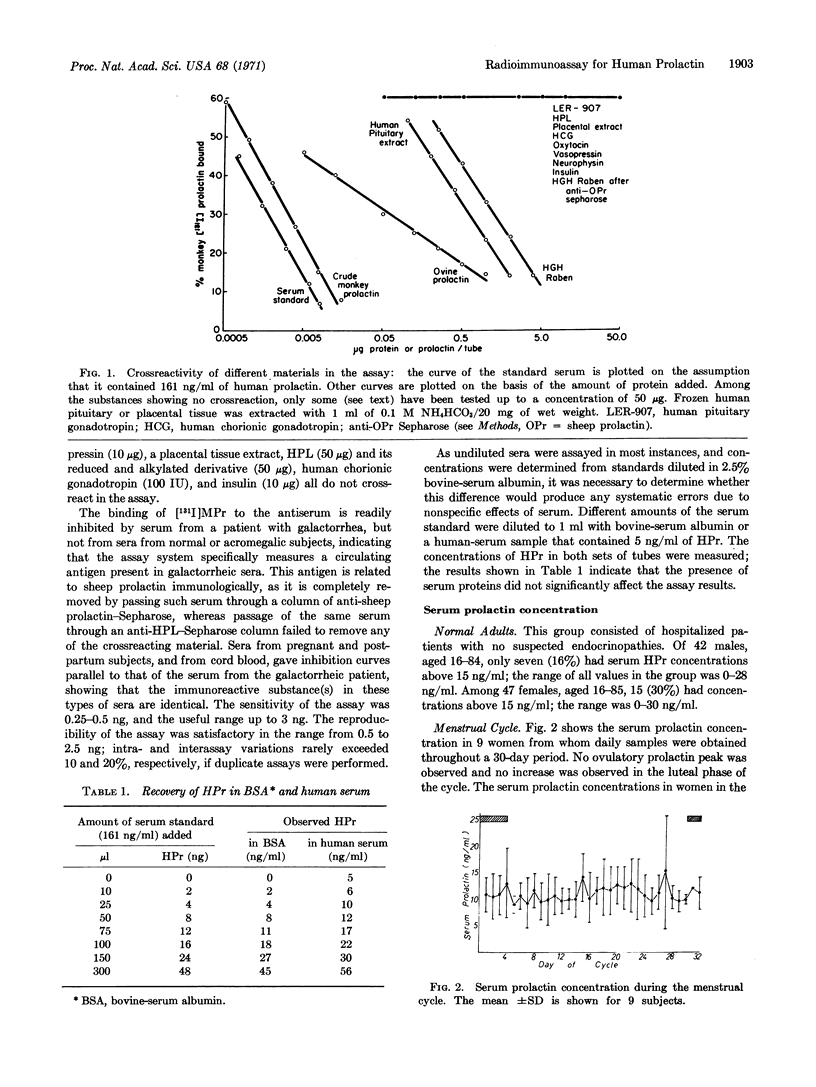
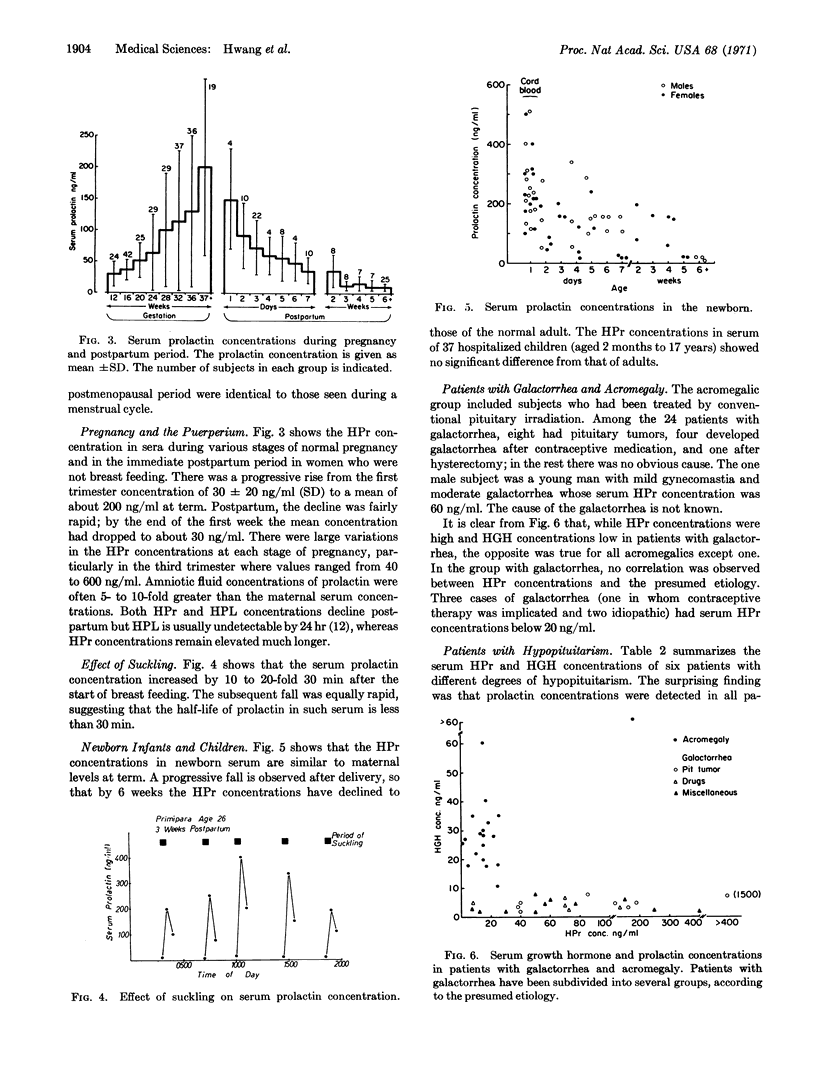
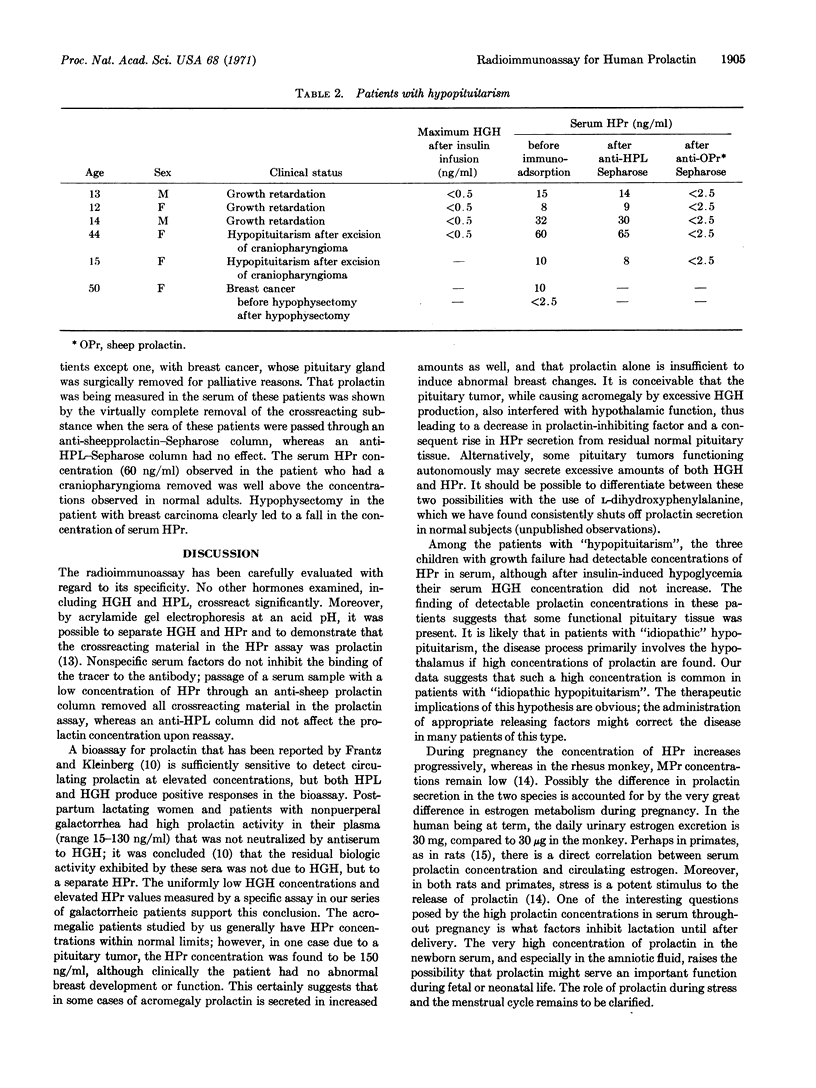
Abstract
A radioimmunoassay for primate prolactin has been developed, with [131I] monkey prolactin, and antibodies to monkey or human prolactin. The assay is specific for prolactin; human growth hormone, and human and monkey placental lactogen show no significant crossreaction. The assay is sensitive enough to measure prolactin concentrations in the sera of most humans studied. The concentration of prolactin in the serum of normal children and adults of either sex was usually below 30 ng/ml, while very high concentrations (up to 500 ng/ml) were observed in newborn infants. The serum prolactin concentration during the menstrual cycle showed no definite increase in the luteal phase. Of 24 patients with galactorrhea, 20 had prolactin concentrations above 30 ng/ml; the highest value observed was 1500 ng/ml. In contrast, 12 of 13 patients with acromegaly had concentrations within the normal range. During pregnancy, the concentration of prolactin in serum rose progressively from an average of 30 ng/ml in the first trimester to 200 ng/ml at term. Postpartum, prolactin concentrations fell to normal levels after 1-2 weeks. Suckling was a potent stimulus to prolactin release, increasing its concentration in serum some 10- to 20-fold.
Keywords: affinity chromatography, galactorrhea, amniotic fluid, menstrual cycle, growth hormone
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cuatrecasas P., Wilchek M., Anfinsen C. B. Selective enzyme purification by affinity chromatography. Proc Natl Acad Sci U S A. 1968 Oct;61(2):636–643. doi: 10.1073/pnas.61.2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz A. G., Kleinberg D. L. Prolactin: evidence that it is separate from growth hormone in human blood. Science. 1970 Nov 13;170(3959):745–747. doi: 10.1126/science.170.3959.745. [DOI] [PubMed] [Google Scholar]
- Friesen H. G., Guyda H. Biosynthesis of monkey growth hormone and prolactin in vitro. Endocrinology. 1971 Jun;88(6):1353–1362. doi: 10.1210/endo-88-6-1353. [DOI] [PubMed] [Google Scholar]
- Guyda H. J., Friesen H. G. The separation of monkey prolactin from monkey growth hormone by affinity chromatography. Biochem Biophys Res Commun. 1971 Mar 19;42(6):1068–1075. doi: 10.1016/0006-291x(71)90013-1. [DOI] [PubMed] [Google Scholar]
- Guyda H., Hwang P., Friesen H. Immunologic evidence for monkey and human prolactin (MPr and HPr). J Clin Endocrinol Metab. 1971 Jan;32(1):120–123. doi: 10.1210/jcem-32-1-120. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Samaan N., Yen S. C., Friesen H., Pearson O. H. Serum placental lactogen levels during pregnancy and in trophoblastic disease. J Clin Endocrinol Metab. 1966 Dec;26(12):1303–1308. doi: 10.1210/jcem-26-12-1303. [DOI] [PubMed] [Google Scholar]
- Sherwood L. M. Human prolactin. N Engl J Med. 1971 Apr 8;284(14):774–777. doi: 10.1056/NEJM197104082841407. [DOI] [PubMed] [Google Scholar]
- Voogt J. L., Chen C. L., Meites J. Serum and pituitary prolactin levels before, during, and after puberty in female rats. Am J Physiol. 1970 Feb;218(2):396–399. doi: 10.1152/ajplegacy.1970.218.2.396. [DOI] [PubMed] [Google Scholar]


