Introduction
A personal history of excessive mucocutaneous bleeding is a key component in the diagnosis of a number of mild bleeding disorders, including von Willebrand disease (VWD), platelet function disorders (PFD), and coagulation factor deficiencies. However, the evaluation of hemorrhagic symptoms is a well-recognized challenge for both patients and physicians, because the reporting and interpretation of bleeding symptoms is subjective. Significant symptoms may be overlooked because they are considered normal and minimal or trivial symptoms may be given undue consideration. The risk of this second issue is highlighted by the high frequency of bleeding symptoms reported by the general population.[1,2] In response to these challenges, a number of attempts have been made to standardize bleeding histories in an effort to 1) improve diagnostic accuracy and thus avoid unwarranted laboratory testing, 2) predict the risk of bleeding in a individual patient, 3) describe symptom severity and 4) inform treatment. In this paper, we will review the evolution of bleeding assessment tools, review the published literature focusing on the application of these tools and discuss remaining challenges.
Bleeding Assessment Tools (BATs)
Over the years, multiple investigators have made attempts to standardize bleeding histories by identifying questions that best distinguish between affected and unaffected individuals. In 1995, Sramek and colleagues published their experience with a bleeding questionnaire that was administered to patients known to have a bleeding disorder and a group of normal controls.[3] The most informative questions in terms of discrimination were about bleeding following traumatic events such as tonsillectomy or dental extraction (but not childbirth) and the presence of a bleeding disorder in a family member. Interestingly, these questions were only discriminatory in a screening setting, not in a referral setting perhaps because a referral population is comprised of a pre-selected group of individuals with highly prevalent symptoms. In 2005, the International Society on Thrombosis and Haemostasis (ISTH) Scientific and Standardization Committee (SSC) on Von Willebrand factor (VWF) established a set of provisional criteria for the diagnosis of VWD type 1 including the threshold that must be met for mucocutaneous bleeding symptoms to be considered significant.[4] Since that time, the field has increasingly focused on quantitative assessments of bleeding, and on the need for standardization.
Vicenza-based BATs
Building on the ISTH provisional criteria, a group of investigators from Vicenza, Italy, led by Rodeghiero, developed and validated a BAT for the diagnosis of Type 1 VWD in a primarily adult population.[5] Each bleeding symptom is scored from 0 (absence or trivial symptoms) to 3 (symptom requiring medical intervention) and the overall bleeding score is determined by summing the scores for all of the bleeding symptoms. The results of this study showed that having at least three hemorrhagic symptoms or a bleeding score of 3 in males and 5 in females was very specific (98%) for the bleeding history of type 1 VWD, although less sensitive (69%).
In an attempt to improve the sensitivity of this bleeding score, the scoring system was revised to increase the range of possible grades from -1 (absence of bleeding after significant hemostatic challenge such as two dental extractions or surgeries) to 4 (symptoms requiring the most significant medical intervention such as infusion of clotting factor concentrates or surgery to control bleeding).[6] This -1 to 4 version was used for the European Molecular and Clinical Markers for the Diagnosis and Management of type 1 VWD (MCMDM-1 VWD) Study and the resultant bleeding score was shown to be strongly inversely correlated with VWF level (p<0.001 based on 3 multiple regression models). Additionally, higher bleeding scores were associated with an increasing likelihood of VWD and scores specifically related to spontaneous mucocutaneous bleeding predicted an increased risk of future bleeding following surgery or dental extraction.
A condensed version of the MCMDM1-VWD Bleeding Questionnaire was then developed by removing all of the details from the full version that do not directly affect the bleeding score. This version was then prospectively analysed in three studies: one in the primary care setting and two in referral populations. In the primary care setting, the Condensed MCMDM-1VWD Bleeding Questionnaire showed a sensitivity of 100%, specificity of 87%, positive predictive value (PPV) of 0.20 and negative predictive value of 1 for the diagnosis of VWD. Inter-observer reliability was confirmed by two observers who administered the questionnaire an average of three months apart (Interclass Correlation Coefficient = 0.81, p<0.001)[7] In a study published by Tosetto et al in 2011, the same condensed bleeding questionnaire was evaluated in a referral population.[8] The data showed that the sensitivity for a mild bleeding disorder varied widely depending on the reason for referral (25 – 47%). The specificity ranged from 81 – 98% in the different referral groups, and the PPV was 0.03 – 0.78. The NPV was again shown to be high (0.82 – 0.99) meaning that a negative or normal bleeding score can help exclude a clinically significant inherited bleeding disorder.[8] The Condensed MCMDM-1VWD Bleeding Questionnaire was also studied in a group of 30 women presenting with menorrhagia and was able to distinguish those with a bleeding disorder from those without a bleeding disorder (sensitivity 85%, specificity 90%, PPV 0.89, NPV 0.86) and was also able to distinguish disease severity; women with Type 3 VWD had the highest bleeding scores. [9]
As mentioned above, an additional area of interest for research involving bleeding quantitation lies in differentiating bleeding severity between different disorders. The data in Table 1 show that in general, mucocutaneous bleeding symptoms are reported more frequently by patients with Type 3 VWD compared with Type 2 and Type 1 VWD patients, although there is a great deal of overlap. Interesting work has evaluated these subtype differences by comparing bleeding symptoms between Type 3 VWD obligate carriers (OC) and normal controls. Type 3 OC reported more epistaxis, cutaneous bleeding and post-surgical bleeding than normal controls further highlighting the heterogeneity of symptoms in VWD.[10]
Table 1.
| Symptoms | Normals n=500 n=341 n=215 | All types VWD n=264 | Type 1 VWD n=671 n=84 | Type 2 VWD n=497 | Type 3 VWD n=348 n=66 |
|---|---|---|---|---|---|
| Epistaxis | 5–11 | 63 | 54–61 | 63 | 66–77 |
| Menorrhagia | 17–44 | 60 | 32–67 | 32 | 56–69 |
| Post-dental extraction bleeding | 5–11 | 52 | 31–72 | 39 | 53–77 |
| Haematomas | 12 | 49 | 13 | 14 | 33 |
| Bleeding from minor wounds | 0.2–5 | 36 | 36–46 | 40 | 50 |
| Gum bleeding | 7–37 | 35 | 31 | 35 | 56 |
| Post-surgical bleeding | 1–6 | 28 | 20–38 | 23 | 41 |
| Postpartum bleeding | 3–23 | 23 | 17–61 | 18 | 15–26 |
| Gastrointestinal bleeding | 1 | 14 | 5 | 8 | 19.2 |
| Joint bleeding | 6 | 8 | 3 | 4 | 37–45 |
| Haematuria | 1–8 | 7 | 2 | 5 | 1–12 |
| Cerebral bleeding | n/a | n/a | 1 | 2 | 9 |
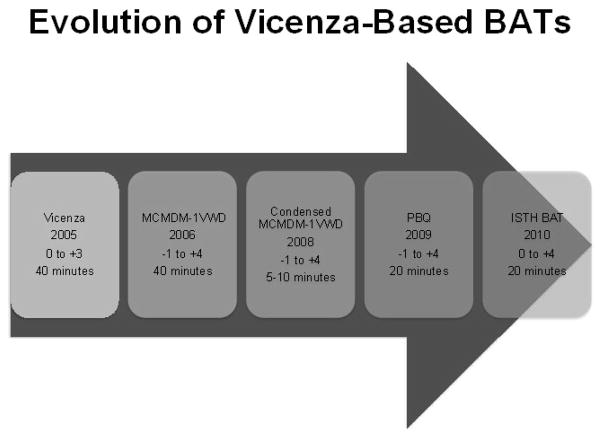
In order to consolidate the knowledge learned from these published studies and the work described below in pediatrics, and to develop a consensus bleeding assessment tool, a Working Party sponsored by the VWF and Perinatal/Pediatric Hemostasis Subcommittees of the ISTH/SSC was established in 2008. This group, with input from the Women’s Health Issues in Thrombosis and Haemostasis SSC, published the ISTH-BAT in 2010. [11] Studies to validate this new tool are ongoing, including the necessary psychometric evaluations. Criticisms of the previously published BATs are based on the scoring of the worst single bleeding episode; as a result there is a lack of accounting for the frequency of bleeding symptoms and a plateau effect is seen if the questionnaire is administered to individuals with severe bleeding disorders. The ISTH-BAT was specifically designed to extend the utility of the earlier BATS by incorporating information on both symptom frequency and severity. A web-based version of the ISTH-BAT is freely available through Rockefeller University with the objective of encouraging investigators to share data (https://bh.rockefeller.edu/ISTH-BATR/). The evolution of the Vicenza-based BATs can be found in Figure 1, a review of the primary publications in Table 2 and a comparison of the different scoring systems in Table 3.
Figure 1.
Shows the original “Vicenza” bleeding score on the left and its subsequent versions over time. The year of publication of the original manuscript is shown, as is the scoring system used and the approximate time of administration.
Table 2.
Primary Vicenza-based and Other Bleeding Assessment Tools
| Lead Author | Tool | Study Population | Sample Size | Scoring System | Ages Studied mean/median, yrs (range) |
|---|---|---|---|---|---|
| Rodeghiero[5] | “Vicenza” Bleeding Questionnaire | Type 1 VWD obligatory carriers (OC) | 341 | semi-quantitative | 45 (n/a) |
| Tosetto[6] | MCMDM-1VWD Bleeding Questionnaire | Type 1 VWD | 907 | quantitative | 37 (1 – 91) |
| Bowman[7] | Condensed MCMDM-1VWD Bleeding Questionnaire | Prospectively investigated for VWD and Type 1, 2 and 3 VWD | 259 | quantitative | 40 (11 – 81) |
| Mauer[2] | RU-BHQ (Rockefeller University –Bleeding History Questionnaire) | Normals | 500 | qualitative | 43 (19 – 86) |
| Higham[20] | PBAC (Pictorial Bleeding Assessment Chart) | Menorrhagia | 28 | quantitative | 39 (27 – 48) |
| Philipp[22] | Screening tool | Menorrhagia | 217 | qualitative | 39 (13 – 53) |
| McKay[13] | CHAT (Clinical History Assessment Tool) | Quebec Platelet Disorder | 127 | quantitative | 34 (1 – 91) |
Table 3.
Comparison of Scoring Systems
The green rows show the scoring system for the Vicenza Bleeding Questionnaire, the blue rows for the Pediatric Bleeding Questionnaire, the white rows for the Condensed MCMDM – 1 VWD bleeding score, and the grey rows show the scoring used for the ISTH-BAT score. [5,7,11,26]
| Score | −1 | 0 | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|---|
| Symtpom | ||||||
| Epistaxis | - | No or trivial | Present | Packing, cauterization | Blood transfusion or Replacement therapy | |
| - | No or trivial (≤ 5) | >5 OR more than 10′ | Consultation only | Packing or Cauterization or Antifibrinolytics | Blood transfusion or Replacement therapy or Desmopressin | |
| -- | No or trivial | > 5 or more than 10′ | Consultation only | Packing or cauterization | Blood transfusion or replacement | |
| No/trivial | - > 5/year or more than 10′ | Consultation only | Packing or cauterization or antifibrinolytic | Blood transfusion or replacement therapy (use of hemostatic blood components and rFVIIa) or desmopressin | ||
| Cutaneous | - | No or trivial | Petechiae or bruises | Hematomas | Consultation | |
| No or trivial (< 1cm) | > 1cm AND no trauma | Consultation only | ||||
| -- | No or trivial | > 1 cm and no trauma | Consultation only | -- | -- | |
| No/trivial | For bruises 5 or more (> 1cm) in exposed areas | Consultation only | Extensive | Spontaneous hematoma requiring blood transfusion | ||
| Minor Wounds | - | No or trivial | Present (1–5 episodes/year) | Consultation | Surgical hemostasis | |
| No or trivial (< 5) | > 5 OR more than 5′ | Consultation only or Steri-strips | Surgical hemostasis or Antifibrinolytics | Blood transfusion or Replacement therapy or Desmopressin | ||
| -- | No or trivial | > 5 or more than 5′ | Consultation only | Surgical hemostasis | Blood transfusion or replacement therapy or desmopressin | |
| No/trivial | - > 5/year or more than 10′ | Consultation only | Surgical hemostasis | Blood transfusion, replacement therapy, or desmopressin | ||
| Oral Cavity | - | No or trivial | Present | Consultation only | Surgical hemostasis/Blood transfusion | |
| No | Reported at least one | Consultation only | Surgical hemostasis or Antifibrinolytics | Blood transfusion or Replacement therapy or Desmopressin | ||
| -- | No | Referred at least one | Consultation only | Surgical hemostasis or antifibrinolytic | Blood transfusion or replacement therapy or desmopressin | |
| No/trivial | Present | Consultation only | Surgical hemostasis or antifibrinolytic | Blood transfusion, replacement therapy or desmopressin | ||
| Menorrhagia | - | No or trivial | Present | Consultation, pill use, iron therapy | Blood transfusion, hysterectomy, D&C | |
| No | Reported or consultation only | Antifibrinolytics or Pill use | D&C, Iron therapy | Blood transfusion or Replacement therapy or Desmopressin or Hysterectomy | ||
| -- | No | Consultation only | Antifibrinolytics, pill use | Dilation & curettage, iron therapy, ablation | Blood transfusion or replacement therapy or desmopressin or hysterectomy | |
| No/trivial | Consultation only* or - Changing pads more frequently than every 2 hours or - Clot and flooding or - PBAC score>100# | - Time off work/school > 2/year or - Requiring antifibrinolytics or hormonal or iron therapy | - Requiring combined treatment with antifibrinolytics and hormonal therapy or - Present since menarche and > 12 months | - Acute menorrhagia requiring hospital admission and emergency treatment or - Requiring blood transfusion, Replacement therapy, Desmopressin, or - Requiring dilatation & curretage or endometrial ablation or hysterectomy) | ||
| Post-partum | - | No or trivial | Present, iron therapy | Blood transfusion, D&C, suturing | Hysterectomy | |
| no bleeding in at least 2 deliveries | No deliveries or no bleeding in 1 delivery | Reported or consultation only | D&C, Iron therapy, Antifibrinolytics | Blood transfusion or Replacement therapy or Desmopressin | Hysterectomy | |
| No bleeding in at least 2 deliveries | None done or no bleeding in 1 delivery | Consultation only | Dilation & curettage, iron therapy, antifibrinolytics | Blood transfusion or replacement therapy or desmopressin | Hysterectomy | |
| No/trivial or no deliveries | Consultation only* or - Use of syntocin or - Lochia > 6 weeks | - Iron therapy or - Antifibrinolytics | - Requiring blood transfusion, replacement therapy, desmopressin or- Requiring examination under anaesthesia and/or the use of uterin balloon/package to tamponade the uterus | - Any procedure requiring critical care or surgical intervention (e.g. hysterectomy, internal iliac artery legation, uterine artery embolization, uterine brace sutures) | ||
| Muscle hematoma | - | No or trivial | Present | Consultation only | Blood transfusion, surgery | |
| Never | Post trauma, no therapy | Spontaneous, no therapy | Spontaneous or traumatic, requiring desmopressin or replacement therapy | Spontaneous or traumatic, requiring surgical intervention or blood transfusion | ||
| Never | Post trauma, no therapy | Spontaneous, no therapy | Spontaneous or traumatic, requiring desmopressin or replacement therapy | Spontaneous or traumatic, requiring surgical intervention or blood transfusion | ||
| Never | Post trauma, no therapy | Spontaneous, no therapy | Spontaneous or traumatic, requiring desmopressin or replacement therapy | Spontaneous or traumatic, requiring surgical intervention or blood transfusion | ||
| Gastrointestinal tract | - | No or trivial | Present | Consultation only | Surgery/Blood transfusion | - |
| No | Identified cause | Consultation or Spontaneous | Surgical hemostasis, Antifibrin., Blood transf., Replacement therapy, Desmopressin | |||
| -- | No | Associated with ulcer, portal hypertension, hemorrhoids, angiodysplasia | Spontaneous | Surgical hemostasis, blood transfusion, replacement therapy, desmopressin, antifibrinolytic | -- | |
| No/trivial | Present (not associated with ulcer, portal hypertension, hemorrhoids, angiodysplasia) | Consultation only* | Surgical hemostasis, antifibrinolytic | Blood transfusion, replacement therapy or desmopressin | ||
| Hematuria | ||||||
| No/trivial | Present (macroscopic) | Consultation only* | Surgical hemostasis, | Blood transfusion, replacement therapy or desmopressin | ||
| Tooth extraction | - | No or trivial | Present | Suturing or packing | Blood transfusion | |
| No bleeding in at least 2 extractions | None done or no bleeding in 1 | Reported, no consultation | Consultation only | Resuturing, Repacking or Antifibrinolytics | Blood transfusion or Replacement therapy or Desmopressin | |
| No bleeding in at least 2 extractions | None done or no bleeding in 1 extraction | Reported, no consultation * | Consultation only | Resuturing or packing | Blood transfusion or replacement therapy or desmopressin | |
| No/trivial or none done | Reported in <25% of all procedures, no intervention* | Reported in >25% of all procedures, no intervention** | Resuturing or packing | Blood transfusion, replacement therapy or desmopressin | ||
| Surgery | - | No or trivial | Present | Suturing or resurgery | Blood transfusion | |
| No bleeding in at least 2 surgeries | None done or no bleeding in 1 | Reported, no consultation | Consultation only | Surgical hemostasis or Antifibrinolytic | Blood transfusion or Replacement therapy or Desmopressin | |
| No bleeding in at least 2 surgeries | None done or no bleeding in 1 surgery | Reported, no consultation | Consultation only | Surgical hemostasis or antifibrinolytic | Blood transfusion or replacement therapy or desmopressin | |
| No/trivial or none done | Reported in <25% of all procedures, no intervention** | Reported in >25% of all procedures, no intervention** | Surgical hemostasis or antifibrinolytic | Blood transfusion, replacement therapy or desmopressin | ||
| Hemarthrosis | - | No or trivial | Present | Consultation only | Blood transfusion, surgery | - |
| Never | Post trauma, no therapy | Spontaneous, no therapy | Spontaneous or traumatic, requiring desmopressin or replacement therapy | Spontaneous or traumatic, requiring surgical intervention or blood transfusion | ||
| Never | Post trauma, no therapy | Spontaneous, no therapy | Spontaneous or traumatic, requiring desmopressin or replacement therapy | Spontaneous or traumatic, requiring surgical intervention or blood transfusion | ||
| Never | Post trauma, no therapy | Spontaneous, no therapy | Spontaneous or traumatic, requiring desmopressin or replacement therapy | Spontaneous or traumatic, requiring surgical intervention or blood transfusion | ||
| Central nervous system | ||||||
| Never | - | - | Subdural, any intervention | Intracerebral, any intervention | ||
| Never | - | - | Subdural, any intervention | Intracerebral, any intervention | ||
| Never | - | - | Subdural, any intervention | Intracerebral, any intervention | ||
| Other Bleedings | ||||||
| No | Reported | Consultation only | Surgical hemostasis, Antifibrinolytics, Iron therapy | Blood transfusion or Replacement therapy or Desmopressin | ||
| No/trivial | Present | Consultation only* | Surgical hemostasis, antifibrinolytics | Blood transfusion or replacement therapy or desmopressin |
Other Bleeding Assessment Tools
In addition to the BATs derived from the Italian group’s work, a number of other tools have been developed and published. A comprehensive ontology-backed system was developed at Rockefeller University (RU-BHQ; Rockefeller University – Bleeding History Questionnaire) that facilitates the collection and collation of detailed, standardized bleeding histories.[12] This bleeding questionnaire is web-based and freely available. To date, the results of the administration of this questionnaire to 500 normal individuals has been reported [2] and data collection on individuals with Type 1 VWD is ongoing. Disease-specific tools have also been studied including a questionnaire specific for the Quebec Platelet Disorder.[13]
Menorrhagia-Specific Tools
Studies have shown that up to 5 – 10% of women seek medical attention for heavy menstrual periods at some point during their reproductive life[14] and that up to 15% of those have an underlying bleeding disorder.[15–18] Despite this, the average delay from onset of bleeding symptoms to the diagnosis of a bleeding disorder has been reported to be 16 years.[19] Additionally, as can be seen in Table 1, menorrhagia is the second most commonly reported bleeding symptom overall by patients with VWD, and the most commonly reported symptom by women. Therefore, tools designed specifically for the assessment of patients with menorrhagia are valuable. The Pictorial Bleeding Assessment Chart (PBAC) allows women to track the number of pads or tampons used for a menstrual period as well as the degree of soiling. Based on that information, a score is generated and PBAC scores ≥100 correlate with menorrhagia as defined as ≥80 mls of menstrual blood loss. [20] More recently, a screening tool for bleeding disorders in women with menorrhagia was developed and tested by Phillipp et al [21,22] on a population of women with PBAC scores ≥100 and normal pelvic exams. The tool, which consists of 11 questions about bleeding symptoms and family history, has a sensitivity of 89% for a bleeding disorder. This was improved to 93% by adding iron deficiency and 95% when the PBAC score was increased to >185. An important detail about this study though, is that of the 217 women enrolled, 154 had a bleeding disorder (which is much higher than the published prevalence of a bleeding disorder in other studies) raising concern about the widespread applicability of the results. A review of these tools can be found in Table 2.
Pediatric Bleeding Assessment Tools
Assessing bleeding symptoms in children presents unique challenges. The issue of overlap of symptoms between normal individuals and those affected with mild bleeding disorders also exist in children, particularly for bruising and epistaxis. An additional consideration is that bleeding symptoms manifest in children in distinctly different ways compared with adults. Some of the classic bleeding symptoms in adults (ie: menorrhagia, post-surgical bleeding) are clearly not prevalent in the pediatric population. A child with a bleeding disorder may not have had surgery, nor (in the case of girls) reached the age of menarche, however, may still have symptoms that cause difficulty and merit treatment. For example, umbilical stump bleeding or bleeding at the time of circumcision may be important early markers of a bleeding disorder, but may be overlooked and not investigated. In order to address these issues, tools have been developed that are specific to pediatrics.
An Epistaxis Scoring System (ESS) was published in 1988 by Katsanis et al.[23] This scoring system results in a child with recurrent nosebleeds being classified as either ‘mild’ or ‘severe’ based on characteristics such as frequency and duration of epistaxis. Children classified as ‘severe’ were more likely to have a family history suggestive of a bleeding diathesis, to be anemic and iron deficient, to have undergone nasal cauterization and to have laboratory coagulation abnormalities identified. In 2000, the hemostasis research group from the Hospital for Sick Children in Toronto, Ontario published their “in house” pediatric bleeding assessment tool [24] and followed this up in 2004 with a second publication confirming the reliability and reproducibility of this questionnaire.[25] After administration of this bleeding questionnaire, children are classified as either ‘bleeders’ or ‘non-bleeders’ depending on whether or not any one of a number of mucocutaneous bleeding symptoms met the criteria to be considered significant (ie: recurrent nose bleeds requiring medical treatment or leading to anemia). This questionnaire was compared with the ISTH provisional consensus criteria for significant mucocutaneous bleeding in a group of children with VWD and was found to be less stringent and therefore perhaps more useful in a pediatric setting.[24]
As a result of the endorsement of the Vicenza-based questionnaires by ISTH, and with the goal of standardization across a range of ages, Bowman et al created the PBQ (Pediatric Bleeding Questionnaire) by adding pediatric-specific bleeding symptoms to the MCMDM1-VWD Bleeding Questionnaire, maintained the same scoring system and tested it in a variety of settings.[26] Their work showed that the PBQ had a sensitivity of 83% and a specificity of 79% for VWD. Additionally, the positive predictive value was low at 0.14, but the negative predictive value was very high at 0.99, making this an effective tool to decide which children do not require blood tests. The receiver operating characteristic (ROC) curve was very good, with an area under the curve of 0.88 (p=0.002), showing that the PBQ can accurately distinguish affected and unaffected children. A review of the main pediatric bleeding questionnaires can be found in Table 4. Subsequently, the PBQ was also tested in children previously known to have an inherited bleeding disorder and was able to: 1) distinguish disease severity in children with different subtypes of VWD (p<0.0001), and 2) highlight age-related increases in bleeding scores in VWD as bleeding challenges are encountered with increasing age. [27] The PBQ was also used to identify the pattern of bleeding symptoms in children with platelet function disorders.[28]
Table 4.
Pediatric-Specific Bleeding Assessment Tools
| Lead Author | Tool | Study Population | Sample Size | Scoring System | Ages Studied mean/median, yrs (range) |
|---|---|---|---|---|---|
| Katsanis[23] | ESS (Epistaxis Severity Score) | recurrent epistaxis | 36 | semi-quantitative | 8.8 (3 – 16) |
| Dean[24] | The Hospital for Sick Children Bleeding Questionnaire PBQ (Pediatric | VWD | 158 | semi-quantitative | 9.4 (1.5 – 18) |
| Bowman[26] | Bleeding Questionnaire) | Prospectively investigated for VWD | 151 | quantitative | 8.3 (6 mos – 17) |
Three independent studies have evaluated the diagnostic utility of the PBQ since its original publication, and while two confirmed its efficacy [29,30], the third did not although their methods of analysis differed.[31]
Evaluating Clinical Utility
When evaluating the clinical utility of the various bleeding assessment tools, it is critically important to keep in mind the specific objective and setting of use. In general, the tools reviewed in this report have been directed towards two main clinical objectives: 1) to act as a screening tool in both the primary and tertiary care settings for individuals being investigated for the first time for an inherited bleeding disorder and 2) to act as a standardized way of describing disease characteristics and of assessing disease severity.
With regards to using BATs as a screening tool for bleeding disorders, it is important to recognize how specific study populations can affect the results, particularly for sensitivity. This is important if symptoms from individuals known to have a bleeding disorder are included after diagnosis, when prophylactic treatments might have been given. Each of the primary Vicenza-based publications dealt with this potential source of bias in different ways; in the original Rodeghiero 2005 publication obligate carriers of Type 1 VWD (rather than index cases) were studied eliminating the possibility of increasing the sensitivity by studying known bleeders.[5] In the 2006 Tosetto paper (full MCMDM-1 VWD), only bleeding symptoms present before the diagnosis of Type 1 VWD were used to compute the bleeding score (or symptoms from individuals who did not receive hemostatic prophylaxis).[6] In both the Bowman 2008 (Condensed MCMDM-1 VWD) and Bowman 2009 (PBQ) studies, individuals presenting for the first time for investigation of VWD were included and lab levels of VWF and FVIII were used as the diagnostic gold standard.[7,26] Additionally, specificity can be affected by the definition of controls; the 2005 Rodeghiero paper used age and gender matched controls that were in good health and had never been referred for evaluation of hemorrhagic symptoms. Normal lab testing was not required.[5] Controls in all three of the other primary Vicenza-based publications were healthy individuals who had never sought medical attention for bleeding symptoms and who had normal VWF levels.[6,7,26]
Undoubtedly, the main focus of the Vicenza-based BATs presented in this review has been VWD but there are a few notable exceptions. The Condensed MCMDM-1 VWD Bleeding Questionnaire has been studied prospectively as a screening tool for platelet function disorders and the sensitivity, specificity, PPV and NPV are 86%, 65%, 0.50 and 0.92 respectively.[32] The 2011 Tosetto paper (which used the Condensed MCMDM-1 VWD Bleeding Questionnaire) evaluated newly referred patients for all hemorrhagic disorders and presents data on VWD, platelet function disorders and FXI deficiency as well as senile purpura and Rendu-Weber-Osler disease.[8] The analysis of diagnostic utility for this study was reviewed previously on page 5. This paper concludes that BATs in conjunction with the aPTT (activated partial thromboplatin time) improve the evaluation of patients suspected of having a mild bleeding disorder even in a low prevalence setting.[8] Finally, as mentioned, the Pediatric Bleeding Questionnaire has been studied in 23 children with platelet function defects; this is purely a descriptive study and analysis of diagnostic utility was not performed.[28]
The original Vicenza bleeding questionnaire was designed to be used before diagnosis, however as mentioned, a number of studies have been performed evaluating the performance of these tools as a standardized way of describing disease severity. Of critical importance for this indication is the impact of the diagnosis of a bleeding disorder on the natural history of the disease. Following diagnosis, patients are typically given hemostatic prophylaxis prior to invasive procedures or surgeries and investigators need to take care not to include these treatments in the calculated bleeding score. Failure to do so will result in false elevations of the overall bleeding score.
There are differences in the published studies reviewed here because of heterogeneity of patient populations and methods of analysis, but in general our ability to predict who is NOT going to bleed is far superior to our ability to predict who is going to bleed. In some settings this may be useful, however in others it challenges us to continue to work to optimize our tools. It is plausible that the expectation that one bleeding assessment tool can serve both clinical objectives well in a variety of clinical settings is far too ambitious. Additionally, many of the existing tools are too long to be of value in a busy clinical practice, and additional study is also required to identify the most discriminatory questions from the perspective of screening, and the most useful questions in terms of assessing disease severity.
On-going Challenges and Future Directions
Despite the well-recognized ideal of standardization of bleeding assessment tools, we have reviewed at least 10 different versions in this report, most with multiple independent publications. Additionally, the best scoring system, even amongst the Vicenza-based BATs remains the subject of debate. To date two publications have addressed this issue; comparing the 0 to +3 with the −1 to +4 scoring system. Neither publication showed clear superiority of one over the other, although when used as a screening tool, particularly in the pediatric population, eliminating the −1 scores was advantageous.[33,34] Further study is necessary to definitively resolve the debate.
An additional issue, particularly for children who have not experienced hemostatic challenges, is the long-term clinical behaviour of patients assessed by BATs. The tools are useful to predict the diagnosis of VWD but studies evaluating whether or not the tools can directly predict future bleeding episodes are lacking. This may be less of a concern for bleeding scores in the adult population, where the clinical behaviour of accumulated exposures to hemostatic challenges is captured.
Our ability to address critical clinical questions such as how to optimize treatment based on the risk of bleeding for various situations is dependent on studies with significant sample sizes. One potential approach to this challenge is to create a system that would allow the merging of existing datasets, rather than setting out to undertake additional prospective studies. Such an approach is currently underway, utilizing the bioinformatics capabilities at Rockefeller University. Through international collaboration, it is possible that our collective legacy data could help direct our future treatment protocols. Ultimately, the goal of this field is to improve care for individuals with inherited bleeding disorders. We envision a web-based system, accessible by interested researchers and clinicians that presents the best questions based on extensive study in the most efficient manner no matter the clinical setting or patient presenting complaint.
References
- 1.Sadler JE. Von Willebrand disease type 1: a diagnosis in search of a disease. Blood. 2003;101:2089–93. doi: 10.1182/blood-2002-09-2892. [DOI] [PubMed] [Google Scholar]
- 2.Mauer AC, Khazanov NA, Levenkova N, Tian S, Barbour EM, Khalida C, Tobin JN, Coller BS. Impact of sex, age, race, ethnicity and aspirin use on bleeding symptoms in healthy adults. Journal of thrombosis and haemostasis. 2011;9:100–8. doi: 10.1111/j.1538-7836.2010.04105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Srámek A, Eikenboom JC, Briët E, Vandenbroucke JP, Rosendaal FR. Usefulness of patient interview in bleeding disorders. Archives of internal medicine. 1995;155:1409–15. [PubMed] [Google Scholar]
- 4.Sadler JE, Rodeghiero F. Provisional criteria for the diagnosis of VWD type 1. Journal of thrombosis and haemostasis. 2005;3:775–7. doi: 10.1111/j.1538-7836.2005.01245.x. [DOI] [PubMed] [Google Scholar]
- 5.Rodeghiero F, Castaman G, Tosetto a, Batlle J, Baudo F, Cappelletti a, Casana P, De Bosch N, Eikenboom JCJ, Federicia B, Lethagen S, Linari S, Srivastava A. The discriminant power of bleeding history for the diagnosis of type 1 von Willebrand disease: an international, multicenter study. Journal of thrombosis and haemostasis. 2005;3:2619–26. doi: 10.1111/j.1538-7836.2005.01663.x. [DOI] [PubMed] [Google Scholar]
- 6.Tosetto A, Rodeghiero F, Castaman G, Goodeve A, Federici AB, Batlle J, Meyer D, Fressinaud E, Mazurier C, Goudemand J, Eikenboom J, Schneppenheim R, Budde U, Ingerslev J, Vorlova Z, Habart D, Holmberg L, Lethagen S, Pasi J, Hill F, Peake I. A quantitative analysis of bleeding symptoms in type 1 von Willebrand disease: results from a multicenter European study (MCMDM-1 VWD) Journal of thrombosis and haemostasis. 2006;4:766–73. doi: 10.1111/j.1538-7836.2006.01847.x. [DOI] [PubMed] [Google Scholar]
- 7.Bowman M, Mundell G, Grabell J, Hopman WM, Rapson D, Lillicrap D, James P. Generation and validation of the Condensed MCMDM-1VWD Bleeding Questionnaire for von Willebrand disease. Journal of thrombosis and haemostasis. 2008;6:2062–6. doi: 10.1111/j.1538-7836.2008.03182.x. [DOI] [PubMed] [Google Scholar]
- 8.Tosetto A, Castaman G, Plug I, Rodeghiero F, Eikenboom J. Prospective evaluation of the clinical utility of quantitative bleeding severity assessment in patients referred for hemostatic evaluation. Journal of thrombosis and haemostasis. 2011;9:1143–8. doi: 10.1111/j.1538-7836.2011.04265.x. [DOI] [PubMed] [Google Scholar]
- 9.Azzam HAG, Goneim HR, El-Saddik AM, Azmy E, Hassan M, El-Sharawy S. The condensed MCMDM-1 VWD bleeding questionnaire as a predictor of bleeding disorders in women with unexplained menorrhagia. Blood coagulation & fibrinolysis. 2012;23:311–5. doi: 10.1097/MBC.0b013e32835274d9. [DOI] [PubMed] [Google Scholar]
- 10.Castaman G, Rodeghiero F, Tosetto a, Cappelletti a, Baudo F, Eikenboom JCJ, Federicia B, Lethagen S, Linari S, Lusher J, Nishino M, Petrini P, Srivastava a, Ungerstedt JS. Hemorrhagic symptoms and bleeding risk in obligatory carriers of type 3 von Willebrand disease: an international, multicenter study. Journal of thrombosis and haemostasis. 2006;4:2164–9. doi: 10.1111/j.1538-7836.2006.02070.x. [DOI] [PubMed] [Google Scholar]
- 11.Rodeghiero F, Tosetto a, Abshire T, Arnold DM, Coller B, James P, Neunert C, Lillicrap D. ISTH/SSC bleeding assessment tool: a standardized questionnaire and a proposal for a new bleeding score for inherited bleeding disorders. Journal of thrombosis and haemostasis. 2010;8:2063–5. doi: 10.1111/j.1538-7836.2010.03975.x. [DOI] [PubMed] [Google Scholar]
- 12.Mauer AC, Barbour EM, Khazanov NA, Levenkova N, Mollah SA, Coller BS. Creating an ontology-based human phenotyping system: The Rockefeller University bleeding history experience. Clinical and translational science. 2009;2:382–5. doi: 10.1111/j.1752-8062.2009.00147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKay H, Derome F, Haq MA, Whittaker S, Arnold E, Adam F, Heddle NM, Rivard GE, Hayward CPM. Bleeding risks associated with inheritance of the Quebec platelet disorder. Blood. 2004;104:159–65. doi: 10.1182/blood-2003-11-4077. [DOI] [PubMed] [Google Scholar]
- 14.Dilley A, Drews C, Miller C, Lally C, Austin H, Ramaswamy D, Lurye D, Evatt B. von Willebrand disease and other inherited bleeding disorders in women with diagnosed menorrhagia. Obstetrics and gynecology. 2001;97:630–6. doi: 10.1016/s0029-7844(00)01224-2. [DOI] [PubMed] [Google Scholar]
- 15.Rees M. Menorrhagia. British medical journal. 1987;294:759–62. doi: 10.1136/bmj.294.6574.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kadir RA, Economides DL, Sabin CA, Owens D, Lee CA. Frequency of inherited bleeding disorders in women with menorrhagia. Lancet. 1998;351:485–9. doi: 10.1016/S0140-6736(97)08248-2. [DOI] [PubMed] [Google Scholar]
- 17.Edlund M, Blombäck M, von Schoultz B, Andersson O. On the value of menorrhagia as a predictor for coagulation disorders. American journal of hematology. 1996;53:234–8. doi: 10.1002/(SICI)1096-8652(199612)53:4<234::AID-AJH4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 18.Philipp CS, Dilley A, Miller CH, Evatt B, Baranwal A, Schwartz R, Bachmann G, Saidi P. Platelet functional defects in women with unexplained menorrhagia. Journal of thrombosis and haemostasis. 2003;1:477–84. doi: 10.1046/j.1538-7836.2003.00061.x. [DOI] [PubMed] [Google Scholar]
- 19.Kirtava A, Crudder S, Dilley A, Lally C, Evatt B. Trends in clinical management of women with von Willebrand disease: a survey of 75 women enrolled in haemophilia treatment centres in the United States. Haemophilia. 2004;10:158–61. doi: 10.1046/j.1351-8216.2003.00832.x. [DOI] [PubMed] [Google Scholar]
- 20.Higham JM, O’Brien PM, Shaw RW. Assessment of menstrual blood loss using a pictorial chart. British journal of obstetrics and gynaecology. 1990;97:734–9. doi: 10.1111/j.1471-0528.1990.tb16249.x. [DOI] [PubMed] [Google Scholar]
- 21.Philipp CS, Faiz A, Dowling NF, Beckman M, Owens S, Ayers C, Bachmann G. Development of a screening tool for identifying women with menorrhagia for hemostatic evaluation. American journal of obstetrics and gynecology. 2008;198:163e1–8. doi: 10.1016/j.ajog.2007.08.070. [DOI] [PubMed] [Google Scholar]
- 22.Philipp CS, Faiz A, Heit JA, Kouides PA, Lukes A, Stein SF, Byams V, Miller CH, Kulkarni R. Evaluation of a screening tool for bleeding disorders in a US multisite cohort of women with menorrhagia. American journal of obstetrics and gynecology. 2011;204:209.e1–7. doi: 10.1016/j.ajog.2010.10.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katsanis E, Luke K, Hsu E, Li M, Lillicrap D. Prevalence and significance of mild bleeding disorders in children with recurrent epistaxis. The Journal of Pediatrics. 1988;113:73–6. doi: 10.1016/s0022-3476(88)80532-8. [DOI] [PubMed] [Google Scholar]
- 24.Dean JA, Blanchette VS, Carcao MD, Stain AM, Sparling CR, Siekmann J, Turecek PL, Lillicrap D, Rand ML. von Willebrand disease in a pediatric-based population--comparison of type 1 diagnostic criteria and use of the PFA-100 and a von Willebrand factor/collagen-binding assay. Thrombosis and haemostasis. 2000;84:401–9. [PubMed] [Google Scholar]
- 25.Hedlund-Treutiger I, Revel-Vilk S, Blanchette VS, Curtin Ja, Lillicrap D, Rand ML. Reliability and reproducibility of classification of children as “bleeders” versus “non-bleeders” using a questionnaire for significant mucocutaneous bleeding. Journal of pediatric hematology/oncology. 2004;26:488–91. doi: 10.1097/01.mph.0000133600.42259.c6. [DOI] [PubMed] [Google Scholar]
- 26.Bowman M, Riddel J, Rand ML, Tosetto a, Silva M, James PD. Evaluation of the diagnostic utility for von Willebrand disease of a pediatric bleeding questionnaire. Journal of thrombosis and haemostasis. 2009;7:1418–21. doi: 10.1111/j.1538-7836.2009.03499.x. [DOI] [PubMed] [Google Scholar]
- 27.Biss TT, Blanchette VS, Clark DS, Bowman M, Wakefield CD, Silva M, Lillicrap D, James PD, Rand ML. Quantitation of bleeding symptoms in children with von Willebrand disease: use of a standardized pediatric bleeding questionnaire. Journal of thrombosis and haemostasis. 2010;8:950–6. doi: 10.1111/j.1538-7836.2010.03796.x. [DOI] [PubMed] [Google Scholar]
- 28.Biss TT, Blanchette VS, Clark DS, Wakefield CD, James PD, Rand ML. Use of a quantitative pediatric bleeding questionnaire to assess mucocutaneous bleeding symptoms in children with a platelet function disorder. Journal of thrombosis and haemostasis. 2010;8:1416–9. doi: 10.1111/j.1538-7836.2010.03846.x. [DOI] [PubMed] [Google Scholar]
- 29.Marcus PD, Nire KG, Grooms L, Klima J, O’Brien SH. The power of a standardized bleeding score in diagnosing paediatric type 1 von Willebrand’s disease and platelet function defects. Haemophilia. 2011;17:223–7. doi: 10.1111/j.1365-2516.2010.02390.x. [DOI] [PubMed] [Google Scholar]
- 30.Bidlingmaier C, Grote V, Budde U, Olivieri M, Kurnik K. Prospective evaluation of a pediaric bleeding questionnaire and the ISTH bleeding assessment tool in children and parents in clinical routine. Journal of thrombosis and haemostasis. 2012;10:1335–1341. doi: 10.1111/j.1538-7836.2012.04775.x. [DOI] [PubMed] [Google Scholar]
- 31.Sidonio RF, Gunawardena S, Shaw PH, Ragni M. Predictors of von Willebrand disease in children. Pediatric blood & cancer. 2012;58:736–40. doi: 10.1002/pbc.23411. [DOI] [PubMed] [Google Scholar]
- 32.James PD, Bowman M, Grabell J, Dwyre L, Rapson D. Prospective validation of the condensed MCMDM-1 VWD bleeding questionnaire for platelet function disorders. Journal of thrombosis and haemostasis. 2011;9 (Suppl 2):549. [Google Scholar]
- 33.Tosetto A, Rodeghiero F, Castaman G, Goodeve A, Federici AB, Batlle J, Meyer D, Goudemand J, Eikenboom J, Schneppenheim R, Budde U, Ingerslev J, Lethagen S, Hill FG, Peake I. A comparison between two semi-quantitative bleeding scales for the diagnosis and assessment of bleeding severity in type 1 von Willebrand disease. Haemophilia. 2011;17:165–6. doi: 10.1111/j.1365-2516.2010.02381.x. [DOI] [PubMed] [Google Scholar]
- 34.James PD, Biss TT, Clark DS, Grabell J, RIddel J, Silva M, Rapson D. Rand M. -1 to +4 vs. 0 to +3?: comparing scoring systems for bleeding symptoms in the condensed MCMDM-1 VWD and the pediatric bleeding questionnaires. Journal of thrombosis and haemostasis. 2011;9(Suppl 2):200. [Google Scholar]
- 35.Silwer J. von Willebrand’s disease in Sweden. Acta paediatrica Scandinavica Supplement. 1973;238:1–159. [PubMed] [Google Scholar]
- 36.Lak M, Peyvandi F, Mannucci PM. Clinical manifestations and complications of childbirth and replacement therapy in 385 Iranian patients with type 3 von Willebrand disease. British journal of haematology. 2000;111:1236–9. doi: 10.1046/j.1365-2141.2000.02507.x. [DOI] [PubMed] [Google Scholar]
- 37.Federici AB. Clinical diagnosis of von Willebrand disease. Haemophilia. 2004;10 (Suppl 4):169–76. doi: 10.1111/j.1365-2516.2004.00991.x. [DOI] [PubMed] [Google Scholar]