Abstract
Increased polyamine signaling in bladder urothelial cells (BUC) may play a role in the pathophysiology of overactive bladder (OAB). We quantitated intracellular polyamine levels in cultured BUC from OAB and asymptomatic (NB) subjects. We assessed whether polyamines modulated rapid intracellular calcium ([Ca2+]i) changes and delayed acetylcholine (ACh) release evoked by oxotremorine (OXO, a muscarinic agonist). BUC were cultured from cystoscopic biopsies. High-performance liquid chromatography (HPLC) quantitated intracellular putrescine, spermidine, and spermine levels. Five-millimeter difluoromethylornithine (DFMO), and one-millimeter methylglyoxalbisguanylhydrazone (MGBG) treatments were used to deplete intracellular polyamines. Ten micrometers of OXO were used to increase [Ca2+]i levels (measured by fura 2 microfluorimetry) and trigger extracellular ACh release (measured by ELISA). Polyamine levels were elevated in OAB compared with NB BUC (0.5 ± 0.15 vs. 0.16 ± 0.03 nmol/mg for putrescine, 2.4 ± 0.21 vs. 1.01 ± 0.13 nmol/mg for spermidine, and 1.90 ± 0.27 vs. 0.86 ± 0.26 nmol/mg for spermine; P < 0.05 for all comparisons). OXO evoked greater [Ca2+]i rise in OAB (205.10 ± 18.82% increase over baseline) compared with in NB BUC (119.54 ± 13.01%; P < 0.05). After polyamine depletion, OXO evoked [Ca2+]i rise decreased in OAB and NB BUC to 43.40 ± 6.45 and 38.82 ± 3.5%, respectively. OXO tended to increase ACh release by OAB vs. NB BUC (9.02 ± 0.1 vs. 7.04 ± 0.09 μM, respectively; P < 0.05). Polyamine depletion reduced ACh release by both OAB and NB BUC. In conclusion, polyamine levels were elevated twofold in OAB BUC. OXO evoked greater increase in [Ca2+]i and ACh release in OAB BUC, although these two events may be unrelated. Depletion of polyamines caused OAB BUC to behave similarly to NB BUC.
Keywords: polyamine, urothelial cells, muscarinic signaling, overactive bladder, intracellular calcium
idiopathic overactive bladder (OAB) is a syndrome characterized by urinary symptoms such as urgency, urge incontinence, frequency, and nocturia without obvious causes such as bacterial cystitis or neurologic diseases. There are numerous theorized pathophysiologic mechanisms including detrusor smooth muscle overactivity, bladder afferent signal augmentation, and loss of central nervous system bladder inhibitory control. Recently, a growing body of evidence indicates that the bladder urothelial cells (BUC) exhibit neuron-like properties that may contribute to bladder sensory function, and BUC play an important role in the regulation of bladder function (2). The ability of the urothelium to exert regulatory control over bladder function was shown in a transgenic mouse with urothelial-specific conditional overexpression of nerve growth factor (NGF; Ref. 24). These observations have important implications in enhancing our knowledge of the pathophysiology of OAB and in identifying relevant biologic mechanisms in the urothelium as potential targets for future urothelial-targeted intervention for OAB.
A possible pathophysiologic mechanism in OAB may be augmented polyamine signaling in BUC. Our previous study showed increased expression of ornithine decarboxylase (ODC), the rate-limiting enzyme in polyamine biosynthesis, in bladder urothelial tissues from OAB subjects (15). We also demonstrated electrophysiologic evidence that OAB BUC had significantly decreased activity of the BK channel (large conductance Ca2+-activated potassium channel). This finding was due to presumed increased intracellular levels of polyamines blocking BK channels. Under physiologic pH, within the intracellular cystosol, the amino groups of polyamines can interact with negative charges nucleic acids and proteins, including ion channels such as the BK channel (27), inward rectifier potassium channels (19), glutamate receptors (11), and transient receptor potential melastatin channels TRPM4 and TRPM7 (12, 17).
An aim of this study was to directly quantitate intracellular polyamine levels of putrescine, spermidine, and spermine in cultured BUC using high-performance liquid chromatography (HPLC). We expect an increased intracellular level of polyamines in OAB BUC. Another aim was to determine whether intracellular polyamines were involved in regulating cytoplasmic Ca2+ and acetylcholine (ACh) release from human BUC triggered by muscarinic receptor activation. These cellular mechanisms were studied to better understand the role of BUC pathophysiology in the context of OAB syndrome. Future therapies for OAB could specifically target BUC.
MATERIALS AND METHODS
Subject selection.
This protocol was approved by the University of Maryland Baltimore Institutional Review Board. The inclusion criteria for OAB subjects were as follows: female gender, age ≥18 yr, urinary frequency >10 in 24 h, ≥1 incontinent episode associated with urge per day, bladder pain and/or discomfort score of 0 on Likert (0–9) scale, and negative urinalysis dip and/or negative urine culture. Exclusion criteria for OAB cohort were as follows: male gender, diagnosis of neurologic disease (stroke, multiple sclerosis, Parkinson's disease, spinal cord injury, etc.), bacterial cystitis, bladder calculi, current active ureteral or urethral calculi, genital herpes within past 12 mo, history of uterine, cervical, vaginal or urethral cancer, symptomatic urethral diverticulum, history of cyclophosphamide use or any type of chemical cystitis, history of tuberculous cystitis, history of pelvic irradiation, history of benign or malignant bladder tumors, active vaginitis, and pregnancy. The inclusion criteria for the NB subjects were as follows: female gender ≥18 yr of age, no voiding symptoms (American Urological Association symptom score ≤7 out of max 35), no bladder pain and/or discomfort (0 on Likert 0–9 scale), denies any urinary incontinence, requirement for concomitant cystoscopy during pelvic surgery such as ureteroscopy, cystoscopy for microhematuria, retrograde pyelogram, hysterectomy, ureteral reimplantation, and negative urinalysis dip and/or negative urine cultures. The exclusion criteria for the NB subjects were identical to the OAB cohort exclusion criteria. Cystoscopic cold-cup biopsies from four OAB and six NB subjects were obtained.
Bladder urothelial cell culture.
Bladder urothelial cultures were performed as previously described (25). Briefly, Bladder biopsies were obtained using the cold-cup biopsy technique from two random areas of the bladder. The samples in normal saline were minced manually into 0.5-mm pieces and placed in uncoated plastic tissue culture plates, and the samples were anchored to the bottom of the well with sterile cover glass slips and incubated in 95% air-5% CO2 at 37°C with buffer containing complete cell medium. The cell medium used was MEM plus l-glutamine supplemented with 1 unit per ml insulin, 10% heat inactivated FBS, 1.25 μg/ml amphotericin B, 100 U/ml penicillin, and 100 μg/ml streptomycin. Once a cell monolayer was present, cells were suspended in cell medium. Epithelial phenotype of the cultures was confirmed by cytokeratin 17-positive staining up to five culture passages.
Immunohistofluorescence.
Bladder biopsies from paraffin embedded 10-μm sections were deparaffinizied according to standard procedure and incubated in 1% H2O2 for 20 min at room temperature. After incubation in blocking solution, the slides and cells were incubated overnight at 4°C with rabbit anti-cytokeratin 17 (1:1,000; Sigma, St. Louis, MO), and then the slides were incubated with FITC-conjugated secondary antibody goat anti- rabbit IgG (1: 400; Molecular Probes, Eugene, OR) for 60 min at room temperature. Nuclei were counterstained with DAPI (1 μg/ml; Sigma). Negative controls without primary antibody were performed on urothelium and cultured urothelial cells to confirm the specificity of immunofluorescent staining.
HPLC measurement for polyamines.
BUC was harvested and homogenized in 700 μl of 0.9% sodium chloride mixed with 10 μl (200 nmol/ml) internal standard (1,7-diaminoheptane). To precipitate proteins, 300 μl of trichloroacetic acid (0.5 M) were added to the homogenate. After 30 min of incubation on ice, the homogenate was centrifuged for 30 min at 14,500 g at 4°C. The supernatant was collected and the derivatization reaction was carried out with 9-fluorenylmethyl chloroformate (Fmoc-Cl) according to the method of Ekegren and Gomes-Trolin (7). The Fmoc-derivatives of polyamines and 1,6-diaminohexane (an internal standard) were separated on a Viva C8 column [150 × 4.6 mm, 5 μm (Resek)] with a linear increase of acetonitrile concentration from 55 to 95% in 50 mM sodium acetate in 30 min, and elution was monitored by fluorescence (Ex = 263 nm and Em = 610 nm; Ref. 20). The flow rate was 1 ml/min and the column was operated at 40°C.
Intracellular calcium measurement.
Recordings of the change of [Ca2+]i were performed within 20 to 36 h after BUC was plated onto individual 5-mm coverslips. The cells were loaded with 2.5 μM fura 2-AM for 30 min and then transferred to the recording chamber mounted on an inverted microscope (Nikon, Tokyo, Japan) and continuously perfused via gravity (1–3 ml/min) with a solution consisting of the following (in mM): 145 NaCl, 5 KCl, 2.5 CaCl2, 1 MgCl2, 10 glucose, and 10 HEPES at pH 7.4. Solution osmolality was adjusted to 325 mosmol/kgH2O. Background corrected fluorescent images were acquired at 1 Hz with a slow scan CCD camera system (Princeton Instruments, Trenton, NJ) through a ×20 Nikon Fluor objective. Fura 2 was excited at 340- and 380-nm wavelengths (λ) using a high speed Lambda 10–2 filter wheel (Sutter Instruments, Novato, CA). Emitted light (510 nm) was acquired with equal exposure time (200 ms) for the excitation wavelengths of 340 and 380 nm. After the subtraction of background fluorescence, the ratio of fluorescence emission for 340/380-nm illumination was calculated by F340/F380 = R. Baseline fura 2 ratios (Rbaseline) were characterized by the average baseline of the 30–45 s before the application of test compounds. Test compounds were applied for 12 s, and the change in R was assessed. The peak increase in fura 2 ratios (Rpeak) was used to determine a percent change from baseline by the formula [(Rpeak − Rbaseline)/Rbaseline × 100]. Percent change in fura 2 fluorescence ratio is directly proportional to [Ca2+]i for concentrations of Ca2+ <10% of the saturating concentration for fura 2 (∼39 μM). Data were collected and analyzed using software Metafluor (Molecular Devices, Sunnyvale, CA) analyzing only cells with ≥20% change in fluorescence ratios. Data were calculated in two ways: 1) maximum fluorescence ratio change from baseline (see Fig. 4A); and 2) percentage of cells responding with ≥20% fluorescence ratio change over baseline (see Fig. 4B).
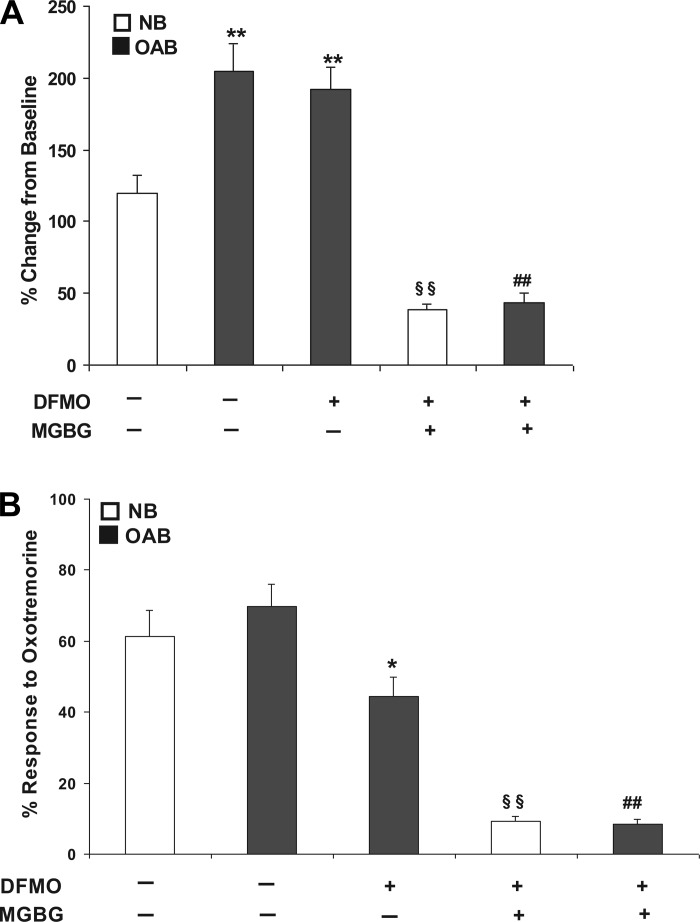
Fig. 4.
A: 10 μM OXO evoked maximum rise in [Ca2+]i in NB-, OAB-, and DFMO-treated OAB and DFMO + MGBG-treated NB and DFMO + MGBG-treated OAB BUC. **P < 0.01 vs. NB, §§P < 0.01 vs. NB, ##P < 0.01 vs. OAB. B: percentage of BUC that responded (see methods for definition of responsive cells) to 10 μM OXO stimulation in NB-, OAB-, DFMO-treated OAB and DFMO + MGBG-treated NB and DFMO + MGBG-treated OAB BUC, *P < 0.05, §§P < 0.01 vs. NB, ##P < 0.01 vs. OAB. Cells were from 3 OAB and 3 NB subjects.
ACh release assay.
Ten micrometers of OXO, a nonspecific muscarinic agonist, were added to untreated NB-, OAB-, and DFMO + MGBG-treated NB, OAB, BUC cultures to stimulate ACh release. ACh quantitation was measured at different time points by the Amplex Red Acetylcholine/Acetylcholinesterase Assay Kit (Invitrogen, Carlsbad, CA) in a fluorescence microplate reader according to manufacturer instructions. The reaction was incubated in Amplex Red reagent for 30 min at room temperature and protected from light, and the fluorescence measured in a fluorescence microplate reader using excitation in the range of 530–560 nm and emission detection at ∼590 nm. For each time point, in addition to duplicate measurements, correction for background fluorescence was performed by subtracting the values derived from the no-ACh control.
Pharmacologic agents.
OXO (10 μM), MGBG (1 mM), and DFMO (5 mM) (Sigma) were dissolved in cell buffer used in the experiments.
Statistical analyses.
Means ± SE values were calculated using number of human subjects as the sample size. Comparisons between the groups were conducted with ANOVA and Student's t-test both for unpaired and paired samples (t-test). Comparison for ANOVA was done with Tukey post hoc test, and differences were considered significant at P < 0.05. Dose-response curves were fitted with a modified Hill equation. Curve fitting was performed with Sigmaplot (version 9.0; Systat, Point Richmond, CA).
RESULTS
Cytokeratin 17 is expressed in human bladder urothelium and cultured urothelial cells.
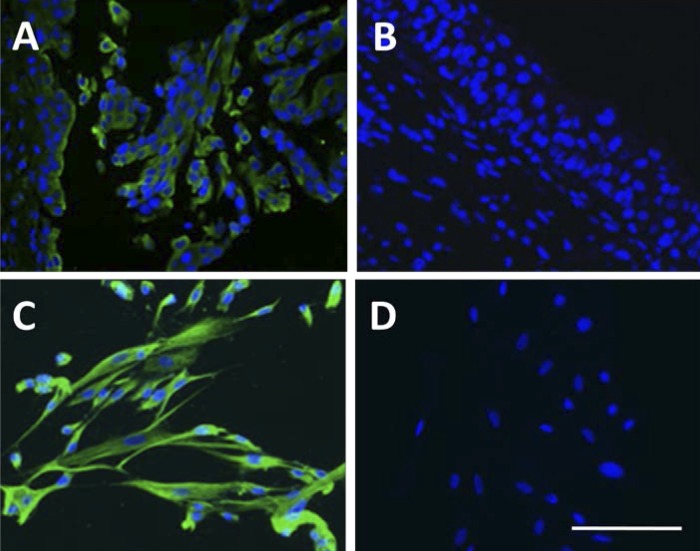
Immunohistofluorescence demonstrated expression of cytokeratin 17 (green signal) in the upper layer of human urothelium biopsies (Fig. 1A) and all the cultured urothelial cells (Fig. 1C). This result confirmed that the cultured urothelial cells used in this study retain the characteristics of BUCs. While there is no any positive cytokeratin 17 signal was observed in negative control human urothelium (Fig. 1B) and cultured urothelial cells (Fig. 1D).
Fig. 1.
Double immunoflurescence staining in human urothelium and cultured urothelial cells. Both cytokeratin 17 (green) and nuclear staining (blue) immunoflurescence staining were observed in urothelium (A) and cultured urothelial cells (C). No expression of cytokeratin 17 was detected in urothelium (B) and urothelial cells (D) using negative control (no primary antibody). Scale bar: 250 μm.
Elevated polyamine levels in human OAB BUC.
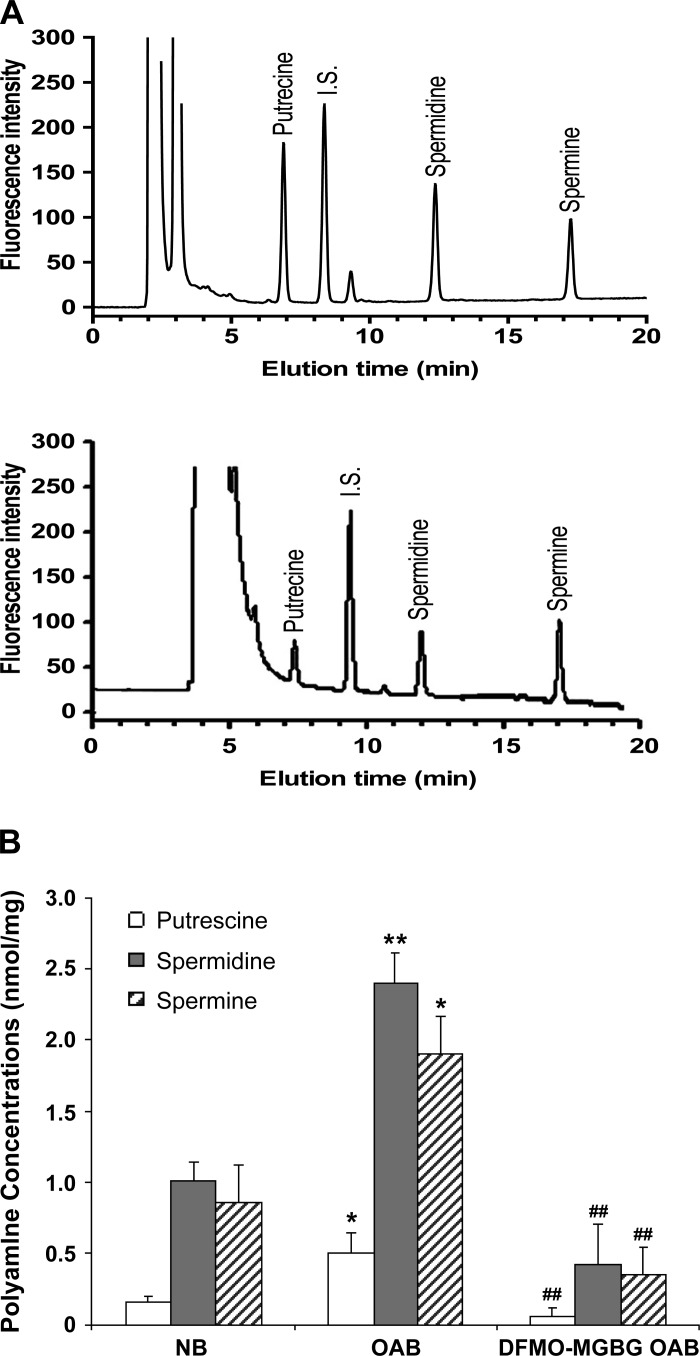
Intracellular putrescine, spermidine, and spermine levels in NB-, OAB-, and MGBG + DFMO-treated OAB BUC were measured by HPLC and normalized for cell weight (mg cell pellet). A sample elution chromatography of a standard run is shown in Fig. 2A. OAB BUC cytosol contained approximately twofold higher levels of all three polyamines than NB BUC (putrescine: 0.50 ± 0.15 vs. 0.16 ± 0.03 nmol/mg; spermidine: 2.4 ± 0.21 vs. 1.0 ± 0.13 nmol/mg; and spermine: 1.9 ± 0.27 vs. 0.86 ± 0.26 nmol/mg from cells derived from 4 OAB and 6 NB subjects; Fig. 2B). After OAB BUC were treated with DFMO + MGBG, intracellular polyamines were significantly decreased (Fig. 2B) but still detectable.
Fig. 2.
A: representative chromatogram showing separation of putrescine, spermidine and spermine of standards (top tracing) and sample (bottom tracing). B: intracellular polyamine quantification in human asymptomatic (NB)-, overactive bladder (OAB)-, and DFMO + methylglyoxalbisguanylhydrazone (MGBG)-treated OAB BUC. *P < 0.05, **P < 0.01 vs. NB bladder urothelial cells (BUC), ##P < 0.01 vs. OAB BUC, cells were from 4 OAB and 6 NB subjects.
Polyamines mediated [Ca2+]i signaling evoked by OXO in BUC.
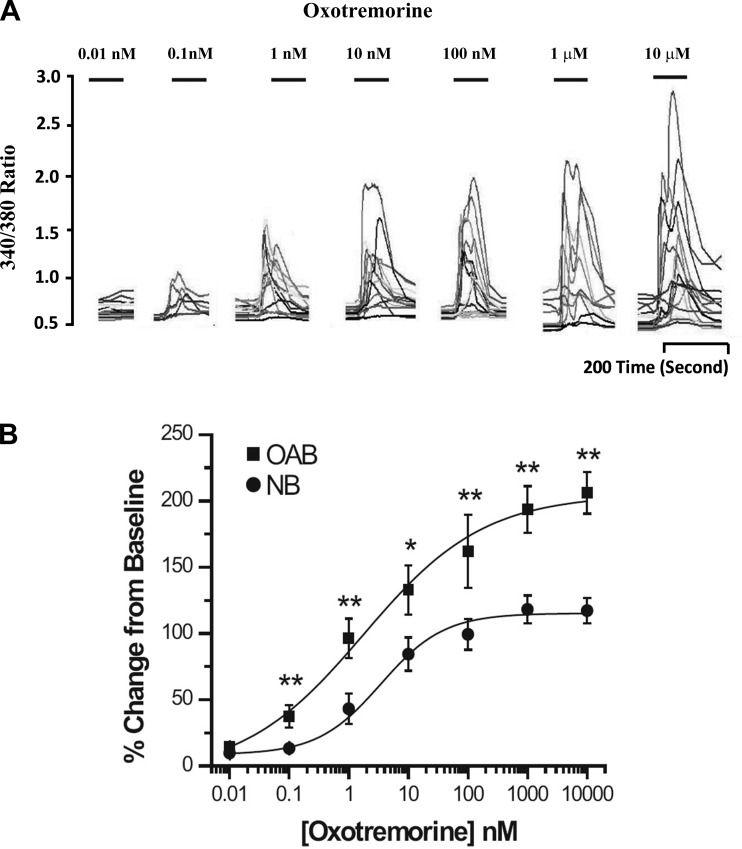
Increasing concentrations of OXO (0.01 nM to 10 μM) were applied to human NB and OAB BUC showing a dose-related increase in maximum [Ca2+]i (Fig. 3A). When this dose-response relationship was plotted and fitted with the Hill equation, the EC50 was 3.62 ± 0.93 nM in NB BUC and 1.78 ± 0.57 nM in OAB BUC (Fig. 3B). As shown in Fig. 4A, the maximal response in [Ca2+]i rise due to OXO was significantly greater in OAB compared with in NB subjects (%change over baseline, 205.10 ± 18.82 vs. 119.54 ± 13.01%; n = 27 cells from 3 OAB subjects and n = 38 cells from 3 NB subjects, respectively). After DFMO + MGBG treatment, maximal [Ca2+]i change evoked by OXO was significantly decreased in OAB (43.4% ± 6.45%; n = 11 cells) and NB (38.82 ± 3.5%; n = 17 cells) BUC (Fig. 4A). When examining the percentage of cells responding to OXO, a similar percentage of OAB and NB cells responded to OXO (Fig. 4B). DFMO treatment alone significantly decreased percentage of OAB BUC responsive to OXO (68.54 ± 6.35 to 44.21 ± 7.10%; P < 0.05). DFMO + MGBG treatment decreased the percentage of NB BUC responsive to OXO (from 61.34 ± 7.42 to 9.2 ± 1.32%; P < 0.01) and of OAB BUC responsive to OXO (from 69.67 ± 6.43 to 8.33 ± 1.45%; P < 0.01) (Fig. 4B).
Fig. 3.
A: representative ratiometric data from human OAB BUC used to generate a OXO dose response curve. B: increasing concentrations of oxotremorine (OXO; 0.01 nM-10 μM) applied to NB and OAB BUC showed a dose-related rise in [Ca2+]i. Data on the 27 cells from 3 NB and 52 cells from 3 OAB subjects were analyzed as a percent change from baseline with EC50 3.62 ± 0.93 nM in NB BUC and 1.78 ± 0.57 nM in OAB BUC (P < 0.05), and OAB cells showed a significant rise in [Ca2+]i compared the NB subjects when OXO applied at the concentration from 0.1 nM to 10 μM. **P < 0.01, *P < 0.05.
Polyamine modulates OXO-evoked ACh release from BUC.
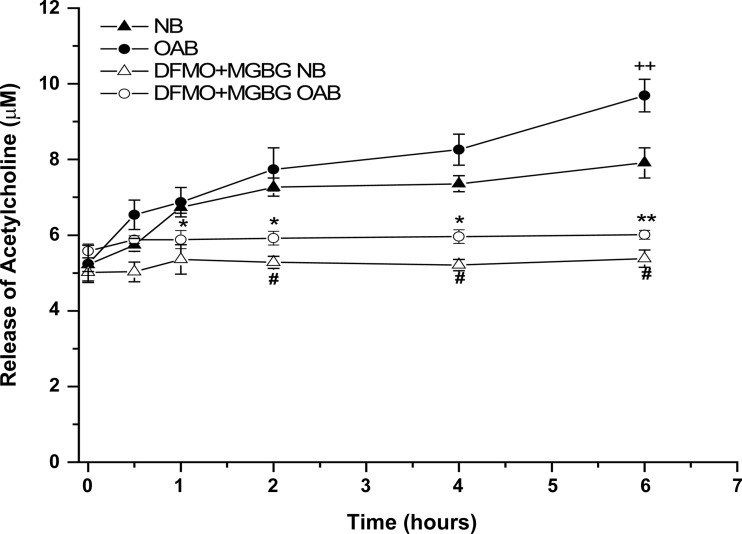
OAB BUC stimulated with OXO released significantly more ACh into the supernatant (Fig. 5) compared with NB BUC at 6 h (9.02 ± 0.1 vs. 7.04 ± 0.09 μM; n = 3 OAB, n = 3 NB subjects; P < 0.05). Treatment of both OAB and NB cells with DFMO + MGBG significantly blunted this ACh release at all time points measured beyond 1 h.
Fig. 5.
OXO (10 μM) stimulated ACh release in human NB-, OAB-, and DFMO + MGBG-treated NB and DFMO + MGBG-treated OAB BUC, ACh release from OAB BUC was significantly increased at hour 6 during the OXO stimulation compared with NB BUC, while the release was significantly decreased in DFMO + MGBG-treated NB and DFMO + MGBG-treated OAB BUC. ++P < 0.05 vs. NB, *P < 0.05, **P < 0.01 vs. OAB, #P < 0.05 vs. NB, cells were from 3 OAB and 3 NB subjects.
DISCUSSION
Our results demonstrated that the intracellular putrescine, spermidine, and spermine, were significantly elevated in cultured human OAB BUC based on quantitative HPLC measurements. This finding is consistent our previous work showing increased ODC expression in OAB urothelium and increased block of BK function, which suggested increased levels of intracellular polyamines (15). Although the function of polyamines in BUC has not been described previously, intracellular polyamines have been tied to a number of important cellular functions, such as cell migration, proliferation, differentiation (16, 26), and regulation of inflammation and pain signaling (8, 22). In gastrointestinal epithelial cells, polyamines have been shown to play a role in regulation of restitution and maintenance of integrity of the epithelial barrier (21). The importance of urothelial cells as a regulator of bladder function is becoming an accepted paradigm. BUC exhibit a number of properties similar to sensory neurons, including release of chemical mediators such as nitric oxide, ATP, ACh, and substance P. These findings suggest that BUC exhibit specialized sensory and signaling properties that could allow reciprocal communication with neighboring urothelial cells as well as nerves or other cells near the urothelium (9, 23). Therefore, it should not be surprising that polyamine signaling would be involved in BUC function, which ultimately can impact bladder function.
OXO evoked greater maximal rise in [Ca2+]i in OAB BUC, and depletion of intracellular polyamines abrogated this phenotype. It has been shown that human BUC possess both muscarinic and nicotinic ACh receptors and these receptors are involved in the regulation of cytoplasmic Ca2+ concentration (1, 5, 13). To avoid the activation of nicotinic receptor, we used a muscarinic-specific agonist OXO to evoke the changes in [Ca2+]i. These results suggest that intracellular polyamines play a role in urothelial Ca2+ signaling from muscarinic receptor activation. It is interesting to note that DFMO alone did not significantly block the OXO evoked [Ca2+]i signal in OAB BUC (Fig. 4A, 3rd bar), although the percentage of OAB BUC responding to OXO did significantly decrease (Fig. 4B, 3rd bar). A possible reason for residual [Ca2+]i signaling is that block of production of intracellular spermine is relatively resistant to DFMO, based on the studies in cultured rabbit corneal epithelial cells and rat intestinal crypt cells (6, 28), While DFMO inhibition of ODC activity led to significantly reduced putrescine and spermidine, it did not reduce spermine concentrations at 2 days; it took 4 days to significantly reduce spermine levels. In this study, we treated cells with DFMO for 3 days, so there may still have been intracellular spermine present at this time point. However, when cells were treated with DFMO + MGBG, there was additional significant decreases in OXO evoked Ca2+ signaling in both OAB and NB BUC (Fig. 4, A and B, 4th and 5th bars). As shown in Fig. 1B, DFMO + MGBG treatment of OAB BUC significantly reduced total intracellular polyamines.
It is well known that muscarinic receptors are abundant in the bladder smooth muscle, and muscarinic cholinergic mechanisms have been recognized as primarily mediating bladder contractility. Accordingly, antimuscarinic drugs are regarded as the standard treatment for detrusor overactivity (urodynamic finding). However, antimuscarinics were subsequently extended to treat symptoms of OAB syndrome (symptom complex) whether or not detrusor overactivity was present. It has been recently hypothesized that antimuscarinics may exert effects at the urothelial level. In pig bladders, the muscarinic receptor density is significantly greater in the urothelium than in the detrusor (10). It was also observed that contractions of human bladder from organ donors was associated with an increase in urothelial muscarinic receptors, indicative of a possible role of the urothelial muscarinic receptors in bladder contractile phenotype (4). Because antimuscarinics are effective during the storage phase of the micturition reflex, at which time parasympathetic nerves are silent, it has been postulated that the ACh released by the urothelium may contribute to detrusor overactivity. Therefore, antimuscarinic drugs may involve modulation of urothelial cell in addition to detrusor smooth muscle function.
Our present studies show that activation of muscarinic receptor also evoked significantly higher ACh release from OAB BUC compared with NB BUC after 6 h. This ACh release may not be involved in neurotransmission as the time frame for release is long (hours vs. minutes). Nonneuronal ACh is known to play a role in homeostasis of keratinocytes and human skin (14), and perhaps BUC-released ACh may play the same role. Polyamines have been shown to be involved in the regulation of neurotransmitter release within certain classes of neurons; for example, DFMO treatment inhibited calcium-stimulated release of aspartate from the brain (3). Limitations to this observation include supraphysiologic doses of OXO utilized in the ACh release experiments and duration of OXO exposure required to see the delayed ACh release. It is unlikely that the acute rise in [Ca2+]I is related to the delayed ACh release. Furthermore, the differences in delayed ACh release, although statistically different, were small.
Although the ultimate physiological relevance of our findings remains to be determined, augmented cytosolic polyamines were measured OAB BUC and polyamines modulated OAB BUC function. Because polyamines also regulate multiple cell functions, our findings of increased intracellular levels of polyamines may have diverse implications for BUC function. The relationship among intracellular polyamines, [Ca2+]i, and release of ACh by BUC is reminiscent of what has been described in pancreatic β-islet cells (18). In these islet cells, decreasing intracellular polyamines decreased cellular release of insulin in response to glucose. These similarities are summarized in Table 1. Further studies are needed to fully elucidate the role of urothelial polyamines in bladder function, and this area of research will represent a new approach at understanding and possibly treating idiopathic OAB syndrome.
Table 1.
Similarities in polyamine/calcium signaling in bladder urothelial cells and β-islet cells
| Bladder Urothelial Cells | β-Islet Cells | |
|---|---|---|
| Reduced intracellular polyamines | ↓Intracellular calcium in response to oxotremorine | ↓Intracellular calcium in response to glucose |
| Reduced intracellular polyamines | Resultant ↓ in acetylcholine release | Resultant ↓ in insulin release |
Conclusions.
Using HPLC, we found that the levels of the three polyamines (putrescine, spermidine and spermine) were all significantly elevated in cultured OAB BUC. Activation of muscarinic signaling by OXO evoked greater rapid rise in [Ca2+]i and delayed ACh release by OAB BUC. Depletion of intracellular polyamines by MGBG plus DFMO treatment abrogated these phenotypic changes in OAB BUC and made them behave more similarly to NB BUC. These findings suggest that targeting OAB polyamine signaling could be a mechanism by which to treat idiopathic OAB.
GRANTS
This work is supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK075728.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.L., Y.S., N.T., and Y.H. performed experiments; M.L., Y.S., and N.T. analyzed data; M.L., Y.S., Y.H., and T.C.C. interpreted results of experiments; M.L. and T.C.C. prepared figures; M.L. and T.C.C. drafted manuscript; M.L., Y.S., and T.C.C. edited and revised manuscript; M.L., Y.S., N.T., Y.H., and T.C.C. approved final version of manuscript; T.C.C. conception and design of research.
REFERENCES
- 1. Beckel JM, Kanai A, Lee SJ, de Groat WC, Birder LA. Expression of functional nicotinic acetylcholine receptors in rat urinary bladder epithelial cells. Am J Physiol Renal Physiol 290: F103–F110, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Birder LA. Auton urothelial signaling. Neuroscience 153: 33–40, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bondy SC, Walker CH. Polyamines contribute to calcium-stimulated release of aspartate from brain particulate fractions. Brain Res 371: 96–100, 1986 [DOI] [PubMed] [Google Scholar]
- 4. Braverman AS, Lebed B, Linder M, Ruggieri MR. M2 mediated contractions of human bladder from organ donors is associated with an increase in urothelial muscarinic receptors. Neurourol Urodyn 26: 63–70, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bschleipfer T, Schukowski K, Weidner W, Grando SA, Schwantes U, Kummer W, Lips KS. Expression and distribution of cholinergic receptors in the human urothelium. Life Sci 80: 2303–2307, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Du H, Viar MJ, Johnson LR, Watsky MA. Polyamines in cultured rabbit corneal cells. Invest Ophthalmol Vis Sci 44: 2512–2517, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Ekegren T, Gomes-Trolin C. Determination of polyamines in human tissues by precolumn derivatization with 9-fluorenylmethyl chloroformate and high-performance liquid chromatography. Anal Biochem 338: 179–185, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Estebe JP, Legay F, Gentili M, Wodey E, Leduc C, Ecoffey C, Moulinoux JP. An evaluation of a polyamine-deficient diet for the treatment of inflammatory pain. Anesth Analg 102: 1781–1788, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Hanna-Mitchell AT, Beckel JM, Barbadora S, Kanai AJ, de Groat WC, Birder LA. Non-neuronal acetylcholine and urinary bladder urothelium. Life Sci 80: 2298–2302, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hawthorn MH, Chapple CR, Cock M, Chess-Williams R. Urothelium-derived inhibitory factor(s) influences on detrusor muscle contractility in vitro. Br J Pharmacol 129: 416–419, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jin L, Miyazaki M, Mizuno S, Takigawa M, Hirose T, Nishimura K, Toida T, Williams K, Kashiwagi K, Igarashi K. The pore region of N-methyl-d-aspartate receptors differentially influences stimulation and block by spermine. J Pharmacol Exp Ther 327: 68–77, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Kerschbaum HH, Kozak JA, Cahalan MD. Polyvalent cations as permeant probes of MIC and TRPM7 pores. Biophys J 84: 2293–2305, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kullmann FA, Artim D, Beckel J, Barrick S, de Groat WC, Birder LA. Heterogeneity of muscarinic receptor-mediated Ca2+ responses in cultured urothelial cells from rat. Am J Physiol Renal Physiol 294: F971–F981, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kurzen H, Wessler I, Kirkpatrick CJ, Kawashima K, Grando SA. The non-neuronal cholinergic system of human skin. Horm Metab Res 39: 125–135, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Li M, Sun Y, Simard JM, Wang JY, Chai TC. Augmented bladder urothelial polyamine signaling and block of BK channel in the pathophysiology of overactive bladder syndrome. Am J Physiol Cell Physiol 297: C1445–C1451, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McCormack SA, Johnson LR. Polyamines and cell migration. J Physiol Pharmacol 52: 327–349, 2001 [PubMed] [Google Scholar]
- 17. Nilius B, Prenen J, Voets T, Droogmans G. Intracellular nucleotides and polyamines inhibit the Ca2+-activated cation channel TRPM4b. Pflügers Arch 448: 70–75, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Ohtani M, Mizuno I, Kojima Y, Ishikawa Y, Sodeno M, Asakura Y, Samejima K, Oka T. Spermidine regulates insulin synthesis and cytoplasmic Ca(2+) in mouse beta-TC6 insulinoma cells. Cell Struct Funct 24: 105–113, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Osawa M, Yokogawa M, Muramatsu T, Kimura T, Mase Y, Shimada I. Evidence for the direct interaction of spermine with the inwardly rectifying potassium channel. J Biol Chem 284: 26117–26126, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Price JR, Metz PA, Veening H. HPLC of 9-Fluorenylmethylchloroformate-polyamine derivatives with fluorescence detection. Chromatographia 24: 795–799, 1987 [Google Scholar]
- 21. Rao JN, Liu L, Zou T, Liu L, Marasa BS, Xiao L, Zeng X, Turner DJ, Wang JY. Polyamines are required for phospholipase C-gamma1 expression promoting intestinal epithelial restitution after wounding. Am J Physiol Gastrointest Liver Physiol 292: G335–G343, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Rivat C, Richebé P, Laboureyras E, Laulin JP, Havouis R, Noble F, Moulinoux JP, Simonnet G. Polyamine deficient diet to relieve pain hypersensitivity. Pain 137: 125–137, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Sadananda P, Shang F, Liu L, Mansfield KJ, Burcher E. Release of ATP from rat urinary bladder mucosa: role of acid, vanilloids and stretch. Br J Pharmacol 158: 1655–1662, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schnegelsberg B, Sun TT, Cain G, Bhattacharya A, Nunn PA, Ford AP, Vizzard MA, Cockayne DA. Overexpression of NGF in mouse urothelium leads to neuronal hyperinnervation, pelvic sensitivity, and changes in urinary bladder function. Am J Physiol Regul Integr Comp Physiol 298: R534–R547, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sun Y, Chai TC. Augmented extracellular ATP signaling in bladder urothelial cells from patients with interstitial cystitis. Am J Physiol Cell Physiol 290: C27–C34, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Wang JY. Polyamines regulate expression of E-cadherin and play an important role in control of intestinal epithelial barrier function. Inflammopharmacology 13: 91–101, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Weiger T, Hermann A. Polyamines block Ca2+-activated K+ channels in pituitary tumor cells (GH3). J Membr Biol 140: 133–142, 1994 [DOI] [PubMed] [Google Scholar]
- 28. Yuan Q, Viar MJ, Ray RM, Johnson LR. Putrescine does not support the migration and growth of IEC-6 cells. Am J Physiol Gastrointest Liver Physiol 278: G49–G56, 2000 [DOI] [PubMed] [Google Scholar]