Abstract
Purpose of review
This review will discuss the evidence both for and against the concept that reactive oxygen species (ROS) play an important role in the regulation of inactivity-induced skeletal muscle atrophy.
Recent findings
It is well established that prolonged skeletal muscle inactivity causes muscle fiber atrophy and a decrease in muscle force production. This disuse-induced muscle atrophy is the consequence of a loss in muscle protein resulting from increased protein degradation and decreased protein synthesis. Recent studies suggest that oxidative stress can influence cell signaling pathways that regulate both muscle protein breakdown and synthesis during prolonged periods of disuse. Specifically, it is feasible that increased ROS production in muscle fibers can promote increased proteolysis and also depress protein synthesis during periods of skeletal muscle inactivity.
Summary
Although it is established that oxidants can participate in the regulation of protein turnover in cells, there remains debate as to whether oxidative stress is required for disuse skeletal muscle atrophy. Nonetheless, based on emerging evidence we conclude that increased ROS production in skeletal muscles significantly contributes to inactivity-induced muscle atrophy.
Keywords: redox, oxidants, antioxidants, proteasome, calpain, caspase-3, reactive oxygen species, muscle protein synthesis
INTRODUCTION
Prolonged periods of skeletal muscle inactivity (e.g., limb immobilization) are characterized by decreases in muscle contractile function and muscle fiber size. Specifically, it is well documented that these inactivity-induced alterations to muscle fibers are the result of both an increase in muscle protein degradation and a decrease in protein synthesis [1,2]. However, many, details regarding the signaling pathways that control muscle protein balance remain unknown. In this regard, the observation that prolonged periods of contractile inactivity also leads to the increased production of reactive oxygen species (ROS) in muscle fibers suggests that ROS could be an important signaling molecule that contributes to disuse muscle atrophy. In theory, increased ROS can accelerate proteolysis and depress protein synthesis [3]. Nonetheless, whether oxidants are a major contributor to disuse muscle atrophy remains controversial. Therefore, the goal of this report is to summarize the evidence both for and against the concept that ROS play an important signaling role in the control of disuse skeletal muscle atrophy. Our approach will be to provide a synopsis of major findings and an analysis of individual studies when warranted. We begin with a historical overview of evidence linking ROS and oxidative stress with inactivity-induced muscle atrophy.
OXIDANT STRESS AND DISUSE MUSCLE ATROPHY: HISTORICAL PERSPECTIVES
The first investigation connecting oxidants to disuse muscle atrophy appeared in the literature over two decades ago [4]. This pioneering study concluded that immobilization-induced skeletal muscle atrophy in the rat is associated with increased biomarkers of oxidative stress (e.g., muscle lipid peroxidation). This report also demonstrated that disuse muscle atrophy can be partially prevented by treatment with the antioxidant vitamin E; this finding led the authors to conclude that oxidative stress contributes to disuse muscle atrophy. Since this early work, numerous animal studies have also concluded that prolonged skeletal muscle disuse promotes oxidative stress in the inactive muscle and a growing number of recent studies suggest that “select” antioxidants can delay muscle atrophy induced by prolonged inactivity [5–7]. Collectively, these results have fueled interest in the role that ROS and redox disturbances play in the signaling events that lead to muscle atrophy induced by disuse.
ROS PRODUCTION IN SKELETAL MUSCLES DURING PROLONGED INACTIVITY
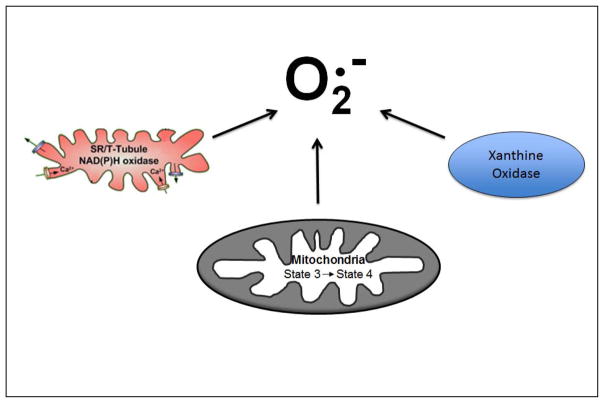
Although numerous definitions exist, oxidative stress is commonly defined as an imbalance of pro-oxidants and antioxidants, with the inequality documented by the accumulation of oxidized molecules in tissues [8]. In this regard, it is widely reported that prolonged muscle disuse results in an accumulation of oxidatively modified proteins and lipids. This inactivity-induced oxidative damage occurs due to both a decrease in antioxidant capacity and increased ROS production within the inactive muscle fibers [9,10]. In reference to the locations of ROS production in inactive skeletal muscle fibers, emerging evidence indicates that mitochondria are a key source of ROS production [10] [5,7]. For example, compared to control (ambulatory) animals, hydrogen peroxide (H2O2) release from mitochondria is increased by ~100% in skeletal muscles of animals exposed to 14 days of hindlimb immobilization [7]. Interestingly, this disuse-induced increase in mitochondrial ROS emission occurs in both atrophying highly oxidative muscles (i.e., soleus) and atrophying muscles primarily composed of type II fibers (i.e., plantaris) [7]. However, it is currently unknown if both populations of mitochondria in skeletal muscle, subsarcolemma and intermyofibrillar, contribute to this increased ROS production. The mechanism(s) responsible for this inactivity-induced increase in mitochondria ROS production are also unknown and remain an active area for research. In addition, some evidence suggests that prolonged muscle inactivity results in low levels of ROS production in cytosolic locations (e.g., xanthine oxidase and/or NADPH oxidase) [11,12] (Fig. 1). Additional research is required to complete our understanding of both the locations and regulation of ROS production in inactive skeletal muscle.
Fig. 1.
Simplified diagram illustrating pathways capable of producing superoxide (O2−) in skeletal muscle during periods of disuse. Candidates for the production of reactive oxygen include NADPH oxidase, xanthine oxidase, and muscle mitochondria.
MECHANISTIC LINKS BETWEEN OXIDANTS AND MUSCLE ATROPHY
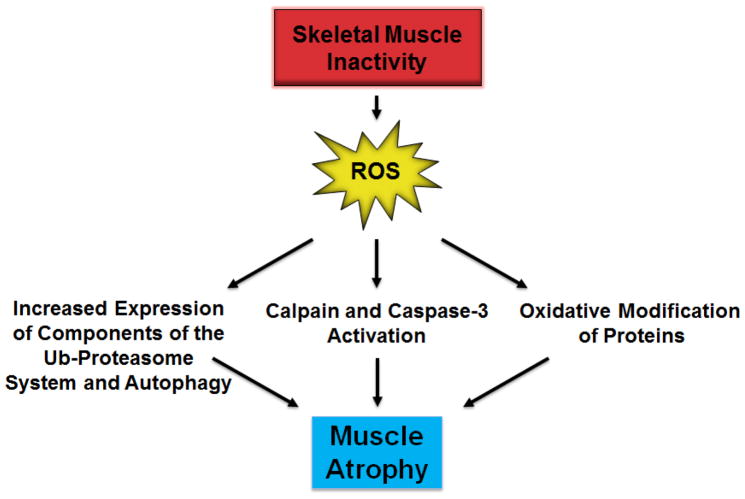
Theoretically, oxidative stress can contribute to disuse muscle atrophy by increasing proteolysis and/or depressing protein synthesis. In regard to ROS and increased proteolysis, growing evidence indicates that oxidative stress can promote muscle protein breakdown in three major ways (Fig. 2). First, altered redox signaling can regulate transcriptional activators and increase gene expression of key components of autophagy and the proteasome system of proteolysis [13,14]. Secondly, inactivity-induced oxidative stress in skeletal muscle can activate both calpain and caspase-3 [13,15–17]. Finally, ROS can also accelerate proteolysis in muscle fibers by oxidizing muscle proteins which enhances their susceptibility to proteolytic processing [18]. For more details on the signaling links between ROS and muscle atrophy, the interested reader is referred to recent reviews on specific aspects of oxidative stress and muscle wasting [1,3,19].
Fig. 2.
In theory, inactivity-induced ROS production in skeletal muscle fibers can increase proteolysis by increasing the expression of components of the ub-proteasome system (e.g., atrogin-1) and autophagy, allosteric activation of proteases, and oxidizing proteins which increases their susceptibility to proteolytic recognition and degradation.
It is also feasible that increased ROS production can contribute to atrophy by impeding muscle protein synthesis. In this regard, cellular protein synthesis occurs via a network of complex signaling pathways that culminate in the translation of mRNA into a specific protein. The rate of protein synthesis is mainly controlled by the efficiency of translation, and emerging evidence suggests that ROS can depress protein synthesis by obstructing mRNA translation at the level of initiation [20–23]. Specifically, oxidants are known for their capacity to reduce the phosphorylation of mammalian target of rapamycin substrates such as eukaryotic initiation factor 4E-binding protein and p70 S6 kinase, thereby inhibiting mRNA translation at the level of initiation [20]. Note, however, that all of the aforementioned protein synthesis studies were performed in cell culture models. Therefore, additional research is required to document that inactivity-induced ROS production can significantly impede protein synthesis in skeletal muscle fibers in vivo.
To summarize, oxidative stress can accelerate proteolysis in several ways and growing evidence suggests that this occurs in inactive skeletal muscles. Further, emerging data suggests that oxidative stress can also depress protein synthesis in cells. In the next segment we address the important question of whether oxidative stress is the cause or simply the sequence of skeletal muscle inactivity.
IS OXIDATIVE STRESS A MAJOR CAUSE OF DISUSE MUSCLE ATROPHY OR SIMPLY A SEQUENCE?
As discussed previously, the concept that ROS contribute to disuse muscle atrophy was first suggested by Kondo et al. in 1991 [4]. During the past two decades many investigations have explored the connection between oxidants and disuse muscle atrophy. Nonetheless, not all studies have concluded that the ROS production in inactive skeletal muscle plays an important role in disuse muscle atrophy. Hence, in the next segments we examine the evidence both for and against the thesis that oxidative stress is a requirement for disuse muscle atrophy.
Arguments against oxidative stress as a causative factor in disuse muscle atrophy
Several recent studies have failed to detect a link between oxidative stress and disuse muscle atrophy. For example, a recent investigation did not detect an increase in biomarkers of oxidant damage in human skeletal muscle following immobilization [24]. Based on this observation, the authors concluded that oxidant pathways do not play a contributory role in disuse muscle atrophy. Similarly, using SOD2 deficient mice, Kuwahara et al. investigated the impact of prolonged oxidative stress on muscle fiber size [25]. The results suggested that although the SOD2 mutant mice showed muscle histological and functional abnormalities, limb muscle weights did not differ between the mutants and wild type mice and therefore, they concluded that oxidative stress does not promote muscle atrophy.
A common approach to investigating the link between ROS and disuse muscle atrophy is to treat animals with antioxidants to abate the signaling effects of oxidants. Using this approach, some authors have deduced that antioxidant supplementation does not prevent disuse muscle atrophy. For example, two recent studies have treated animals with the antioxidant trolox (i.e., water soluble vitamin E analog) and failed to observe protection against disuse muscle atrophy [26,27]. Together, these studies illustrate that not all antioxidant treatments can protect against disuse muscle atrophy. Further, although vitamin E has been shown to blunt disuse muscle atrophy, a recent study suggests that the protective effect of vitamin E could be due to modulation of muscle proteolysis-related genes rather than its antioxidant function [28]. More specifically, this report demonstrated that vitamin E treatment was linked to increased expression of heat shock protein 72 and decreased expression of several key proteases including calpain and caspase-3 in the inactive skeletal muscles [28]. The authors concluded that while vitamin E can protect against disuse muscle atrophy, this protection could be achieved by alterations in the expression of muscle proteolysis-related genes and not necessarily the antioxidant function of vitamin E.
Collectively, the aforementioned reports have questioned the importance of ROS in promoting disuse muscle atrophy. In contrast to this conclusion, a growing body of literature has provided support for a causative link between oxidants and inactivity-induced muscle atrophy. A brief summary of these studies follows.
Arguments for oxidative stress as a causative factor in disuse muscle atrophy
As mentioned previously, the concept that ROS contribute to disuse muscle atrophy is over 20 years old [4]. In this regard, there are five major lines of reasoning to support the notion that oxidants can and do contribute to disuse muscle atrophy. First, although some studies have failed to detect evidence of oxidative stress in inactive skeletal muscles, many recent studies have concluded that prolonged inactivity is associated with increased biomarkers of oxidative stress in both human and animal skeletal muscles [5,7,12,16,29–33]. This increase in oxidant damage appears to be due, at least in part, to increased ROS production in mitochondrial and cytosolic sources [5,7,10,34 ]. Therefore, in contrast to the aforementioned negative findings, the majority of investigations have concluded that disuse muscle atrophy is accompanied with oxidant damage.
The observation that the exposure of myotubes to ROS (i.e., H2O2) results in fiber atrophy provides a second line of evidence to support the notion that oxidants are capable of promoting muscle atrophy [13,17,35]. While these cell culture studies do not provide direct evidence that inactivity-induced ROS production is responsible for disuse muscle atrophy in vivo, these studies provide proof of concept that exposure of cells to ROS can promote muscle fiber atrophy.
A third line of logic to support the notion that ROS production contributes to disuse muscle atrophy is the finding that oxidative stress promotes the expression and allosteric regulation of proteases involved in muscle atrophy. For example, exposure of myotubes to H2O2 has been shown to increase FoxO3a signaling and the expression of important E3 ligases involved in the ubiquitin-proteasome system of proteolysis and autophagy genes [17]. Similarly, inactivity-induced oxidant stress is a requirement for the increased expression of the E3 ligases atrogin-1 and MurF-1 in skeletal muscle in vivo [5,7]. Further, oxidants appear to be required to activate both calpain and caspase-3 in locomotor and respiratory muscles during prolonged periods of inactivity [5,16].
In addition to protease activation, ROS-induced protein oxidation provides a fourth line of evidence to link oxidative stress to accelerated muscle protein breakdown. Indeed, it is established that oxidative modification of cellular proteins results in protein unfolding and enhances their susceptibility to proteolytic degradation [3]. This is illustrated by the observation that compared to non-oxidized proteins, oxidized proteins are more rapidly degraded by the 20S proteasome[3]. Further, a recent study demonstrates that oxidized myofibrillar proteins are more rapidly degraded by both calpains and caspase-3 [18]. Collectively, these reports reveal that oxidative stress can accelerate the rate of protein breakdown in skeletal muscles.
The final and perhaps the strongest line of reasoning to support the notion that oxidative stress is linked to disuse muscle atrophy comes from studies indicating that prevention of inactivity-induced oxidative stress can delay or prevent disuse muscle atrophy. In this regard, numerous studies using a variety of oxidant scavenging compounds have demonstrated that treatment of animals with antioxidants can impede disuse muscle atrophy [4,5,7,16,29,36,37]. Further, genetic overexpression of the antioxidant enzyme catalase significantly attenuates disuse muscle atrophy [14]. The mechanism to explain why antioxidants provide protection against disuse muscle atrophy appears to be due, at least in part, to the prevention of protease expression and activation, which may be mediated via antioxidant modulation of specific transcription factors [14]. Indeed, several studies demonstrate that antioxidants can prevent the activation of the ubiquitin-proteasome system, calpain, and caspase-3 in inactive skeletal muscles [3]. Finally, although it is feasible that oxidative stress can depress protein synthesis in muscle, direct evidence demonstrating that disuse-induced oxidative stress can depress muscle protein synthesis in vivo is not currently available.
CONCLUSIONS
Disuse skeletal muscle atrophy occurs in a variety of conditions including prolonged bed rest, limb immobilization, mechanical ventilation, and space flight. Although there is growing evidence to suggest that inactivity-induced production of ROS play an important role in the rate of disuse skeletal muscle atrophy, this topic remains debated. Nonetheless, in our view, the evidence to support a causal relationship between reactive oxygen species and disuse muscle atrophy is robust. Therefore, investigations into the signaling pathways that connect ROS to disuse muscle atrophy remains an important topic for future research as these studies could lead to the identification of biological targets for therapeutic intervention to protect against inactivity-induced skeletal muscle atrophy.
Key points.
Disuse muscle atrophy is associated with an increase in the production of reactive oxygen species and the accumulation of biomarkers of oxidative damage.
Increased oxidant production in muscle fibers promotes activation of proteases and depresses cellular protein synthesis leading to fiber atrophy.
Mitochondria are reported to be a major source of oxidant production in skeletal muscles during prolonged periods of inactivity.
Recent studies reveal that mitochondrial-targeted antioxidants are protective against disuse muscle atrophy.
Acknowledgments
Our work in this research area has been supported by National Heart, and Lung Institute grants R01 HL-062361, R01 HL-072789, and HL-087839 awarded to Scott K. Powers and National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant R03AR056418 awarded to Andrew R. Judge.
References and recommended reading
Papers of particular interest, published within the annual period of this review, have been highlighted as:
* of special interest
**of outstanding interest
- 1.Powers SK, Kavazis AN, McClung JM. Oxidative stress and disuse muscle atrophy. J Appl Physiol. 2007;102(6):2389–2397. doi: 10.1152/japplphysiol.01202.2006. [DOI] [PubMed] [Google Scholar]
- 2.Powers SK, Kavazis AN, DeRuisseau KC. Mechanisms of disuse muscle atrophy: role of oxidative stress. Am J Physiol Regul Integr Comp Physiol. 2005;288(2):R337–344. doi: 10.1152/ajpregu.00469.2004. [DOI] [PubMed] [Google Scholar]
- 3**.Powers SK, Smuder AJ, Criswell DS. Mechanistic links between oxidative stress and disuse muscle atrophy. Antioxid Redox Signal. 2011;15(9):2519–2528. doi: 10.1089/ars.2011.3973. Review that summarizes the biological signaling connections between reactive oxygen species and disuse muscle atrophy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kondo H, Miura M, Itokawa Y. Oxidative stress in skeletal muscle atrophied by immobilization. Acta Physiol Scand. 1991;142(4):527–528. doi: 10.1111/j.1748-1716.1991.tb09191.x. [DOI] [PubMed] [Google Scholar]
- 5**.Powers SK, Hudson MB, Nelson WB, Talbert EE, Min K, Szeto HH, Kavazis AN, Smuder AJ. Mitochondria-targeted antioxidants protect against mechanical ventilation-induced diaphragm weakness. Crit Care Med. 2011;39(7):1749–1759. doi: 10.1097/CCM.0b013e3182190b62. First study to demonstrate that mitochondria are an important source of reactive oxygen species in diaphragm muscle during disuse and that mitochondrial-targeted antioxidants can protect diaphragm muscle from atrophy during prolonged mechanical ventilation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McClung JM, Whidden MA, Kavazis AN, Falk DJ, Deruisseau KC, Powers SK. Redox regulation of diaphragm proteolysis during mechanical ventilation. Am J Physiol Regul Integr Comp Physiol. 2008;294(5):R1608–1617. doi: 10.1152/ajpregu.00044.2008. [DOI] [PubMed] [Google Scholar]
- 7**.Min K, Smuder AJ, Kwon OS, Kavazis AN, Szeto HH, Powers SK. Mitochondrial-targeted antioxidants protect skeletal muscle against immobilization-induced muscle atrophy. J Appl Physiol. 2011;111(5):1459–1466. doi: 10.1152/japplphysiol.00591.2011. This report concludes that mitochondria are an important source of reactive oxygen species in inactive locomotor skeletal muscle and that mitochondrial-targeted antioxidants can protect inactive fibers from atrophy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Powers SK, Smuder AJ, Kavazis AN, Hudson MB. Experimental guidelines for studies designed to investigate the impact of antioxidant supplementation on exercise performance. International journal of sport nutrition and exercise metabolism. 2011;20(1):2–14. doi: 10.1123/ijsnem.20.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falk DJ, Deruisseau KC, Van Gammeren DL, Deering MA, Kavazis AN, Powers SK. Mechanical ventilation promotes redox status alterations in the diaphragm. J Appl Physiol. 2006;101:1017–1024. doi: 10.1152/japplphysiol.00104.2006. [DOI] [PubMed] [Google Scholar]
- 10.Kavazis AN, Talbert EE, Smuder AJ, Hudson MB, Nelson WB, Powers SK. Mechanical ventilation induces diaphragmatic mitochondrial dysfunction and increased oxidant production. Free Radic Biol Med. 2009;46(6):842–850. doi: 10.1016/j.freeradbiomed.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McClung JM, Van Gammeren D, Whidden MA, Falk DJ, Kavazis AN, Hudson MB, Gayan-Ramirez G, Decramer M, DeRuisseau KC, Powers SK. Apocynin attenuates diaphragm oxidative stress and protease activation during prolonged mechanical ventilation. Crit Care Med. 2009;37(4):1373–1379. doi: 10.1097/CCM.0b013e31819cef63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whidden MA, McClung JM, Falk DJ, Hudson MB, Smuder AJ, Nelson WB, Powers SK. Xanthine oxidase contributes to mechanical ventilation-induced diaphragmatic oxidative stress and contractile dysfunction. J Appl Physiol. 2009;106(2):385–394. doi: 10.1152/japplphysiol.91106.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McClung JM, Judge AR, Talbert EE, Powers SK. Calpain-1 is required for hydrogen peroxide-induced myotube atrophy. Am J Physiol Cell Physiol. 2009;296(2):C363–371. doi: 10.1152/ajpcell.00497.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14*.Dodd SL, Gagnon BJ, Senf SM, Hain BA, Judge AR. Ros-mediated activation of NF-kappaB and Foxo during muscle disuse. Muscle Nerve. 2010;41(1):110–113. doi: 10.1002/mus.21526. Study demonstrating that reactive oxygen species can activate both NF-kappaB and FoxO signaling in inactive skeletal muscles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smuder AJ, Hudson MB, Nelson WB, Kavazis AN, Powers SK. Nuclear factor-kappaB signaling contributes to mechanical ventilation-induced diaphragm weakness. Crit Care Med. 2011 doi: 10.1097/CCM.0b013e3182374a84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whidden MA, Smuder AJ, Wu M, Hudson MB, Nelson WB, Powers SK. Oxidative stress is required for mechanical ventilation-induced protease activation in the diaphragm. J Appl Physiol. 2010;108(5):1376–1382. doi: 10.1152/japplphysiol.00098.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McClung JM, Judge AR, Powers SK, Yan Z. p38 MAPK links oxidative stress to autophagy-related gene expression in cachectic muscle wasting. Am J Physiol Cell Physiol. 2010;298(3):C542–549. doi: 10.1152/ajpcell.00192.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smuder AJ, Kavazis AN, Hudson MB, Nelson WB, Powers SK. Oxidation enhances myofibrillar protein degradation via calpain and caspase-3. Free Radic Biol Med. 2010;49(7):1152–1160. doi: 10.1016/j.freeradbiomed.2010.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19**.Pellegrino MA, Desaphy JF, Brocca L, Pierno S, Camerino DC, Bottinelli R. Redox homeostasis, oxidative stress and disuse muscle atrophy. J Physiol. 2011;589(Pt 9):2147–2160. doi: 10.1113/jphysiol.2010.203232. Review paper that discusses the role of redox homeostasis and oxidants play in disuse muscle atrophy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20**.Zhang L, Kimball SR, Jefferson LS, Shenberger JS. Hydrogen peroxide impairs insulin-stimulated assembly of mTORC1. Free Radic Biol Med. 2009;46 (11):1500–1509. doi: 10.1016/j.freeradbiomed.2009.03.001. Study demonstrating that oxidants can impair anabolic signaling in muscle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shenton D, Smirnova JB, Selley JN, Carroll K, Hubbard SJ, Pavitt GD, Ashe MP, Grant CM. Global translational responses to oxidative stress impact upon multiple levels of protein synthesis. J Biol Chem. 2006;281(39):29011–29021. doi: 10.1074/jbc.M601545200. [DOI] [PubMed] [Google Scholar]
- 22.O’Loghlen A, Perez-Morgado MI, Salinas M, Martin ME. N-acetyl-cysteine abolishes hydrogen peroxide-induced modification of eukaryotic initiation factor 4F activity via distinct signalling pathways. Cellular signalling. 2006;18 (1):21–31. doi: 10.1016/j.cellsig.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 23.Kimball SR, Jefferson LS. New functions for amino acids: effects on gene transcription and translation. The American journal of clinical nutrition. 2006;83 (2):500S–507S. doi: 10.1093/ajcn/83.2.500S. [DOI] [PubMed] [Google Scholar]
- 24.Glover EI, Yasuda N, Tarnopolsky MA, Abadi A, Phillips SM. Little change in markers of protein breakdown and oxidative stress in humans in immobilization-induced skeletal muscle atrophy. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme. 2010;35(2):125–133. doi: 10.1139/H09-137. [DOI] [PubMed] [Google Scholar]
- 25.Kuwahara H, Horie T, Ishikawa S, Tsuda C, Kawakami S, Noda Y, Kaneko T, Tahara S, Tachibana T, Okabe M, Melki J, et al. Oxidative stress in skeletal muscle causes severe disturbance of exercise activity without muscle atrophy. Free Radic Biol Med. 2010;48(9):1252–1262. doi: 10.1016/j.freeradbiomed.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 26.Desaphy JF, Pierno S, Liantonio A, Giannuzzi V, Digennaro C, Dinardo MM, Camerino GM, Ricciuti P, Brocca L, Pellegrino MA, Bottinelli R, et al. Antioxidant treatment of hindlimb-unloaded mouse counteracts fiber type transition but not atrophy of disused muscles. Pharmacol Res. 2010;61(6):553–563. doi: 10.1016/j.phrs.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 27.Brocca L, Pellegrino MA, Desaphy JF, Pierno S, Camerino DC, Bottinelli R. Is oxidative stress a cause or consequence of disuse muscle atrophy in mice? A proteomic approach in hindlimb-unloaded mice. Exp Physiol. 2010;95 (2):331–350. doi: 10.1113/expphysiol.2009.050245. [DOI] [PubMed] [Google Scholar]
- 28.Servais S, Letexier D, Favier R, Duchamp C, Desplanches D. Prevention of unloading-induced atrophy by vitamin E supplementation: links between oxidative stress and soleus muscle proteolysis? Free Radic Biol Med. 2007;42 (5):627–635. doi: 10.1016/j.freeradbiomed.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arbogast S, Smith J, Matuszczak Y, Hardin BJ, Moylan JS, Smith JD, Ware J, Kennedy AR, Reid MB. Bowman-Birk inhibitor concentrate prevents atrophy, weakness, and oxidative stress in soleus muscle of hindlimb-unloaded mice. J Appl Physiol. 2007;102(3):956–964. doi: 10.1152/japplphysiol.00538.2006. [DOI] [PubMed] [Google Scholar]
- 30.Levine S, Nguyen T, Taylor N, Friscia ME, Budak MT, Rothenberg P, Zhu J, Sachdeva R, Sonnad S, Kaiser LR, Rubinstein NA, et al. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med. 2008;358(13):1327–1335. doi: 10.1056/NEJMoa070447. [DOI] [PubMed] [Google Scholar]
- 31.Falk DJ, Kavazis AN, Whidden MA, Smuder AJ, McClung JM, Hudson MB, Powers SK. Mechanical ventilation-induced oxidative stress in the diaphragm: role of heme oxygenase-1. Chest. 2011;139(4):816–824. doi: 10.1378/chest.09-2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jaber S, Petrof BJ, Jung B, Chanques G, Berthet JP, Rabuel C, Bouyabrine H, Courouble P, Koechlin-Ramonatxo C, Sebbane M, Similowski T, et al. Rapidly progressive diaphragmatic weakness and injury during mechanical ventilation in humans. Am J Respir Crit Care Med. 2011;183(3):364–371. doi: 10.1164/rccm.201004-0670OC. [DOI] [PubMed] [Google Scholar]
- 33.Hussain SN, Mofarrahi M, Sigala I, Kim HC, Vassilakopoulos T, Maltais F, Bellenis I, Chaturvedi R, Gottfried SB, Metrakos P, Danialou G, et al. Mechanical ventilation-induced diaphragm disuse in humans triggers autophagy. Am J Respir Crit Care Med. 2010;182(11):1377–1386. doi: 10.1164/rccm.201002-0234OC. [DOI] [PubMed] [Google Scholar]
- 34.Muller FL, Song W, Jang YC, Liu Y, Sabia M, Richardson A, Van Remmen H. Denervation-induced skeletal muscle atrophy is associated with increased mitochondrial ROS production. Am J Physiol Regul Integr Comp Physiol. 2007;293(3):R1159–1168. doi: 10.1152/ajpregu.00767.2006. [DOI] [PubMed] [Google Scholar]
- 35*.Gilliam LA, Moylan JS, Patterson EW, Smith JD, Wilson AS, Rabbani Z, Reid MB. Doxorubicin acts via mitochondrial ROS to stimulate catabolism in C2C12 myotubes. Am J Physiol Cell Physiol. 2011 doi: 10.1152/ajpcell.00217.2011. This investigation demonstrates that doxorubicin-induced myotube atrophy occurs due to increased production of reactive oxygen species in mitochondria. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agten A, Maes K, Smuder A, Powers SK, Decramer M, Gayan-Ramirez G. N-Acetylcysteine protects the rat diaphragm from the decreased contractility associated with controlled mechanical ventilation. Crit Care Med. 2011;39 (4):777–782. doi: 10.1097/CCM.0b013e318206cca9. [DOI] [PubMed] [Google Scholar]
- 37.McClung JM, Kavazis AN, Whidden MA, DeRuisseau KC, Falk DJ, Criswell DS, Powers SK. Antioxidant administration attenuates mechanical ventilation-induced rat diaphragm muscle atrophy independent of protein kinase B (PKB Akt) signalling. J Physiol. 2007;585(Pt 1):203–215. doi: 10.1113/jphysiol.2007.141119. [DOI] [PMC free article] [PubMed] [Google Scholar]