Abstract
During development, Schwann cells extend lamellipodia-like processes to segregate large- and small-caliber axons during the process of radial sorting. Radial sorting is a prerequisite for myelination and is arrested in human neuropathies because of laminin deficiency. Experiments in mice using targeted mutagenesis have confirmed that laminins 211, 411, and receptors containing the β1 integrin subunit are required for radial sorting; however, which of the 11 α integrins that can pair with β1 forms the functional receptor is unknown. Here we conditionally deleted all the α subunits that form predominant laminin-binding β1 integrins in Schwann cells and show that only α6β1 and α7β1 integrins are required and that α7β1 compensates for the absence of α6β1 during development. The absence of either α7β1 or α6β1 integrin impairs the ability of Schwann cells to spread and to bind laminin 211 or 411, potentially explaining the failure to extend cytoplasmic processes around axons to sort them. However, double α6/α7 integrin mutants show only a subset of the abnormalities found in mutants lacking all β1 integrins, and a milder phenotype. Double-mutant Schwann cells can properly activate all the major signaling pathways associated with radial sorting and show normal Schwann cell proliferation and survival. Thus, α6β1 and α7β1 are the laminin-binding integrins required for axonal sorting, but other Schwann cell β1 integrins, possibly those that do not bind laminins, may also contribute to radial sorting during peripheral nerve development.
Introduction
Schwann cells synthesize extensive spiraling membranes containing specific proteins and lipids to generate myelin, which safeguards axons and ensures fast conduction of action potentials. Before myelination, Schwann cells engage in a 1:1 relationship with large-caliber axons, which they achieve during a multistep process called radial sorting (Webster et al., 1973). Before radial sorting, Schwann cells deposit a basal lamina, which contain laminins. Laminins are trimeric glycoproteins in which different α-, β-, and γ- subunits combine with remarkable tissue specificity (Miner and Yurchenco, 2004). The basal lamina of Schwann cells contains laminin 211 (α2β1γ1), 411 (α4β1γ1), and 511 (α5β1γ1); laminin 511 is specifically localized around nodes of Ranvier (Occhi et al., 2005). Laminins 211 and 411 have both redundant and specific functions in axonal sorting. Mutations in the α2 chain of laminin 211 cause congenital muscular dystrophy 1A (CMD1A), which includes a muscular dystrophy, a peripheral neuropathy, and central nervous system abnormalities (Helbling-Leclerc et al., 1995). The peripheral neuropathy has been studied mostly in the dystrophic (dy/dy) mutant mice and is characterized by a partial arrest in radial sorting (for review, see Feltri and Wrabetz, 2005). Similarly, laminin α4 (411) deficient mice have modest impairment in radial sorting (Wallquist et al., 2005; Yang et al., 2005). However, axonal sorting is entirely arrested in combined α2/α4 double laminin mutants, with axons remaining naked and amyelinated (Yang et al., 2005; Yu et al., 2005). This synergistic interaction between laminins 211 and 411 suggests distinct roles, which could be explained by specific interactions with dedicated receptors. Schwann cells express integrins and dystroglycan laminin receptors. Deletion of the β1 integrin subunit in Schwann cells almost completely arrests sorting (Feltri et al., 2002), and deletion of β1 integrins and dystroglycan in Schwann cells completely impairs the process (Berti et al., 2011). β1 integrins are heterodimers formed by one of 11 possible α subunits that combine with the β1 chain to form dimers with various ligand specificities. Which β1 dimer is required for radial sorting in Schwann cells is unknown. Because the abnormalities resulting from deletion of β1 integrin resemble those resulting from deletion of laminins, we focused on the αβ1 integrins that bind laminins. Schwann cells in vivo express three β1 integrins that are structurally similar (do not contain the αI domain) and are mainly laminin receptors (α3β1, α6β1, and α7β1), and two αI-containing, hybrid integrins (α1β1, α2β1), which also bind collagen (Previtali et al., 2003a, b). Whether these receptors are used interchangeably and are redundant or they have unique ligand specificities and functions in Schwann cells and other cell types is largely unknown. Here we show that α6β1 and α7β1 are required for radial sorting. Deletion of α6β1 or α7β1 integrins causes different effects on Schwann cell development and on their ability to bind laminins 211 and 411, suggesting specific roles for laminin-integrin receptor pairs in Schwann cells.
Materials and Methods
Transgenic mice.
All experiments involving animals followed experimental protocols approved by the San Raffaele Scientific Institute and Roswell Park Cancer Institute Animal Care and Use Committees. α6 integrin “floxed” (F) mice will be described (A. De Arcangelis et al., manuscript in preparation); α7 integrin knock-out and α3 integrin floxed mice have been described (Mayer et al., 1997; Liu et al., 2009). α6 integrin constitutive heterozygous null mice were the progeny of animals obtained by crossing α6 integrinF/+ animals and CMV-Cre animal. P0Cre transgenic mice have been previously characterized (Feltri et al., 1999, 2002). α6 integrinF/F, α6−/−, and α7 integrin−/− mice were congenic in C57BL/6 background. α3 integrin floxed mice were in a 129Sv/B6 mixed background; the progeny in this study resulted from parents that were N3–N4 generations congenic for C57BL6. Double α6/α7 integrin mutant mice were derived from parents that were N8 generations congenic for C57BL6. In the case of non-congenic genetic background, only littermates were compared. Mice of either sex were used. Genotyping of mutant mice was performed by PCR on tail genomic DNA, as described previously (Feltri et al., 1999, 2002; Liu et al., 2009; Germain et al., 2010).
Extraction of genomic DNA from sciatic nerves.
Sciatic nerves were dissected from P1 and P28 WT and α6 integrin mutant mice. The perineurium was dissected away, and the nerves were digested overnight at 55°C in 500 μl SNET (Tris, pH 8.0, 20 mm, EDTA, pH 8.0, 5 mm, NaCl 0.4 m, SDS 1% in distilled water) and 10 μl of Proteinase K (20 mg/ml). The following day, genomic DNA extraction with phenol/chloroform and PCR analysis was performed.
Mouse DRG explant cultures.
Explants were dissected from E13.5 embryos generated by mating α6 integrin+/− or α7 integrin+/− mice, plated onto rat collagen-I (Cultrex) coated glass coverslips, and maintained for 1 week in NB medium (B27 supplement, d-glucose 4 g/L, l-glutamine 2 mm, NGF 50 ng/ml in Neurobasal medium; Invitrogen); myelination was induced for 10 d with ascorbic acid (50 μg/ml) in C-medium (FCS 10%, l-glutamine 2 mm, d-glucose 4 g/L, NGF 50 ng/ml in MEM; Invitrogen). Explants were then fixed in 4% PFA for 20′ or nonfixed, washed, permeabilized with cold methanol for 5′, and stained according to standard immunohistochemistry protocols. For myelin internode quantification, the number of MBP-positive internodes in 10–15 images taken for each DRG was counted. This analysis was performed on at least three coverslips per embryo and on three embryos per genotype.
RNA extraction and semiquantitative RT-PCR analysis.
Sciatic nerves from P5 wild-type and α6 mutant mice were frozen in liquid nitrogen. Total RNA was prepared with TRIzol (Roche Diagnostic), and 1 μg of RNA was reverse transcribed using 1 mm dNTPs, 2.5 ng/ml random examers, 40 units Rnasin, and SuperScriptII RNase H-Reverse Transcriptase (Invitrogen) as per the manufacturer's instructions.
TaqMan quantitative PCR analysis.
Total RNA (1 μg) was retrotranscribed, and quantitative PCR was performed according to manufacturer's instructions (TaqMan, PE Applied Biosystems) on an ABI PRISM 7700 sequence detection system (Applied Biosystems). The relative standard curve method was applied using wild-type mice as reference. Normalization was performed using 18S rRNA as a reference gene. Target and reference gene PCR amplification was performed in separate tubes with Assays on Demand (Applied Biosystems): 18S assay: Hs99999901_s1; and α7 integrin assay: Mm 00434400_m1.
Western blotting and Rac1 assay.
Frozen sciatic nerves dissected from P1, P5, and P28 mice were pulverized, sonicated in lysis buffer (95 mm NaCl, 25 mm Tris-HCl, pH 7.4, 10 mm EDTA, 2% SDS, 1 mm Na3VO4, 1 mm NaF), and 1:100 Protease Inhibitor Cocktail (Sigma-Aldrich), boiled for 5 min, and centrifuged at 14,000 × rpm in a microcentrifuge for 10 min at 16°C to eliminate insoluble material. The protein concentration in supernatants was determined by BCA protein assay (Thermo Scientific) according to the manufacturer's instructions. DRG explants were rinsed three times in PBS on ice and then suspended in lysis buffer (95 mm NaCl, 25 mm Tris-HCl, pH 7.4, 10 mm EDTA, 2% SDS, 1 mm Na3VO4, 1 mm NaF, and 1:100 Protease Inhibitor Cocktail; Sigma-Aldrich). After lysis, samples were sonicated in a water sonicator with two cycles of 20 s at maximum power. Equal amounts of homogenates were loaded with standard reducing or nonreducing sample buffer. The samples were denatured, resolved on SDS-polyacrylamide gel, and electroblotted onto PVDF membrane. To verify equal loading of protein, membranes were stained with Ponceau red. Blots were then blocked with 5% dry milk in PBS 1× 0.1% Tween or BSA 5% in PBS 1× 0.1% Tween and incubated with the appropriate antibody. Blots were developed with ECL or ECL plus (GE Healthcare), and band intensity was quantified from the films using ImageJ software. GST pull-down assay for Rac1-GTP was performed as described previously (Nodari et al., 2007).
Immunoprecipitation.
Sciatic nerves from wild-type and α6 mutant mice were dissected and sonicated in lysis buffer (150 mm NaCl, 50 mm Tris-HCl, pH 7.4, 1 mm EDTA, 1 mm EGTA, 1 mm Na3VO4, 1 mm NaF, 1:100 Protease Inhibitor Cocktail [Sigma-Aldrich], 1% NP-40, 0.25% sodium deoxycholate, and 0.1% SDS). After lysis, sciatic nerve extracts were incubated with hamster anti-β1 integrin antibodies (BD Biosciences) O/N at 4°C on a rotating wheel. The following day, they were incubated with protein G-agarose (Sigma) for 1 h at 4°C. After washing with lysis buffer with or without detergents, beads were suspended in a solution 1:1 lysis buffer: standard sample buffer 2× (with β-mercaptoethanol), and followed by Western blotting.
Mass spectrometry analysis.
After immunoprecipitation, samples were loaded and resolved by 7.5% SDS-PAGE. The gel was then stained with a silver stain mass spectrometry kit (Pierce, as per the manufacturer's instruction). Subsequently, mass spectrometry analysis was performed on discrete isolated bands from the silver-stained gel. Briefly, bands of interest were excised from gel, subjected to reduction by 10 mm dithiothreitol and alkylation by 55 mm iodoacetamide, and digested overnight with trypsin (Roche). Supernatant mixtures from the digestion (10 μl) were acidified with formic acid up to a final concentration of 10% (v/v), desalted and concentrated with Stage Tip μC18 (Proxeon Biosystems), and then separated on a NanoLC (EasyLC, Proxeon Biosystems). Peptide separation occurred on a homemade reverse-phase 15 cm spraying fused silica capillary column (75 mm i.d.), packed in house with 3 mm ReproSil-Pur 120C18-AQ (Dr. Maisch). A gradient of eluents A (H2O with 2% v/v acetonitrile, 0.5% v/v acetic acid) and B (80% acetonitrile with 0.5% v/v acetic acid) was used to achieve separation, from 7% B (at 0 min 0.2 μL/min flow rate) to 40% B (in 60 min, at 0.2 μL/min flow rate). The LC system was connected to an LTQ-Orbitrap mass spectrometer (Thermo Fisher Scientific) equipped with a nanoelectrospray ion source (Proxeon Biosystems). MS and MS/MS spectra were acquired selecting the 10 most intense ions per survey spectrum acquired in the orbitrap from m/z 300–1750 with 30,000 resolution. Target ions selected for the MS/MS were fragmented in the ion trap and dynamically excluded for 60 s. For accurate mass measurements, the lock-mass option was used (Olsen et al., 2005). Peptides were identified from the MS/MS spectra searched against IPI MOUSE database (version 3.65) using Mascot 2.1 search engine. Cysteine carbamidomethylation was used as fixed modification, methionine oxidation, and protein N-terminal acetylation as variable modifications. The initial mass tolerance in MS mode was set to 5 ppm and MS/MS mass tolerance was 0.5 Da; a maximum of two missed cleavages was allowed. Peptides and proteins were accepted with two minimum peptides identified per protein one of which was unique.
Immunohistochemistry.
Postnatal day 1, 5, and 28 sciatic nerves were dissected and either fixed in 4% PFA for 30 min at room temperature or nonfixed, cryopreserved with 5% sucrose and 20% sucrose in PBS, embedded in OCT (Miles), and snap frozen in liquid nitrogen. Transverse or longitudinal 10-μm-thick sciatic nerve cryosections were permeabilized in cold acetone or methanol for 5 min and then blocked in 20% FCS, 2% BSA, and 0.1% Triton X-100 in PBS for 1 h at room temperature. Primary antibody incubation was done for 2 h at room temperature or O/N at 4°C using antibodies diluted in blocking solution. Sections were then rinsed in PBS and incubated for 1 h with secondary antibodies, stained with DAPI, mounted with Vectashield (Vector Laboratories), and examined on a confocal microscope (PerkinElmer Ultraview).
Morphologic analysis.
Mutant and control littermates were killed at the indicated ages, and sciatic nerves were dissected. Semithin section and electron microscopic analyses of sciatic nerves were performed as previously described (Wrabetz et al., 2006). Morphometric studies were performed on 17 random EM sections form 3 α6 integrin mutant sciatic nerves and 3 controls, at a magnification of 1840×. The fraction of hypomyelinated (g-ratio > 0.8) or hypermyelinated fibers (g-ratio < 0.6)/total number of fibers was calculated and expressed in percentage. Fibers with g-ratio of 0.69 ± 0.031 were considered normal. The density of unsorted axons, of axons with caliber <1 μm that were myelinated, or of axons with caliber >1 μm that were not myelinated, was calculated as the number of axons per unit area.
TUNEL and proliferation assays.
TUNEL was performed as described previously (Feltri et al., 2002). For proliferation assays, sections were permeabilized with 0.2% Triton X-100, blocked with ADS buffer (10% FCS, 0.1 m lysine, and 0.02% sodium azide in 0.1 m PBS), and incubated with a rabbit anti-PH3 primary antibody (Millipore) as described previously (Berti et al., 2011). Nuclei were counterstained with DAPI and analyzed with a fluorescence microscope. For both techniques, three different mice per genotype and four different slides per animal were analyzed.
Laminin production and purification.
Human embryonic kidney cells (HEK293) stably expressing α2, β1, and γ1 laminin chains (laminin 211) or α4, β1, and γ1 laminin chains (laminin 411) were produced and cultured as described previously (McKee et al., 2007). Recombinant laminins were then purified from media on anti-HA (for the 211 protein) or anti-FLAG (for the 411 protein) matrices (Sigma) packed in a Bio-Rad column.
Laminin-binding assay.
Schwann cell–DRG neuron explant cultures were generated as described above and maintained for 5 d in culture in NB medium. Recombinant laminins 211 and 411 were added to the cell culture medium at the final concentration of 20 μg/ml. After 3 h at 37°C, explants were rinsed three times in PBS, fixed in PFA 4%, and immunostained as described above. To quantify the relative amount of fluorescence in different samples, we used ImageJ to measure the fluorescent signals coming from laminin staining and from DAPI, in 20 fields per genotype, in each of three experiments. The laminin signal was normalized to the signal from DAPI.
Antibodies.
The following antibodies were used for Western blotting: goat anti-α6 integrin (Santa Cruz Biotechnology); rabbit anti-α7 integrin (a gift from Guido Tarone, Department of Genetics, Biology and Biochemistry, University of Torino, Molecular Biotechnology Center, Torino, Italy); rabbit anti-α3 integrin (a gift from Michael DiPersio, Center for Cell Biology and Cancer Research, Albany Medical College, Albany, New York); mouse anti-β1 integrin (BD Biosciences); rabbit anti-p42/44 and phospho-p42/44 (Thr202/Tyr204); rabbit anti-AKT and phospho-AKT (Ser473); rabbit anti-FAK and phospho-FAK (Tyr397); rabbit anti-Src and phospho-Src (Tyr416) (all from Cell Signaling Technology); chicken anti-P0 (Aves Laboratories); and rabbit anti-calnexin, mouse anti-β-tubulin, and peroxidase-conjugated secondary antibodies (Sigma) were used. For immunohistochemistry: rat anti-α6 integrin (GoH3, a gift from Arnoud Sonnenberg, Division of Cell Biology, The Netherlands Cancer Institute); rabbit anti-α7 integrin (from Ulrike Mayer); rat anti-α2 laminin (Alexis); rabbit anti-α4 laminin (a gift from Lydia Sorokin, Institute of Physiological Chemistry and Pathobiochemistry, University of Münster, Münster, Germany); rabbit anti-Neurofilament H (Millipore Bioscience Research Reagents); and rat anti-Neurofilament (TA-51) and rat anti-MBP (gifts from V. Lee, University of Pennsylvania, Philadelphia). Secondary antibodies: Dy Light 488 or 549-conjugated secondary antibody (Jackson ImmunoResearch Laboratories).
Results
Deletion of α6 integrin in Schwann cells does not impair axonal sorting
The most abundant laminin receptor present in Schwann cells during axonal sorting is α6β1 integrin (Previtali et al., 2003b). We therefore produced and characterized mice with a deletion of α6 integrin specifically in Schwann cells (α6cKO), by crossing α6 floxed mice, in which the genomic regions coding for the transmembrane portion are flanked by two loxP sites (A. De Arcangelis et al., manuscript in preparation), with the Schwann cell-specific mP0TOTCre mice (from here on called P0Cre). P0Cre activates Cre expression in the Schwann cell lineage starting at embryonic day 13.5 (E13.5) (Feltri and Wrabetz, unpublished observation), with ablation of the target protein a few days later (Feltri et al., 2002). When crossed with ubiquitous CMV-Cre mice, α6F/F mice showed the expected perinatal lethality resulting from skin blistering (Georges-Labouesse et al., 1996) (Fig. 1A), showing that the floxed α6 allele is successfully recombined. In contrast, mice with Schwann cell-specific deletion of α6 integrin are viable and fertile. Genomic DNA extracted from mutant sciatic nerves at postnatal day 1 (P1, the earliest time tested) showed the expected 1000 bp recombination band after amplification of the α6 integrin locus, and only in the presence of P0Cre. A nonrecombined band was also visible, which could derive from nonrecombined Schwann cells, fibroblasts, or axons (Fig. 1B). Western blot and immunohistochemistry on developing sciatic nerves showed that the level of α6 integrin protein was significantly reduced in mutant Schwann cells at P1 and P5 and completely absent in adult Schwann cells. α6 integrin was still present in perineurial and endothelial cells, as expected (Fig. 1D). To evaluate the effect of α6 integrin ablation on axonal sorting and myelination, we analyzed developing sciatic nerves by semithin sections. Mutant nerves showed normal morphology with no evidence of radial sorting delay at P5 or radial sorting arrest at P28 (Fig. 2). Because the arrest of radial sorting in laminin 211 mutants is most evident in spinal roots (Bradley and Jenkison, 1975), we also analyzed dorsal and ventral spinal roots, which showed no overt phenotype (Fig. 2). Other aspects of nerve development, including the number of myelinated fibers, the endoneurial extracellular matrix content, or the perineurium, were preserved. To exclude the possibility that the absence of a pathological phenotype was the result of low levels of residual α6 integrin protein in early development, we asked whether DRG explants from α6 ubiquitous null embryos (α6 integrin−/−, transmitted via the germline from α6 integrinF/F CMVCre) were able to proceed normally through radial sorting and myelinate in vitro. Similar DRG explants prepared from β1 integrinF/F P0cre mice showed significant impairment in myelination, likely resulting from the radial sorting arrest, even if β1 integrin protein levels were only reduced in 50% of Schwann cells (Nodari et al., 2007). DRGs were explanted from α6 integrin−/− mice at E14.5, to bypass the perinatal letalithy, and cultured in myelinating conditions. Despite the complete absence of α6 integrin protein in α6-null explants (Fig. 3A), there were no differences in the number of myelin internodes and in the amount of Myelin Protein Zero synthesized in mutant versus wild-type explants (Fig. 3). These in vitro observations paralleled the in vivo data and showed that the absence of α6 integrin does not impede radial sorting and myelination.
Figure 1.
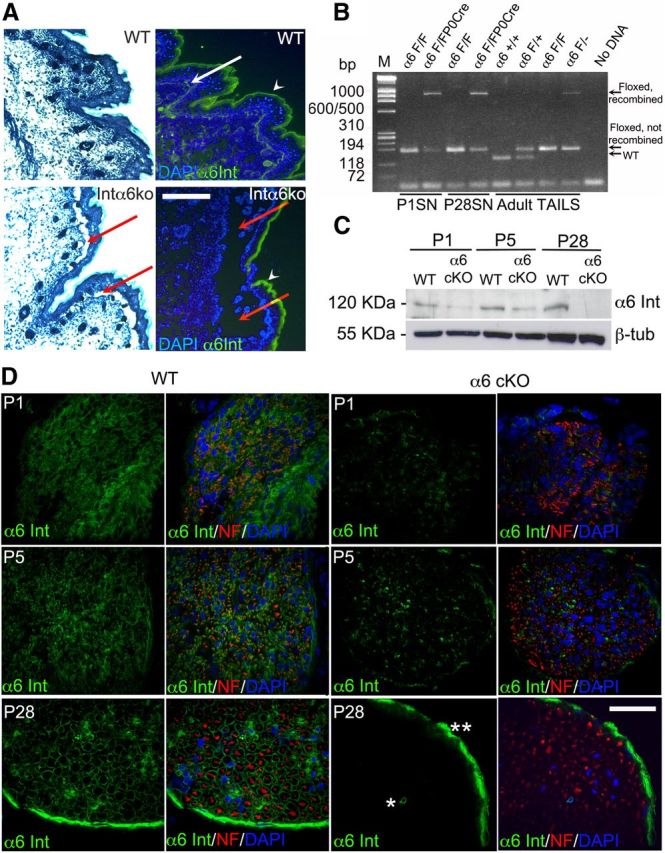
Efficient genomic recombination and Schwann cell-specific deletion of α6 integrin in α6 integrinF/F P0Cre mutant nerves. A, Constitutive deletion of the α6 allele in α6−/− mice causes detachment between epidermis and dermis (epidermolysis bullosa) resulting from the absence of α6 integrin (red arrows). The white arrow in wild-type indicates the normal α6 staining at the dermal–epidermal junction. Arrowheads point to a non-specific staining in the stratum corneus. B, Genomic DNA from tail samples of wild-type (α6+/+), α6F/+, α6F/F, and α6F/− mice were amplified using primers AH065 (placed upstream of the 5′ loxP site), AHM4 and AHM3 (flanking the 3′ loxP site) and generated the expected WT (120 base pairs), unrecombined floxed (154 base pair), and recombined floxed (1000 base pair) bands. PCR on genomic DNA from α6 integrinF/F and α6 integrinF/F P0Cre sciatic nerves (SN) at P1 and P28 amplified the floxed band and the recombined floxed band only in the presence of P0Cre at P1 and P28. C, D, Western blotting and immunohistochemistry in α6 integrinF/F P0Cre+/− and control sciatic nerves at P1, P5, and P28. α6 integrin is absent in mutant nerves at P28 and markedly reduced by P1. One representative experiment of three is shown; different animals or pools of animals were used for each experiment. C, β-tubulin was used as a loading control. D, Green represents α6 integrin. Red represents neurofilament. Blue represents nuclei (DAPI). Of note, α6 integrin in mutant adult nerves is still present in blood vessels (*) and in the perineurium (**), where P0Cre is not expressed. Scale bars: A, 300 μm; D, 35 μm.
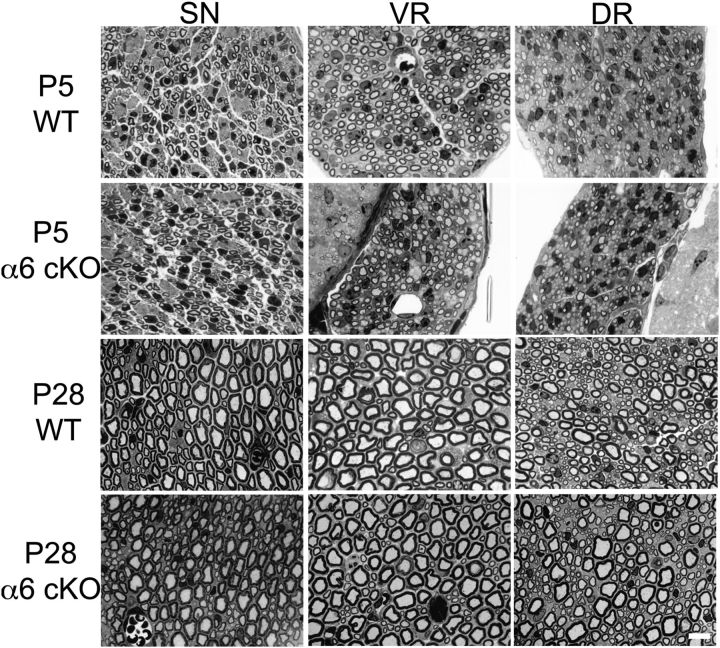
Figure 2.
α6 integrin ablation in Schwann cells does not lead to radial sorting defects in α6 integrinF/F P0Cre sciatic nerves and spinal roots. Semithin sections of sciatic nerves (SN), dorsal (DR), and ventral roots (VR) from wild-type and α6 integrinF/F P0Cre mutant mice (cKO) at P5 and P28 are shown. No bundles of naked axons are present in mutant mice, indicating normal radial sorting. Scale bar, 10 μm.
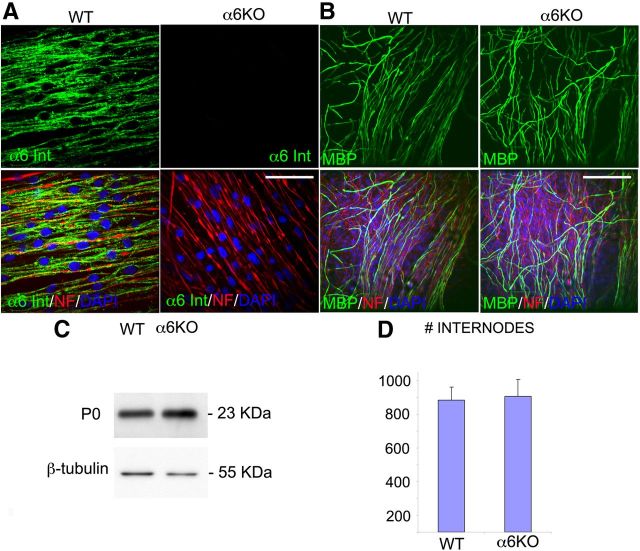
Figure 3.
Absence of α6 integrin does not lead to radial sorting arrest and subsequent myelination defects in Schwann cell-DRG neuron explants. Schwann cell–DRG neuron explants were prepared from wild-type and α6 integrin constitutive null embryos, and induced to myelinate for 10 d. A, Wild-type and α6-deficient (α6KO) explants were immunostained for α6 integrin (green), neurofilament (red), and nuclei (DAPI, blue); α6 protein is expressed in Schwann cells from wild-type explants, but it is absent in Schwann cells from deficient mice. B, Wild-type and α6 integrin-null explants were immunostained for MBP (green), neurofilament (red), and nuclei (DAPI, blue). Both wild-type and α6 integrin-null Schwann cells can myelinate axons to a similar extent. One representative of three experiments is shown. C, Western blot analysis of total protein extracts from wild-type and α6 integrin mutant explants shows a similar amount of P0 protein in null samples and controls. β-tubulin was used as a loading control. One representative of three experiments is shown. D, Quantification of myelin internodes in wild-type and α6 integrin-null explants in three independent experiments; in each experiment, different pools of DRGs were used. The number of myelin segments is comparable between the two genotypes. Error bars indicate SEM. Statistical significance was measured by Student's t test. Scale bars: A, 35 μm; B, 130 μm.
Absence of α6 integrin from Schwann cells causes minor ensheathment and myelin abnormalities
Analysis of Schwann cell-specific α6 null nerves by electron microscopy at P28 revealed minor, but frequent, abnormalities in myelination. Remak bundles often contained abnormal axons that were >1 μm in diameter (Fig. 4C,D, asterisks), suggesting that they were not properly recognized as destined for myelination, or not sorted out of bundles. Other times, large-caliber axons were sorted by promyelinating Schwann cells, but not myelinated, suggesting a delay in myelination (Fig. 4H, asterisks). In contrast, some axons <1 μm were abnormally myelinated (Fig. 4E, arrows, F,G, arrowheads), 25% of axons had overly thick (Fig. 4A,B, white arrowheads) and 5% overly thin (Fig. 4C,F, double asterisk) myelin for the caliber of the axon, and some fibers showed occasional axonal degeneration (Fig. 4I, J, arrows). These observations could reflect an impairment of α6 integrin-null Schwann cells to properly distinguish the threshold of 1 μm above which an axon should be myelinated, and later also to regulate the proper amount of myelin for a given axonal caliber. Indeed, these alterations may indicate a deregulated radial sorting, suggesting a role for α6 integrin in this process. Overall, these data suggest that α6 integrin is important for axonal sorting and the formation of the correct amount of myelin, but its role may be redundant or compensated for by other molecules.
Figure 4.
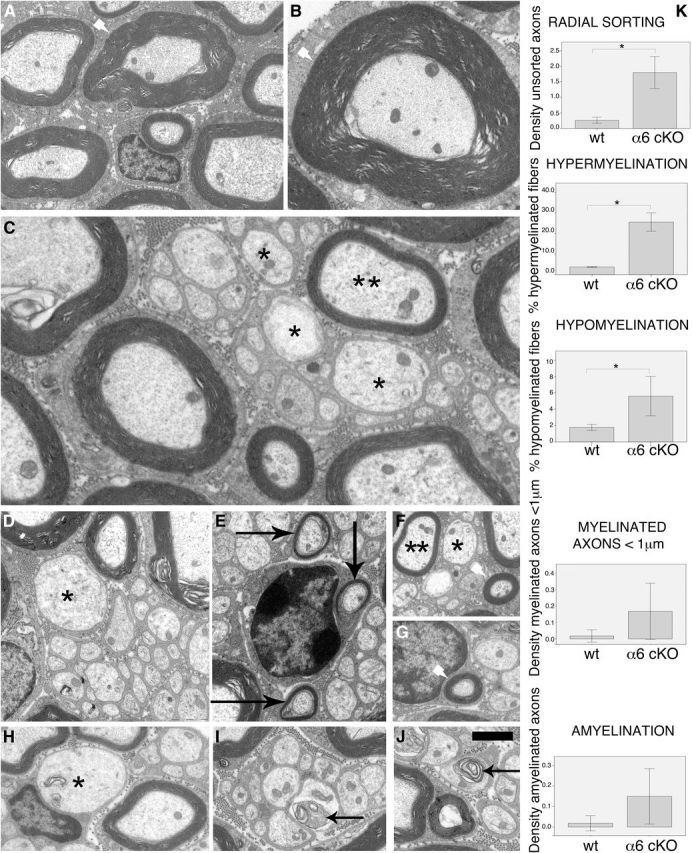
The absence of α6 integrin in Schwann cells causes ensheathment abnormalities in sciatic nerves. Electron microscopy analysis performed on α6 integrinF/F P0Cre sciatic nerves at P28 revealed the presence of hypermyelination (A, B, arrowheads), hypomyelination (C, F, **), axons <1 μm inappropriately myelinated (E, arrows), sometimes by a thick myelin sheath (F, G, arrowheads), Remak bundles containing axons >1 μm (C, D, F, *), axons >1 μm that were sorted but not myelinated (H, *), and occasional signs of axonal degeneration (I, L, arrows). K, Quantification of these abnormalities showed that ∼25% of fibers were hypermyelinated (g-ratio < 0.6) and ∼5% of fibers were hypomyelinated (g-ratio > 0.8) in mutant nerves. Densities are indicated per 100 μm2. Error bars indicate 2 SEs. *p < 0.05 (Mann–Whitney test). Scale bars: A–C, 2 μm; D–K, 1 μm.
α3 Integrin is not required for radial sorting and myelination, and its role is not redundant with that of α6 integrin
α3β1, α6β1, and α7β1 integrins could be redundant because they constitute a group of very similar integrins, which bind preferentially laminins and share structural characteristics, such as the absence of an αI-containing domain (for review, see Humphries et al., 2006). Previous studies showed that α7β1 integrin is not expressed at the time of radial sorting, and its deletion does not impair nerve development (Previtali et al., 2003a), whereas the role and expression of α3β1 integrin were never carefully assessed. Similarly, α1β1 and α2β1, other integrins that can bind laminin, but also collagen, are barely detectable by premyelinating Schwann cells in vivo (Stewart et al., 1997; Previtali et al., 2003a, 2003b). Collectively, these studies left the role of only one β1 laminin-binding integrin unexplored, α3β1, suggesting that it may be redundant or compensating for α6β1 integrin during Schwann cell development. In addition, α3β1 and α6β1 integrins have redundant functions during formation of the ectodermal ridge and in organogenesis (De Arcangelis et al., 1999). By Western blot, we showed that α3 integrin is present in developing and mature sciatic nerves, but its expression is not increased when Schwann cells lack α6β1 integrin (Fig. 5B), suggesting lack of compensation, but not excluding redundancy. To investigate a possible role for α3 integrin in axonal sorting, we thus produced α3 conditional null mice (α3cKO) by crossing α3 integrin floxed (Liu et al., 2009) and P0Cre mice (α3F/F P0Cre), and double α3 and α6 integrins mutant animals (α3F/Fα6F/F P0Cre). Mutant animals showed absence of the α3 integrin protein in sciatic nerves by P28 (Fig. 5A). Semithin sections of single- and double-mutant sciatic nerves and spinal roots revealed a normal morphology: axons were normally sorted and myelinated (Fig. 5C,D). Therefore, α3β1 integrin is neither redundant nor compensatory for α6β1 integrin in sciatic nerves.
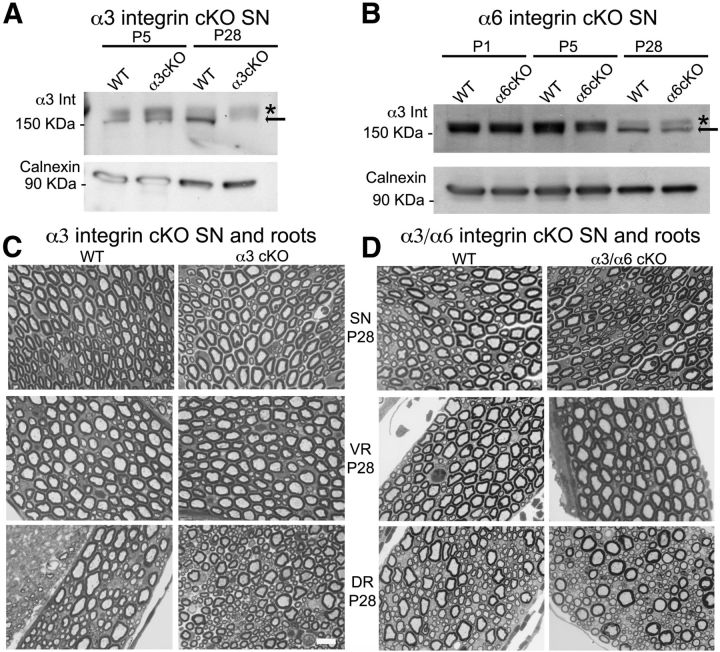
Figure 5.
α3β1 integrin is dispensable in peripheral nerve development and is neither redundant nor compensatory for α6 integrin. A, Western blot analysis of total protein extracts from wild-type and α3 integrinF/F P0Cre sciatic nerves shows that the α3 protein (arrow) is absent from mutant sciatic nerves at P28, but a residual level of the protein is still present in P5 mutant nerves. *An aspecific band was detected in all samples. Calnexin was used as a loading control. Sciatic nerve pools from different animals were used for each sample. B, Western blot analysis of total protein extracts from wild-type and α6 integrinF/F P0Cre sciatic nerves shows that α3 integrin expression is comparable between wild-type and mutant sciatic nerves; thus, the absence of α6 integrin does not cause a compensatory upregulation of α3 integrin. Calnexin was used as a loading control. Sciatic nerve pools from different animals were used for each sample. C, D, Semithin sections of sciatic nerves (SN), dorsal (DR), and ventral roots (VR) from wild-type, α3, and double α3/α6 integrin conditional null mice (α3 integrinF/F, α6 integrinF/F P0Cre) at P28. Single α3 and double α3/α6 integrin mutant sciatic nerve and root morphology is comparable with control nerves. Scale bar, 10 μm.
α7β1 integrin compensates for α6β1 integrin in Schwann cells
To ask by unbiased means which subunit partners with β1 integrin in the absence of α6, we immunoprecipitated β1 integrin from wild-type and mutant α6 integrin sciatic nerves at P5, a time when radial sorting is still ongoing, and identified its partners by mass spectrometry. The β1 integrin subunit could be readily immunoprecipitated from both wild-type and mutant nerves (Fig. 6A), indicating that the overall amount of β1 integrins was not reduced when α6 was deleted. Immunoprecipitates from mutant and wild-type nerves were separated on acrylamide gel, silver stained, and four areas containing bands of different intensity excised and analyzed by mass spectrometry. Interestingly, we found that β1 integrin coimmunoprecipitated with α7 integrin only in α6 integrin mutant sciatic nerves, but not in wild-type nerve. The absence of α7 integrin wild-type nerves at P5 is in agreement with our previous report that most α7 integrin protein in Schwann cells is detected only after myelination, starting around P6, whereas in embryonic and perinatal nerves some α7 integrin is expressed by axons (Previtali et al., 2003a). To validate that α7 integrin was expressed earlier in nerves in the absence of α6 integrin, we analyzed developing sciatic nerves from wild-type and α6 integrin mutant mice by Western blotting and immunohistochemistry. Strikingly, whereas α7 integrin was absent in the endoneurium and in Schwann cells at P1 and P5, mutant nerves showed significant amounts of α7 integrin in Schwann cells already at P1 (Fig. 6E). α7 integrin was not only expressed earlier but also significantly upregulated by Western blot analysis in mutant sciatic nerves at P1 and P5, during the time of radial sorting (Fig. 6B). Interestingly, this upregulation occurred at the post-translational level because α7 mRNA levels were comparable in mutant and wild-type nerves (Fig. 6D), and it was also detectable in α6 integrin-null explant cultures (Fig. 6C), indicating that the stimulus that triggers compensatory α7 expression was also present in the in vitro system that we used to assess myelination.
Figure 6.
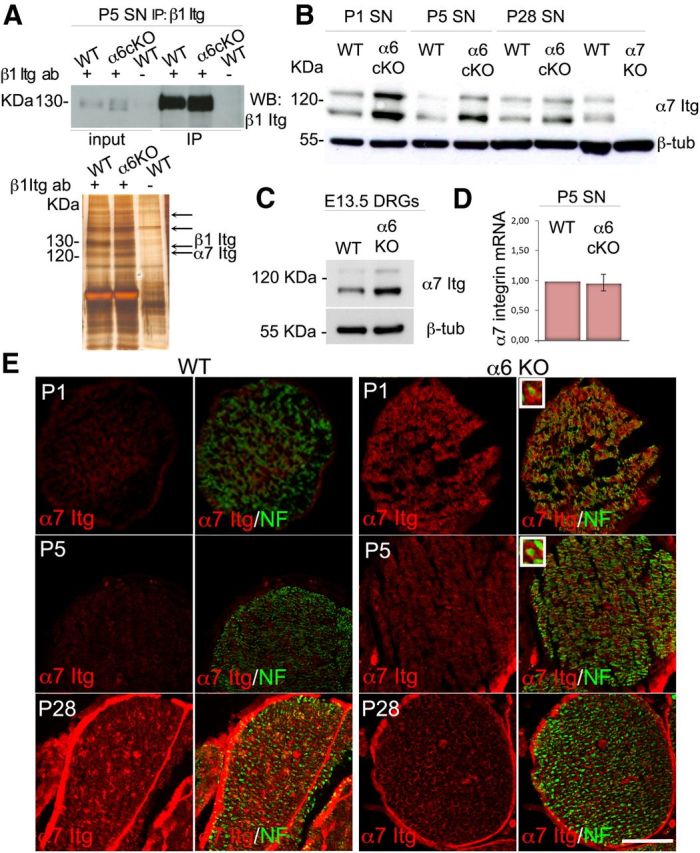
α7 integrin protein is expressed earlier and upregulated in sciatic nerves from α6 conditional null mice during development. A, Top, Immunoprecipitation of β1 integrin from wild-type and α6 conditional-null sciatic nerves at P5; blotting with β1 integrin antibody shows similar amounts of β1 integrin in wild-type and mutant sciatic nerves (SN) and significant enrichment compared with inputs (first two lanes). Omission of the primary antibody on wild-type samples was used as a negative control. Bottom, Mass spectrometry analysis was performed on four bands identified on the silver-stained immunoprecipitates (arrows). α7 was identified by mass spectrometry only in α6 mutant samples. B, C, Western blot analysis of total protein lysates from wild-type and mutant sciatic nerves (B) or DRG explants (C) validates a strong upregulation of α7 integrin when α6 integrin is absent in vivo and in vitro. α6 cKO, Schwann cell conditional knockout in B; α6 KO, general knockout in C. B, α7 integrin-deficient sciatic nerve lysate (α7 KO) was used as negative control for α7 integrin. One representative experiment of three is shown; nerves (or DRGs) were pooled from different animals in each experiment. D, RNA extraction was performed on P5 wild-type and mutant sciatic nerves; and α7 integrin mRNA levels were assessed by real-time PCR. Error bars indicate SEM. The experiment was done in triplicate; nerves were pooled from different animals in each experiment. E, Frozen sections of wild-type and α6 mutant sciatic nerves at P1, P5, and P28 immunostained for α7 integrin (red) and neurofilament (green). α7 integrin is expressed in Schwann cells only after myelination in WT nerves, but beginning at P1 in α6 integrin-null nerves. Magnified insets, Endoneurial α7 integrin staining comes from Schwann cells. Scale bar, 35 μm.
Double α6 and α7 integrin mutants have radial sorting defects
The upregulation of α7 integrin protein in α6 mutant sciatic nerves at the time of radial sorting prompted us to ablate both integrins in Schwann cells. We generated double α6/α7 integrin-deficient mice by crossing α6 integrin conditional-null mice and α7 null mice (α6F/Fα7−/−P0Cre). α7 integrin deficiency in mice causes a muscular dystrophy, with no peripheral neuropathy at the behavioral or morphological levels (Mayer et al., 1997; Previtali et al., 2003a). In contrast, morphological analysis of double α6-α7 integrin-deficient nerves revealed an arrest in radial sorting. Double-mutant sciatic nerves showed a delay in axonal sorting already apparent at P5 (Fig. 7D), and several bundles of naked axons, the hallmark of axonal sorting arrest, at P15 and P28 (arrows in Fig. 7D,H,L). Electron microscopy confirmed that the unsorted bundles of double α6/α7 mutant nerves contained mixed caliber axons that were naked, without Schwann cell cytoplasm ensheathing them (Fig. 7M,N), and redundant and detached basal lamina, suggesting either the inability to anchor the basal lamina or the retraction of Schwann cell processes (Fig. 7O, arrows). These features are very similar to those observed in β1 integrin conditional-null nerves (Feltri et al., 2002). Similar to single α6 integrin mutants, hypermyelinated axons and signs of axonal degeneration (Fig. 7N,P) were also observed.
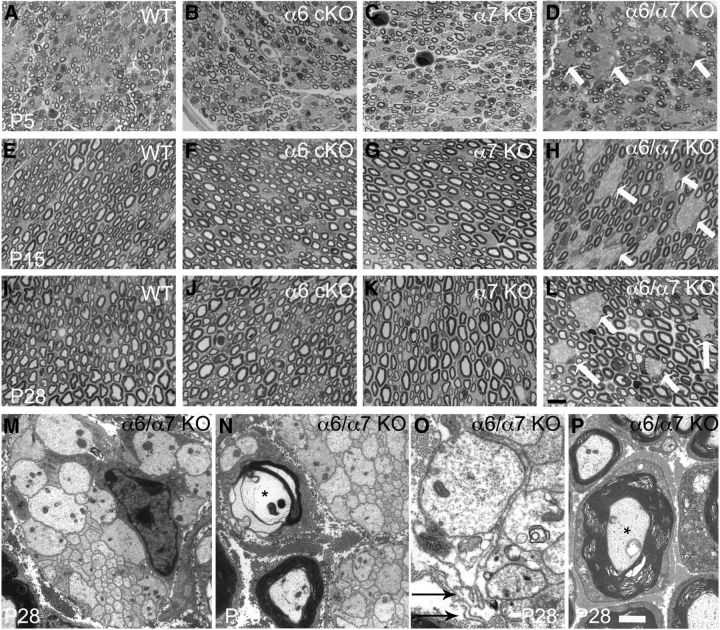
Figure 7.
Double α6/α7 mutant sciatic nerves have defective radial sorting throughout development. A–L, Semithin sections from wild-type, α6 integrin Schwann cell conditional null nerves (α6 cKO), α7 integrin-null nerves (α7 KO), and double α6/α7 integrin mutant nerves (α6/α7 KO) at P5, P15, and P28. In contrast to the normal morphology of single-mutant nerves, double-mutant sciatic nerves present many bundles of naked axons (D, H, L, arrows), evidence of defective radial sorting. Scale bar, 10 μm. B, Electron microscopy analysis of double α6/α7 mutant sciatic nerves at P28 reveals the presence of bundles of naked and unsorted axons of mixed caliber (M, N), basal lamina detachment (O, arrows), hypermyelinated axons (P, *), and occasional axonal degeneration (N, *). Scale bars: M, N, P, 2 μm; O, 1 μm.
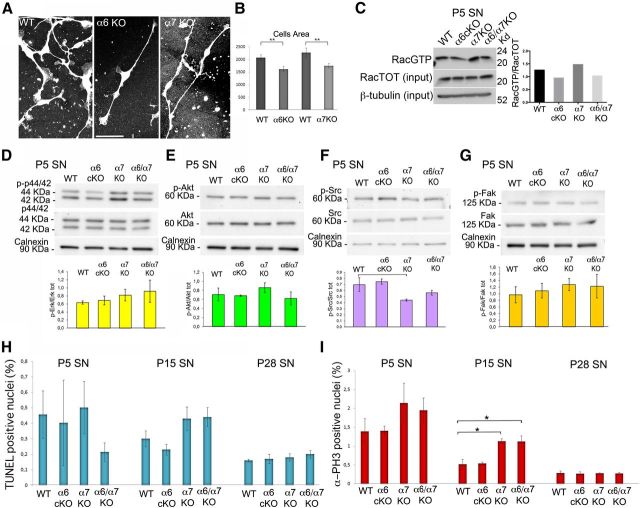
α6- and α7-deficient Schwann cells spread less when plated on laminin but can activate Rac1
During radial sorting, Schwann cells must expand their surface area many-fold to extend cytoplasmic protrusions that contact and enwrap axons. The molecular mechanisms that mediate these dramatic cytoskeletal rearrangements are not completely understood, but similar to other cell types, the actin cytoskeleton plays an important role, as exemplified by the sorting and myelination defects resulting from deletion of Fak, ILK, Rac1, Cdc42, and N-Wasp in Schwann cells (Benninger et al., 2007; Grove et al., 2007; Nodari et al., 2007; Pereira et al., 2009; Jin et al., 2011; Novak et al., 2011). We previously showed that β1 integrins are required for Schwann cell spreading, formation of lamellipodia, and activation of Rac1. To ask whether α6 or α7 integrins are also required in Schwann cells to expand their cytoplasm in response to laminin engagement, we analyzed the ability of α6 or α7 integrin-null Schwann cells to spread and activate Rac1. Schwann cells were isolated from DRG neurons explants from α6 or α7 null embryos and plated on laminin 111-coated coverslips to induce cell spreading (Nodari et al., 2007). We found that both α6 and α7 null Schwann cells spread significantly less than wild-type cells when plated on laminin (Fig. 8A,B). However, pull-down of Rac1-GTP from P5 sciatic nerves did not reveal significant differences between the levels of active Rac1 in mutant nerves, except for a trend toward less activation in the absence of α6 and more activation in the absence of α7 integrin (Fig. 8C). Thus, either α6 or α7 integrins is required for Schwann cells to spread in response to laminin, which may explain the defect in radial sorting.
Figure 8.
Double α6/α7 mutant sciatic nerves do not show significant alterations in signaling, proliferation, and survival at the time of axonal sorting. A, B, Schwann cells from α6 and α7 integrin-null mice were isolated from DRG explant cultures, plated on laminin 111, and stained for S100. Schwann cells area was measured by ImageJ software: both α6 and α7 null Schwann cells spread significantly less than wild-type cells on laminin. **p < 0.01 (Student's t test). C, Pull-down assay of Pak-binding-domain GST for Rac1 was performed on pools of 6–10 P5 sciatic nerves from WT and mutant mice. Active proteins were normalized to total Rac1, and equal loading was verified by β-tubulin. One of two experiments is shown. D–G, Western blot analysis on total protein extracts from three pools of 10–16 P5 sciatic nerves from mice of the indicated genotype reveals no significant alterations in the phosphorylation levels of ERK (D), AKT (Ser473) (E), Src (Tyr416) (F), and Fak (Tyr397) (G) in double-mutants compared with controls. Calnexin was used as a loading control. One representative experiment of three is shown. Error bars indicate SEM. The statistical significance of the various replicates was measured by Student's t test. H, I, Frozen sections of mutant sciatic nerves at P5, P15, and P28 were analyzed by TUNEL or immunostained for phosphorylated histone3 (P-H3). The percentage of P-H3 (H) or TUNEL (G) positive nuclei was calculated for each genotype. No differences in the level of Schwann cell apoptosis or proliferation were observed, except for an increase in Schwann cell proliferation at P15 in α7 null and double α6/α7 null nerves. Experiments were performed in triplicate; different pools of animals for each experiment were used. Error bars indicate SEM. *p < 0.05 (Student's t test).
All other major signaling pathways examined are activated normally in double-mutant nerves
Several signaling pathways have been implicated in radial sorting. β1 integrin mutant nerves present early impairment in multiple pathways involved in Schwann cell survival, proliferation, and cytoskeletal rearrangements, such as PI-3K/AKT, RhoA and RhoE, and Src (Nodari et al., 2007; Berti et al., 2011). Activation of AKT is defective at the time of sorting in both laminin and β1 integrin mutants, but this is probably the consequence, rather than the cause, of defective axonal contact (Yu et al., 2005; Berti et al., 2011). Deletion of the integrin adaptors Fak and ILK causes a severe axonal sorting phenotype reminiscent of the one observed in laminin and β1 integrin-null mice (Grove et al., 2007; Pereira et al., 2009). Finally, Src is important downstream of neuregulin and Shp2 phosphatase (Grossmann et al., 2009), and its phosphorylation is decreased in β1 integrin-deficient Schwann cells (Berti et al., 2011). To ask whether α6β1, α7β1, or both are required for the activation of these signaling cascades, we measured the levels of phosphorylation of these proteins in sciatic nerves from mutant mice at P5, during axonal sorting. Surprisingly, we did not detect significant differences in the activation of any of these pathways in single- or double-mutants (Fig. 8D–G), suggesting either that the signal coming from normally myelinating Schwann cells in the nerve was masking a small difference or that other α integrins that dimerize with β1 are responsible for activating these signaling pathways.
Schwann cells proliferate and survive normally in the absence of α6 and α7 integrins
Mice mutants for laminins, Cdc42, Fak, or β1 integrin present a moderate decrease in Schwann cell proliferation and/or an increase in apoptosis rate (Yang et al., 2005; Yu et al., 2005; Benninger et al., 2007; Grove et al., 2007; Berti et al., 2011). For these mutants, the reduced proliferation and survival could be a consequence of the failure to contact axons and thus to access proliferative cues coming from neuregulins on axons (Berti et al., 2011). In turn, reduced Schwann cell proliferation with concomitantly increased apoptosis could combine to reduce the number of Schwann cells available to engage in a 1:1 relationship with axons, aggravating the failure in axonal sorting. To verify whether Schwann cells showed reduced proliferation and survival in double α6/α7 integrin mutant nerves, we measured the rate of apoptotic and proliferating cells by TUNEL and anti-phospho-histone3 staining. As shown in Figure 8H, I, no significant differences in Schwann cell proliferation or apoptosis could be detected among the four genotypes analyzed, apart from an increase in proliferation of α7 integrin-null Schwann cells. These data indicate that the sorting defects observed in double α6/α7 integrin mutant nerves are not caused by an insufficient number of Schwann cells to match axons.
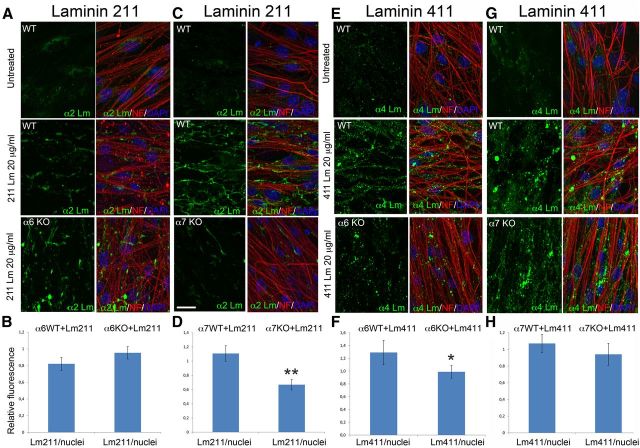
Absence of α6 or α7 integrin in Schwann cells decreases the binding of specific laminin isoforms
Laminins 211 and 411 are both required for axonal sorting, and genetic evidence suggests that they may have additive functions in Schwann cells because compound heterozygous nulls for laminins 211 and 411 (Lam α4−/+Dy2J/+ mice) are normal (Yang et al., 2005), whereas ablation of either laminin α2 or α4 produces partial defects in radial sorting, and ablation of both laminins completely prevents the process (Yang et al., 2005; Yu et al., 2005). It has been proposed that this relates to the function of specific laminin-integrin pairs in Schwann cells because α6β1 integrin is required for adhesion to laminin 411 (Yang et al., 2005), whereas α7β1 integrin is a specific receptor for laminin 211 (Chernousov et al., 2007). However, the degree of specificity between individual laminins and integrins is a matter of debate. When the affinity of laminins for soluble integrins is measured in a recombinant in vitro system, all integrins show promiscuous binding, and laminin 411 binds poorly to both receptors (Nishiuchi et al., 2006). In addition, laminin 411 was not able to promote myelination in DRG explants, which required instead high concentrations of laminin 211, with an intact polymerization and integrin binding domain (McKee et al., 2012). To address whether laminins 211 and 411 have preferential affinity for α6β1 or α7β1 integrins in Schwann cells, we took advantage of mutant DRG explant cultures that contain Schwann cells in contact with axons, a prerequisite for basal lamina synthesis and differentiation (for review, see Bunge et al., 1986), and a tridimensional orientation closer to the in vivo situation. Before the addition of ascorbic acid to induce myelination, these cells have an exposed basal surface that can be used to probe the binding of recombinant laminins. DRGs were explanted from embryos deficient in α6 or α7 integrins and used within 5 d, a time in which only low levels of endogenous laminins were detected (Fig. 9). Next, we added recombinant laminin 211 or 411, and after several washes we revealed the amount and organization of laminins bound to the Schwann cell surface, using antibodies specific to the α2 and α4 laminin chains. After addition of recombinant laminins, a network of polymerized laminin was evident on the surface of Schwann cells in wild-type explants, similar to what was described on cultured myotube cells (Colognato et al., 1999; Li et al., 2005). Interestingly, α7 integrin-deficient Schwann cells assembled a less prominent 211 laminin network, and α6 integrin-deficient Schwann cells assembled a less evident 411 network. Of note, the organization of 411 laminin on the surface of α6 null Schwann cells also seemed more disordered compared with controls. These data support the idea that specific laminins may preferentially bind specific integrins on Schwann cells.
Figure 9.
Deletion of α6 or α7 integrin in Schwann cells reduces binding to laminins 411 and 211, respectively. DRG explant cultures from wild-type, α6 integrin (A, E), and α7 integrin (C, G) null embryos were cultured for 5 d, then treated with laminin 211 or 411 at 37°C for 3 h to allow binding of laminins to the basal Schwann cell surface. After washing, cells were immunostained for α2 or α4 laminin chains (green), neurofilament (red), and DAPI (blue). Staining of untreated cells detected low levels of endogenous laminins. Laminin 211 binds less to α7 null explants, and laminin 411 binds less to α6 null samples. One representative experiment of three is shown. B, H, Semiquantitative estimation of the relative amount of fluorescence (green represents laminin; blue represents DAPI) in WT, α6KO, and α7KO explants to which laminins were added was performed with ImageJ software. Scale bar, 15 μm. Error bars indicate SEM. *p < 0.05 (Student's t test). **p < 0.001 (Student's t test).
Discussion
In this paper, we identify the integrin receptors involved in radial sorting. We had previously shown that integrins of the β1 class were essential for radial sorting of axons; however, which of the 11 possible α integrins dimerizes with β1 to form the functional receptor was unknown. Here we show that α6β1 integrin is the receptor required, but its function in vivo can be compensated by α7β1 integrin. α6β1 integrin is probably the receptor that mediates radial sorting in physiological conditions because it is highly expressed at the appropriate time, in contrast to α7β1 integrin, which is normally not present in early Schwann cell development (Previtali et al., 2003a). In addition, only deletion of α6 integrin causes abnormalities that, albeit minor, can be ascribed to the inability to correctly assign axons to the myelinated or nonmyelinated phenotype. These abnormalities include the presence of axons >1 μm in Remak bundles, or conversely the inappropriate deposition of thick myelin sheaths around axons <1 μm. In oligodendrocytes, β1 integrins are thought to contribute to the recognition of the axonal threshold for myelination because mice expressing dominant-negative β1 integrins require slightly larger axons than normal to become myelinated (Câmara et al., 2009). Similarly, it appears that, in the absence of α6β1 integrin, Schwann cells are more prone to misjudge the size of axons near the threshold for myelination. Thus, α6β1 integrins may act as molecular caliber in myelinating glia that properly “read” the size of axons, and in Schwann cells sort the ones to be myelinated. The molecular mechanisms are unknown but may be related to the ability of integrins to probe mechanical forces and constraints related to axonal size (for review, see Schiller and Fassler, 2013), or may be mediated by the amounts of Neuregulin 1 type III on axons, which is the quantitative signal that triggers myelin fate in Schwann cells (Michailov et al., 2004; Taveggia et al., 2005).
α6 and α7 integrins have distinct functions in Schwann cells
Ablation of either α6β1 or α7β1 integrin is not sufficient to show a radial sorting phenotype in vivo because α7β1 can compensate for the absence of α6β1 integrin. However, the deletion of either α6β1 or α7β1 integrin causes detectable abnormalities in Schwann cells in vitro, namely, decreased binding of laminin 211 or 411, and reduced ability to spread. These abnormalities probably explain the partial radial sorting arrest that is observed when both α integrins are deleted in vivo. One outstanding question is why mammals have such an extensive repertoire of apparently partially redundant laminin isoforms and integrin receptors. One possibility is that a high density of laminin and integrin molecules is required for basal lamina assembly and function. This hypothesis is consistent with data showing that PNS myelination in vivo is particularly sensitive to laminin dosage (McKee et al., 2012) and in vitro high concentrations of laminins are necessary to polymerize and bind integrins required for myelination, whereas lower concentrations induce Schwann cell proliferation (Eldridge et al., 1989; McKee et al., 2012). Still, the fact that different laminins and integrin receptors are used suggests that some specificity exists in their function during radial sorting. Indeed, even if α7 integrin compensation is able to prevent an overt phenotype when α6 integrin is deleted, we showed that the absence of α6β1 reduces binding of laminin 411, whereas the absence of α7β1 reduces binding of laminin 211 on Schwann cells. Of note, the reduced binding was evident despite the presence of dystroglycan, which binds laminin 211 with high affinity. This suggests a certain degree of ligand–receptor specificity in Schwann cells and is in agreement with previous data proposing that α6β1 and α7β1 integrin are preferred receptors for 411 and 211 laminin, respectively (Wallquist et al., 2005; Yang et al., 2005; Chernousov et al., 2007). Overall, these data suggest that, in physiological conditions, laminin 211/integrin α7β1 and laminin411/integrin α6β1 are preferred ligand–receptor pairs with specific functions in Schwann cells, but in pathological conditions one pair is able to substitute for the other to sort and myelinate axons.
Radial sorting can be impaired, even if enough Schwann cells are available to match the number of axons
Axonal sorting requires that Schwann cells can interact with axons and are in sufficient number to ensheath all axons. Interestingly, whereas α6β1 integrin is required for proper survival of oligodendrocyte precursors, α6β1 is dispensable for survival in Schwann cells. The survival of myelinating glia is regulated by axonal contact, which ensures the correct matching of the necessary number of glial cells in the CNS and peripheral nervous system (Barres et al., 1992; Grinspan et al., 1996; Trapp et al., 1997). Thus, decreased Schwann cell number is a possible reason for defect in axonal sorting, which is evident in mutants characterized by massive Schwann cell death, such as in transgenic mice expressing diphtheria toxin in Schwann cells, or deficient in Erb3 receptors (Messing et al., 1992; Riethmacher et al., 1997). In less extreme cases, when molecules that regulate both Schwann cell number and the cytoskeletal rearrangements required to interact and enwrap axons are deleted, both of these factors contribute to defects in radial sorting. This is the case for laminins, Cdc42, Fak, and β1 integrin mutants in C57BL/6 background (Yang et al., 2005; Yu et al., 2005; Benninger et al., 2007; Grove et al., 2007; Berti et al., 2011) that show a moderate decrease in Schwann cell proliferation and a moderate increase in apoptosis. Even if subtle, a reduced proliferation with concomitantly increased apoptosis could contribute to diminish the number of cells available for axon ensheathment.
Many cases have also been described where radial sorting is partially or completely arrested, even in the presence of normal or excess Schwann cells, which are, however, incapable of interacting with axons. This is the case of β1 integrin mutants in mixed C57BL/6//129 background, Rac1, ILK, or N-WASP mutants (Benninger et al., 2007; Nodari et al., 2007; Pereira et al., 2009; Jin et al., 2011; Novak et al., 2011; Guo et al., 2012), and the α6/α7 integrin-null mice described here. Similarly, in zebrafish, the role of ErbB2 in regulating process extension during radial sorting can be separated by its proliferative effect (Raphael et al., 2011). Thus, control of Schwann cell cytoskeleton and number are independently required for radial sorting of axons.
Deletion of α6 and α7 integrins causes only a subset of the abnormalities caused by deletion of all β1 integrins
Even if α6/α7 integrin double-mutants manifest a clear radial sorting phenotype, it is less severe than the phenotype obtained with deletion of all β1 integrins (Feltri et al., 2002; Berti et al., 2011), and Schwann cells in the double-mutants show significantly less impairment of signaling. Several possibilities may explain this. The first is technical because a small residue of α6 is still present during early phases of postnatal development. This is surprising given the early activation of the P0Cre gene (E13.5) and is specific to α integrins, as it was never seen with any of the many proteins previously conditionally deleted in Schwann cells using P0Cre. The reason for this is unknown but may be related to a longer half-life of α integrins in Schwann cells. We do not believe that this explains the mild phenotype because complete absence of α6 integrin in vitro, a model usually more permissive to reveal a phenotype, did not impair radial sorting and myelination. Interestingly, the absence of α6 integrin in vitro and the significant down- regulation of α6 integrin in early development in vivo were both sufficient to induce earlier expression of α7 integrin. This indirectly shows that the decrease of α6 integrin was “sensed” by developing Schwann cells, which compensated by increasing expression of α7 integrin. Interestingly, α7 integrin can also compensate for members of the dystroglycan–dystrophin complex in human or mouse muscles (Hodges et al., 1997; Côté et al., 2002; Allikian et al., 2004), but in these cases α7 integrin is upregulated at the mRNA level. In contrast, we observe a post-translational increase in α7 integrin protein expression, which is, to our knowledge, unique among integrins.
We postulate instead that the function of α6 and α7 integrin might be compensated by non–laminin-binding β1 integrins. The β1 integrin subunit can interact with other 9 α chains (α1, α2, α4, α5, α8, α9, α10, α11, and αV), many of which are expressed by Schwann cells (Lefcort et al., 1992; Milner et al., 1997; Stewart et al., 1997; Previtali et al., 2003b) and bind collagen, fibronectin, vitronectin, or cellular ligands. Conditional deletion of the fibronectin receptor α5β1 does not cause radial sorting abnormalities (M.P. and M.L.F., unpublished observations). However, it is possible that a collagen-binding integrin (α1, α2, α10, or α11) is compensating or redundant because deficiency of collagen XV aggravates the radial sorting phenotype due to laminin 411 deficiency (Rasi et al., 2010). Alternatively, we recently reported that, in early development, β1 integrins are located also near axons by immune-electron microscopy (Berti et al., 2011); so we could speculate that a subset of αβ1 integrins act independently of laminins and may directly interact with axons at the adaxonal surface. Despite the milder phenotype, our data conclusively show that α6 and α7 are the laminin integrins required for radial sorting of axons.
Footnotes
This work was supported by National Institute of Neurological Disorders and Stroke R01NS045630 to M.L.F., R01NS055256 to L.W., and R01DK36425 to P.D.Y., Telethon Italia (GPP10007A to M.L.F. and GG10007C to L.W.), and the European Community's Seventh Framework Programme (FP7/2007-2013) HEALTH-F2-2008-201535, UE-FP7 (NGIDD). We thank Guido Tarone (University of Torino), Michael DiPersio (Albany Medical College), Arnoud Sonnenberg (The Netherlands Cancer Institute), Lydia Sorokin (University of Münster), and Virginia Lee (University of Pennsylvania) for antibodies; Angela Bachi (San Raffaele Scientific Institute) for useful suggestions and access to the proteomic facility; and Nilo Riva (San Raffaele Hospital) for help with morphometry.
This work would not have been possible without the contribution of Elisabeth Georges-Labouesse who provided α6-floxed mice. Sadly, Elisabeth passed away prematurely on July 21, 2012.
The authors declare no competing financial interests.
References
- Allikian MJ, Hack AA, Mewborn S, Mayer U, McNally EM. Genetic compensation for sarcoglycan loss by integrin alpha7beta1 in muscle. J Cell Sci. 2004;117:3821–3830. doi: 10.1242/jcs.01234. [DOI] [PubMed] [Google Scholar]
- Barres BA, Hart IK, Coles HS, Burne JF, Voyvodic JT, Richardson WD, Raff MC. Cell death and control of cell survival in the oligodendrocyte lineage. Cell. 1992;70:31–46. doi: 10.1016/0092-8674(92)90531-G. [DOI] [PubMed] [Google Scholar]
- Benninger Y, Thurnherr T, Pereira JA, Krause S, Wu X, Chrostek-Grashoff A, Herzog D, Nave KA, Franklin RJ, Meijer D, Brakebusch C, Suter U, Relvas JB. Essential and distinct roles for cdc42 and rac1 in the regulation of Schwann cell biology during peripheral nervous system development. J Cell Biol. 2007;177:1051–1061. doi: 10.1083/jcb.200610108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berti C, Bartesaghi L, Ghidinelli M, Zambroni D, Figlia G, Chen ZL, Quattrini A, Wrabetz L, Feltri ML. Non-redundant function of dystroglycan and beta1 integrins in radial sorting of axons. Development. 2011;138:4025–4037. doi: 10.1242/dev.065490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley WG, Jenkison M. Neural abnormalities in the dystrophic mouse. J Neurol Sci. 1975;25:249–255. doi: 10.1016/0022-510X(75)90144-6. [DOI] [PubMed] [Google Scholar]
- Bunge RP, Bunge MB, Eldridge CF. Linkage between axonal ensheathment and basal lamina production by Schwann cells. Annu Rev Neurosci. 1986;9:305–328. doi: 10.1146/annurev.ne.09.030186.001513. [DOI] [PubMed] [Google Scholar]
- Câmara J, Wang Z, Nunes-Fonseca C, Friedman HC, Grove M, Sherman DL, Komiyama NH, Grant SG, Brophy PJ, Peterson A, ffrench-Constant C. Integrin-mediated axoglial interactions initiate myelination in the central nervous system. J Cell Biol. 2009;185:699–712. doi: 10.1083/jcb.200807010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernousov MA, Kaufman SJ, Stahl RC, Rothblum K, Carey DJ. Alpha7beta1 integrin is a receptor for laminin-2 on Schwann cells. Glia. 2007;55:1134–1144. doi: 10.1002/glia.20536. [DOI] [PubMed] [Google Scholar]
- Colognato H, Winkelmann DA, Yurchenco PD. Laminin polymerization induces a receptor-cytoskeleton network. J Cell Biol. 1999;145:619–631. doi: 10.1083/jcb.145.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Côté PD, Moukhles H, Carbonetto S. Dystroglycan is not required for localization of dystrophin, syntrophin, and neuronal nitric-oxide synthase at the sarcolemma but regulates integrin alpha 7B expression and caveolin-3 distribution. J Biol Chem. 2002;277:4672–4679. doi: 10.1074/jbc.M106879200. [DOI] [PubMed] [Google Scholar]
- De Arcangelis A, Mark M, Kreidberg J, Sorokin L, Georges-Labouesse E. Synergistic activities of alpha3 and alpha6 integrins are required during apical ectodermal ridge formation and organogenesis in the mouse. Development. 1999;126:3957–3968. doi: 10.1242/dev.126.17.3957. [DOI] [PubMed] [Google Scholar]
- Eldridge CF, Bunge MB, Bunge RP. Differentiation of axon-related Schwann cells in vitro: II. Control of myelin formation by basal lamina. J Neurosci. 1989;9:625–638. doi: 10.1523/JNEUROSCI.09-02-00625.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltri ML, Wrabetz L. Laminins and their receptors in Schwann cells and hereditary neuropathies. J Peripher Nerv Syst. 2005;10:128–143. doi: 10.1111/j.1085-9489.2005.0010204.x. [DOI] [PubMed] [Google Scholar]
- Feltri ML, D'Antonio M, Previtali S, Fasolini M, Messing A, Wrabetz L. P0-Cre transgenic mice for inactivation of adhesion molecules in Schwann cells. Ann N Y Acad Sci. 1999;883:116–123. doi: 10.1111/j.1749-6632.1999.tb08574.x. [DOI] [PubMed] [Google Scholar]
- Feltri ML, Graus Porta D, Previtali SC, Nodari A, Migliavacca B, Cassetti A, Littlewood-Evans A, Reichardt LF, Messing A, Quattrini A, Mueller U, Wrabetz L. Conditional disruption of beta 1 integrin in Schwann cells impedes interactions with axons. J Cell Biol. 2002;156:199–209. doi: 10.1083/jcb.200109021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges-Labouesse E, Messaddeq N, Yehia G, Cadalbert L, Dierich A, Le Meur M. Absence of integrin alpha 6 leads to epidermolysis bullosa and neonatal death in mice. Nat Genet. 1996;13:370–373. doi: 10.1038/ng0796-370. [DOI] [PubMed] [Google Scholar]
- Germain M, De Arcangelis A, Robinson SD, Baker M, Tavora B, D'Amico G, Silva R, Kostourou V, Reynolds LE, Watson A, Jones JL, Georges-Labouesse E, Hodivala-Dilke K. Genetic ablation of the alpha 6-integrin subunit in Tie1Cre mice enhances tumour angiogenesis. J Pathol. 2010;220:370–381. doi: 10.1002/path.2654. [DOI] [PubMed] [Google Scholar]
- Grinspan JB, Marchionni MA, Reeves M, Coulaloglou M, Scherer SS. Axonal interactions regulate Schwann cell apoptosis in developing peripheral nerve: neuregulin receptors and the role of neuregulins. J Neurosci. 1996;16:6107–6118. doi: 10.1523/JNEUROSCI.16-19-06107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann KS, Wende H, Paul FE, Cheret C, Garratt AN, Zurborg S, Feinberg K, Besser D, Schulz H, Peles E, Selbach M, Birchmeier W, Birchmeier C. The tyrosine phosphatase Shp2 (PTPN11) directs Neuregulin-1/ErbB signaling throughout Schwann cell development. Proc Natl Acad Sci U S A. 2009;106:16704–16709. doi: 10.1073/pnas.0904336106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove M, Komiyama NH, Nave KA, Grant SG, Sherman DL, Brophy PJ. FAK is required for axonal sorting by Schwann cells. J Cell Biol. 2007;176:277–282. doi: 10.1083/jcb.200609021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Moon C, Niehaus K, Zheng Y, Ratner N. Rac1 controls Schwann cell myelination through cAMP and NF2/merlin. J Neurosci. 2012;32:17251–17261. doi: 10.1523/JNEUROSCI.2461-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helbling-Leclerc A, Zhang X, Topaloglu H, Cruaud C, Tesson F, Weissenbach J, Tomé FM, Schwartz K, Fardeau M, Tryggvason K. Mutations in the laminin alpha 2-chain gene (LAMA2) cause merosin-deficient congenital muscular dystrophy. Nat Genet. 1995;11:216–218. doi: 10.1038/ng1095-216. [DOI] [PubMed] [Google Scholar]
- Hodges BL, Hayashi YK, Nonaka I, Wang W, Arahata K, Kaufman SJ. Altered expression of the alpha7beta1 integrin in human and murine muscular dystrophies. J Cell Sci. 1997;110:2873–2881. doi: 10.1242/jcs.110.22.2873. [DOI] [PubMed] [Google Scholar]
- Humphries JD, Byron A, Humphries MJ. Integrin ligands at a glance. J Cell Sci. 2006;119:3901–3903. doi: 10.1242/jcs.03098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin F, Dong B, Georgiou J, Jiang Q, Zhang J, Bharioke A, Qiu F, Lommel S, Feltri ML, Wrabetz L, Roder JC, Eyer J, Chen X, Peterson AC, Siminovitch KA. N-WASp is required for Schwann cell cytoskeletal dynamics, normal myelin gene expression and peripheral nerve myelination. Development. 2011;138:1329–1337. doi: 10.1242/dev.058677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefcort F, Venstrom K, McDonald JA, Reichardt LF. Regulation of expression of fibronectin and its receptor, alpha 5 beta 1, during development and regeneration of peripheral nerve. Development. 1992;116:767–782. doi: 10.1242/dev.116.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Liquari P, McKee KK, Harrison D, Patel R, Lee S, Yurchenco PD. Laminin-sulfatide binding initiates basement membrane assembly and enables receptor signaling in Schwann cells and fibroblasts. J Cell Biol. 2005;169:179–189. doi: 10.1083/jcb.200501098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Chattopadhyay N, Qin S, Szekeres C, Vasylyeva T, Mahoney ZX, Taglienti M, Bates CM, Chapman HA, Miner JH, Kreidberg JA. Coordinate integrin and c-Met signaling regulate Wnt gene expression during epithelial morphogenesis. Development. 2009;136:843–853. doi: 10.1242/dev.027805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer U, Saher G, Fässler R, Bornemann A, Echtermeyer F, von der Mark H, Miosge N, Pöschl E, von der Mark K. Absence of integrin alpha 7 causes a novel form of muscular dystrophy. Nat Genet. 1997;17:318–323. doi: 10.1038/ng1197-318. [DOI] [PubMed] [Google Scholar]
- McKee KK, Harrison D, Capizzi S, Yurchenco PD. Role of laminin terminal globular domains in basement membrane assembly. J Biol Chem. 2007;282:21437–21447. doi: 10.1074/jbc.M702963200. [DOI] [PubMed] [Google Scholar]
- McKee KK, Yang DH, Patel R, Chen ZL, Strickland S, Takagi J, Sekiguchi K, Yurchenco PD. Schwann cell myelination requires integration of laminin activities. J Cell Sci. 2012;125:4609–4619. doi: 10.1242/jcs.107995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing A, Behringer RR, Hammang JP, Palmiter RD, Brinster RL, Lemke G. P0 promoter directs expression of reporter and toxin genes to Schwann cells of transgenic mice. Neuron. 1992;8:507–520. doi: 10.1016/0896-6273(92)90279-M. [DOI] [PubMed] [Google Scholar]
- Michailov GV, Sereda MW, Brinkmann BG, Fischer TM, Haug B, Birchmeier C, Role L, Lai C, Schwab MH, Nave KA. Axonal neuregulin-1 regulates myelin sheath thickness. Science. 2004;304:700–703. doi: 10.1126/science.1095862. [DOI] [PubMed] [Google Scholar]
- Milner R, Wilby M, Nishimura S, Boylen K, Edwards G, Fawcett J, Streuli C, Pytela R, ffrench-Constant C. Division of labor of Schwann cell integrins during migration on peripheral nerve extracellular matrix ligands. Dev Biol. 1997;185:215–228. doi: 10.1006/dbio.1997.8547. [DOI] [PubMed] [Google Scholar]
- Miner JH, Yurchenco PD. Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol. 2004;20:255–284. doi: 10.1146/annurev.cellbio.20.010403.094555. [DOI] [PubMed] [Google Scholar]
- Nishiuchi R, Takagi J, Hayashi M, Ido H, Yagi Y, Sanzen N, Tsuji T, Yamada M, Sekiguchi K. Ligand-binding specificities of laminin-binding integrins: a comprehensive survey of laminin-integrin interactions using recombinant alpha3beta1, alpha6beta1, alpha7beta1 and alpha6beta4 integrins. Matrix Biol. 2006;25:189–197. doi: 10.1016/j.matbio.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Nodari A, Zambroni D, Quattrini A, Court FA, D'Urso A, Recchia A, Tybulewicz VL, Wrabetz L, Feltri ML. Beta1 integrin activates Rac1 in Schwann cells to generate radial lamellae during axonal sorting and myelination. J Cell Biol. 2007;177:1063–1075. doi: 10.1083/jcb.200610014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak N, Bar V, Sabanay H, Frechter S, Jaegle M, Snapper SB, Meijer D, Peles E. N-WASP is required for membrane wrapping and myelination by Schwann cells. J Cell Biol. 2011;192:243–250. doi: 10.1083/jcb.201010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Occhi S, Zambroni D, Del Carro U, Amadio S, Sirkowski EE, Scherer SS, Campbell KP, Moore SA, Chen ZL, Strickland S, Di Muzio A, Uncini A, Wrabetz L, Feltri ML. Both laminin and Schwann cell dystroglycan are necessary for proper clustering of sodium channels at nodes of Ranvier. J Neurosci. 2005;25:9418–9427. doi: 10.1523/JNEUROSCI.2068-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen JV, de Godoy LM, Li G, Macek B, Mortensen P, Pesch R, Makarov A, Lange O, Horning S, Mann M. Parts per million mass accuracy on an Orbitrap mass spectrometer via lock mass injection into a C-trap. Mol Cell Proteomics. 2005;4:2010–2021. doi: 10.1074/mcp.T500030-MCP200. [DOI] [PubMed] [Google Scholar]
- Pereira JA, Benninger Y, Baumann R, Gonçalves AF, Ozçelik M, Thurnherr T, Tricaud N, Meijer D, Fässler R, Suter U, Relvas JB. Integrin-linked kinase is required for radial sorting of axons and Schwann cell remyelination in the peripheral nervous system. J Cell Biol. 2009;185:147–161. doi: 10.1083/jcb.200809008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Previtali SC, Dina G, Nodari A, Fasolini M, Wrabetz L, Mayer U, Feltri ML, Quattrini A. Schwann cells synthesize alpha7beta1 integrin which is dispensable for peripheral nerve development and myelination. Mol Cell Neurosci. 2003a;23:210–218. doi: 10.1016/S1044-7431(03)00014-9. [DOI] [PubMed] [Google Scholar]
- Previtali SC, Nodari A, Taveggia C, Pardini C, Dina G, Villa A, Wrabetz L, Quattrini A, Feltri ML. Expression of laminin receptors in Schwann cell differentiation: evidence for distinct roles. J Neurosci. 2003b;23:5520–5530. doi: 10.1523/JNEUROSCI.23-13-05520.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raphael AR, Lyons DA, Talbot WS. ErbB signaling has a role in radial sorting independent of Schwann cell number. Glia. 2011;59:1047–1055. doi: 10.1002/glia.21175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasi K, Hurskainen M, Kallio M, Stavén S, Sormunen R, Heape AM, Avila RL, Kirschner D, Muona A, Tolonen U, Tanila H, Huhtala P, Soininen R, Pihlajaniemi T. Lack of collagen XV impairs peripheral nerve maturation and, when combined with laminin-411 deficiency, leads to basement membrane abnormalities and sensorimotor dysfunction. J Neurosci. 2010;30:14490–14501. doi: 10.1523/JNEUROSCI.2644-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riethmacher D, Sonnenberg-Riethmacher E, Brinkmann V, Yamaai T, Lewin GR, Birchmeier C. Severe neuropathies in mice with targeted mutations in the ErbB3 receptor. Nature. 1997;389:725–730. doi: 10.1038/39593. [DOI] [PubMed] [Google Scholar]
- Schiller HB, Fässler R. Mechanosensitivity and compositional dynamics of cell-matrix adhesions. EMBO Rep. 2013;14:509–519. doi: 10.1038/embor.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart HJ, Turner D, Jessen KR, Mirsky R. Expression and regulation of alpha1beta1 integrin in Schwann cells. J Neurobiol. 1997;33:914–928. doi: 10.1002/(SICI)1097-4695(199712)33:7<914::AID-NEU4>3.0.CO%3B2-B. [DOI] [PubMed] [Google Scholar]
- Taveggia C, Zanazzi G, Petrylak A, Yano H, Rosenbluth J, Einheber S, Xu X, Esper RM, Loeb JA, Shrager P, Chao MV, Falls DL, Role L, Salzer JL. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron. 2005;47:681–694. doi: 10.1016/j.neuron.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp BD, Nishiyama A, Cheng D, Macklin W. Differentiation and death of premyelinating oligodendrocytes in developing rodent brain. J Cell Biol. 1997;137:459–468. doi: 10.1083/jcb.137.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallquist W, Plantman S, Thams S, Thyboll J, Kortesmaa J, Lännergren J, Domogatskaya A, Ogren SO, Risling M, Hammarberg H, Tryggvason K, Cullheim S. Impeded interaction between Schwann cells and axons in the absence of laminin alpha4. J Neurosci. 2005;25:3692–3700. doi: 10.1523/JNEUROSCI.5225-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster HD, Martin R, O'Connell MF. The relationships between interphase Schwann cells and axons before myelination: a quantitative electron microscopic study. Dev Biol. 1973;32:401–416. doi: 10.1016/0012-1606(73)90250-9. [DOI] [PubMed] [Google Scholar]
- Wrabetz L, D'Antonio M, Pennuto M, Dati G, Tinelli E, Fratta P, Previtali S, Imperiale D, Zielasek J, Toyka K, Avila RL, Kirschner DA, Messing A, Feltri ML, Quattrini A. Different intracellular pathomechanisms produce diverse Myelin Protein Zero neuropathies in transgenic mice. J Neurosci. 2006;26:2358–2368. doi: 10.1523/JNEUROSCI.3819-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Bierman J, Tarumi YS, Zhong YP, Rangwala R, Proctor TM, Miyagoe-Suzuki Y, Takeda S, Miner JH, Sherman LS, Gold BG, Patton BL. Coordinate control of axon defasciculation and myelination by laminin-2 and -8. J Cell Biol. 2005;168:655–666. doi: 10.1083/jcb.200411158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu WM, Feltri ML, Wrabetz L, Strickland S, Chen ZL. Schwann cell-specific ablation of laminin gamma1 causes apoptosis and prevents proliferation. J Neurosci. 2005;25:4463–4472. doi: 10.1523/JNEUROSCI.5032-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]