upon the transect data, a far less precise estimate is obtained (16–48 species, see Table 3). Thus, even if the R2 is relatively high, at sites with high heterogeneity, the error about the coefficient of determination may be large; hence the range of predicted species richness may be so great that it becomes non-informative. Furthermore, if species-rich genera dominate the community, the strength of the generic-species richness relationship diminishes. Lastly, in the context of threatened species, generic richness data will not provide the necessary information to enable species population trends to be monitored.
Conclusion
If logistic or budgetary constraints prohibit ongoing species-level monitoring of coral biodiversity, measuring generic richness on replicated belt transects provides a meaningful way to monitor and detect critical changes in phylogenetic diversity and to predict species richness with reasonable amount of certainty. It is important to note however, that prior to incorporating GRb into monitoring programs, species level data should first be collected to confirm that generic richness provides a robust and precise estimate of species richness at the target location. Furthermore, the most appropriate scale of analysis must be resolved and the performance of the proxy metric must be regularly evaluated, particularly after local disturbances. Overall, if preventing coral biodiversity loss is a priority to coral reef management authorities, it is essential that monitoring programs are adapted to include a suitable proxy for species richness that could complement broad-scale habitat data.
Supporting Information
Composition of the coral communities at the three study locations. (a) Total number of colonies on belt transects within each genus; (b) Species richness within the 10 most species-rich genera.
(TIF)
ANOVA for examining the performance of predictor variables across scales of analysis (site versus transect).
(DOCX)
Acknowledgments
We thank Don Hess from the Marshall Islands College and staff of the Marshall Island Marine Resource Authority for supporting our work at Majuro Atoll. Thank you to Maria Beger for fieldwork collaboration at Majuro Atoll and Ashmore Reef. Thanks to Andy George, Osamu and Marston from the Kosrae Conservation and Safety Organization for field support at Kosrae. Ashmore-Cartier Reef data was collected during the 2009 National Marine Reserve Survey and we thank officers of the RV Walcott for their support in the field. The write-up phase of the project was partly supported by Woodside Energy.
Funding Statement
Funding was provided by the Australian Museum Foundation (http://australianmuseum.net.au/The-Australian-Museum-Foundation); The NOAA coral reef conservation programme (http://coastalmanagement.noaa.gov/programs/coral.html); and by the Australian Department of Environment, Arts and Heritage. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Botkin DB, Saxe H, Araujo MB, Betts R, Bradshaw RHW, et al. (2007) Forecasting the effects of global warming on biodiversity. BioSci 57: 227–236. [Google Scholar]
- 2. Butchart SHM (2010) Global biodiversity: Indicators of recent declines. Science 328: 1164–68. [DOI] [PubMed] [Google Scholar]
- 3. Hughes TP, Baird AH, Bellwood DR, Card M, Connolly SR, et al. (2003) Climate change, Human Impacts, and the Resilience of Coral Reefs. Science 301: 929–933. [DOI] [PubMed] [Google Scholar]
- 4. Halpern BS, Walbridge S, Selkoe KA, Kappel CV, Micheli F, et al. (2008) A global map of human impact on marine ecosystems. Science 319: 948–952. [DOI] [PubMed] [Google Scholar]
- 5.Knowlton N, Brainard RE, Fisher R, Moews M, Plaisance L, et al.. (2010) Coral reef biodiversity. In: Mcintyre AD, editor. Life in the World's Oceans: Diversity, Distribution and Abundance, Wiley-Blackwell: Oxford. Pp. 65–74. [Google Scholar]
- 6. Wilkinson C (2004) Status of coral reefs of the world. Australian Institute of Marine Science [Google Scholar]
- 7. Carpenter KE, Abrar M, Aeby G, Aronson RB, Banks S, et al. (2008) One-Third of Reef-Building Corals Face Elevated Extinction Risk from Climate Change and Local Impacts. Science 321: 560–563. [DOI] [PubMed] [Google Scholar]
- 8. Cardinale BJ, Duffy E, Gonzalez A, Hooper DU, Perrings C, et al. (2012) Biodiversity loss and its impact on humanity. Nature 486: 59–67. [DOI] [PubMed] [Google Scholar]
- 9. Richards ZT, Syms C, Wallace C, Muir P, Willis B (2013) Multiple occupancy-abundance patterns in staghorn coral communites. Div Dist 19: 884–895. [Google Scholar]
- 10. Bracken MES, Low NHN (2012) Realistic losses of rare species disproportionately impact higher trophic levels. Ecol Lett 15: 461–467. [DOI] [PubMed] [Google Scholar]
- 11. Mouillot D, Bellwood DR, Baraloto C, Chave J, Galzin R, et al. (2013) Rare Species Support Vulnerable Functions in High-Diversity Ecosystems. PLoS Biol 11 (5) e1001569 doi:10.1371/journal.pbio.1001569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chapin FS III, Zavaleta ES, Eviner VT, Naylor RL, Vitousek PM, et al. (2000) Consequences of changing biodiversity. Nature 405: 234–242. [DOI] [PubMed] [Google Scholar]
- 13. Balmford A, Crane P, Dobson, Green RE, Mace GM (2005) The 2010 challenge: data availability information needs and extraterrestrial insights. Phil. Trans. Roy. Soc (London) Bio Sci 360: 221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hendriks IE, Duarte CM, Heip CHR (2006) Biodiversity Research Still Grounded. Science 312: 1715. [DOI] [PubMed] [Google Scholar]
- 15. Knowlton N, Jackson JBC (2008) Shifting baselines, local impacts and global change on coral reefs. PLoS Biology 6 (2) e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Richardson AJ, Poloczanska ES (2008) Under-Resourced, Under Threat. Science 32: 1294–95. [DOI] [PubMed] [Google Scholar]
- 17. Gardner TA, Barlow J, Araujo IS, Ávila-Pires TC, Bonaldo AB, et al. (2008) The cost-effectiveness of biodiversity surveys in tropical forests. Ecol Lett 11: 139–150. [DOI] [PubMed] [Google Scholar]
- 18. Rodrigues ASL, Brooks TM (2007) Shortcuts for biodiversity conservation planning: The effectiveness of surrogates. ARES 38: 713–737. [Google Scholar]
- 19. Dalleau M, Andréfouët S, Wabnitz CC, Payri C, Wantiez L, et al. (2010) Use of habitats as surrogates of biodiversity for efficient coral reef conservation planning in Pacific Ocean islands. Cons Biol 24: 541–552. [DOI] [PubMed] [Google Scholar]
- 20. Baillie JEM, Collen B, Amin R, Akcakaya HR, Butchart SHM, et al. (2008) Towards monitoring global biodiversity. Cons Lett 1: 18–26. [Google Scholar]
- 21. Humphries CJ, Williams PH, Wanewright RI (1995) Measuring biodiversity value for conservation. ARES 26: 93–111. [Google Scholar]
- 22. Favareau JM, Drew CA, Hess GR, Rubino MJ, Koch FH, et al. (2006) Recommendations for assessing the effectiveness of surrogate species approaches. Biol Cons 15: 3949–3969. [Google Scholar]
- 23. Araújo MB, Humphries CJ, Densham PJ, Lampinen R, Hagemeijer WJM, et al. (2001) Would environmental diversity be a good surrogate for species diversity? Ecography 24: 103–10. [Google Scholar]
- 24. Pressey RL (2004) Conservation planning and biodiversity: Assembling the best data for the job. Cons Biol 18: 1677–1681. [Google Scholar]
- 25. Andleman SL, Fagen WF (2000) Umbrellas and flagships: efficient conservation surrogates or expensive mistakes? Proc Natl Acad Sci USA 97: 5954–5959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vellend M, Lilley PL, Starzomski BM (2008) Using subsets of species in biodiversity surveys. J App Ecol 45: 161–169. [Google Scholar]
- 27. Bruno J, Selig ER (2007) Regional decline of coral cover in the Indo-Pacific: Timing, extent and subregional comparisons. PloS One 8: e711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eakin CM, Nim CJ, Brainard RE, Aubrecht C, Elvidge C, et al. (2010) Monitoring coral reefs from space. Oceanog 23: 118–133. [Google Scholar]
- 29. De'ath G, Fabricius KE, Sweatman H, Poutinen M (2012) The 27-year decline of coral cover on the Great Barrier Reef and its causes. Proc Natl Acad Sci USA 109: 17995–17999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Richards ZT (2013) A comparison of proxy performance in coral biodiversity monitoring. Coral Reefs 32: 287–292. [Google Scholar]
- 31. Williams PH, Gaston KJ (1994) Measuring more of biodiversity: can higher-taxon richness predict wholesale species richness? Biol Cons 67: 211–217. [Google Scholar]
- 32. Balmford A, Green MJB, Murray MG (1996a) Using higher-taxon richness as a surrogate for species richness: I. Regional Tests. Roy Soc Proc B 263: 1267–1274. [Google Scholar]
- 33. Balmford A, Jayasuriya AHM, Green MJB (1996b) Using higher-taxon richness as a surrogate for species richness: II. Local Applications. Roy Soc Proc B 263: 1571–1575. [Google Scholar]
- 34. Balmford A, Lyon AJE, Lang RM (2000) Testing the higher-taxon approach to conservation planning in a megadiverse group: the macrofungi. Biol Cons 93: 209–217. [Google Scholar]
- 35. Niemelä J (2000) Biodiversity monitoring for decision-making. Ann Zool Fenn 37: 307–317. [Google Scholar]
- 36. Villasenor JL, Ibarra-Manríquez G, Meave JA, Ortiz E (2005) Higher taxa as surrogates of plant biodiversity in a megadiverse country. Cons Biol 19: 232–238. [Google Scholar]
- 37. Lovell S, Hamer M, Slotow R, Herbert D (2007) Assessment of congruency across invertebrate taxa and taxonomic levels to identify potential surrogates. Biol Cons 139: 113–125. [Google Scholar]
- 38. Lewandowski AS, Noss RF, Parson DR (2010) The effectiveness of surrogate taxa for the representation of biodiversity. Cons Biol 24: 1367–1377. [DOI] [PubMed] [Google Scholar]
- 39.Veron JEN (1995) Corals in Space and Time. The biogeography and evolution of the Scleracinia. UNSW Press, Sydney, Australia. Pp 48 & 156.
- 40.R Core Team (2013) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
- 41. Bellwood DR, Hoey AS, Choat JH (2003) Limited functional redundancy in high diversity systems: resilience and ecosystem function on coral reefs. Ecol Lett 6: 281–285. [Google Scholar]
- 42. Hooper DU, Chapin FS, Ewel JJ, Hector J, Inchausti P, et al. (2005) Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol Mono 75: 3–35. [Google Scholar]
- 43. Danovaro R, Gambi C, Dell'Anno A, Corinaidesi C, Fraschetti S, et al. (2008) Exponential decline of deep-sea ecosystem functioning linked to benthic biodiversity loss. Current Biology 18: 1–8. [DOI] [PubMed] [Google Scholar]
- 44. Hughes TP (1994) Catastrophes, phase-shifts, and large-scale degradation of a Caribbean coral reef. Science 265: 1547–1551. [DOI] [PubMed] [Google Scholar]
- 45. Bellwood DR, Hughes TP, Hoey AS (2006) Sleeping functional group drives coral reef recovery. Current Biology 16: 2434–2439. [DOI] [PubMed] [Google Scholar]
- 46. Micheli F, Halpern BS (2005) Low functional redundancy in coastal marine assemblages. Ecology Letters 8: 391–400. [Google Scholar]
- 47. Burkepile DE, Hay M (2008) Herbivore species richness and feeding complementarity affect community structure and function on a coral reef. Proc Natl Acad Sci USA 105: 16201–16206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ateweberhan M, McClanahan TR, Graham NAJ, Sheppard CRC (2011) Episodic heterogenous decline and recovery of coral cover in the Indian Ocean. Coral Reefs 30: 739–752. [Google Scholar]
- 49. Graham NAJ, Nash KL, Kool JT (2011) Coral reef recovery dynamics in a changing world. Coral Reefs 30: 283–294. [Google Scholar]
- 50. Sweatman H, Delean S, Syms C (2011) Assessing loss of coral cover on Australia's Great Barrier Reef over two decades, with implication for longer-term trends. Coral Reefs 30: 521–531. [Google Scholar]
- 51. Mumby PJ, Skirving W, Strong AE, Hardy JT, Le Drew EF, et al. (2004) Remote sensing of coral reefs and their physical environment. Mar Poll Bull 48: 219–228. [DOI] [PubMed] [Google Scholar]
- 52. Connell JH (1978) Diversity in tropical rain forests and coral reefs. Science 199: 1302–1310. [DOI] [PubMed] [Google Scholar]
- 53. Bruno JF, Selig ER, Casey KS, Page CA, Willis BL, et al. (2007) Thermal stress and coral cover as drivers of coral disease outbreaks. PloS Biol 5: e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jones GP, McCormick MI, Srinivasan M, Eagle JV (2004) Coral decline threatens fish biodiversity in marine reserves. Proc Natl Acad Sci USA 101: 8251–8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Faith DP (1992) Conservation evaluation and phylogenetic diversity. Biol Cons 61: 1–10. [Google Scholar]
- 56. Mace GM, Gittleman JL, Purvis A (2003) Preserving the tree of life. Science 300: 1707–1709. [DOI] [PubMed] [Google Scholar]
- 57. Roy K, Jablonski D, Valentine JW (1996) Higher taxa in biodiversity studies: patterns from eastern Pacific marine molluscs. Phil Trans Roy Soc B 351: 1605–1613. [Google Scholar]
- 58. Vanderklift MA, Ward TJ, Phillips JC (1998) Use of assemblages derived from different taxonomic levels to select areas for conserving marine biodiversity. Biol Cons 86: 307–315. [Google Scholar]
- 59. Magierowski RH, Johnson CR (2006) Robustness of surrogates of biodiversity in marine benthic communities. Ecol Appl 16: 2264–2275. [DOI] [PubMed] [Google Scholar]
- 60. Mellin C, Delean S, Caley J, Edgar G, Meekan, et al. (2011) Effectiveness of biological surrogates for predicting patterns of marine biodiversity: a global meta-analysis. PloS One 6: e20141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Composition of the coral communities at the three study locations. (a) Total number of colonies on belt transects within each genus; (b) Species richness within the 10 most species-rich genera.
(TIF)
ANOVA for examining the performance of predictor variables across scales of analysis (site versus transect).
(DOCX)