Abstract
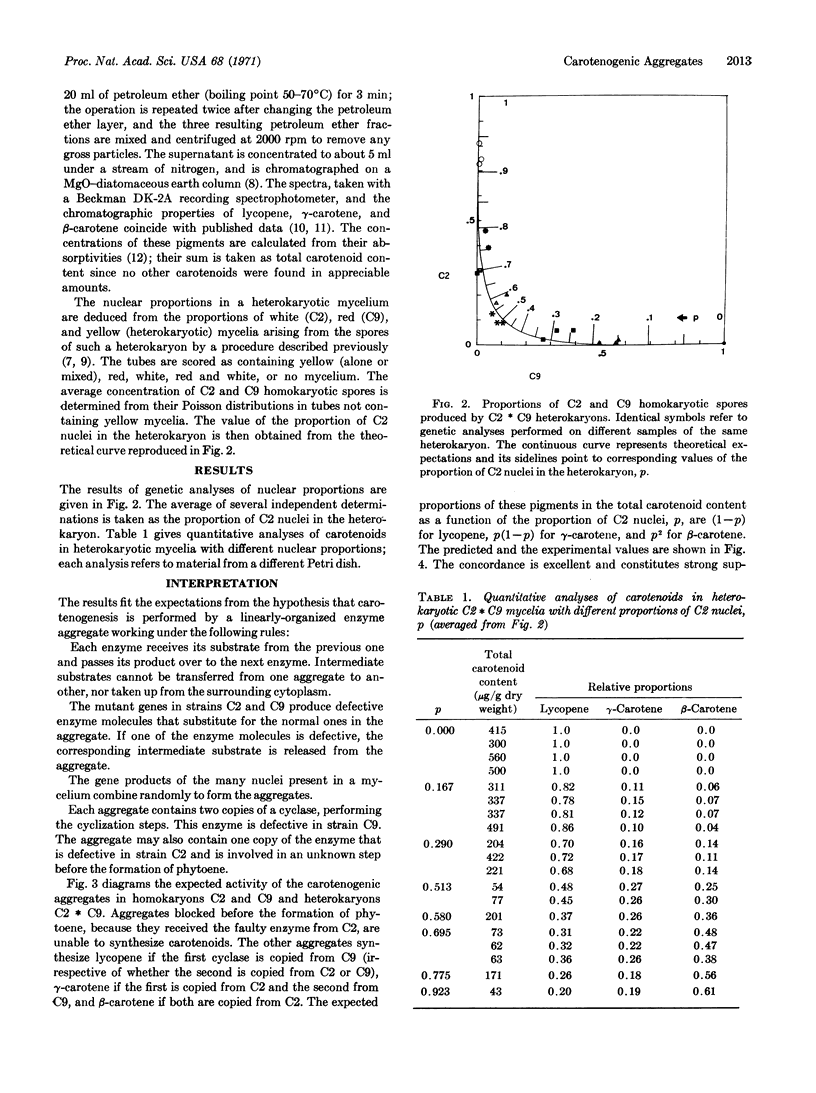
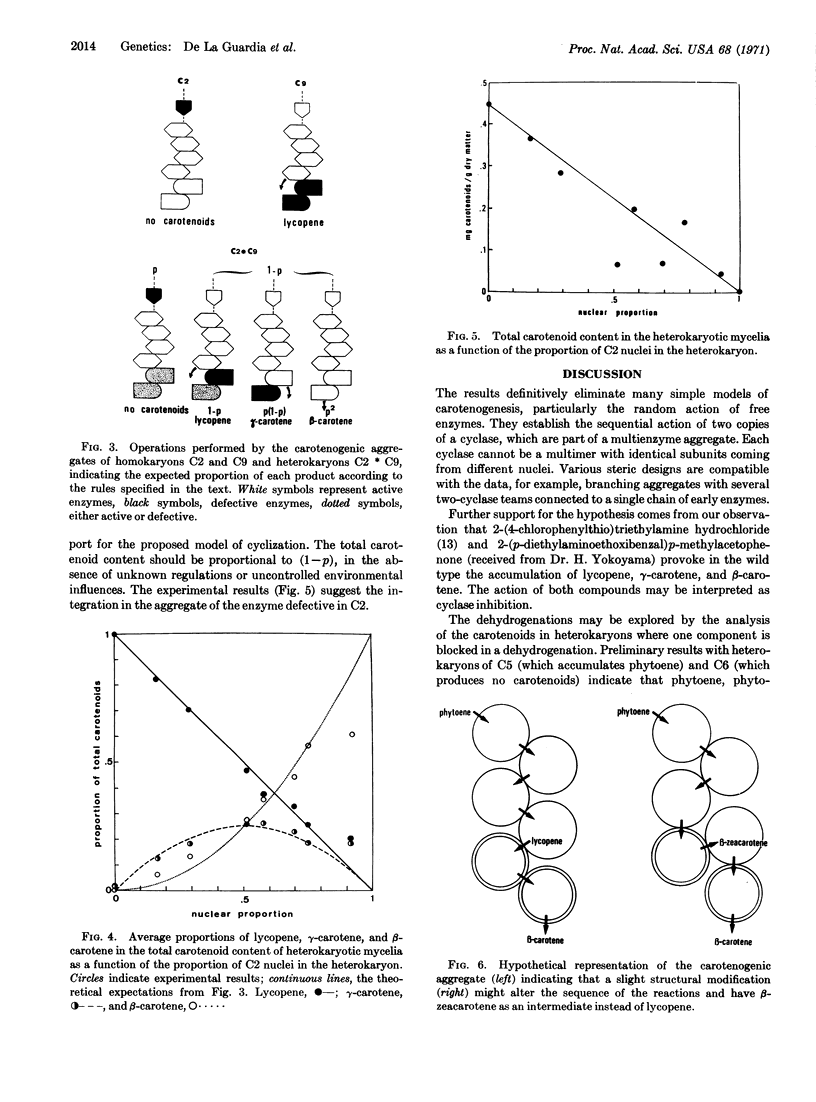
Wild-type Phycomyces blakesleeanus accumulates β-carotene, while the mutant strain C2 is unable to synthesize carotenoids, and the mutant strain C9 accumulates lycopene. Heterokaryons containing a proportion, p, of C2 nuclei and (l — p) of C9 nuclei accumulate lycopene, γ-carotene, and β-carotene in the relative amounts (l — p), p(l — p), and p2, respectively, for different values of p.
It is shown that these results are expected from the operation of a carotenogenic enzyme aggregate that works as an assembly line and contains two copies of a cyclase, which is defective in strain C9, as well as other enzymes.
Keywords: heterokaryons, mutants, lycopene, phytoene
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Case M. E., Giles N. H. Partial enzyme aggregates formed by pleiotropic mutants in the arom gene cluster of Neurospora crassa. Proc Natl Acad Sci U S A. 1971 Jan;68(1):58–62. doi: 10.1073/pnas.68.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerdá-Olmedo E., Reau P. Genetic classification of the lethal effects of various agents on heterokaryotic spores of Phycomyces. Mutat Res. 1970 Apr;9(4):369–384. doi: 10.1016/0027-5107(70)90019-9. [DOI] [PubMed] [Google Scholar]
- Coggins C. W., Jr, Henning G. L., Yokoyama H. Lycopene accumulation induced by 2-(4-chlorophenylthio)-triethylamine hydrochloride. Science. 1970 Jun 26;168(3939):1589–1590. doi: 10.1126/science.168.3939.1589. [DOI] [PubMed] [Google Scholar]
- GOODWIN T. W. Studies in carotenogenesis. III. Identification of the minor polyene components of the fungus Phycomyces blakesleeanus and a study of their synthesis under various cultural conditions. Biochem J. 1952 Feb;50(4):550–558. doi: 10.1042/bj0500550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg A., Stadtman E. R. Multienzyme systems. Annu Rev Biochem. 1970;39:429–472. doi: 10.1146/annurev.bi.39.070170.002241. [DOI] [PubMed] [Google Scholar]
- Heisenberg M., Cerdá-Olmedo E. Segregation of heterokaryons in the asexual cycle of Phycomyces. Mol Gen Genet. 1968;102(3):187–195. doi: 10.1007/BF00385973. [DOI] [PubMed] [Google Scholar]
- Meissner G., Delbruck M. Carotenes and retinal in Phycomyces mutants. Plant Physiol. 1968 Aug;43(8):1279–1283. doi: 10.1104/pp.43.8.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subden R. E., Turian G. A mechanism for carotenoid synthesis in fungi involving a multifunctional enzyme complex. Mol Gen Genet. 1970;108(4):358–364. doi: 10.1007/BF00267773. [DOI] [PubMed] [Google Scholar]


