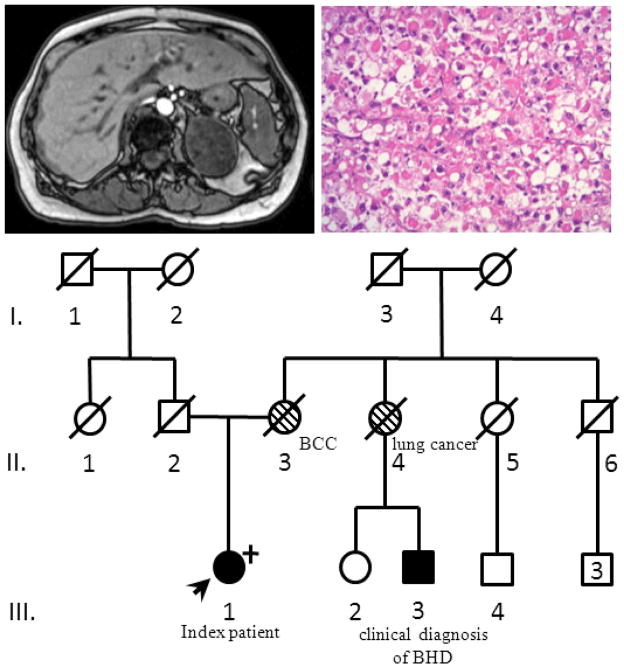
A 62-year old female patient was initially diagnosed with a histologically confirmed trichodiscoma. Due to the association of trichodiscomas with Birt-Hogg-Dubé Syndrome (BHD), the patient underwent screening for renal tumors, which revealed a heterogeneous right adrenal mass (Figure 1a). The patient did not have any clinical hormone excess. Adrenalectomy revealed a tumor measuring 6.2cm and weighing 104g with features diagnostic for an adrenal oncocytoma (Figure 1b). While the tumor did not fulfill the established criteria for malignancy in oncocytic adrenal neoplasms, the borderline Ki67 labeling of 5% suggested the diagnosis of at least uncertain malignant behavior rather than a benign lesion 1. The traditional Weiss scoring system to differentiate benign and malignant lesion fails in oncocytomas as they almost invariably are positive for at least two of the criteria, leading to overdiagnosis of malignant lesions 1.The patient remains disease free 24 months following surgery. Review of a chest CT imaging revealed two small cysts. The patient’s family history was suggestive of BHD, with a maternal cousin with a history of spontaneous pneumothorax (Figure 1c). The patient’s mother had a diagnosis of basal cell carcinoma and one maternal aunt died of lung cancer. The patient’s father died of pancreatic cancer. The patient underwent germline genetic testing, revealing a deleterious mutation in exon 11 of FLCN (c.1252delC; p.Leu418TrpfsX50) leading to a premature stop codon and confirming the diagnosis of BHD. Unfortunately, insufficient tumor tissue precluded loss of heterozygosity analysis.
Figure 1.
(a) heterogeneous adrenal mass on T1 weighted MRI. (b) oncocytic adrenal neoplasm with diffuse growth pattern. (c) Pedigree. Proband (III.1) indicated with the arrow. A maternal cousin fulfilled clinical criteria for BHD (III.3), making the patient’s mother (II.3) and aunt (II.4) obligate carriers.
Adrenocortical tumors, benign and malignant, are well known to occur as part of hereditary cancer susceptibility syndromes2. Adrenocortical carcinoma (ACC) is a core malignancy of Li Fraumeni syndrome. ACC and adrenocortical adenomas are observed in Beckwith Wiedemann syndrome, Multiple Endocrine Neoplasia type 1 and Familial Adenomatous Polyposis. Oncocytic adrenal tumors are rare, have biological behavior which can be difficult to predict and have not been reported in association with hereditary syndromes1.
BHD was first described in 1977 with the classical manifestations of benign hair follicle associated tumors. In the decades to follow, BHD has been defined as an autosomal dominant hereditary syndrome, characterized by the cutaneous triad of fibrofolliculomas, trichodiscomas and acrochordons, lung cysts and renal tumors. BHD is caused by mutations in FLCN (Online Mendelian Inheritance in Man #13510, http://omim.org/entry/135150, for photograph of typical skin lesions see Ref. 3). Renal oncocytomas, as well as chromophobe and mixed chromophobe oncocytic adrenal tumors, are typical for BHD 3.
In order to explore an adrenal phenotype in BHD patients, we identified 14 patients from 11 unique families with genetically confirmed FLCN mutations in the University of Michigan Cancer Genetics Registry. A stable 1.2cm adrenal nodule was observed in a 22 year old male patient. Next, we retrospectively reviewed 359 patients diagnosed with ACC using the Michigan Endocrine Oncology Repository. None of the 359 patients had reported history of trichodiscoma or fibrofolliculoma. One patient reportedly had 2 lung cysts sized 1.2cm. Three patients had histories of renal tumors, including one oncocytoma. Five patients reported first-degree relatives with kidney cancer. No patient fulfilled clinical criteria for BHD, had undergone germline genetic testing for FLCN mutations, or had a reported family history of BHD. In summary, adrenal tumors may be more common in BHD patients, but BHD is not common amongst ACC patients.
Review of the literature yielded three reports of adrenal tumors in BHD patients. First, a study of 23 BHD patients with confirmed FLCN mutations presenting with lung cysts reported one patient with an adrenal nodule4. This adrenal nodule was small (1.5 x 2.0cm) and non-hormone-secreting (personal communication Dr. Seyama). Second, Juszczak et al. reported a large oncocytic adrenal tumor in a 36 year old female patient with BHD and confirmed FLCN mutation 5. The third report of an adrenal tumor describes a presumed ectopic renal tumor in the adrenal gland of a BHD patient 6.
Our case, together with the case described by Juszczak et al,. suggest that adrenal oncocytomas might be part of the BHD tumor spectrum. With the exception of trichodiscoma, fibrofolliculoma and typical kidney tumors, no specific association of other tumors with BHD has been proven, despite several reports of benign and malignant tumors in BHD patients3. As is often the case with rare cancer susceptibility syndromes, predisposition to tumors other than the core malignancies is not well-defined. Furthermore, the phenotype of BHD can be subtle, leading to underdiagnosis. Suspicion of BHD should be raised in patients with adrenal oncocytomas. Dermatologic exam and review of chest imaging can reveal other hallmark clinical features of BHD.
Acknowledgments
We appreciate Dr. Seyama’s interest in sharing information on the patient with an adrenal tumor he had reported in a recent case series. Tobias Else is supported by NIH T32-DK007245.
Footnotes
Disclosure Statement: The authors have nothing to disclose
References
- 1.Bisceglia M, Ludovico O, Di Mattia A, Ben-Dor D, Sandbank J, Pasquinelli G, Lau SK, Weiss LM. Adrenocortical oncocytic tumors: report of 10 cases and review of the literature. Int J Surg Pathol. 2004;12:231–243. doi: 10.1177/106689690401200304. [DOI] [PubMed] [Google Scholar]
- 2.Else T. Association of adrenocortical carcinoma with familial cancer susceptibility syndromes. Mol Cell Endocrinol. 2012;351:66–70. doi: 10.1016/j.mce.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Menko FH, van Steensel MA, Giraud S, Friis-Hansen L, Richard S, Ungari S, Nordenskjold M, Hansen TV, Solly J, Maher ER. Birt-Hogg-Dube syndrome: diagnosis and management. Lancet Oncol. 2009;10:1199–1206. doi: 10.1016/S1470-2045(09)70188-3. (This publication contains clinical photographs of the typical skin lesions found in BHD Syndrome) [DOI] [PubMed] [Google Scholar]
- 4.Juszczak A, Halliday D, Mihai R, Ali A. European Congress of Endocrinology. Rotterdam, Netherlands: 2011. A large adrenal tumour as a phenotypic manifestation of the Birt–Hogg–Dube syndrome; p. P58. Endocrine Abstracts. [Google Scholar]
- 5.Kunogi M, Kurihara M, Ikegami TS, Kobayashi T, Shindo N, Kumasaka T, Gunji Y, Kikkawa M, Iwakami S, Hino O, Takahashi K, Seyama K. Clinical and genetic spectrum of Birt-Hogg-Dube syndrome patients in whom pneumothorax and/or multiple lung cysts are the presenting feature. J Med Genet. 2010;47:281–287. doi: 10.1136/jmg.2009.070565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reese E, Sluzevich J, Kluijt I, Teertstra HJ, De Jong D, Horenblas S, Ryu J. Birt-Hogg-Dube Syndrome. 2009 [PubMed] [Google Scholar]