Abstract
Objective/Hypothesis
The objective of this study was to examine sub-types of individuals with Myalgic Encephalomyelitis/chronic fatigue syndrome (ME/CFS) based upon variables that are theoretically associated with the Energy Envelope Theory (EET), and to examine the role of coping strategies in explaining the differences found between the subtypes.
Methods
Cluster analysis was used. Grouping variables included physical functioning, post-exertional malaise severity, and the extent to which an individual was outside of the EE. These clusters were evaluated using discriminant function analysis to determine whether they could be differentiated based on coping styles.
Results
Cluster analysis identified three groups. Clusters 1 and 2 were consistent with the EET. However, Cluster 3 was characterized by patients with the most impairment, but they were to a lesser extent exceeding their EE. Coping strategies explained a small percentage (10%) of the variance in differentiating the clusters.
Discussion
Energy maintenance may be associated with improved functioning and less severe symptoms for some. However, patients in Cluster 3 were closer to remaining within their EE and also used higher levels of adaptive coping, but were more impaired than Cluster 2. This suggests that adaptive coping strategies were not associated with improved health, as members of Cluster 3 were severely limited in functioning.
Keywords: Myalgic Encephalomyelitis, chronic fatigue syndrome, energy envelope, pacing, coping
Myalgic Encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a debilitating and chronic illness [1]. Although contested, the most frequently used case definition is referred to as the Fukuda case definition [2]. This definition stipulates that an individual must experience six or more months of chronic, unexplained fatigue of new/definite onset that is not alleviated by rest and not the result of ongoing exertion. Further, to meet criteria for a diagnosis, an individual must experience at least 4 out of 8 definitional symptoms concurrently with this fatigue: sore throat, lymph node pain, muscle pain, joint pain, post-exertional malaise (PEM), headaches, memory and concentration issues, and unrefreshing sleep. Unfortunately, PEM is not a required symptom, even though it is considered a cardinal symptom of ME/CFS, and several alternative case definitions require this symptom for a diagnosis [3, 4, 5].
PEM is a debilitating exhaustion/flu-like sickness that individuals with ME/CFS experience after physical or mental exertion. It typically often lasts for more than 24 hours [3]. It is associated with a worsening of other symptoms and reduced social, occupational, physical and emotional functioning. The experience of PEM has been found to help discriminate individuals with ME/CFS from those individuals with a primary Major Depressive Disorder. Hawk, Jason and Torres-Harding found that 100% of a sample of individuals with ME/CFS reported PEM, while only 20% of individuals with Major Depressive Disorder and 6% of healthy controls reported this symptom [6]. Further, Jason and Taylor performed a cluster analysis on individuals with ME/CFS and controls, and found that ME/CFS patient clusters were best distinguished from healthy controls based upon their reported levels of PEM severity [7].
As indicated above, the majority of patients with ME/CFS experience PEM. Individuals with ME/CFS have a limited energy capacity, and this lower threshold may make it easier to expend more energy than is readily available, thus contributing to their energy “crashes” [8]. PEM is typically triggered by over-exertion. However, walking a few blocks to the store or doing the dishes could characterize “over-exertion” for a patient with ME/CFS. Pacing and staying within one’s energy envelope have been advocated as one part of a treatment approach for patients with ME/CFS dealing with PEM [9]. The energy envelope theory postulates that patients who maintain their expended energy at a level consistent with their available energy will have better health outcomes and quality of life compared to those who over-expend their energy levels [10]. Individuals who consistently exceed their energy envelope report poorer quality of life due to severe fatigue, increased disability, sleep difficulties, depression and anxiety [8]. In contrast, those who can stay within this energy envelope report improvements in their physical functioning and decreased fatigue severity [10]. Brown, Khorana, and Jason also found that those who were within their energy envelope before treatment showed more improvement in physical functioning and fatigue compared with those outside of their energy envelope [11].
Lipowski describes illness-specific coping as behaviors individuals employ during recovery, and also behaviors employed to compensate for limitations their illness places on them [12]. Differences in coping strategies in ME/CFS populations have been found based on ethnicity [13], gender, and employment status [14]. Brown, Brown and Jason found that individuals who had a longer illness duration employed adaptive coping strategies significantly more than those who had recently developed the illness [15]. Camacho and Jason studied patients who had recovered from ME/CFS, those who had not recovered from ME/CFS, and a non-ME/CFS healthy control group. No significant differences were found between groups on measures of optimism, stress, and social support; however, those who had recovered spent less time focusing on symptoms than those who had not recovered [16]. In addition, those who had recovered in comparison to healthy controls more often used positive reinterpretation and growth strategies and thus may have benefitted from the experience of being ill in some ways. Some patients with ME/CFS might use less adaptive coping strategies [17, 18] such as avoidance [19], and less adaptive coping strategies may be associated with increased disability [20]. A few cognitive-behavioral treatment studies have incorporated strategies that help individuals with ME/CFS reduce perfectionism [21, 22]. It has been suggested that some aspects of perfectionism lead to a higher use of maladaptive coping strategies, predisposing individuals to fatigue [23]. However, this proposed association between fatigue and perfectionism has not consistently been found in ME/CFS populations [24]. There have been no previous investigations of the relationship between the energy envelope theory and perfectionism.
The proposed study aims to investigate distinct ME/CFS subgroups based on self-reported levels of post-exertional malaise severity, level of physical functioning, and the ability to stay within the “energy envelope.” In this exploratory study, we wanted to assess whether coping strategies would explain a significant portion of the difference observed between these subgroups. It was hypothesized that one subgroup would be outside their energy envelope, with more severe post-exertional malaise and poorer physical functioning.
Method
Participants
Participants with ME/CFS were recruited for a non-pharmacological treatment trial [25] from the larger Chicago metropolitan area, relying mostly on physician referrals. Additionally, announcements were placed in newspapers and support groups were visited. In total, 114 individuals were included. Of these 114, 46% were referred by physicians, 34% through media advertisements, and 20% from other sources (e.g. heard about the study through a friend or family member). Participants recruited from these difference sources did not differ sociodemographically. All participants were at least 18 years old, not pregnant, English speaking, and were physically capable of attending scheduled sessions. Written consent was received from all participants and DePaul University’s Institutional Review Board approved the study. Of the sample of 114, 16.7 percent were male and 83.3 percent were female. The average age at baseline was 43.8 years. In terms of race/ethnicity, 87.7 percent self-identified as White, 4.4 percent identified as African-American, 4.4 percent identified as Latino, and 3.5 percent identified as Asian-American. All data used in the present analyses come from baseline assessment and only 91 of the total 114 participants were included due to missing data on key variables.
Measures
Physical Functioning
A subscale from the Medical Outcomes Study Short-Form 36 [26] was used to assess physical functioning. The SF-36 is a self-report measure of functional status related to health. Scores range from 0–100, with higher scores indicating less disability and better functioning. Adequate reliability has been found for this measure.
Post-Exertional Malaise Severity
An item from the Chronic Fatigue Syndrome Medical Questionnaire [27] stating “Rate the severity of your post-exertional malaise over the past 6 months” was used to measure PEM severity. Scores range from 0–100 with higher scores indicating greater severity. Hawk and colleagues found this item had adequate test-retest reliability [28].
Energy Envelope Quotient
Participants were asked to rate weekly perceived energy and expended energy on a 100-point scale (0= no energy; 100= abundant energy). Perceived energy referred to the participant’s approximation of his or her own energy capacity (over the previous week). Expended energy referred to the participant’s approximation of the total amount of energy expended (over the previous week). An energy quotient score was calculated by dividing the perceived available energy by the amount of expended energy and then multiplying by 100. Scores greater than 100 indicate that the participant pushes himself or herself beyond his/her energy resources and the higher the number is beyond 100, the more the person is outside of the energy envelope. The energy quotient was used to determine whether or not the participants remained within their available energy resources. Hawk and colleagues found this measure had adequate test-retest reliability [28].
Coping
Coping strategies were assessed using the Brief Coping Orientation to Problems Experienced Scale (bCOPE) [29]. This is a frequently used scale with adequate reliability and assesses different ways of coping with stress. The bCOPE assesses problem-focused coping and emotion-focused coping strategies. A total of 28 items are rated on 4-point scales, with scores ranging from 1 (not engaging in the coping strategy) to 4 (engaging in the coping strategy a lot). Fourteen distinct coping strategies are measured. Relevant subscales for the present study included self-distraction, active coping, behavioral disengagement, planning, acceptance, and self-blame. Self-distraction, behavioral disengagement, and self-blame subscales were combined and averaged to form a “less adaptive coping” scale. We use the word “less adaptive” as it is unclear whether or not some of these strategies might be helpful for individuals with severe illness, and who might be using distraction and disengagement to reduce symptoms. The active coping, planning, and acceptance subscales were combined and averaged to form an “adaptive coping” scale. We use the word “adaptive coping” as there is research suggesting that these strategies are related to better outcomes with patients. The adaptive and less adaptive scales were not significantly correlated with one another. The following scales and reliability coefficients were reported by Carver [29]: Self-Distraction (α = .71), Active Coping (α = .68), Behavioral Disengagement (α = .65), Planning (α = .73), Acceptance (α = .57), and Self-Blame (α = .69).
Results
Analytic Strategy
A two-step cluster analysis was run to explore potential clusters of individuals with ME/CFS on physical functioning, post-exertional malaise severity, and energy envelope quotient. We used these three measures as they tap three distinct characteristics of patients, overall physical functioning, the extent that one of the classic symptoms of this illness (PEM) is experienced, and the extent that patients exceed their energy envelope. These three variables are all theoretically related to the Energy Envelope Theory. All variables were standardized before the clustering procedure. To establish an appropriate initial number of clusters, Ward’s Hierarchical clustering method was employed. Next, a K-Means non-hierarchical approach was used to examine multiple cluster solutions. Follow-up discriminant analyses were conducted in order to determine whether cluster groups could be differentiated by adaptive and less adaptive coping styles.
Cluster Analysis
An appropriate number of clusters was determined by examining the following indices: R2, partial R2, and the average standard deviation. Given the abrupt jump in values for each of these indices between a 3 and 4-cluster solution, a 3-cluster solution was selected to minimize within cluster variance and maximize between cluster variance. Using the K-Means method, 2, 3 and 4-cluster solutions were examined. The 3-cluster solution was easily interpretable and identified 3 very distinct subgroups of patients with ME/CFS, and thus it was selected for further interpretation.
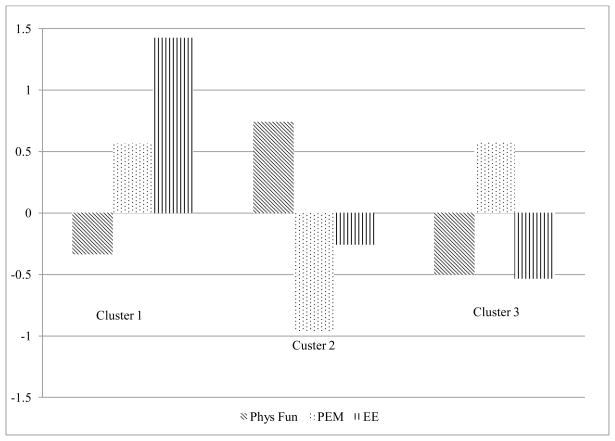
Cluster 1 (n=20) was named “Symptomatic and Highly Overextended” as this cluster was characterized by impaired physical functioning, high levels of post-exertional malaise, and exceeding their energy envelope. Cluster 2 (n=34) was named “Less symptomatic and Moderately Overextended” and was characterized by better physical functioning, low or mild post-exertional malaise, and moderately exceeding their energy envelope. Cluster 3 (n=37) was named “Symptomatic and Mildly Overextended” and was characterized by impaired physical functioning, high post-exertional malaise, and only minimally exceeding their energy envelope (see Figure 1 for a graphical representation of the cluster solution using standardized values, and see Table 1 for descriptive statistics for the clusters on each of the un-standardized grouping variables). The clusters did not differ sociodemographically.
Figure 1.
3-Cluster Solution for Physical Functioning, Post-Exertional Malaise Severity, and Energy Envelope Quotient
Table 1.
Means and Standard Deviations on Cluster Variables
| Variable | Cluster 1 | Cluster 2 | Cluster 3 | |||
|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | |
| Phys. Functioning | 39.44a | 19.46 | 64.79ab | 18.20 | 35.54b | 20.44 |
| PEM Severity | 85.13a | 8.13 | 61.03ab | 11.40 | 85.34b | 10.87 |
| Energy Envelope | 318.54ac | 72.76 | 156.42a | 61.47 | 129.85c | 55.90 |
Similiar superscript letters across row variables indicate significant differences at the p < .05 level.
Discriminant Analysis
A descriptive discriminant analysis was conducted to investigate whether the use of different coping strategies (adaptive and less adaptive) could discriminate the 3 clusters (see Table 2 for descriptive statistics for the clusters on each of the coping strategies). To test the assumption of homogeneity of within covariance matrices, Box’s Test was used. An alpha level of .001 was set. The test was non-significant, X2[6] = 11.21, p = .08. Therefore, a pooled covariance matrix was used in the discriminant function.
Table 2.
Average Coping Strategy Use in Three Clusters
| Adaptive | Less adaptive | ||||
|---|---|---|---|---|---|
|
| |||||
| Cluster | n | M | SD | M | SD |
| 1 | 20 | 3.05 | 0.72 | 1.28 | 0.36 |
| 2 | 34 | 2.76* | 0.70 | 1.46 | 0.47 |
| 3 | 37 | 3.18* | 0.60 | 1.57 | 0.50 |
Statistically significant difference at the p < .05 level.
Two functions emerged to separate the clusters, both of which are described in Table 3. Function 1 was significant, F[4] = 3.31, p = .01, and could account for 10.3% of the variance between groups, as determined by an Rc value of 0.32. Function 2 was non-significant, F[1] = 3.37, p = .07, and accounted for only 4% of the variance between groups, as determined by an Rc value of 0.19. Only Function 1 was selected for further interpretation.
Table 3.
Canonical Discriminant Functions for the Three Cluster Groups
| Function | F | df | p | Rc | Rc2 |
|---|---|---|---|---|---|
| 1–2 | 3.31 | 4 | .012 | .32 | 10.3% |
| 2 | 3.37 | 1 | .07 | .19 | 4.0% |
Structure coefficients were examined next for Function 1, and reported for Function 2 (see Table 4 for the standardized discriminant function and structure coefficients for the three clusters). All of the coefficients for Function 1 were greater than .30, indicating that each coping strategy was significantly correlated with the function. Adaptive coping accounted for 56% of the variance explained by the function, as determined by an rs value of .75, and less adaptive coping accounted for 25% of the variance, as determined by an rs value of .50. By examining the standardized coefficients for Function 1, we can examine the importance of each coping strategy while controlling for the other. Adaptive coping was correlated at 0.88 with the function, suggesting that this measure is predominantly driving the function.
Table 4.
Standardized Discriminant Function and Structure Coefficients for Three Clusters
| Scale | Coefficient | rs | rs2 |
|---|---|---|---|
| Function 1 | |||
| Adaptive | .88 | .75 | 56.0% |
| Less adaptive | .67 | .50 | 25.0% |
| Function 2 | |||
| Adaptive | −.51 | −.66 | 44.0% |
| Less adaptive | .77 | .87 | 76.0% |
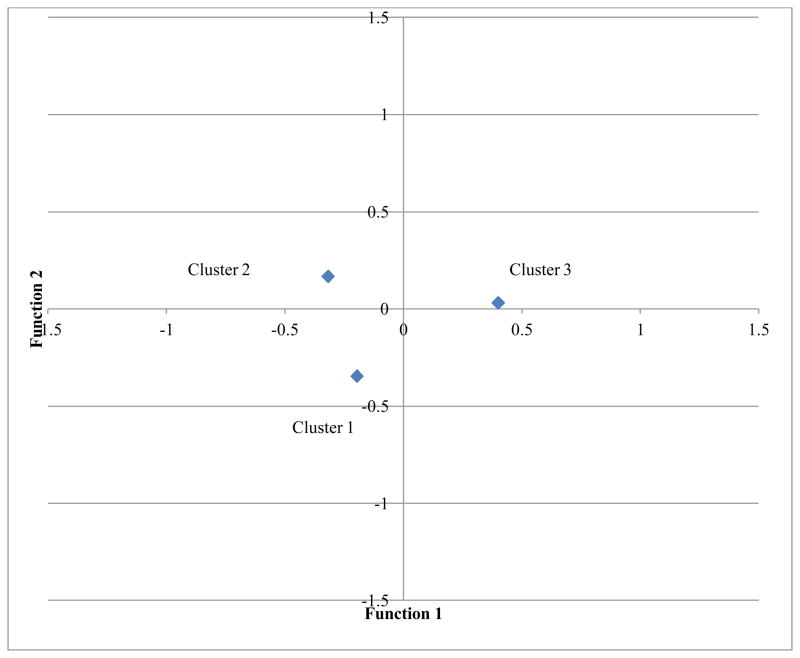
Finally, the group centroids were examined to understand which clusters used each type of coping strategy. Function 1 is largely driven by adaptive coping, and Cluster 3, the Symptomatic and Mildly Overextended group, are high in function 1; therefore, this group was high in adaptive coping. Function 1 separates Cluster 3 from the other two clusters (see Figure 2 for a graphical representation of the group centroids means for each function).
Figure 2.
Group Centroid Means
Discussion
In the current study, using a two-step clustering procedure, three distinct ME/CFS subgroups were identified based on participants’ self-reported levels of physical functioning, post-exertional malaise severity, and energy envelope maintenance. Cluster 1 (Symptomatic and Highly Overextended) included individuals highly outside the energy envelope who experienced high levels of post-exertional malaise severity and impaired physical functioning. This cluster pattern is consistent with the energy envelope theory, which states that individuals who expend more energy than is available will experience worse health outcomes. This is consistent with previous literature that has showed that those who engage in activity pacing and energy monitoring are better at staying within the energy envelope and also experience improvements in health, such as lower levels of post-exertional malaise severity, depression, anxiety, and improved physical functioning and quality of life [10].
Cluster 2 (Less symptomatic and Moderately Overextended) is also consistent with the energy envelope theory. Participants within this group were better at staying within their energy envelope compared to Cluster 1, experienced higher levels of physical functioning, and were lower on post-exertional malaise severity. Although this group was still moderately overextended beyond their energy envelope, they were better at maintaining appropriate energy levels and had better PEM symptom and physical functioning outcomes in comparison to Cluster 1
Cluster 3 (Symptomatic and Mildly Overextended) was comprised of individuals who were the least outside of their energy envelope, but were impaired on physical functioning, and had high levels of post-exertional malaise. Paradoxically, at least according to the energy envelope, individuals in this subgroup were very impaired even though they stayed closer to within their energy envelope.
Through descriptive discriminant analysis, it was revealed that coping strategies explained a small percentage (10%) of the variance in differentiating the three cluster groups. Interestingly, the Symptomatic and Mildly Overextended group (Cluster 3) was primarily driving the cluster separation. Furthermore, this group endorsed using more adaptive coping strategies than all other groups (significantly more than Cluster 2 but only directionally more than Cluster 1). Cluster 3 was about as impaired as Cluster 1, but was comprised of individuals who endorsed the highest use of adaptive coping strategies. When the adaptive coping strategies were examined individually, Cluster 3 was not significantly different than Clusters 1 or 2.
It is possible that energy maintenance and pacing may be associated with improved functioning and less severe symptoms for some individuals (as evidenced by patients in Cluster 2 in contrast to Cluster 1). However, patients in Cluster 3 were closer to remaining within their energy envelope and also used higher levels of adaptive coping, but were more impaired than Cluster 2. It is possible that the patients in Cluster 3 have severe limitations, and have learned to modulate their energy reserves and use a variety of coping strategies in order to deal with their illness.
Camacho and Jason found that patients who had recovered from ME/CFS, those who had not recovered from ME/CFS, and a non-ME/CFS healthy control group were indistinguishable based upon levels of optimism, stress or social support [16]. Likewise, the current results indicate that the use of more adaptive coping strategies was not associated with decreased symptomatology and increased physical functioning. Many proponents of cognitive-behavioral therapy posit that teaching individuals with ME/CFS how to better cope with their illness will lead to better health outcomes. The current findings suggest that adaptive coping strategies were not associated with improved health. In fact, members of Cluster 3 used the highest rates of adaptive coping but were severely limited in functioning.
In an attempt to further explain the differences between the three clusters, the clusters were compared on the remaining SF-36 subscales. Cluster 3 had significantly worse Bodily Pain when compared to Cluster 2. It is possible that there is an association between the severe symptomatology and functional impairment observed in Cluster 3 and the amount of bodily pain experienced by these individuals. Based on the Energy Envelope theory, Cluster 3’s quotient should be associated with higher functioning than what was observed: the most severe debilitation. Future research could examine pain as a moderating factor between one’s energy envelope quotient and other health outcomes.
It should be noted that the distinction between adaptive and less adaptive may be more complex for this patient group than for other groups. Given the documented benefits of and patient preference for pacing and staying within one’s energy envelope, it is possible that strategies which may be considered “maladaptive” for other groups such as avoidance, behavioral disengagement, or self-distraction are, for this population, part of an “adaptive” response to low energy and high fatigue levels. Additional studies are needed to determine the validity of the adaptive/maladaptive construct for this patient population.
The current findings may have implications for graded exercise therapy (GET) as an appropriate treatment for ME/CFS. GET, which consists of supervised exercise and promotes gradual increases in physical activity, has been found to be effective in multiple studies [30, 31, 32]. If a GET participant is experiencing increased fatigue, they are encouraged to continue exercising at their current level but not increase their activity until the fatigue has subsided [30]. According to the Energy Envelope Theory, fatigue is a product of over extension and can be potentially managed by reducing activity. Cluster 2 provides evidence that pacing, rather than pushing one’s self to increase activity, is associated with better physical functioning. Further, Cluster 1 suggests that a failure to pace is associated with poor functioning. Interestingly, Cluster 3 was functioning poorly even while pacing most effectively. It is unclear whether Cluster 3 participants would benefit from increased activity levels. However, the general pattern of results does not suggest that pushing beyond one’s limits would be beneficial with regards to symptomatology or functionality.
There are several limitations to the present study. For example, the sample was recruited primarily through physician referrals and represents a tertiary-care subgroup of patients. This study needs to be replicated with a community-based sample, as prevalence studies have shown that minorities and individuals of low SES experience similar or higher rates of ME/CFS compared to White individuals of higher SES [33,34]. Further, the energy envelope quotient is a self-reported assessment of perceived and expended energy and may represent a potential measurement issue. Additionally, further research might attempt to replicate this cluster solution with a larger sample. Future research should also focus on investigating other factors that could discriminate these clusters, beyond coping strategies. More biologically based work that examines potential immune, autonomic, and neurological dysfunction within this patient population could be integrated into this type of research.
Acknowledgments
The authors appreciate the funding provided by NIAID (grant numbers AI 49720).
References
- 1.Afari N, Buchwald D. Chronic fatigue syndrome: A review. American Journal of Psychiatry. 2003;160:221–236. doi: 10.1176/appi.ajp.160.2.221. [DOI] [PubMed] [Google Scholar]
- 2.Fukuda K, Straus SE, Hickie I, et al. The chronic fatigue syndrome: A comprehensive approach to its definition and study. Annals of Internal Medicine. 1994;121:953–959. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- 3.Carruthers BM, Jain AK, DeMeirleir KL, et al. Myalgic encephalomyelitis/chronic fatigue syndrome: Clinical working case definition, diagnostic and treatments protocols. Journal of Chronic Fatigue Syndrome. 2003;11:7–115. [Google Scholar]
- 4.Carruthers BM, van de Sande MI, De Meirleir KL, et al. Myalgic encephalomyelitis: International consensus criteria. Journal of Internal Medicine. 2011;270(4):327–338. doi: 10.1111/j.1365-2796.2011.02428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jason LA, Evans M, Porter N, et al. The development of a revised Canadian Myalgic encephalomyelitis/chronic fatigue syndrome case definition. American Journal of Biochemistry and Biotechnology. 2010;6(2):120–135. [Google Scholar]
- 6.Hawk C, Jason LA, Torres-Harding S. Differential diagnosis of chronic fatigue syndrome and major depressive disorder. International Journal of Behavioral Medicine. 2006;13(3):244–251. doi: 10.1207/s15327558ijbm1303_8. [DOI] [PubMed] [Google Scholar]
- 7.Jason LA, Taylor RR. Applying cluster analysis to define a typology of chronic fatigue syndrome in a medically-evaluated, random community sample. Psychology and Health. 17(3):323–337. [Google Scholar]
- 8.Jason L, Muldowney K, Torres-Harding S. The energy envelope theory and Myalgic encephalomyelitis/chronic fatigue syndrome. American Association of Occupational Health Nurses. 2008;56(5):186–195. doi: 10.3928/08910162-20080501-06. [DOI] [PubMed] [Google Scholar]
- 9.Goudsmit EM, Jason LA, Nijs J, Wallman KE. Pacing as a strategy to improve energy management in myalgic encephalomyelitis/chronic fatigue syndrome: A consensus document. Disability and Rehabilitation. 2012;34(13):1140–1147. doi: 10.3109/09638288.2011.635746. [DOI] [PubMed] [Google Scholar]
- 10.Jason L, Benton M, Torres-Harding S, Muldowney K. The impact of energy modulation on physical functioning and fatigue severity among patients with ME/CFS. Patient Education and Counseling. 2009;77(2):237–241. doi: 10.1016/j.pec.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown M, Khorana N, Jason LA. The role of changes in activity as a function of perceived available and expended energy in non-pharmacological treatment outcomes for ME/CFS. Journal of Clinical Psychology. 2011;67:253–260. doi: 10.1002/jclp.20744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lipowski ZJ. Physical illness, the individual, and the coping process. Psychiatric Medicine. 1970;1:91–102. doi: 10.2190/19q3-9ql8-xyv1-8xc2. [DOI] [PubMed] [Google Scholar]
- 13.Njoku MGC, Jason LA, Torres-Harding SR. The relationships among coping styles and fatigue in an ethnically diverse sample. Ethnicity and Health. 2005;10:263–278. doi: 10.1080/13557850500138613. [DOI] [PubMed] [Google Scholar]
- 14.Jason LA, Taylor RR, Kennedy CL, et al. Chronic fatigue syndrome: Sociodemographic subtypes in a community-based sample. Evaluation and the Health Professions. 2000;23:243–263. doi: 10.1177/01632780022034598. [DOI] [PubMed] [Google Scholar]
- 15.Brown MM, Brown AA, Jason LA. Illness duration and coping style in chronic fatigue syndrome. Psychological Reports. 2010;106(2):383–393. doi: 10.2466/pr0.106.2.383-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Camacho J, Jason LA. Psychosocial factors show little relationship to chronic fatigue syndrome recovery. Journal of Psychology and the Behavioral Sciences. 1998;12:60–70. [Google Scholar]
- 17.Creswell C, Chalder T. Defensive coping styles in chronic fatigue syndrome. Journal of Psychosomatic Research. 2001;51:607–610. doi: 10.1016/s0022-3999(01)00267-7. [DOI] [PubMed] [Google Scholar]
- 18.Moss-Morris R, Petrie KJ, Weinman J. Functioning in chronic fatigue syndrome: Do illness perceptions play a regulatory role? British Journal of Health Psychology. 1996;1:15–25. [Google Scholar]
- 19.Ax S, Gregg VH, Jones D. Coping and illness cognitions: chronic fatigue syndrome. Clinical Psychological Review. 2001;21:161–182. doi: 10.1016/s0272-7358(99)00031-8. [DOI] [PubMed] [Google Scholar]
- 20.Ray C, Jefferies S, Weir WC. Coping and other predictors of outcome in chronic fatigue syndrome: A 1-year follow-up. Journal of Psychosomatic Research. 1997;43(4):405–415. doi: 10.1016/s0022-3999(97)00111-6. [DOI] [PubMed] [Google Scholar]
- 21.Sharpe M, Hawton K, Simkin S, et al. Cognitive behavior therapy for the chronic fatigue syndrome: A randomized controlled trial. British Medical Journal. 1996;312:22–26. doi: 10.1136/bmj.312.7022.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deale A, Chalder T, Marks I, et al. Cognitive behavior therapy for chronic fatigue syndrome: A randomized controlled trial. The American Journal of Psychiatry. 1997;154:408–414. doi: 10.1176/ajp.154.3.408. [DOI] [PubMed] [Google Scholar]
- 23.Magnusson AE, Nias DKB, White PD. Is perfectionism associated with fatigue? Journal of Psychosomatic Research. 1996;41:377–383. doi: 10.1016/s0022-3999(96)00189-4. [DOI] [PubMed] [Google Scholar]
- 24.Blenkiron P, Edwards R, Lynch S. Associations between perfectionism, mood and fatigue in chronic fatigue syndrome: A pilot study. Journal of Nervous and Mental Disease. 1999;187:566–570. doi: 10.1097/00005053-199909000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Jason LA, Torres-Harding SR, Friedberg F, et al. Non-pharmacologic interventions for CFS: A randomized trial. Journal of Clinical Psychology in Medical Settings. 2007;14:275–296. [Google Scholar]
- 26.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Medical Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 27.Jason LA, Ropacki MT, Santoro NB, et al. A screening scale for chronic fatigue syndrome: Reliability and validity. Journal of Chronic Fatigue Syndrome. 1997;3:39–59. [Google Scholar]
- 28.Hawk C, Jason LA, Torres-Harding S. Reliability of a chronic fatigue syndrome questionnaire. Journal of Chronic Fatigue Syndrome. 2007;13(4):41–66. [Google Scholar]
- 29.Carver CS, Scheier MF, Weintraub JK. Assessing coping strategies: A theoretically based approach. Journal of Personality and Social Psychology. 1989;56(2):267–283. doi: 10.1037//0022-3514.56.2.267. [DOI] [PubMed] [Google Scholar]
- 30.Fulcher KY, White PD. Randomised controlled trial of graded exercise in patients with the chronic fatigue syndrome. British Medical Journal. 1997;314:1647. doi: 10.1136/bmj.314.7095.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Powell P, Bentall RP, Nye FJ, et al. Randomised controlled trial of patient education to encourage graded exercise in chronic fatigue syndrome. British Medical Journal. 2001;322:387. doi: 10.1136/bmj.322.7283.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White PD, Goldsmith KA, Johnson AL, et al. Comparison of adaptive pacing therapy, cognitive behavior therapy, graded exercise therapy, and specialist medical care for chronic fatigue syndrome (PACE): A randomized trial. The Lancet. 2011;377:823–836. doi: 10.1016/S0140-6736(11)60096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jason LA, Richman JA, Rademaker AW, et al. A community-based study of chronic fatigue syndrome. Archives of Internal Medicine. 1999;159(18):2129–2137. doi: 10.1001/archinte.159.18.2129. [DOI] [PubMed] [Google Scholar]
- 34.Reyes M, Nisenbaum R, Hoaglin DC, et al. Prevalence and incidence of chronic fatigue synrome in Wichita, Kansas. Archives of Internal Medicine. 2003;163:1530–1536. doi: 10.1001/archinte.163.13.1530. [DOI] [PubMed] [Google Scholar]