Abstract
Fc engineering is a promising approach to enhance the antitumor efficacy of monoclonal antibodies (mAbs) through antibody-dependent cell-mediated cytotoxicity (ADCC). Glyco- and protein-Fc engineering have been employed to enhance FcγR binding and ADCC activity of mAbs; the drawbacks of previous approaches lie in their binding affinity to both FcγRIIIa allotypes, the ratio of activating FcγR binding to inhibitory FcγR binding (A/I ratio) or the melting temperature (TM) of the CH2 domain. To date, no engineered Fc variant has been reported that satisfies all these points. Herein, we present a novel Fc engineering approach that introduces different substitutions in each Fc domain asymmetrically, conferring optimal binding affinity to FcγR and specificity to the activating FcγR without impairing the stability. We successfully designed an asymmetric Fc variant with the highest binding affinity for both FcγRIIIa allotypes and the highest A/I ratio compared with previously reported symmetrically engineered Fc variants, and superior or at least comparable in vitro ADCC activity compared with afucosylated Fc variants. In addition, the asymmetric Fc engineering approach offered higher stability by minimizing the use of substitutions that reduce the TM of the CH2 domain compared with the symmetric approach. These results demonstrate that the asymmetric Fc engineering platform provides best-in-class effector function for therapeutic antibodies against tumor antigens.
Keywords: A/I ratio, ADCC, Fc engineering, FcγR, antibody engineering
Introduction
Monoclonal antibodies (mAbs) have enormous potential as anticancer therapeutics. MAbs promote elimination of tumor cells by Fab-dependent and Fc-dependent mechanisms, such as interference with signaling pathways, apoptosis induction, complement-dependent cytotoxicity, antibody-dependent cell-mediated phagocytosis and antibody-dependent cell-mediated cytotoxicity (ADCC).
ADCC is induced when effector cells are recruited by the Fc domain engaging with a member of the Fcγ receptor family, which is comprised in humans of FcγRI, FcγRIIa, FcγRIIb, FcγRIIc, FcγRIIIa and FcγRIIIb isoforms. FcγRI, FcγRIIa, FcγRIIc and FcγRIIIa are activating receptors characterized by the immunoreceptor tyrosine-based activation motif (ITAM), and FcγRIIb is the only inhibitory receptor characterized by ITIM. The receptors are expressed on a variety of immune cells, such as NK cells, monocytes, macrophages and dendritic cells.1
Increasing affinity for FcγR enhances ADCC, so Fc engineering is considered to be a promising means of increasing the antitumor potency of mAbs.2 Previous reports described that follicular lymphoma patients treated with rituximab had, on average, significantly prolonged progression-free survival if they possessed two copies of the high-affinity FcγRIIIa allele, FcγRIIIaV158.3,4 This result suggests that the efficacy of rituximab is mediated by FcγRIIIa-expressing cells, such as NK cells, and that higher affinity to FcγRIIIa improves the efficacy. Several strategies have been employed to enhance the FcγR binding of mAbs. The first strategy was engineering the glycan moiety attached to Asn297 residue in the Fc domain. Afucosylated IgG1 antibody, which is a mAb without fucose in the N-linked glycan at Asn297, binds to FcγRIIIa with higher affinity and mediates superior ADCC compared with wild-type fucosylated IgG1 antibody.5,6 The second strategy was introducing amino acid substitutions into the Fc domain. mAbs with triple substitutions, S239D/A330L/I332E, bind to FcγRIIIa with higher affinity and have shown superior ADCC activity than wild-type IgG.7 In addition to enhancing the binding to activating FcγRs, minimizing the interaction with inhibitory FcγR, namely FcγRIIb, is another strategy to enhance the potential of the antibody.8 Enhanced cancer elimination was observed in FcγRIIb knockout mice compared with that observed in mice expressing FcγRIIb when they were treated with anti-Her2/neu mAb or anti-E-cadherin mAb,9,10 demonstrating that the ratio of activating FcγR binding to inhibitory FcγR binding (A/I ratio) is an important factor determining the therapeutic efficacy of antitumor antibody.11 mAb with five substitutions, L235V/F243L/R292P/Y300L/P396L, showed enhanced binding to FcγRIIIa, but not to FcγRIIb, which improved the A/I ratio.8 In terms of improving the selectivity for a specific FcγR, antibody variants with selectively enhanced binding for FcγRI were reported.12
Although these glyco- and protein-engineering approaches have successfully enhanced the effector function of mAbs, each technology has issues to overcome. First, afucosylated antibodies bind with lower affinity to FcγRIIIaF158 than to FcγRIIIaV158. As a consequence, the resulting ADCC mediated by NK cells bearing the lower-affinity FcγRIIIa allotype is lower than that mediated by NK cells bearing the higher-affinity allotype, suggesting that afucosylated Fc may not achieve maximum ADCC activity for patients having the lower-affinity allotype.13 Second, because activating and inhibitory FcγRs have high homology, the S239D/A330L/I332E variant also increased binding affinity against inhibitory FcγRIIb, which would be undesirable for achieving maximum antitumor efficacy considering the A/I ratio. Moreover, the TM in the CH2 domain of the S239D/A330L/I332E variant was significantly reduced (by more than 20°C), which could be an issue when the variant is developed as a pharmaceutical product.14 Third, although the L235V/F243L/R292P/Y300L/P396L variant did not increase the binding affinity against inhibitory FcγRIIb, it had only 10-fold increased binding affinity to FcγRIIIa, which is substantially less than the S239D/A330L/I332E variant, thereby achieving only a moderate A/I ratio.
To date, neither glyco- nor protein-engineering has been able to overcome all these issues. Ideal therapeutic use requires an antibody Fc variant that has higher binding affinity to both FcγRIIIaF158 and FcγRIIIaV158 and better stability of the CH2 domain, but that does not increase binding affinity to inhibitory FcγRIIb to maintain a higher A/I ratio. To overcome these issues, in this study we focused on the fact that homodimeric and symmetric Fc domain recognizes monomeric FcγR asymmetrically, which was previously revealed by the structural analysis of Fc fragment with FcγR.15 Considering that Fc and FcγR interact asymmetrically, we hypothesized that asymmetric Fc engineering would make it possible to design a novel Fc variant with improved affinity against both low- and high-affinity FcγRIIIa allotypes, enhancing ADCC activity compared with previously known protein- or glyco-engineering. In addition, asymmetric Fc engineering would result in fewer substitutions or avoidance of the need for stability-reducing substitutions to minimize the reduction of the TM of the CH2 domain. Moreover, asymmetric Fc engineering would allow us to optimize the Fc-FcγR interaction more precisely so as not to increase binding affinity to inhibitory FcγRIIb and to have a higher A/I ratio by discriminating activating FcγRs from inhibitory FcγR.
We designed antibody variants with an asymmetrically engineered Fc domain (asym-mAb) by introducing different substitutions in each Fc domain. Comprehensive mutagenesis in the CH2 domain has identified several substitutions that increase the binding affinity for FcγRs more strongly when they are introduced in one Fc domain than in both chains. We successfully designed an asym-mAb with higher affinity for both FcγRIIIa allotypes and superior or at least comparable ADCC than the previously reported symmetrically engineered antibody (sym-mAb), without increasing the affinity for FcγRIIb or substantially reducing the stability of the antibody. Our results demonstrated a novel approach for optimizing the interaction between Fc and FcγR and confirmed the advantage of that approach when applied therapeutically.
Results
Comparing the binding affinity for FcγRIIIa of asym-mAb and sym-mAb
We screened a set of over 1,000 asym- and sym-mAbs, each with a single substitution in the lower hinge and CH2 domain, for binding to human FcγRIIIaF158 to identify substitutions that enhance FcγRIIIa binding only when they were introduced in one Fc domain. The effect of substitutions in both sym- and asym-mAbs was evaluated using surface plasmon resonance (SPR). We identified several unique substitutions to meet our criteria (binding affinity of asym-mAb > that of sym-mAb). Of them, we selected three single substitutions, L234Y, G236W and S298A, and designed a variant with L234Y/G236W/S298A (YWA) substitutions, to investigate whether asymmetric Fc engineering has any advantages over symmetric Fc engineering. As an example of symmetric Fc engineering, we utilized S239D/A330L/I332E (DLE) substitutions, which were previously reported to increase affinity to FcγRIIIa.7 We prepared five variants: hemi-DLE variant, variant with DLE substitutions in only one Fc domain; homo-DLE variant, variant with DLE in both Fc domains; hemi-YWA variant, variant with YWA substitutions in only one Fc domain; homo-YWA variant, variant with YWA in both Fc domains and DLE/YWA variant with DLE in one Fc domain and YWA in the other Fc domain. We evaluated the affinity for FcγRIIIaF158 of each variant (Table 1). The representative sensorgrams are depicted in Figure S1.
Table 1. Affinity for FcγRIIIaF158 and TM of antibody variants.
| Fc variants | Substitutions in heavy chain A |
Substitutions in heavy chain B |
KD (µmol/L) |
Fold | TM (°C) |
ΔTM (°C) |
|---|---|---|---|---|---|---|
| control mAb1 | - | - | 1.4 ± 0.3 | 1 | 68 | - |
| hemi-YWA | - | L234Y/G236W/S298A | 0.28 ± 0.04 | 5.0 | 68 | 0 |
| hemi-DLE | S239D/A330L/I332E | - | 0.046 ± 0.009 | 30 | 60 | -8 |
| homo-YWA | L234Y/G236W/S298A | L234Y/G236W/S298A | 3.0 ± 0.6 | 0.47 | 68 | 0 |
| homo-DLE | S239D/A330L/I332E | S239D/A330L/I332E | 0.0055 ± 0.0005 | 255 | 48 | -20 |
| DLE/YWA | S239D/A330L/I332E | L234Y/G236W/S298A | 0.0042 ± 0.0003 | 333 | 59 | -9 |
KD = KD for FcγRIIIaF158
Fold = KD (control mAb1)/KD (Fc variants)
TM means TM of the CH2 domain.
ΔTM = TM (Fc variants) - TM (control mAb1)
KD was represented as mean ± SD (n = 3)
First, we compared homo- and hemi-DLE variants to evaluate the effect of DLE substitutions in symmetric Fc engineering or asymmetric Fc engineering. The homo-DLE variant increased the affinity for FcγRIIIa 255-fold compared with control mAb1, which only has substitutions to facilitate heterodimerization of two heavy chains, while the hemi-DLE variant increased it only 30-fold. Next, we evaluated the other substitutions, YWA. The homo-YWA variant reduced the affinity 0.47-fold, but the hemi-YWA variant increased it 5.0-fold. YWA substitutions showed a distinctly different effect on the Fc-FcγRIIIa interaction when introduced in one Fc domain than when introduced in both Fc domains. The DLE/YWA variant showed the highest affinity among evaluated variants, even higher than the homo-DLE variant.
ADCC of antibody variants with asymmetrically engineered Fc
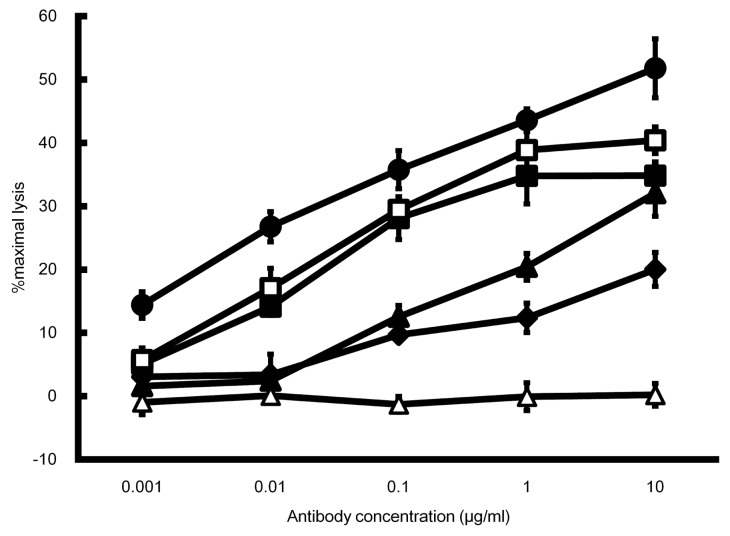
The cellular cytotoxicity of asym-mAb and sym-mAb to tumor antigen X with enhanced FcγRIIIa binding was evaluated using SK-Hep-1 cells expressing tumor antigen X and human PBMC (Fig. 1). Homo- and hemi-DLE variants showed higher ADCC than control mAb1, while ADCC of the homo-DLE variant was slightly higher than that of hemi-DLE. On the other hand, the homo-YWA variant showed no detectable ADCC, but the hemi-YWA showed higher ADCC than control mAb1. In ADCC assay, YWA substitutions showed this opposite effect whether they were introduced in both Fc domains or in only one Fc domain. The DLE/YWA variant with the highest FcγRIIIa binding showed the highest ADCC. These results from the ADCC assay were consistent with those obtained in the kinetic analyses of the variants.
Figure 1. ADCC comparison of asym-mAbs and sym-mAbs. ADCC was determined by percent lysis of SK-Hep-1 cells expressing tumor antigen X at varying concentrations of antibody Fc variants to tumor antigen X using PBMC as effector cells. Mean ± SD of triplicate wells. Black diamond, control mAb1; white square, homo-DLE; black square, hemi-DLE; white triangle, homo-YWA; black triangle, hemi-YWA and black circle, DLE/YWA.
Thermostability and accelerated stability study of antibody variants with asymmetrically engineered Fc
TM of the CH2 domain of hemi-DLE, homo-DLE, hemi-YWA, homo-YWA and DLE/YWA variants was measured by thermal shift assay (Table 1). The TM of the CH2 domain of hemi-DLE variant decreased by 8°C from control mAb1 and that of homo-DLE by 20°C. On the other hand, the TM of hemi-YWA and homo-YWA variants was not significantly reduced, and that of the DLE/YWA variant decreased to the same degree as that of hemi-DLE variant.
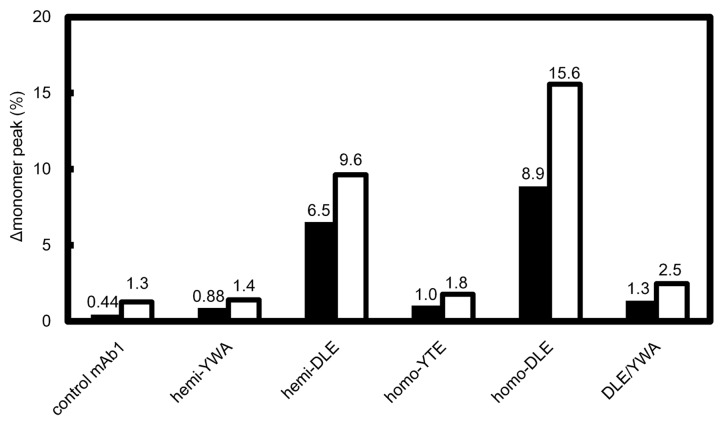
After storage for two and four weeks at 40°C at a concentration of 1 mg/ml, the reduction of a monomer peak of each antibody in size-exclusion chromatography was compared (Fig. 2). The monomer peak of hemi-DLE variant decreased ~10% after 4-week storage and that of homo-DLE decreased 16%. The same tendency was observed after 2-week storage. On the other hand, the reduction of monomer peaks of other variants, including DLE/YWA, was comparable with that of control mAb1.
Figure 2. Stability of antibody Fc variants in accelerated stability study. Percentage of the reduction of a monomer peak (Δmonomer peak (%)) of each variant after storage at 40°C in size-exclusion chromatography is shown. Black and white bars represent the average reduction in monomer peaks after 2-week and 4-week storage, respectively. Each experiment was performed twice and individual data were shown in Table S2.
Further optimization of asymmetrically engineered Fc
We further designed more potent asym-mAbs with higher binding affinity to active FcγRs. Further optimization to enhance FcγRIIIa binding was performed based on the result of comprehensive mutagenesis. As a result, we obtained asym-mAb1 containing L234Y/L235Q/G236W/S239M/H268D/D270E/S298A substitutions in one Fc domain and D270E/K326D/A330M/K334E substitutions in the other. We also prepared the reported protein- and glyco-engineered Fc variants to compare their affinity for FcγRs with asym-mAb1. Protein-engineered Fc variants were prepared by control mAb2, an antibody with only substitutions to facilitate heterodimerization of the two heavy chains. Afucosylated IgG1, afucosyl mAb and the homo-DLE variant were prepared as antibodies with enhanced FcγRIIIa binding, and the homo-L235V/F243L/R292P/Y300L/P396L (VLPYLL) variant was also prepared as an antibody with a high A/I ratio, without increased affinity for FcγRIIb.8 Their increase of affinity for human FcγRs, A/I ratio and the reduction of TM in CH2 domain are shown in Table 2. KD and TM in CH2 domain of these variants are summarized in Table S1.
Table 2. Relative affinity for FcγRs and TM in the CH2 domain of Fc variants.
| Fc variants | FcγRIa | FcγRIIaR131 | FcγRIIaH131 | FcγRIIb | FcγRIIIaF158 | FcγRIIIaV158 | ΔTM (°C) |
||
|---|---|---|---|---|---|---|---|---|---|
| Fold KD |
Fold KD |
Fold KD |
Fold KD |
Fold KD |
Fold A/I |
Fold KD |
Fold A/I |
||
| afucosyl mAb | 0.53 | 1.8 | 0.85 | 2.3 | 18 | 7.7 | 45 | 20 | -2 |
| homo-DLE | 3.4 | 2.9 | 1.4 | 6.7 | 286 | 43 | 126 | 18 | -21 |
| homo-VLPYLL | 0.36 | 0.32 | 2.2 | 0.54 | 63 | 119 | 33 | 59 | -1 |
| asym-mAb1 | 1.0 | 2.6 | 4.9 | 1.0 | 2167 | 2188 | 1054 | 1032 | -6 |
Fold KD = KD (control)/KD (Fc variants)
A/I = (KD for FcγRIIb)/(KD for FcγRIIIaF158) or (KD for FcγRIIb)/(KD for FcγRIIIaV158)
Fold A/I = A/I (Fc variants)/A/I (control)
ΔTM = TM in CH2 domain (control) - TM in CH2 domain (Fc variants)
In calculating the parameters of afucosyl mAb and protein-engineered Fc variants, those of IgG1 and control mAb2 were used as a control, respectively.
Compared with the afucosyl mAb, homo-DLE variant and homo-VLPYLL variant, asym-mAb1 demonstrated greatly enhanced affinity for FcγRIIIa. Asym-mAb1 increased affinity for FcγRIIIaF158 by 2000-fold and for FcγRIIIaV158 by 1000-fold. On the other hand, the afucosyl mAb, homo-DLE variant and homo-VLPYLL variant enhanced affinity for FcγRIIIaF158 only by 18-, 286- and 63-fold and affinity for FcγRIIIaV158 only by 45-, 126- and 33-fold, respectively. As for the binding to FcγRIIb, the affinity for FcγRIIb of asym-mAb1 and of VLPYLL variant was comparable with that of control mab2.
Asym-mAb1 also demonstrated the highest A/I ratio. Asym-mAb1 increased A/I ratio for FcγRIIIaF158 2000-fold and for FcγRIIIaV158 1000-fold, while the afucosyl mAb, homo-DLE variant and homo-VLPYLL variant enhanced A/I ratio for FcγRIIIaF158 only by 7.7-, 43- and 119-fold and that for FcγRIIIaV158 only by 20-, 18- and 59-fold, respectively.
Homo-DLE variant reduced the TM in CH2 domain by 21°C, while other engineered Fc variants reduced it only by less than 10°C.
ADCC of further optimized asym-mAb
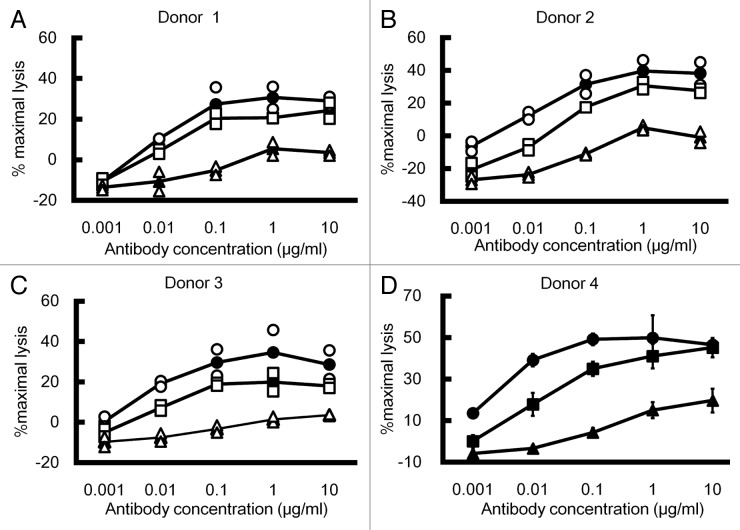
ADCC activity of the optimized asym-mAb was compared with that of afucosyl mAb using human PBMC obtained from four different donors. Asym-mAb1 showed remarkably greater ADCC activity than IgG1, and comparable or slightly higher ADCC activity compared with afucosyl mAb, as shown in Figure 3 (A, B, C and D).
Figure 3. ADCC of antibody Fc variants. ADCC of IgG1, afucosyl mAb and asym-mAb1 was determined by percent lysis of DLD-1 cells expressing tumor antigen Y opsonized at varying concentrations of antibody Fc variants to tumor antigen Y using PBMC obtained from four different donors as effector cells with 10 mg/ml human IgG (A, B and C) or without it (D). Triangle, IgG1; square, afucosyl mAb and circle, asym-mAb1. (A, B and C) Open markers indicate individual data and closed makers with lines indicate average (n = 2). (D) Mean ± SD of triplicate wells.
Discussion
Despite the fact that the Fc domain recognizes FcγRs asymmetrically with two distinct interfaces,15 previous approaches for modifying Fc-FcγR interaction, such as alanine scanning mutagenesis, a protein structure design algorithm and a yeast surface displayed random mutant library screening, focused on modifying the Fc domain in a symmetric manner,7,8,16 making it difficult to identify substitutions that enhance FcγR binding when they are introduced in only one Fc domain. We investigated such substitutions by comparing single-substituted asymmetric variants and the corresponding symmetric variants in comprehensive mutagenesis and combined the substitutions, L234Y, G236W and S298A, with the desired property that we identified through the investigation.
We evaluated the FcγR binding and ADCC of homo-YWA, hemi-YWA, homo-DLE, hemi-DLE and DLE/YWA variants. In a previous analysis, DLE substitutions were thought to improve FcγR interaction mainly in one Fc domain.7 In our results, hemi- and homo-DLE variant enhanced affinity for FcγRIIIa by 30- and 255-fold, respectively, compared with the wild-type antibody. While DLE substitutions in one Fc domain enhanced the binding affinity 30-fold, the gain of affinity by DLE substitution when introduced in the other Fc domain was only 8.5-fold, suggesting that DLE substitutions in each Fc domain contribute positively, but to differing degrees, to enhancing the binding affinity for FcγRIIIa. In contrast to DLE substitutions, YWA substitutions showed a distinct effect. While hemi-YWA variant enhanced affinity for FcγRIIIa 5.0-fold, homo-YWA variant substantially reduced the affinity. This result implies that YWA substitutions stabilize the interaction in one of the two interfaces with FcγRIIIa, but substantially destabilize the interaction in the other interface. The sum of the interactions in each Fc domain was not energetically beneficial, and, as a result, when YWA substitutions were introduced in both Fc domains, they substantially reduced the binding affinity to FcγRIIIa. Although the hemi-YWA variant has enhanced binding affinity for FcγRIIIa, the binding affinity was weaker than that of the hemi-DLE variant. These results indicate that YWA substitutions are less potent than DLE substitutions both in hemi-manner and homo-manner; however, the DLE/YWA variant unexpectedly exhibited even higher affinity for FcγRIIIa than homo-DLE variant, suggesting that DLE and YWA enhance FcγRIIIa binding synergistically (not just additively) by stabilizing each of the Fc-FcγRIIIa interfaces simultaneously in a distinct structural environment. Importantly, although the homo-DLE variant was the most potent Fc variant reported to date, the effect of DLE/YWA variant demonstrated that Fc-FcγRIIIa interactions could be further optimized by the novel asymmetric engineering approach. Consistent with the affinity for FcγRIIIa, the DLE/YWA variant exerted stronger ADCC activity than homo-DLE. Among the evaluated variants, higher ADCC showed a general correlation with higher affinity for FcγRIIIa. This correlation demonstrates that asymmetrically engineered Fc could interact with FcγRIIIa expressed on effector cells in a similar manner to symmetrically engineered Fc.
DLE substitutions were reported to decrease TM of the CH2 domain by more than 20°C in a homo-DLE variant.14 In our analysis, the homo-DLE variant did reduce the TM by 20°C, but the hemi-DLE variant reduced it by only 8°C, less than half of the reduction by homo-DLE. On the other hand, even when YWA substitutions were introduced in both Fc domains, they did not decrease the TM, and the DLE/YWA variant showed almost the same reduction in TM as hemi-DLE. These results suggest that substitutions in each Fc domain reduce the TM of the CH2 domain independently and that the net reduction is the sum of them. To further clarify the storage stability of asym-mAb, we investigated the storage stability of each variant under accelerated conditions. The monomer peak of the homo-DLE variant was reduced by 16% after 4 weeks of accelerated storage, while that of hemi-DLE was reduced by about 10%, suggesting that DLE substitutions additively reduced the storage stability of the variants. On the other hand, the reduction in monomer peaks of homo-YWA, hemi-YWA and even DLE/YWA variants was comparable with that of wild-type antibody. These studies demonstrate that asymmetric engineering cannot only offer Fc variants with superior ADCC activity compared with the symmetric one, but can also offer Fc variants with higher stability.
By further optimizing the Fc domain in an asymmetric manner, we successfully generated asym-mAb1 variant. During the optimization of the DLE/YWA variant to generate asym-mAb1, we removed DLE substitutions because, despite the fact that DLE substitution significantly contributes to the increased binding affinity to FcγRIIIa, DLE substitutions even in one of the Fc domains significantly reduced the TM of the CH2 domain. To the best of our knowledge, asym-mAb1 binds to FcγRIIIaF158 and FcγRIIIaV158 with the highest affinity among any reported Fc engineered mAbs (KD = 1.2 nM and 0.37 nM, respectively). Consistent with this increased binding affinity to FcγRIIIa, asym-mAb1 demonstrated significantly higher ADCC activity than IgG1, and comparable or slightly superior ADCC activity compared with afucosyl mAb. Asym-mAb1 showed slightly superior ADCC activity than afucosyl mAb in some donors, who might have lower-affinity FcγRIIIa genotype. Notably, asym-mAb1 increased FcγRIIIa binding affinity 1000-fold while maintaining inhibitory FcγRIIb binding comparable with wild-type IgG1. We assumed that this selectivity against FcγRIIb binding was achieved by fine-tuning each Fc-FcγR interaction, resulting in an A/I ratio of 1000 to 2000, which is far superior to the other Fc variants. Moreover, the TM of the CH2 domain in asym-mAb1 was 64°C, significantly higher than that of the homo-DLE variant and, though it is slightly lower than wild-type IgG, high enough for pharmaceutical development (Table S1). These results demonstrate that our novel asymmetric engineering provides an antibody Fc variant that has the strongest binding affinity for both FcγRIIIaF158 and FcγRIIIaV158 with no increased binding affinity to inhibitory FcγRIIb and with high stability of the CH2 domain.
Although asym-mAb1 has a human FcγR binding property superior to other Fc variants, it is challenging to precisely evaluate and compare the therapeutic effects of these Fc-engineered antibody in human using an in vivo pre-clinical murine model system. This is because the structural diversity and expression patterns of murine FcγRs do not correspond to those of human FcγRs.1 As expected, asym-mAb1 and other Fc variants bound to murine FcγRs with a different specificity and affinity (data not shown), making it impossible to predict their efficacy in human based on results from the murine model. It would be possible to predict the effect mediated by human FcγRIIIa by using human FcγRIIIa-transgenic mice as previously described, but it is still difficult to evaluate the effect mediated through other FcγRs, including the therapeutic advantage of superior A/I ratio.17 A mouse whose FcγRs were replaced with human FcγRs was recently developed. This mouse recapitulated the unique expression pattern and the functions of human FcγRs that are mediated by human IgG18 and might enable us to evaluate the effect of our asymmetrically engineered antibody in human more accurately.
Asymmetric bispecific IgG antibody, which binds to different antigens with each arm, was recently reported to be valuable in the field of hemophilia A19 and approaches to overcome the problems involved in manufacturing this type of IgG antibody has been recently reviewed.20 Cancer-targeting bispecific antibodies, although not in IgG form, have been investigated to enhance efficacy by targeting two different tumor antigens.21 Since asymmetric bispecific IgG antibodies targeting two different tumor antigens inevitably require hetero-dimerization of the two heavy chains, our asymmetrically engineered Fc could be applied to such bispecific antibodies without any difficulty. Bispecific IgG antibody is a promising antibody format for next-generation antibody therapeutics, and our novel asymmetrically engineered Fc can be easily applied to the format to achieve maximum anti-tumor efficacy.
In conclusion, we demonstrated that asymmetric Fc engineering provides more effective optimization of the Fc-FcγR interaction and identified an asymmetric Fc variant with the highest binding affinity for both FcγRIIIa allotypes, the highest A/I ratio, and slightly higher ADCC activity than previously reported symmetric Fc variants. In addition, asymmetric Fc engineering minimized the use of substitutions that reduce the TM of the CH2 domain. Therefore, our asymmetric Fc engineering platform provides best-in-class effector function for maximizing the therapeutic potential of either monospecific or bispecific IgG antibodies against tumor antigens.
Materials and methods
Preparation of antibodies
The antibody variants used in the experiments were expressed transiently in FreeStyleTM 293 cells (Invitrogen) transfected with plasmids encoding heavy and light chains and purified from culture supernatants using rProtein A Sepharose 4 Fast Flow or rProtein G Sepharose 4 Fast Flow (GE Healthcare). Site-directed mutagenesis of the constant regions of mAbs was performed using QuikChange Site-Directed Mutagenesis Kit (Stratagene) or In-Fusion HD Cloning Kit (Clontech) and the sequence was confirmed by DNA sequencing. The substitutions to facilitate Fc heterodimerization were introduced to obtain asym-mAbs consisting of different heavy chains.20 Asym-mAbs were generated by FreestyleTM 293 cells transfected with three plasmids encoding a light chain and two different heavy chains. Sym-mAbs were generated by FreestyleTM 293 cells transfected with plasmids encoding a light chain and a heavy chain. Afucosylated antibody was prepared as previously described.22
Construction, expression and purification of FcγRs
The sequence information of genes encoding the extracellular region of human FcγRs was obtained from the National Center for Biotechnology Information (NCBI) and the genes were synthesized. FcγRs were fused with 6x His-tag at the C terminus. Vectors containing FcγRs were transfected into FreeStyleTM 293 cells (Invitrogen). Media were harvested and receptors were purified using cation exchange chromatography, nickel affinity chromatography and size exclusion chromatography. Instead of cation exchange chromatography, anion exchange chromatography was used to purify FcγRIa.
Comprehensive mutagenesis of the Fc domain of IgG1 and evaluation of its binding
Sym-mAb and corresponding asym-mab against tumor antigen X, each with a single substitution in only one heavy chain, were prepared for comprehensive mutagenesis assay. They were designed by substituting each of the residues 234–239, 265–271, 285, 296, 298, 300 and 324–337 (EU numbering) in the lower hinge and CH2 domain with other 18 amino acids excluding cysteine. The Fc variants were expressed in 6-well cell culture plate (Becton, Dickinson and Company) transiently in FreeStyleTM 293 cells (Invitrogen) and purified from the culture supernatants using rProtein A Sepharose 4 Fast Flow or rProtein G Sepharose 4 Fast Flow (GE Healthcare) in a 96-well format. The concentrations of purified the Fc variants were determined by NanoDrop 8000 (Thermo Scientific). The binding activity of those variants to FcγRIIIa158F was quantified by a Biacore instrument. The variants were captured on the CM5 sensor chip (GE Healthcare) on which antigen peptide was immobilized, followed by injection of FcγRs. The binding of each antibody to each FcγR was normalized by the captured amount of each variant on the sensor chip and was expressed as a percentage of that of the antibody without the substitutions.
Kinetic analysis by surface plasmon resonance
The kinetic analysis of antibody variants for human FcγRs was monitored by SPR using a Biacore instrument (GE Healthcare), as previously described.23 A recombinant protein L (ACTIGEN) was immobilized on CM5 sensor chip (GE Healthcare) using a standard primary amine-coupling protocol. Antibody variants were captured on the chip, followed by injection of FcγRs.
Thermal shift assay
TM of the CH2 domain of an antibody was measured as previously described.24 The SYPRO orange dye (Invitrogen) was diluted into phosphate buffered saline (PBS; Sigma-Aldrich), before being added to the 0.3 mg/ml protein solutions. Fluorescence measurements were employed using a real-time polymerase chain reaction (RT-PCR) instrument, Rotor-Gene Q (QIAGEN). Rotor-Disc 72 was used with 20 μL of solution per well. The fluorescence emission was collected at 555 nm with a fixed excitation wavelength at 470 nm. During the measurement, the temperature was increased from 30°C to 99°C at a heating rate of 4°C/min.
Stability study under accelerated conditions
Antibodies were dialyzed against PBS and diluted to 1 mg/ml. The antibody solutions were stored at 40°C and analyzed before the treatment and after 2 weeks and 4 weeks. A monomer peak area of each antibody was analyzed with size-exclusion chromatography TSK-GEL G3000SWXL column (TOSOH) by SEC-HPLC with UV detection (Waters). The percentage of reduction from initial monomer peak area was calculated and reported using Empower Waters software.
ADCC assay
Cytotoxicity of antibody against antigen X and antigen Y was measured using a standard 4-h 51Cr release assay and calcein-AM release assay, respectively.6,25,26 Peripheral blood mononuclear cells (PBMC) were purified from whole human blood of healthy donors and used as effector cells. For 51Cr-release assay, we used SK-Hep-1 cells transfected with tumor antigen X as target cells. Target cells were labeled with 1.85 MBq of 51Cr at 37°C for 1 h in a CO2 incubator. For calcein-AM release assay, DLD-1 cells expressing tumor antigen Y were labeled with calcein solution at 37°C for 2 h in a CO2 incubator. 10 mg/ml human IgG (Sanglopor, CSL Behring K.K.) was added to mimic endogenous IgG in human. The number of tumor antigen X expressed on the cell surface was 9.8 × 104 per cell and that of tumor antigen Y was 3.7 × 105 per cell.
Antibody solution was mixed with target cells (1 × 104 cells) and then effector cells were added to the solution at 50:1 PBMC/target cell ratio. The solution was incubated in a CO2 incubator at 37°C for 4 h. Supernatant was harvested and its radioactivity (in 51Cr release assay) or the fluorescence emitted from its released calcein (in calcein-AM release assay) was quantified. Calculating the percentage of specific cell lysis from experiments was done using the following equation: % specific lysis = 100 × (mean experimental release − mean spontaneous release) ÷ (mean maximal release − mean spontaneous release). “Mean experimental release” is radioactivity in 51Cr release assay or fluorescent emission in calcein-AM release assay of the supernatant from the reaction solution with antibody variants. “Mean spontaneous release” is radioactivity in 51Cr release assay or fluorescent emission in calcein-AM release assay of the supernatant from the reaction solution without antibody. “Mean maximal release” is measured from the prepared supernatant by lysing the target cells with 2% NP-40.
Supplementary Material
Acknowledgments
We thank our colleagues at Chugai Research Institute for Medical Science, Inc. and Chugai Pharmaceutical Co. Ltd., M. Fujii, Y. Nakata, A. Maeno and S. Masujima for antibody generation; M. Saito for carrying out SPR analysis and A. Sakamoto, M. Okamoto and M. Endo for carrying out preparation of human FcγRs. This work was fully supported by Chugai Pharmaceutical Co. Ltd.
Glossary
Abbreviations:
- ADCC
antibody-dependent cell-mediated cytotoxicity
- TM
melting temperature
- mAb
monoclonal antibody
- NK
natural killer
- ITAM
immunoreceptor tyrosine-based activation motif
- ITIM
immunoreceptor tyrosine-based inhibition motif
- SPR
surface plasmon resonance
- PBMC
peripheral blood mononuclear cells
- FcγR
Fc gamma receptor
Disclosure of Potential Conflicts of Interest
The authors have no conflicts of interests to disclose.
Footnotes
Previously published online: www.landesbioscience.com/journals/mabs/article/23452
References
- 1.Bruhns P. Properties of mouse and human IgG receptors and their contribution to disease models. Blood. 2012;119:5640–9. doi: 10.1182/blood-2012-01-380121. [DOI] [PubMed] [Google Scholar]
- 2.Scott AM, Allison JP, Wolchok JD. Monoclonal antibodies in cancer therapy. Cancer Immun. 2012;12:14. [PMC free article] [PubMed] [Google Scholar]
- 3.Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99:754–8. doi: 10.1182/blood.V99.3.754. [DOI] [PubMed] [Google Scholar]
- 4.Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol. 2003;21:3940–7. doi: 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Shields RL, Lai J, Keck R, O’Connell LY, Hong K, Meng YG, et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J Biol Chem. 2002;277:26733–40. doi: 10.1074/jbc.M202069200. [DOI] [PubMed] [Google Scholar]
- 6.Shinkawa T, Nakamura K, Yamane N, Shoji-Hosaka E, Kanda Y, Sakurada M, et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J Biol Chem. 2003;278:3466–73. doi: 10.1074/jbc.M210665200. [DOI] [PubMed] [Google Scholar]
- 7.Lazar GA, Dang W, Karki S, Vafa O, Peng JS, Hyun L, et al. Engineered antibody Fc variants with enhanced effector function. Proc Natl Acad Sci U S A. 2006;103:4005–10. doi: 10.1073/pnas.0508123103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stavenhagen JB, Gorlatov S, Tuaillon N, Rankin CT, Li H, Burke S, et al. Fc optimization of therapeutic antibodies enhances their ability to kill tumor cells in vitro and controls tumor expansion in vivo via low-affinity activating Fcgamma receptors. Cancer Res. 2007;67:8882–90. doi: 10.1158/0008-5472.CAN-07-0696. [DOI] [PubMed] [Google Scholar]
- 9.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6:443–6. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 10.Green SK, Karlsson MC, Ravetch JV, Kerbel RS. Disruption of cell-cell adhesion enhances antibody-dependent cellular cytotoxicity: implications for antibody-based therapeutics of cancer. Cancer Res. 2002;62:6891–900. [PubMed] [Google Scholar]
- 11.Nimmerjahn F, Ravetch JV. Fcgamma receptors: old friends and new family members. Immunity. 2006;24:19–28. doi: 10.1016/j.immuni.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 12.Jung ST, Reddy ST, Kang TH, Borrok MJ, Sandlie I, Tucker PW, et al. Aglycosylated IgG variants expressed in bacteria that selectively bind FcgammaRI potentiate tumor cell killing by monocyte-dendritic cells. Proc Natl Acad Sci U S A. 2010;107:604–9. doi: 10.1073/pnas.0908590107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niwa R, Hatanaka S, Shoji-Hosaka E, Sakurada M, Kobayashi Y, Uehara A, et al. Enhancement of the antibody-dependent cellular cytotoxicity of low-fucose IgG1 Is independent of FcgammaRIIIa functional polymorphism. Clin Cancer Res. 2004;10:6248–55. doi: 10.1158/1078-0432.CCR-04-0850. [DOI] [PubMed] [Google Scholar]
- 14.Oganesyan V, Damschroder MM, Leach W, Wu H, Dall’Acqua WF. Structural characterization of a mutated, ADCC-enhanced human Fc fragment. Mol Immunol. 2008;45:1872–82. doi: 10.1016/j.molimm.2007.10.042. [DOI] [PubMed] [Google Scholar]
- 15.Radaev S, Motyka S, Fridman WH, Sautes-Fridman C, Sun PD. The structure of a human type III Fcgamma receptor in complex with Fc. J Biol Chem. 2001;276:16469–77. doi: 10.1074/jbc.M100350200. [DOI] [PubMed] [Google Scholar]
- 16.Shields RL, Namenuk AK, Hong K, Meng YG, Rae J, Briggs J, et al. High resolution mapping of the binding site on human IgG1 for Fc gamma RI, Fc gamma RII, Fc gamma RIII, and FcRn and design of IgG1 variants with improved binding to the Fc gamma R. J Biol Chem. 2001;276:6591–604. doi: 10.1074/jbc.M009483200. [DOI] [PubMed] [Google Scholar]
- 17.Junttila TT, Parsons K, Olsson C, Lu Y, Xin Y, Theriault J, et al. Superior in vivo efficacy of afucosylated trastuzumab in the treatment of HER2-amplified breast cancer. Cancer Res. 2010;70:4481–9. doi: 10.1158/0008-5472.CAN-09-3704. [DOI] [PubMed] [Google Scholar]
- 18.Smith P, DiLillo DJ, Bournazos S, Li F, Ravetch JV. Mouse model recapitulating human Fcγ receptor structural and functional diversity. Proc Natl Acad Sci U S A. 2012;109:6181–6. doi: 10.1073/pnas.1203954109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitazawa T, Igawa T, Sampei Z, Muto A, Kojima T, Soeda T, et al. A bispecific antibody to factors IXa and X restores factor VIII hemostatic activity in a hemophilia A model. Nat Med. 2012;18:1570–4. doi: 10.1038/nm.2942. [DOI] [PubMed] [Google Scholar]
- 20.Klein C, Sustmann C, Thomas M, Stubenrauch K, Croasdale R, Schanzer J, et al. Progress in overcoming the chain association issue in bispecific heterodimeric IgG antibodies. MAbs. 2012;4:653–63. doi: 10.4161/mabs.21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kontermann R. Dual targeting strategies with bispecific antibodies. MAbs. 2012;4:182–97. doi: 10.4161/mabs.4.2.19000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrara C, Grau S, Jäger C, Sondermann P, Brünker P, Waldhauer I, et al. Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcgammaRIII and antibodies lacking core fucose. Proc Natl Acad Sci U S A. 2011;108:12669–74. doi: 10.1073/pnas.1108455108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richards JO, Karki S, Lazar GA, Chen H, Dang W, Desjarlais JR. Optimization of antibody binding to FcgammaRIIa enhances macrophage phagocytosis of tumor cells. Mol Cancer Ther. 2008;7:2517–27. doi: 10.1158/1535-7163.MCT-08-0201. [DOI] [PubMed] [Google Scholar]
- 24.He F, Hogan S, Latypov RF, Narhi LO, Razinkov VI. High throughput thermostability screening of monoclonal antibody formulations. J Pharm Sci. 2010;99:1707–20. doi: 10.1002/jps.21955. [DOI] [PubMed] [Google Scholar]
- 25.Neri S, Mariani E, Meneghetti A, Cattini L, Facchini A. Calcein-acetyoxymethyl cytotoxicity assay: standardization of a method allowing additional analyses on recovered effector cells and supernatants. Clin Diagn Lab Immunol. 2001;8:1131–5. doi: 10.1128/CDLI.8.6.1131-1135.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roden MM, Lee KH, Panelli MC, Marincola FM. A novel cytolysis assay using fluorescent labeling and quantitative fluorescent scanning technology. J Immunol Methods. 1999;226:29–41. doi: 10.1016/S0022-1759(99)00039-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.