Abstract
Brain tumors are a major cause of cancer-related mortality in children. Overexpression of epidermal growth factor receptor (EGFR) is detected in pediatric brain tumors and receptor density appears to increase with tumor grading. Nimotuzumab is an IgG1 antibody that targets EGFR. Twenty-three children with high-grade glioma (HGG) were enrolled in an expanded access program in which nimotuzumab was administered alone or with radio-chemotherapy. The mean number of doses was 39. Nimotuzumab was well-tolerated and treatment with the antibody yielded a survival benefit: median survival time was 32.66 mo and the 2-y survival rate was 54.2%. This study demonstrated the feasibility of prolonged administration of nimotuzumab and showed preliminary evidence of clinical benefit in HGG patients with poor prognosis.
Keywords: High grade glioma, nimotuzumab, monoclonal antibody, children glioma, anaplastic astrocytoma, glioblastoma multiforme
Introduction
Brain tumors are a major cause of cancer-related mortality in children. In particular, low-grade gliomas (LGG), high-grade gliomas (HGG) and diffuse intrinsic pontine gliomas (DIPG) comprise 30–50%, ~20% and ~15%, respectively, of all central nervous tumors in children. Ependymomas represent 6–12% of all intracranial tumors in children.1,2 In Cuba, ~40 new cases of brain tumors are diagnosed every year in children younger than 15 y, which calculates to a rate of 13.8 per 100 000 inhabitants.3
Treatment of HGG remains a challenge for neurosurgeons, radiotherapists and medical oncologists because of their dismal prognosis.4 Different therapeutic strategies with radiotherapy combined or not with chemotherapy have been investigated without significant benefit in terms of survival.5-8 Despite intensive investigation, there is no standard chemotherapy regimen that is universally acknowledged in the setting of pediatric HGG.9
While the role of epidermal growth factor receptor (EGFR) in the genesis and progression of adult HGG is well-validated,10 its relevance in pediatric brain tumors is less established. Despite the lack of universal expression in all pediatric brain tumors, overexpression of EGFR is indeed found in HGG by immunohistochemistry.11-13 Furthermore, relative to pediatric LGG, significant overexpression of EGFR has been observed in pediatric HGG, and it is been claimed that EGFR expression increases with tumor grading.14
Other authors have reported a high degree of immunopositivity for wild type EGFR in pediatric glioma, together with low expression of EGFRvIII.15,16 Nimotuzumab is a humanized IgG1 monoclonal antibody that targets EGFR. Its preclinical activity has been summarized previously17 and more than 30,000 patients bearing epithelial-derived tumors have been treated worldwide with nimotuzumab. Compared with other marketed anti-EGFR monoclonal antibodies such as cetuximab and panitumumab, nimotuzumab has an intermediate affinity and it binds preferentially to tissues with high receptor density, e.g., epithelial tumors.18 Thus, nimotuzumab spares normal tissue, thereby avoiding unwanted toxicities.
The antibody has been previously shown to accumulate in primary and secondary malignant brain tumors. Technetium 99-labeled ior egf/r3, nimotuzumab’s parental antibody, has been administered intravenously to cancer patients in a diagnostic clinical trial using immunoscintigraphy.19 Sensitivity was 100% for glioma patients, as evidenced by the accumulation of antibody in all patients with confirmed brain tumors.19 Nimotuzumab itself, labeled with the same radioisotope, accumulated in the brain tumors of patients with persistent glioblastoma multiforme or anaplastic astrocytomas after treatment with irradiation and nimotuzumab.20 The objective responses (complete and partial responses) seen after treating relapsing glioma patients with ior egf/r321 or nimotuzumab alone,22 indicates antibody penetration and effect at the tumor site.
Several studies with nimotuzumab in children have been completed. A Phase 2 trial evaluated nimotuzumab in 47 pediatric patients (4–17 y) with refractory or relapsed pediatric HGG. Nimotuzumab was infused at 150 mg/m2 weekly for 6 weeks followed by a consolidation therapy of 4 infusions every 3 weeks in the absence of progressive disease. Objective response was achieved in 14 of 46 patients. Median overall survival was extended for responders (10 mo) compared with non-responders (4 mo).22
Children with newly diagnosed DIPG have also been treated with nimotuzumab in combination with radiotherapy or radiotherapy/vinorelbine. A Phase 3 open-label, single-arm trial was done to assess the safety and efficacy of nimotuzumab in newly-diagnosed DIPG patients in combination with radiotherapy. The best responses included partial remission in 4 patients (9.8%) and stable disease in 27 children (65.8%). The median overall survival was 9.6 mo.23 In another study, Massimino et al. treated DIPG patients with nimotuzumab, vinorelbine and radiation, followed by consolidation courses of the antibody every 2 weeks. Preliminary results for 12 children, age range 3–13 y were recently reported.24 After a mean follow-up of 10 mo, their progression free survival (PFS) at 9 mo was 69 ± 21% and their overall survival at 12 mo was 81.5 ± 12%.
Nimotuzumab has been approved in Cuba as a treatment for children with relapsing glioma since 2007. Here, we report the results of an expanded access program that included 23 pediatric patients with newly diagnosed HGG that were treated with nimotuzumab in combination with radiotherapy, chemotherapy or with the antibody alone.
Results
From 2007 to 2011, 23 pediatric patients with documented HGG were included in the nimotuzumab expanded access program conducted at the Juan Manuel Marquez Hospital. The mean age was 12.4 y (range 2–18 y). All patients had a Karnofsky or Lansky performance status of 40 or more. Five patients received nimotuzumab in combination with radiotherapy followed by chemotherapy. Sixteen patients who were already irradiated prior to joining the expanded access program received nimotuzumab combined with chemotherapy (procarbazine, cyclophosphamide, cisplatin and prednisone) and 3 patients were treated with the antibody alone because they had previously finished radio-chemotherapy. Considering the poor prognosis of the paitents, treatment with nimotuzumab continued after radio- and chemotherapy.
The mean number of doses of nimotuzumab administered was 39 (median 19, range 8–106). Eleven patients (47.8%) received more than 20 doses of nimotuzumab and 7 patients (30.4%) received more than 60 antibody doses. Treatment was not discontinued at the moment of radiologic disease progression, but was discontinued if there was a severe deterioration of the performance status (Karnofsky or Lansky performance status ≤ 20), in case of unmanageable toxicity or after a maximum treatment period of 4 y. In total, 4 patients were treated for the maximum period, and did not have progressive disease, significant clinical deterioration or severe toxicity. Patient, disease and treatment characteristics are shown in Table 1
Table 1. Patient, disease and treatment characteristics of the patients enrolled in nimotuzumab expanded access program.
| Pt Id | Age | Sex | Diagnose | Tumor location | Previous therapy | Nimotuzumab administration (Combination) | Number of doses | Best response | Survival time (months) |
|---|---|---|---|---|---|---|---|---|---|
| DVM-01 | 14 | F | AA | Temporal | Surgery/RT | CTP | 106 | CR | 63.87 |
| JLGP-02 | 11 | M | AA | Occipital | Surgery/RT | CTP | 18 | CR | 43.77 |
| ARU-03 | 13 | M | AA | Fronto-temporal | Surgery/RT | CTP | 10 | SD | 64.07 |
| AAF-04 | 14 | M | AA | Intraventricular | Surgery/RT | CTP | 87 | CR | 49.57 |
| VRG-05 | 18 | F | AA | Temporo-occipital | Surgery/RT | CTP | 82 | CR | 57.73 |
| AFR-06 | 16 | F | AA | Frontal | Surgery/RT | CTP | 100 | PR | 59.53 |
| LARI-07 | 18 | M | AA | Occipital | Surgery/RT | CTP | 104 | CR | 61.60 |
| YGH-08 | 13 | F | GBM | Parietal | Surgery/RT | CTP | 67 | CR | 32.67 |
| GLM-09 | 17 | F | AA | Occipital | Surgery/RT | CTP | 8 | PR | 46.20 |
| LPS-10 | 16 | F | AA | Thalamus | Surgery/RT | CTP | 10 | PD | 6.50 |
| MRQ-11 | 4 | F | AA | Fourth ventricle | Surgery/RT | CTP | 20 | PD | 9.17 |
| ACR-12 | 8 | F | GBM | Parieto-occipital | Surgery | RT/CTP | 14 | CR | 5.27 |
| MTR-13 | 2 | F | GBM | Intraventricular | Surgery | CTP | 12 | PD | 9.37 |
| RSP-14 | 16 | F | AA | Parietal | Surgery/RT/CTP | monotherapy | 16 | PD | 6.0 |
| OPI-15 | 15 | F | GBM | Frontal | Surgery | RT/CTP | 20 | PD | 19.93 |
| RHE-16 | 8 | F | AA | Thalamus | Surgery/RT/CTP | monotherapy | 8 | PD | 2.07 |
| AAF-17 | 4 | F | AA | Thalamus | Surgery/RT/CTP | monotherapy | 10 | PD | 3.07 |
| SSP-18 | 14 | F | AA | Frontal | Surgery/RT | CTP | 26 | PR | 27.70 |
| RAJD-19 | 13 | F | AA | Temporal | Surgery/RT | CTP | 106 | CR | 56.50 |
| AIT-20 | 11 | F | AA | Spinal cord Level C3-C6 | Surgery | RT/CTP | 30 | SD | 15.20 |
| CJBO-21 | 15 | M | AA | Fronto-parietal | Surgery | RT/CTP | 19 | CR | 7.33 |
| EVL-22 | 10 | M | AA | Temporo-occipital | Surgery | RT/CTP | 19 | PR | 7.33 |
| KYSB-23 | 7 | M | AA | Parietal | Surgery/RT | CTP | 9 | SD | 1.00 |
GBM, glioblastoma multiforme; AA, anaplastic astrocytoma; RT, radiotherapy; CTP, chemotherapy; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
Remarkably, at the time this report was prepared, none of the patients has disease progression after treatment discontinuation and no delayed adverse events (AEs) related to nimotuzumab exposure were detected.
In spite of the prolonged therapy, nimotuzumab was well-tolerated. The frequency of AEs did not increase with repeated treatment, confirming the lack of cumulative toxicity. One patient experienced grade 4 mucositis that did not result in treatment discontinuation. Another patient had vomiting and a vasovagal episode after administration of a total of 23 nimotuzumab doses, leading to voluntary withdrawal. Two patients developed mild to moderate skin rash (4 episodes), while 2 patients had grade 1 or 2 mucositis.
Cardiac function was evaluated through electrocardiograms and echocardiography to estimate the ventricular ejection fraction before and after treatment. None of the patients had evidence of cardiac toxicity. No other alterations were detected either in the hepatic or renal function assessed through conventional laboratory tests (bilirubin, transaminases, urea, creatinine and uric acid) and by hepatic and renal ultrasound. Patients were evaluated up to 1 y of finishing therapy.
Overall, children reacted very favorably to the antibody infusions. Apart from chemotherapy that causes a lot of discomfort, nimotuzumab infusions were very well tolerated and children did not need hospitalization. Twenty out of 23 patients were older than 6 y and many of them, could re-start school activities during nimotuzumab treatment period. Only one 16 y-old patient complained about bi-weekly visits to the hospital. In addition, children received strong psychological support by the hospital experts that contributed to the acceptance of the lengthy therapy.
Treatment response was as follows: 9 patients achieved complete response, 3 had partial response, 4 had disease stabilization and 6 patients had progressive disease as best response. The objective response rate (complete + partial response) was 52.2% while the disease control rate (complete + partial response + stable disease) was 69.5%. Figure 1 shows two images of a patient showing prolonged disease stabilization after 4 y of treatment.
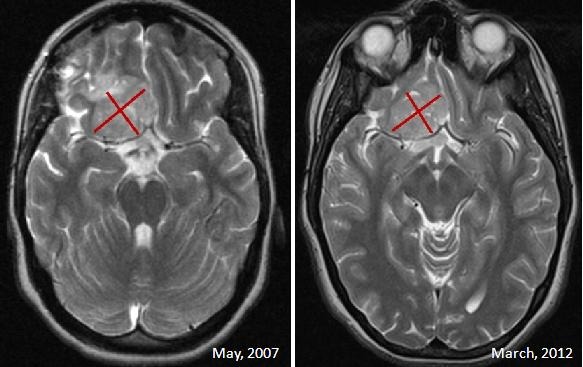
Figure 1. Patient showing prolonged disease stabilization after 100 doses of nimotuzumab over 4 y.
Overall survival was estimated from the beginning of the antibody treatment: mean and median overall survival was 36.44 and 32.66 mo, respectively. The 1, 2 and 3 y survival rate was 64%, 54.2% and 48.8%, respectively. No correlation was found between age and survival as per a Cox regression.
Discussion
The management of brain tumors in children remains a challenge for neuro-oncologists. HGG consists of anaplastic astrocytoma [AA; World Health Organization (WHO) grade III] and glioblastoma multiforme (GBM; WHO grade IV).6 They occur most commonly in the supratentorial region or in the brainstem.
We present here the results of an expanded access program that enrolled 23 pediatric patients with non-brainstem, pathology confirmed-high grade astrocytomas. All patients had residual tumors after surgery. The expanded access program was launched because of the poor patient prognosis and the previous clinical evidences of nimotuzumab activity in the recurrent scenario. Evaluation of EGFR expression in the primary tumor was not done as part of the study.
This study established the safety of prolonged (up to 4 y) administration of nimotuzumab and provided preliminary evidences of survival benefit. To our knowledge, this study shows one of the highest cumulative monoclonal antibody exposure ever achieved in pediatric population. The mean nimotuzumab number of doses was 39 and one third of the children received more than 60 antibody infusions after prolonged stabilization or clinically irrelevant progressive disease. Long-term treatment with nimotuzumab yielded initial evidences of survival benefit: the 2-y survival rate was 54.2%, compared with the < 10–30% historical 2-y survival rate after aggressive multimodality therapeutic approach (surgery, radiation and chemotherapy).
Several strategies that employ high-dose chemotherapy have been used so far without significant impact in overall survival.6-9 The most common regimens consist of combinations of prednisone, lomustine and vincristine (PCV) and radiotherapy or temozolomide and irradiation. Study CCG 945 compared radiotherapy and PCV chemotherapy to a combination of local irradiation and 8-in-1 chemotherapy administered pre-and post-radiotherapy.10 The study enrolled 172 newly diagnosed children with HGG who had undergone surgical resection. After a central pathologic review, the 5-y survival estimates were 19% ± 5% (standard) and 23% ± 5% (8 in 1 chemotherapy).9
A second study conducted by ACNS0126 where 107 patients with AA, GBM, or gliosarcoma underwent concomitant chemoradiotherapy with temozolomide followed by adjuvant temozolomide, yielded a 3-y overall survival rates of 22 ± 5%. The authors concluded that temozolomide failed to improve outcome in children with high-grade astrocytomas.6 Another multi-institutional study coordinated by St. Jude Children’s Research Hospital between 1999 and 2002 tested the efficacy of temozolomide in patients with non-brainstem HGG. Following surgery, patients received radiotherapy (RT) and 6 cycles of temozolomide. The study also included an optional window therapy of irinotecan. Thirty-one patients were enrolled and the 1- and 2-y overall survival estimates were 63% ± 8% and 21% ± 7%, respectively.9 In a different study of temozolomide and RT, 23 newly-diagnosed HGG patients underwent surgical resection with radiation therapy and temozolomide for 1 y. The median overall survival for invasive or focal HGG patients was 13 and 24 mo, respectively.25 Both chemotherapy regimens (PCV or temozolomide) have been associated with severe hematologic toxicity.6-9,25 Previous treatment intensification using conventional nonspecific chemotherapy has thus resulted in additional toxicity without major improvement in survival.
In our study, encouraging survival and long-lasting disease control was attributed to the prolonged treatment with nimotuzumab, which was only discontinued after severe deterioration of the performance status, unmanageable toxicity or after4 y of treatment. We speculate that cancer can be transformed from a rapid fatal disease to a chronic condition, potentially compatible with many years of quality life, by the use of lengthy treatment with drugs that target relevant antigens for oncogenesis that are not overexpressed in normal tissues. This is the case of pediatric glioma and nimotuzumab. This antibody has an excellent safety profile that is unique in its class.22,23 Because the antibody has intermediate affinity for EGFR, it preferentially accumulates in malignant tumors that overexpress EGFR, avoiding the severe skin rash, hypomagnesemia or diarrhea usually seen with cetuximab, panitumumab, erlotinib or gefitinib.26,27
Other monoclonal antibodies such as bevacizumab, an anti-VEGF molecule, have been used in the treatment of children with brain tumors at significantly lower cumulative doses.28-31 Children were administered bevacizumab at 10 or 15 mg/kg every 2 or 3 weeks as long as they did not show unacceptable toxicity or disease progression. In general, toxicities reported for bevacizumab were grade 1–2 hypertension, proteinuria, lymphopenia, wound healing delay, grade 1 to 3 fatigue, grade 1 central nervous system (CNS) hemorrhage and grade 4 CNS ischemia.28-31
In summary, the feasibility and safety of prolonged administration of nimotuzumab in a pediatric population has been demonstrated, and preliminary evidences of clinical benefit in 23 HGG patients with very poor prognosis was observed. New trials in the same indication evaluating different combination schemes together with biomarker correlatives studies with nimotuzumab are warranted.
Patients and methods
Children bearing newly diagnosed supratentorial HGG were included in the nimotuzumab expanded access program conducted at the onco-hematology service of the Juan Manuel Marquez Hospital (Havana, Cuba) between 2007 and 2011. Histology confirmation was mandatory for enrollment. The tumors were graded using the WHO criteria32 in which an AA exhibits focal or diffuse anaplasia with increased cellularity, pleomorphism, nuclear atypia and mitotic activity, and a GBM exhibits all the above features in conjunction with prominent vascular proliferation and/or necrosis. All patients had poor prognosis due to confirmed residual tumors after surgery.
Other inclusion criteria were age between 2–18 y; patients had measurable lesions, defined as those that can be exactly measured in at least 2 dimensions (2 perpendicular larger diameters) using conventional imaging techniques (CT scan, MRI); female patients had a negative pregnancy test and used effective contraceptive methods if they were fertile and sexually active; life expectancy higher than 12 weeks; general performance status ≥ 40% (Karnosfsky for patients older than 16 y and Lansky for patients younger than 16); laboratory parameters within normal limits defined as: hemoglobin ≥ 10 g/L, total leukocytes ≥ 2 × 109 cells/L, platelets ≥ 100 × 109/L, functional hepatic tests within normal limits demonstrated by GPT, GOT ≤ 2.5 × the upper reference value, renal function: seric creatinine ≤ 1.5 the upper reference value. Parents and legal guardians were required agree to the participation of the patient and to sign informed consent documents. The protocol was approved by the Institutional Review Board of Hospital. The study was conducted under the principles embodied in the Declaration of Helsinki.
Patients were treated with nimotuzumab alone or in combination with irradiation or chemotherapy. Nimotuzumab was added to the conventional therapy according the national guideline, which consisted of radiotherapy equivalent to 5,400 cGy, followed by chemotherapy consisting of procarbazine 100 mg/m2 (days 1–10), cyclophosphamide 1 g/m2 (day 1), cisplatin 30 mg/m2 (days 1–4) and prednisone 40 mg/m2 (days 1–14) (4 to 6 cycles). Nimotuzumab was administered in combination with radiotherapy, chemotherapy or alone, according the treatment stage of each patient at the time of enrollment in the expanded access program.
Patients received 12 weekly doses of nimotuzumab (induction therapy) at the dose of 150 mg/m2. After the induction, patients received the same dose, every 14 d. Nimotuzumab was administered intravenously (antecubital vein) in 250 ml of saline solution for 1 h. Treatment was interrupted only in case of serious or severe toxicity or after severe deterioration of the patients’ performance status.
All AEs were assessed, recorded and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 3.0. All patients who received at least 1 dose of nimotuzumab qualified as evaluable for response and safety. Objective response (complete +partial response) was evaluated according the updated response assessment criteria for HGG proposed by the Neuro-Oncology Working Group.33 Overall survival was estimated using the Kaplan Meier method at the time of nimotuzumab treatment initiation.
Acknowledgments
We thank our patients and parents for their absolute support.
Glossary
Abbreviations:
- AA
anaplastic astrocytoma
- ASCO
American Society of Clinical Oncology
- CNS
central nervous system
- CT
computer tomography
- CTCAE
common terminology criteria for adverse events
- DIPG
diffuse intrinsic pontine gliomas
- EGFR
epidermal growth factor Receptor
- GBM
glioblastoma multiforme
- GOT
glutamic oxalacetic transaminase
- GPT
glutamic piruvic transaminase
- HGG
high-grade glioma
- LGG
low-grade gliomas
- MAb
monoclonal antibody
- MRI
magnetic resonance imaging
- PCV
prednisone, lomustine and vincristine
- PFS
progression free survival
- RT
radiotherapy
- WHO
World Health Organization
Disclosure of Potential Conflicts of Interest
Giselle Saurez, Patricia Lorenzo Luaces and Tania Crombet are employees of the Center of Molecular Immunology, the research institution that patented and manufactures nimotuzumab.
Footnotes
Previously published online: www.landesbioscience.com/journals/mabs/article/22970
References
- 1.Lashkari HP, Saso S, Moreno L, Athanasiou T, Zacharoulis S. Using different schedules of Temozolomide to treat low grade gliomas: systematic review of their efficacy and toxicity. J Neurooncol. 2011;105:135–47. doi: 10.1007/s11060-011-0657-7. [DOI] [PubMed] [Google Scholar]
- 2.Khatua S, Sadighi ZS, Pearlman ML, Bochare S, Vats TS. Brain tumors in children--current therapies and newer directions. Indian J Pediatr. 2012;79:922–7. doi: 10.1007/s12098-012-0689-9. [DOI] [PubMed] [Google Scholar]
- 3.Anuario Estadístico de Cuba 2010. Incidencia de cáncer en menores de 15 años según grupos diagnósticos y edad año 2007. Disponible en: http//bvs.sld.cu/cgi-bin/wxis/anuario
- 4.MacDonald TJ, Aguilera D, Kramm CM. Treatment of high-grade glioma in children and adolescents. Neuro Oncol. 2011;13:1049–58. doi: 10.1093/neuonc/nor092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen KJ, Pollack IF, Zhou T, Buxton A, Holmes EJ, Burger PC, et al. Temozolomide in the treatment of high-grade gliomas in children: a report from the Children’s Oncology Group. Neuro Oncol. 2011;13:317–23. doi: 10.1093/neuonc/noq191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fogh SE, Andrews DW, Glass J, Curran W, Glass C, Champ C, et al. Hypofractionated stereotactic radiation therapy: an effective therapy for recurrent high-grade gliomas. J Clin Oncol. 2010;28:3048–53. doi: 10.1200/JCO.2009.25.6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolff JE, Driever PH, Erdlenbruch B, Kortmann RD, Rutkowski S, Pietsch T, et al. Intensive chemotherapy improves survival in pediatric high-grade glioma after gross total resection: results of the HIT-GBM-C protocol. Cancer. 2010;116:705–12. doi: 10.1002/cncr.24730. [DOI] [PubMed] [Google Scholar]
- 8.Gottardo NG, Gajjar A. Chemotherapy for malignant brain tumors of childhood. J Child Neurol. 2008;23:1149–59. doi: 10.1177/0883073808321765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hargrave D, Bartels U, Bouffet E. Diffuse brainstem glioma in children: critical review of clinical trials. Lancet Oncol. 2006;7:241–8. doi: 10.1016/S1470-2045(06)70615-5. [DOI] [PubMed] [Google Scholar]
- 10.Van den Eynde M, Baurain JF, Mazzeo F, Machiels JP. Epidermal growth factor receptor targeted therapies for solid tumours. Acta Clin Belg. 2011;66:10–7. doi: 10.2143/ACB.66.1.2062508. [DOI] [PubMed] [Google Scholar]
- 11.Bredel M, Pollack IF, Hamilton RL, James CD. Epidermal growth factor receptor expression and gene amplification in high-grade non-brainstem gliomas of childhood. Clin Cancer Res. 1999;5:1786–92. [PubMed] [Google Scholar]
- 12.Suri V, Das P, Pathak P, Jain A, Sharma MC, Borkar SA, et al. Pediatric glioblastomas: a histopathological and molecular genetic study. Neuro Oncol. 2009;11:274–80. doi: 10.1215/15228517-2008-092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fouladi M, Stewart CF, Blaney SM, Onar-Thomas A, Schaiquevich P, Packer RJ, et al. Phase I trial of lapatinib in children with refractory CNS malignancies: a Pediatric Brain Tumor Consortium study. J Clin Oncol. 2010;28:4221–7. doi: 10.1200/JCO.2010.28.4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui Y, Zhao JZ. [Expression of epidermal growth factor receptor and Ki-67 antigen in brain ependymoma and the correlation between them] Zhonghua Yi Xue Za Zhi. 2008;88:3356–8. [PubMed] [Google Scholar]
- 15.Bax DA, Gaspar N, Little SE, Marshall L, Perryman L, Regairaz M, et al. EGFRvIII deletion mutations in pediatric high-grade glioma and response to targeted therapy in pediatric glioma cell lines. Clin Cancer Res. 2009;15:5753–61. doi: 10.1158/1078-0432.CCR-08-3210. [DOI] [PubMed] [Google Scholar]
- 16.Liang ML, Ma J, Ho M, Solomon L, Bouffet E, Rutka JT, et al. Tyrosine kinase expression in pediatric high grade astrocytoma. J Neurooncol. 2008;87:247–53. doi: 10.1007/s11060-007-9513-1. [DOI] [PubMed] [Google Scholar]
- 17.Massimino M, Bode U, Biassoni V, Fleischhack G. Nimotuzumab for pediatric diffuse intrinsic pontine gliomas. Expert Opin Biol Ther. 2011;11:247–56. doi: 10.1517/14712598.2011.546341. [DOI] [PubMed] [Google Scholar]
- 18.Garrido G, Tikhomirov IA, Rabasa A, Yang E, Gracia E, Iznaga N, et al. Bivalent binding by intermediate affinity of nimotuzumab: a contribution to explain antibody clinical profile. Cancer Biol Ther. 2011;11:373–82. doi: 10.4161/cbt.11.4.14097. [DOI] [PubMed] [Google Scholar]
- 19.Ramos-Suzarte M, Rodríguez N, Oliva JP, Iznaga-Escobar N, Perera A, Morales A, et al. 99mTc-labeled antihuman epidermal growth factor receptor antibody in patients with tumors of epithelial origin: Part III. Clinical trials safety and diagnostic efficacy. J Nucl Med. 1999;40:768–75. [PubMed] [Google Scholar]
- 20.Ramos TC, Figueredo J, Catala M, González S, Selva JC, Cruz TM, et al. Treatment of high-grade glioma patients with the humanized anti-epidermal growth factor receptor (EGFR) antibody h-R3: report from a phase I/II trial. Cancer Biol Ther. 2006;5:375–9. doi: 10.4161/cbt.5.4.2522. [DOI] [PubMed] [Google Scholar]
- 21.Crombet T, Torres O, Rodríguez V, Menéndez A, Stevenson A, Ramos M, et al. Phase I clinical evaluation of a neutralizing monoclonal antibody against epidermal growth factor receptor in advanced brain tumor patients: preliminary study. Hybridoma. 2001;20:131–6. doi: 10.1089/02724570152057634. [DOI] [PubMed] [Google Scholar]
- 22.Bode U, Buchen S, Warmuth-Metz M, Pietsch T, Bach F, Fleischhack G. Final report of a phase II trial of nimotuzumab in the treatment of refractory and relapsed high-grade gliomas in children and adolescents. Journal of Clinical Oncology, 2007 ASCO Annual Meeting Proceedings Part I. Vol 25, No. 18S (June 20 Supplement), 2007: 2006. [Google Scholar]
- 23.Bode U, Windelberg M, Massimino M, Khuhlaeva E, Warmuth-Metz M, Kortmann RD. Phase III trial of nimotuzumab for the treatment of newly diagnosed diffuse intrinsic pontine gliomas in children and adolescents. J Clin Oncol. 2008;26:2058. [Google Scholar]
- 24.Massimino M, Biassoni V, Gandola L, Schiavello E, Pecori E, Bach F. Nimotuzumab and vinorelbine concomitantly to radiation and as maintenance for diffuse pontine glioma in childhood: Results from a series of 12 patients. J Clin Oncol. 2011;26:9557. [Google Scholar]
- 25.Narayana A, Perretta D, Kunnakkat S, Gruber D, Golfinos J, Parker E, et al. Invasion is not an independent prognostic factor in high-grade glioma. J Cancer Res Ther. 2011;7:331–5. doi: 10.4103/0973-1482.87039. [DOI] [PubMed] [Google Scholar]
- 26.Pinto C, Barone CA, Girolomoni G, Russi EG, Merlano MC, Ferrari D, et al. American Society of Clinical Oncology. European Society of Medical Oncology Management of skin toxicity associated with cetuximab treatment in combination with chemotherapy or radiotherapy. Oncologist. 2011;16:228–38. doi: 10.1634/theoncologist.2010-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pomerantz RG, Mirvish ED, Geskin LJ. Cutaneous reactions to epidermal growth factor receptor inhibitors. J Drugs Dermatol. 2010;9:1229–34. [PubMed] [Google Scholar]
- 28.Couec ML, André N, Thebaud E, Minckes O, Rialland X, Corradini N, et al. Comité Pharmacologie of the SFCE Bevacizumab and irinotecan in children with recurrent or refractory brain tumors: toxicity and efficacy trends. Pediatr Blood Cancer. 2012;59:34–8. doi: 10.1002/pbc.24066. [DOI] [PubMed] [Google Scholar]
- 29.Gururangan S, Chi SN, Young Poussaint T, Onar-Thomas A, Gilbertson RJ, Vajapeyam S, et al. Lack of efficacy of bevacizumab plus irinotecan in children with recurrent malignant glioma and diffuse brainstem glioma: a Pediatric Brain Tumor Consortium study. J Clin Oncol. 2010;28:3069–75. doi: 10.1200/JCO.2009.26.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parekh C, Jubran R, Erdreich-Epstein A, Panigrahy A, Bluml S, Finlay J, et al. Treatment of children with recurrent high grade gliomas with a bevacizumab containing regimen. J Neurooncol. 2011;103:673–80. doi: 10.1007/s11060-010-0444-x. [DOI] [PubMed] [Google Scholar]
- 31.Narayana A, Kunnakkat S, Chacko-Mathew J, Gardner S, Karajannis M, Raza S, et al. Bevacizumab in recurrent high-grade pediatric gliomas. Neuro Oncol. 2010;12:985–90. doi: 10.1093/neuonc/noq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kleihues P, Burger PC, Scheithauer BW. Histological typing of tumours of the central nervous system In: SobinLH, editor. International histological classification of tumours. Geneva: Springer-Verlag, 1993: 11–16 [Google Scholar]
- 33.Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963–72. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]


