Abstract
Severe lower respiratory tract infection in infants and small children is commonly caused by respiratory syncytial virus (RSV). Palivizumab (Synagis®), a humanized IgG1 monoclonal antibody (mAb) approved for RSV immunoprophylaxis in at-risk neonates, is highly effective, but pharmacoeconomic analyses suggest its use may not be cost-effective. Previously described potent RSV neutralizers (human Fab R19 and F2–5; human IgG RF-1 and RF-2) were produced in IgG format in a rapid and inexpensive Nicotiana-based manufacturing system for comparison with palivizumab. Both plant-derived (palivizumab-N) and commercial palivizumab, which is produced in a mouse myeloma cell line, showed protection in prophylactic (p < 0.001 for both mAbs) and therapeutic protocols (p < 0.001 and p < 0.05 respectively). The additional plant-derived human mAbs directed against alternative epitopes displayed neutralizing activity, but conferred less protection in vivo than palivizumab-N or palivizumab. Palivizumab remains one of the most efficacious RSV mAbs described to date. Production in plants may reduce manufacturing costs and improve the pharmacoeconomics of RSV immunoprophylaxis and therapy.
Keywords: RSV, monoclonal, antibody, prophylaxis, therapy, Nicotiana
Introduction
Respiratory syncytial virus (RSV), a member of the Pneumovirus genus of the Paramyxoviridae family, is commonly the cause of severe lower respiratory tract infection in infants and small children. Nearly all children have had at least one RSV infection by the age of two. Of the infants who have primary RSV-mediated infections, nearly one-third develop lower respiratory tract infections. This lower respiratory tract illness in early childhood is associated with subsequent development of wheezing in children up to age 11 y.1 Each year, RSV is estimated to be responsible for up to 125,000 pediatric hospitalizations, with an associated mortality rate of ~2%.2 Among hospitalized infants with chronic lung or congenital heart disease (CLD or CHD), the rate of mortality due to RSV may reach 5%. RSV also causes significant morbidity and mortality in certain adult populations, such as elderly patients in long-term care facilities. Each year, ~5–10% of these patients develop RSV infections, and the rates of subsequent development of pneumonia or of death are 10–20% and 2–5%, respectively.3 RSV is also a common lower respiratory infection in hematopoietic stem cell transplant patients. In these patients, the incidence of RSV infection typically ranges from 1–15% and rates of pneumonia following RSV infection can be as high as 80%. Stem cell transplant patients with RSV-related pneumonia typically have death rates of 70–80%.4 There are an estimated 175,000 adults hospitalized annually in the US alone with RSV infection.5
Palivizumab (Synagis®; MedImmune) entered the market in September 1998. Palivizumab is a humanized RSV IgG1 mAb (anti-F-protein) that is routinely used to prevent serious lower respiratory tract disease caused by RSV in pediatric patients at high risk of RSV disease. The prophylactic regimen consists of monthly intramuscular injections (thigh muscle) during the course of the RSV season. In pivotal clinical trials, palivizumab immunoprophylaxis resulted in a 55% reduction in hospitalizations among high-risk pediatric patients.6 Although palivizumab is effective for RSV immunoprophylaxis in at-risk neonates, ongoing analyses on the pharmacoeconomics demonstrate that palivizumab is not cost-effective in actual use, even when its use is tightly restricted to its labeled population.7-13 It has been estimated that 16–18 patients must be treated to prevent one RSV-related hospitalization.14,15 A National Institute for Research assessment found that prophylaxis with palivizumab did not provide adequate benefit for the cost, based on the incremental cost-effectiveness ratio threshold, when used unselectively in children without CLD/CHD or children with CLD or CHD.16
Several human mAbs against glycoprotein F (gF) that could be alternatives to palivizumab have been described. RF-1 and RF-2 were isolated from human SPL-SCID mice and reported to have IC50 ranging from 8–165 ng/mL and 30–1000 ng/mL, respectively, against 13 RSV strains (representing both subgroup A and B).17 These mAbs bound to overlapping conformational epitopes on gF.17 Human Fabs 19 and F2–5 were isolated from human phage display libraries and had IC60 ranging from 0.2–3.0 μg/mL (tested against 19 isolates of subgroup A and B) and 0.1–1.4 μg/mL (11 isolates), respectively.18,19 These Fabs were shown to bind to different gF epitopes and both were found to significantly reduce lung viral titer when applied topically to RSV-infected mice.19,20 None of the four antibodies (RF-1, RF-2, Fab 19 or F2–5) has yet been tested in the cotton rat model that was used for the development of palivizumab.21
Manufacturing of mAbs in plants, although first reported in 1989 as a potential means of cheaper production,22 has been slow to develop as a viable alternative to traditional manufacturing in bioreactors using mammalian cell culture. Nicotiana-based manufacturing of antibodies has recently advanced because of two technological breakthroughs: (1) viral based transient expression allowing high accumulation of antibody in days;23,24 (2) transgenic plants with altered glycosylation pathways that can produce antibodies with mammalian glycoforms.25 Although purification costs are similar to mammalian production of mAbs, raw materials and capital expenses are substantially reduced for plant manufacturing of mAbs using these advances.26 Lowering the cost of anti-RSV mAb could expand the patient populations in which immunoprophylaxis would be cost-effective,7,12 and could make immunotherapy pharmacoeconomically viable.
Results
Production of mAbs
All molecules (including those previously expressed only as Fabs) were expressed as IgG1 mAbs using a viral-based transient expression system (magnICON). Transgenic Nicotiana benthamiana, in which plant-specific N-glycans (with core α1,3 fucose and β1,2 xylose) are reduced by RNAi inhibition of plant-specific glycosyl-transferases,25 were used as the host plant. Plants (~1 kg) were infiltrated with the mAb vectors and after 7–8 d, the plants were homogenized with a commercial juicer. Extract was clarified and IgG purified by protein A chromatography (Table 1). Purified mAbs were greater than 95% pure as assessed by SDS-PAGE and HPLC-SEC (Fig. S1), and all mAbs except for F2–5 had low levels of aggregate (≤ 3%). An additional purification step was performed on F2–5 resulting in elimination of detectable aggregates (Table 1). Palivizumab (MedImmune) was purchased from a commercial supplier.
Table 1. Purification yield and % aggregate of Nicotiana-derived mAbs.
| Yield or aggregates | mAb tested | ||||
|---|---|---|---|---|---|
| RSV19 | F2–5 | RF-1 | RF-2 | palivizumab-N | |
| Yield post-protein A (mg/kg) | 24 | 58 | 117 | 54 | 180 |
| % aggregate1 post protein A | 0 | 15/02 | 0 | 0 | 3 |
Palivizumab-n = An antibody with the palivizumab amino acid sequence produced in Nicotiana. 1 As determined by HPLC size exclusion chromatography.2 Ceramic hydroxyapatite chromatography was used for the F2–5 sample to remove aggregates from the post-protein A preparation.
Neutralization testing of mAbs
Over the course of these studies, mAbs were tested in three laboratories (Barnard, Utah State University; Crowe, Vanderbilt University Medical Center; Piedra, Baylor College of Medicine) using standard neutralization assays against a variety of strains (Table 2). All mAbs displayed neutralizing activity, although mAbs RSV19 and RF-1 were in general the least potent. RF-2 displayed activity similar to palivizumab and Nicotiana-derived palivizumab (palivizumab-N) although its activity was not as broad-spectrum. F2–5 exhibited activity similar to palivizumab or palivizumab-N, although it was tested against fewer strains.
Table 2. Neutralization activity and antigen binding affinities of the RSV mAbs.
| Value | Testing Lab | Viral Strain | Viral Subgroup | mAb | |||||
|---|---|---|---|---|---|---|---|---|---|
| RSV19 | F2–5 | RF-1 | RF-2 | palivizumab-N | palivizumab | ||||
| Neutralization IC50 (μg/mL) | Lab A | Tracy | A | 7.3 | 3.6 | 190 | 21 | 1.9 ± 0.81 | 1.5 ± 0.81 |
| 18537 | B | 5.2 | 1.8 | 190 | 60 | 3.0 | - | ||
| Lab B | A2 | A | 3 | 0.07 | - | - | - | 0.6 | |
| Lab C | A2 | A | - | - | > 10 | 0.14 | 0.08 | 0.08 | |
| 9320 | B | - | - | 0.3 | 0.1 | 0.39 | 0.15 | ||
| Long | A | - | - | > 10 | 1.8 | 0.23 | 0.23 | ||
|
KD (x 10- 10M) |
Biacore | A2 | A | 160 ± 110 | 4.9 ± 1.7 | 1.0 ± 0.1 | 0.8 ± 0.1 | 1.6 ± 0.2 | 2.0 ± 0.5 |
“-“ = not tested.1 Neutralization mediated by antibodies in these samples was tested 7 times – once before each cotton rat study. Averages and standard deviations are shown.2 Average KD (n=3) for binding to gF (A2 strain) with standard deviations are shown. Lab A = Piedra; Lab B = Crowe; Lab C = Barnard
Kinetic analyses of mAb binding to recombinant F protein
Dissociation constants (KD) were determined using surface plasmon resonance (Biacore) with an NTA chip capturing His-tagged RSV gF (based on RSV strain A2; Fig. S2). A summary of the results are shown in Table 2. The affinities of the mAbs for RSV gF did not correlate with neutralizing activity. F2–5 was a more potent neutralizer of the A2 strain than palivizumab, but had a weaker binding affinity. Conversely, RF-1 and RF-2 had superior binding affinities than palivizumab, but poorer neutralizing activity.
Prophylaxis in the cotton rat model
Prophylaxis studies (Table 3) followed the protocol used in the development of palivizumab.21 Cotton rats received intramuscular injections of mAb 1 d prior to challenge with the subgroup A strain RSV/Tracy.27 Nasal and lung lavage samples were taken upon necropsy on day four post-challenge. Only palivizumab and Nicotiana-derived palivizumab (palivizumab-N) provided significant reductions in the nasal viral titer (p < 0.05 to p < 0.001 depending upon the experiment), although these reductions were relatively minor (2–5-fold). In contrast, all of the mAbs tested in vivo provided significant (p < 0.01 to p < 0.001) reductions in lung viral titer compared with untreated controls; RSV19 and RF-1 were not tested in cotton rats due to their poor neutralization activity relative to palivizumab. The reduction provided by palivizumab or palivizumab-N was superior to that seen for F2–5 and RF-2. Given its weak in vitro neutralizing activity against the challenge strain (Table 2), the weak protection conferred by RF-2 was not surprising. F2–5, however, had similar neutralizing activity (and superior antigen binding activity), so the poor protection observed was unexpected. Figure 1 combines the results from multiple experiments, and displays the relative reduction in lung titer compared with untreated controls for the mAbs tested at different doses.
Table 3. Prevention of RSV replication by prophylactic mAb treatment in cotton rats.
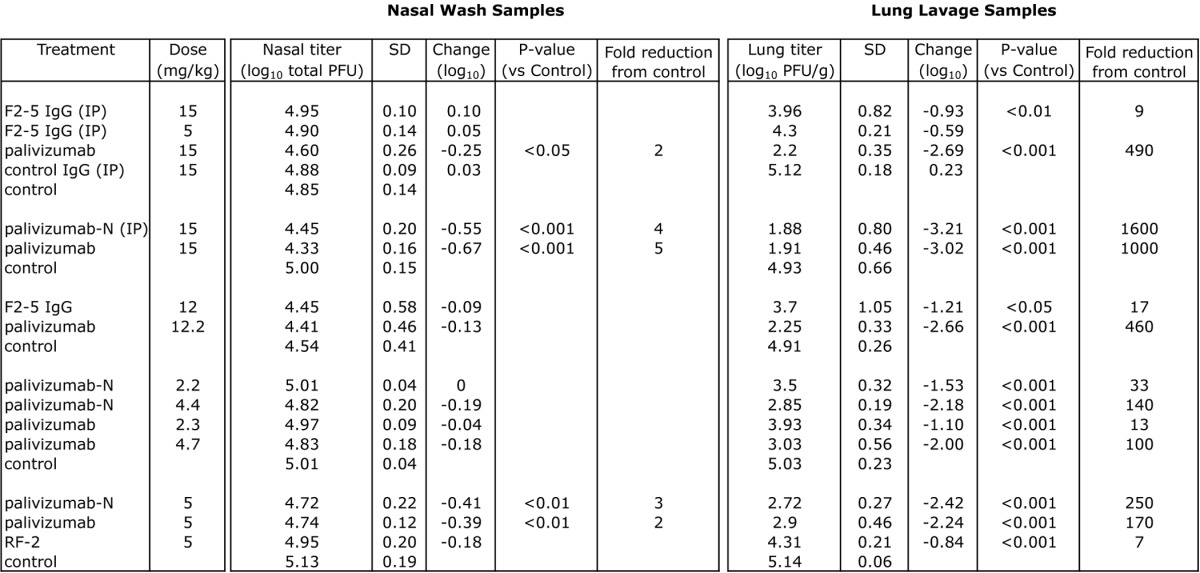
Groups consisting of 5–6 cotton rats treated with mAb or PBS (control) 1 d prior to intranasal challenge with 2.25 x 105 pfu of RSV (Tracy) in 0.1 ml. Unless otherwise indicated, all animals received mAb intramuscularly. Viral titer was assessed 4 d post-challenge. IP = intraperitonal, SD = standard deviation. P-values were determined by ANOVA with a follow-up Dunnett’s pairwise comparison test.
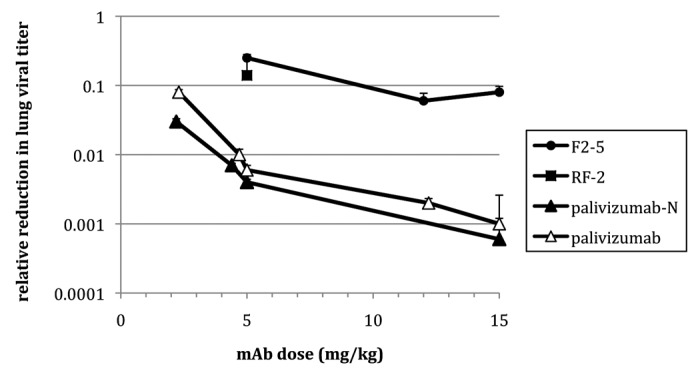
Figure 1. Relative reduction in RSV lung titer in cotton rats receiving mAb prophylactically. Animals received mAb 1 d prior to challenge (RSV strain Tracy) and viral titer was determined 4 d post-challenge. Error bars denote standard deviation. Note that RF-2 was tested at a single dose (5 mg/kg).
Serum pharmacokinetics
ELISA testing demonstrated that palivizumab and palivizumab-N had indistinguishable serum pharmacokinetics (Fig. 2), with serum half-lives of ~7 d.
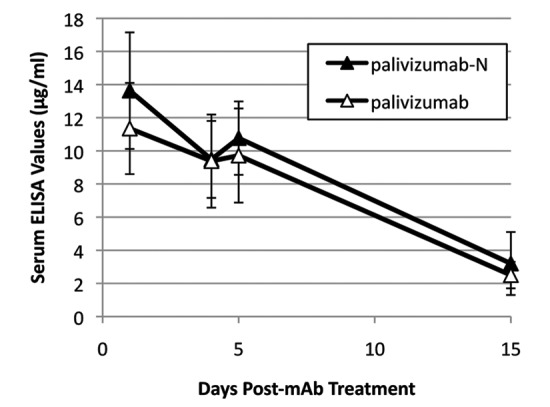
Figure 2. Serum levels of palivizumab and palivizumab-N in cotton rats. Animals (n = 6) were treated with 5 mg/kg of mAb intramuscularly and serum level of mAb assessed by ELISA. Error bars denote standard deviation.
Therapy
RF-2, palivizumab and palivizumab-N were tested therapeutically (Table 4) to determine the potential utility of mAb treatment after an infection is established. When delivered 1 d after viral challenge, 4.6 mg/kg RF-2 failed to reduce lung titers significantly. In contrast, 4.6 mg/kg of palivizumab or 4.2 mg/kg of palivizumab-N provided significant reductions (8- and 25-fold, respectively). Increasing the concentration resulted in a dose-dependent reduction in lung titer for palivizumab-N (Fig. 3) with up to 540-fold reduction at the highest dose tested; palivizumab was not tested at higher doses due to budgetary constraints. Significant reductions (p < 0.001) also were seen when palivizumab-N was administered a full 2 d after viral challenge.
Table 4. Reduction of RSV replication mediated by mAb therapy in cotton rats.
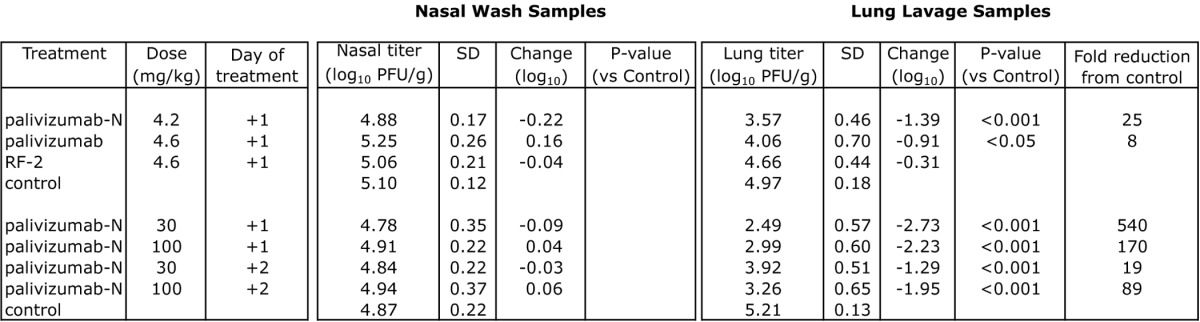
Groups consisting of 5–6 cotton rats challenged intranasally with 2.25 x 105 pfu of RSV (Tracy) were treated at +1 or +2 d with mAb or PBS (control) intramuscularly. Viral titer was assessed 4 d post-challenge. SD = standard deviation. P-values were determined by ANOVA with a follow-up Dunnett’s pairwise comparison test.
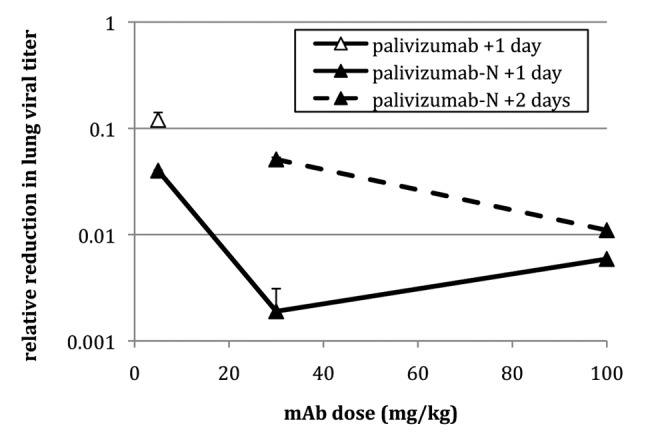
Figure 3. Relative reduction in RSV lung titer in cotton rats treated therapeutically. Animals received mAb (5, 30, or 100 mg/kg) either one (+1) or two (+2) days after challenge (RSV strain Tracy) and viral titer was determined 4 d post-challenge. Error bars denote standard deviation.
Discussion
Production of mAbs using transient expression systems in Nicotiana is becoming routine in research laboratories and for at least one contract manufacturer.26 All of the mAbs expressed for this study had reasonable yields compared with other Nicotiana-derived mAbs described in the literature, especially considering that no attempts were made to optimize expression or purification. All of the Nicotiana-derived mAbs tested had significant neutralizing activity in vitro and high affinity for recombinant gF (Table 2). Of the mAbs tested in vivo, all reduced lung viral titer in the cotton rat, but none except palivizumab-N were as effective as palivizumab. This observation of functional equivalence between palivizumab-N and palivizumab, combined with previous reports of Nicotiana-derived mAbs having binding activity indistinguishable from parental mAbs,28-31 would suggest the inferiority of the other mAbs tested was unrelated to the expression system. Indeed, the only demonstrated difference to date between mammalian-derived and plant-derived mAbs is glycosylation. For the mAbs produced here, a transgenic N. benthamiana line was used that yields mAbs with mammalian N-glycans predominantly of the GnGn glycoform.25 Although this glycoform can enhance antibody-dependent cell-mediated cytotoxicity via higher affinity binding to the FcγRIII receptor,32 it does not influence other effector functions or the long serum half-life of IgG conferred by binding to the FcRn receptor. This pattern was confirmed by the pharmacokinetics seen in the cotton rat (Fig. 2); rodent FcRn is known to be promiscuous in its binding of IgG from other species.33
Although there are an estimated 175,000 adults hospitalized annually with RSV infection in the US alone,5 data on the therapeutic usefulness of palivizumab in this setting are very limited.34 Studies in hematopoietic stem cell transplant patients (where mortality rates from RSV infection can be extremely high) are inconclusive and likely have been limited by dosing costs.34 With current pricing, a 15 mg/kg dose of palivizumab for a 50 kg adult costs ~$125,000. We are unaware of any reports of therapeutic testing of palivizumab in the cotton rat model. In this study, we found palivizumab and palivizumab-N both provided significant reductions in lung viral titer; however, doses of 6x and 20x the prophylactic dose (5 mg/kg) were required to provide similar reductions of viral lung titer (100-fold) when delivered 1 and 2 d, respectively, after viral challenge. This is not surprising because the therapeutically applied mAb was targeting an established viral infection.
A large proportion of the world’s population does not have access to palivizumab, and for those populations that do, the pharmacoeconomic benefit is questionable. At commercial scale, production of mAbs in the Nicotiana transient expression system is expected to reduce costs 2–5-fold compared with production in mammalian cell culture.24,35 We have found contract manufacturing of plant mAb for preclinical and Phase 1 testing to be 3–5 times less expensive than contract manufacturing in cell culture. The reduced costs are primarily the result of the comparatively inexpensive infrastructure associated with producing the plant raw material. Within the last several years, Nicotiana-derived mAbs have been made under Good Manufacturing Practices (GMPs) and tested clinically.26,36 This manufacturing platform may serve as an alternative production system that could reduce costs and thus expand the patient populations that can benefit from this potent mAb product.
Methods
N. benthamiana expression vectors
RF-1 and RF-2 variable region amino acid sequences (VR) were used as reported and codon-optimized for N. benthamiana and synthesized (GeneArt, AG; RF-1 VL, KC283069; RF-1 VH, KC283070; RF-2 VL, KC283071; RF-2 VH, KC283072).37 The F2–5 variable regions were originally reported by Crowe et al.19 and deposited in GenBank (accession nos. L41061 and L41062). The VH gene sequence was used as reported. The VL (lambda) FR1 residues 1–3 and 8 were altered to be consistent with genomic sequences according to Kabat database. Both genes were codon-optimized for N. benthamiana (VL, KC283073; VH, KC283074). The RSV19 VRs were derived from RSV Fab 19.18 The VH sequence was used as reported. The VL (kappa) FR1 residues 1–4 and FR4 residues 103–107 were altered to be consistent with Kabat, and both genes were codon-optimized for N. benthamiana (VL, KC283075; VH, KC283076). Palivizumab VR sequences were used as reported and codon-optimized as above (VL, KC283077; VH, KC283078).21 Genes were codon optimized and synthesized and subsequently cloned into plant (TMV and PVX) expression vectors (Icon Genetics, GmbH), followed by transformation into Agrobacterium tumefaciens strain ICF320.36
Production of anti-RSV mAbs in N. benthamiana
We used the “magnifection” procedure,23 with minor modifications. Plants grown for 4 weeks in an enclosed growth room (20–23°C) were used for vacuum infiltration. Equal volumes of Agrobacterium cultures grown overnight were mixed in infiltration buffer (10 mM MES/10 mM MgSO4 pH 5.5) resulting in a 1:1000 dilution for each individual culture. Infiltration solution was transferred into a 20 L custom built (Kentucky Bioprocessing) vacuum chamber. Aerial parts of plants were inverted into the bacterial/buffer solution. A vacuum of 0.5 bars was applied for 2 min and plants were returned to the growth room. Seven days post-infiltration, leaf tissue was extracted in a juicer (Green Star, Model GS-1000), using 250 ml of chilled extraction buffer (100 mM Tris/40 mM ascorbic acid/1 mM EDTA) per kg of green leaf tissue. The extract was clarified by lowering the pH to 4.8 with 1 M phosphoric acid then re-adjusting to pH 7.5 with 2 M Tris base to insolubilize plant debris. The supernatant was transferred and centrifuged at 16,000 x g for 30 min. The clarified extract was filtered (0.2 µm) prior to concentration via Minim Tangential Flow Filtration System (Pall) then 0.2 µm filtered immediately prior to loading onto 5 ml HiTrap MabSelect SuRe (GE Healthcare) Protein A column at 2 ml/min. The column was washed with running buffer (50 mM HEPES/100 mM NaCl, pH 7.5) and eluted with 0.1 M acetic acid, pH 3.0. The resulting eluate was neutralized to pH 7 using 2 M Tris, pH 8.0 and supplemented with Tween 80 to 0.01%. The mAb solution was polished via Q filtration (Mustang Acrodisc Q membrane; Pall), aliquoted and stored at -80°C until use. All mAbs were fully assembled and of greater than 98% purity as determined by SDS-PAGE. Endotoxin levels were measured with Endosafe PTS (Charles River) and were < 100 EU/mg.
Kinetic testing of mAb binding to recombinant RSV F protein
The kinetics of RSV F recombinant protein binding by mAbs were measured on a Biacore X100. An NTA chip was used to capture recombinant gF (strain A2). Each mAb (diluted in NTA running buffer) was then flowed over the chip at 5 different concentrations (with the highest concentration having an Rmax between 30 and 80 RUs) and kinetic analyses performed using BIAEvaluation software (1:1 fit). Fast flow rates and controls, including a flow cell with no gF, and immobilized gF with flow of buffer only, were performed to insure against acquiring mass transfer-limited data.
Labs A and C: Microneutralization assay
Tests were performed in 96-well microtiter plates with HEp-2 cells prepared in 2% FBS-MEM. Plaque purified RSV strains were used in the microneutralization assay. Serial 2-fold dilutions (50 µL) in duplicate starting at a 1:8 dilution were performed to determine the neutralizing titer for each sample. The neutralizing antibody titer was defined as the serum dilution at which > 50% reduction in viral cytopathic effect (CPE) is observed. CPE was determined visually after the wells were fixed with 10% neutral buffered formalin and stained with crystal violet. Neutralizing antibody titers were categorical log numbers and not continuous values. The lowest detectable titer was 2.5 log2. Samples with non-detectable titers were assigned a value of 2 log2.
Lab B: Neutralization assay
The neutralizing activity of the antibodies was measured by a plaque reduction assay using HEp-2 cell monolayers. The activity was measured in µg/ml and calculated as the lowest concentration that reduced plaque number by 60%. One hundred twenty-five µl of diluted of RSV calculated to yield 50 plaques per well was mixed with 125 µl of 1:4 dilutions of mAb, and incubated at 37°C for 60 min. Cell monolayers in 24-well tissue culture plates at 80–90% confluency were inoculated in triplicate by replacing media in each well with 100 µl of virus-mAb mixture. After incubation at 37°C for 1 h, the monolayer in each well was then overlaid with 0.75% methylcellulose in Opti-MEM I (Invitrogen) supplemented with 2% FBS, 320 µg/mL l-glutamine, 2.7 µg/mL amphotericin B, and 45 µg/mL gentamicin. Cultures were incubated for 4 d at 37°C in 5% CO2 after which the overlay was removed and the monolayers were fixed in 80% cold methanol. Plaques were stained and quantified by an immunoperoxidase staining procedure. Plaques for each mAb dilution were counted, triplicates averaged, and mAb dilution vs. plaque number plotted. The plaque reduction neutralizing activity (µg/ml) was determined by regression curve analysis.
Cotton rat testing
Cotton rats (Sigmoden hispidis of either sex, 60–125 g body weight) were bred and housed in the Baylor College of Medicine’s (BCM) vivarium in cages covered with barrier filters and given food and water ad libitum. Experiments performed for these studies were performed utilizing NIH and USDA guidelines and experimental protocols approved by the BCM Investigational Animal Care and Use Committee. Groups of 5–6 animals were used in all studies. mAbs were injected either intraperitoneally or intramuscularly (see Table 3). Control IgG was a Nicotiana-derived human IgG against C. difficile toxin A. Challenge virus (RSV-Tracy, 2 x 105 PFU) was administered intranasally (100 μL) to cotton rats lightly anesthetized with isoflurane. Animals were euthanized 4 d after challenge. Following euthanasia with CO2, each cotton rat was weighed and the sex and age recorded. The left and one of the large right lobes of the lungs was removed, rinsed in sterile water to remove external blood contamination and weighed. The left lobe was transpleurally lavaged using 3 mL of Iscove’s medium, with 15% glycerin mixed with 2% FBS-MEM (1:1, v:v) in a 3 mL syringe with a 26G x 3/8 inch needle and injecting at multiple sites to totally inflate the lobe. Lavage fluid was recovered by gently pressing the inflated lobe flat and used to transpleurally lavage the right lobe following the same technique. The lavage fluid was collected and stored on ice until titered. For nasal washes of the upper respiratory tract, the jaw was disarticulated. The head was removed and 1 mL of Iscove’s medium with 15% glycerin mixed with 2% FBS-MEM (1:1, v:v) was pushed through each nare (total of 2 mL). The effluent was collected from the posterior opening of the pallet and stored on ice until titered at the end of all sample collections. RSV Tracy lung lavage titers (PFU/gm lung) and nasal wash titers (total PFU) were determined by plaque assay. Plaque assays were performed using 24-well tissue culture plates containing nearly confluent monolayers (20 to 40 x 104 cells/well) of HEp-2 cells prepared in 10% FBS 24 h prior to start of assay. At the start of each assay, dilutions (serial log10) were made of the test samples. 0.2 mL of each sample was added to wells in duplicate and allowed to adsorb for 90 min in a 36°C, 5% CO2 incubator with occasional gentle agitation. After the inoculum was removed, monolayers were overlayed with 0.75% methylcellulose in 2% FBS-MEM containing antibiotics, vitamins and other nutrients. Tissue culture and positive virus controls were included in each assay. The plates were placed in a 36°C, 5% CO2 incubator. Day 6 + 1 d later, plates were stained with 0.01% crystal violet/10% formalin solution (~1.5 mL/well) and allowed to sit for 24–48 h at room temperature. Wells were rinsed with water. All of the plaques in wells containing ~20 to 80 plaques were enumerated, averaged and the virus titers calculated as total log10 PFU for nasal wash fluid or log10 PFU/g of tissue for lungs. The lower limit of detection by this method was ~0.7 total log10 PFU for nasal wash fluid or 1.5 log10 PFU/g lung tissue. Comparisons of the groups for each experiment were performed by ANOVA. When ANOVA indicated there was at least one treatment that differed significantly from the control, then a follow up Dunnett’s pairwise comparison test was performed. Statistical analyses were performed using Prism 5 for Mac OS X.
Supplementary Material
Acknowledgments
The authors thank Sonnie Kim Grossman, M.S., for her assistance with this research, Dr. Yuri Gleba for providing access to magnICON, and Dr. Steinkellner for access to the glycosylation modified plants. The authors also thank Annette Machado, David Derubeis, Alan Jewell and Letisha Aideyan for their technical assistance. This work was supported by the National Institutes of Health (R41AI063681 to L.Z., NO1-AI-30048 to D.L.B. and HHSN272201000004I to B.E.G.).
Disclosure of Potential Conflicts of Interest
K.W. and L.Z. have an ownership position in Mapp Biopharmaceutical.
Supplementary Materials
Supplemental materials may be found here: www.landesbioscience.com/journals/mabs/article/23281
Footnotes
Previously published online: www.landesbioscience.com/journals/mabs/article/23281
References
- 1.Stein RT, Sherrill D, Morgan WJ, Holberg CJ, Halonen M, Taussig LM, et al. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354:541–5. doi: 10.1016/S0140-6736(98)10321-5. [DOI] [PubMed] [Google Scholar]
- 2.Leader S, Kohlhase K. Recent trends in severe respiratory syncytial virus (RSV) among US infants, 1997 to 2000. J Pediatr. 2003;143(Suppl):S127–32. doi: 10.1067/S0022-3476(03)00510-9. [DOI] [PubMed] [Google Scholar]
- 3.Falsey AR, Walsh EE. Respiratory syncytial virus infection in adults. Clin Microbiol Rev. 2000;13:371–84. doi: 10.1128/CMR.13.3.371-384.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowden RA. Respiratory virus infections after marrow transplant: the Fred Hutchinson Cancer Research Center experience. Am J Med. 1997;102(3A):27–30, discussion 42-3. doi: 10.1016/S0002-9343(97)00007-7. [DOI] [PubMed] [Google Scholar]
- 5.Storey S. Respiratory syncytial virus market. Nat Rev Drug Discov. 2010;9:15–6. doi: 10.1038/nrd3075. [DOI] [PubMed] [Google Scholar]
- 6.The IMpact-RSV Study Group Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics. 1998;102:531–7. doi: 10.1542/peds.102.3.531. [DOI] [PubMed] [Google Scholar]
- 7.Joffe S, Ray GT, Escobar GJ, Black SB, Lieu TA. Cost-effectiveness of respiratory syncytial virus prophylaxis among preterm infants. Pediatrics. 1999;104:419–27. doi: 10.1542/peds.104.3.419. [DOI] [PubMed] [Google Scholar]
- 8.Lofland JH, O’Connor JP, Chatterton ML, Moxey ED, Paddock LE, Nash DB, et al. Palivizumab for respiratory syncytial virus prophylaxis in high-risk infants: a cost-effectiveness analysis. Clin Ther. 2000;22:1357–69. doi: 10.1016/S0149-2918(00)83032-5. [DOI] [PubMed] [Google Scholar]
- 9.Numa A. Outcome of respiratory syncytial virus infection and a cost-benefit analysis of prophylaxis. J Paediatr Child Health. 2000;36:422–7. doi: 10.1046/j.1440-1754.2000.00303.x. [DOI] [PubMed] [Google Scholar]
- 10.Barton LL, Grant KL, Lemen RJ. Respiratory syncytial virus immune globulin: decisions and costs. Pediatr Pulmonol. 2001;32:20–8. doi: 10.1002/ppul.1084. [DOI] [PubMed] [Google Scholar]
- 11.Vogel AM, McKinlay MJ, Ashton T, Lennon DR, Harding JE, Pinnock R, et al. Cost-effectiveness of palivizumab in New Zealand. J Paediatr Child Health. 2002;38:352–7. doi: 10.1046/j.1440-1754.2002.00790.x. [DOI] [PubMed] [Google Scholar]
- 12.Roeckl-Wiedmann I, Liese JG, Grill E, Fischer B, Carr D, Belohradsky BH. Economic evaluation of possible prevention of RSV-related hospitalizations in premature infants in Germany. Eur J Pediatr. 2003;162:237–44. doi: 10.1007/s00431-002-1106-6. [DOI] [PubMed] [Google Scholar]
- 13.Rietveld E, Steyerberg EW, Polder JJ, Veeze HJ, Vergouwe Y, Huysman MW, et al. Passive immunisation against respiratory syncytial virus: a cost-effectiveness analysis. Arch Dis Child. 2010;95:493–8. doi: 10.1136/adc.2008.155556. [DOI] [PubMed] [Google Scholar]
- 14.Storch GA. Humanized monoclonal antibody for prevention of respiratory syncytial virus infection. Pediatrics. 1998;102:648–51. doi: 10.1542/peds.102.3.648. [DOI] [PubMed] [Google Scholar]
- 15.The PREVENT Study Group Reduction of respiratory syncytial virus hospitalization among premature infants and infants with bronchopulmonary dysplasia using respiratory syncytial virus immune globulin prophylaxis. Pediatrics. 1997;99:93–9. doi: 10.1542/peds.99.1.93. [DOI] [PubMed] [Google Scholar]
- 16.Wang D, Bayliss S, Meads C. Palivizumab for immunoprophylaxis of respiratory syncytial virus (RSV) bronchiolitis in high-risk infants and young children: a systematic review and additional economic modelling of subgroup analyses. Health Technol Assess. 2011;15:iii–iv, 1-124. doi: 10.3310/hta15050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chamat S, Walsh EE, Anderson D, Osta M, Awaraji C, Pan LZ, et al. Human monoclonal antibodies isolated from spontaneous Epstein-Barr virus-transformed tumors of Hu-SPL-SCID mice and specific for fusion protein display broad neutralizing activity toward respiratory syncytial virus. J Infect Dis. 1999;180:268–77. doi: 10.1086/314876. [DOI] [PubMed] [Google Scholar]
- 18.Barbas CF, 3rd, Crowe JE, Jr., Cababa D, Jones TM, Zebedee SL, Murphy BR, et al. Human monoclonal Fab fragments derived from a combinatorial library bind to respiratory syncytial virus F glycoprotein and neutralize infectivity. Proc Natl Acad Sci U S A. 1992;89:10164–8. doi: 10.1073/pnas.89.21.10164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crowe JE, Jr., Gilmour PS, Murphy BR, Chanock RM, Duan L, Pomerantz RJ, et al. Isolation of a second recombinant human respiratory syncytial virus monoclonal antibody fragment (Fab RSVF2-5) that exhibits therapeutic efficacy in vivo. J Infect Dis. 1998;177:1073–6. doi: 10.1086/517397. [DOI] [PubMed] [Google Scholar]
- 20.Crowe JE, Jr., Murphy BR, Chanock RM, Williamson RA, Barbas CF, 3rd, Burton DR. Recombinant human respiratory syncytial virus (RSV) monoclonal antibody Fab is effective therapeutically when introduced directly into the lungs of RSV-infected mice. Proc Natl Acad Sci U S A. 1994;91:1386–90. doi: 10.1073/pnas.91.4.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson S, Oliver C, Prince GA, Hemming VG, Pfarr DS, Wang SC, et al. Development of a humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus. J Infect Dis. 1997;176:1215–24. doi: 10.1086/514115. [DOI] [PubMed] [Google Scholar]
- 22.Hiatt A, Cafferkey R, Bowdish K. Production of antibodies in transgenic plants. Nature. 1989;342:76–8. doi: 10.1038/342076a0. [DOI] [PubMed] [Google Scholar]
- 23.Marillonnet S, Thoeringer C, Kandzia R, Klimyuk V, Gleba Y. Systemic Agrobacterium tumefaciens-mediated transfection of viral replicons for efficient transient expression in plants. Nat Biotechnol. 2005;23:718–23. doi: 10.1038/nbt1094. [DOI] [PubMed] [Google Scholar]
- 24.Hiatt A, Pauly M. Monoclonal antibodies from plants: a new speed record. Proc Natl Acad Sci U S A. 2006;103:14645–6. doi: 10.1073/pnas.0607089103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strasser R, Stadlmann J, Schähs M, Stiegler G, Quendler H, Mach L, et al. Generation of glyco-engineered Nicotiana benthamiana for the production of monoclonal antibodies with a homogeneous human-like N-glycan structure. Plant Biotechnol J. 2008;6:392–402. doi: 10.1111/j.1467-7652.2008.00330.x. [DOI] [PubMed] [Google Scholar]
- 26.Pogue GP, Vojdani F, Palmer KE, Hiatt E, Hume S, Phelps J, et al. Production of pharmaceutical-grade recombinant aprotinin and a monoclonal antibody product using plant-based transient expression systems. Plant Biotechnol J. 2010;8:638–54. doi: 10.1111/j.1467-7652.2009.00495.x. [DOI] [PubMed] [Google Scholar]
- 27.Piedra PA, Wyde PR, Castleman WL, Ambrose MW, Jewell AM, Speelman DJ, et al. Enhanced pulmonary pathology associated with the use of formalin-inactivated respiratory syncytial virus vaccine in cotton rats is not a unique viral phenomenon. Vaccine. 1993;11:1415–23. doi: 10.1016/0264-410X(93)90170-3. [DOI] [PubMed] [Google Scholar]
- 28.Whaley KJ, Hiatt A, Zeitlin L. Emerging antibody products and Nicotiana manufacturing. Hum Vaccin. 2011;7:349–56. doi: 10.4161/hv.7.3.14266. [Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yusibov V, Streatfield SJ, Kushnir N. Clinical development of plant-produced recombinant pharmaceuticals: vaccines, antibodies and beyond. Hum Vaccin. 2011;7:313–21. doi: 10.4161/hv.7.3.14207. [Review] [DOI] [PubMed] [Google Scholar]
- 30.Zeitlin L, Olmsted SS, Moench TR, Co MS, Martinell BJ, Paradkar VM, et al. A humanized monoclonal antibody produced in transgenic plants for immunoprotection of the vagina against genital herpes. Nat Biotechnol. 1998;16:1361–4. doi: 10.1038/4344. [DOI] [PubMed] [Google Scholar]
- 31.Ko K, Koprowski H. Plant biopharming of monoclonal antibodies. Virus Res. 2005;111:93–100. doi: 10.1016/j.virusres.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 32.Zeitlin L, Pettitt J, Scully C, Bohorova N, Kim D, Pauly M, et al. Enhanced potency of a fucose-free monoclonal antibody being developed as an Ebola virus immunoprotectant. Proc Natl Acad Sci U S A. 2011;108:20690–4. doi: 10.1073/pnas.1108360108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ober RJ, Radu CG, Ghetie V, Ward ES. Differences in promiscuity for antibody-FcRn interactions across species: implications for therapeutic antibodies. Int Immunol. 2001;13:1551–9. doi: 10.1093/intimm/13.12.1551. [DOI] [PubMed] [Google Scholar]
- 34.Shah JN, Chemaly RF. Management of RSV infections in adult recipients of hematopoietic stem cell transplantation. Blood. 2011;117:2755–63. doi: 10.1182/blood-2010-08-263400. [DOI] [PubMed] [Google Scholar]
- 35.Farid SS. Process economics of industrial monoclonal antibody manufacture. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;848:8–18. doi: 10.1016/j.jchromb.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 36.Bendandi M, Marillonnet S, Kandzia R, Thieme F, Nickstadt A, Herz S, et al. Rapid, high-yield production in plants of individualized idiotype vaccines for non-Hodgkin’s lymphoma. Ann Oncol. 2010;21:2420–7. doi: 10.1093/annonc/mdq256. [DOI] [PubMed] [Google Scholar]
- 37.Heard C, Brams P, Walsh E, Huynh T, Chamat S, Reff M, et al. Two neutralizing human anti-RSV antibodies: cloning, expression, and characterization. Mol Med. 1999;5:35–45. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


