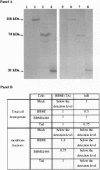
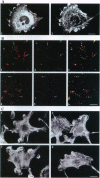
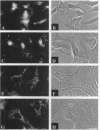
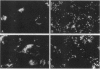
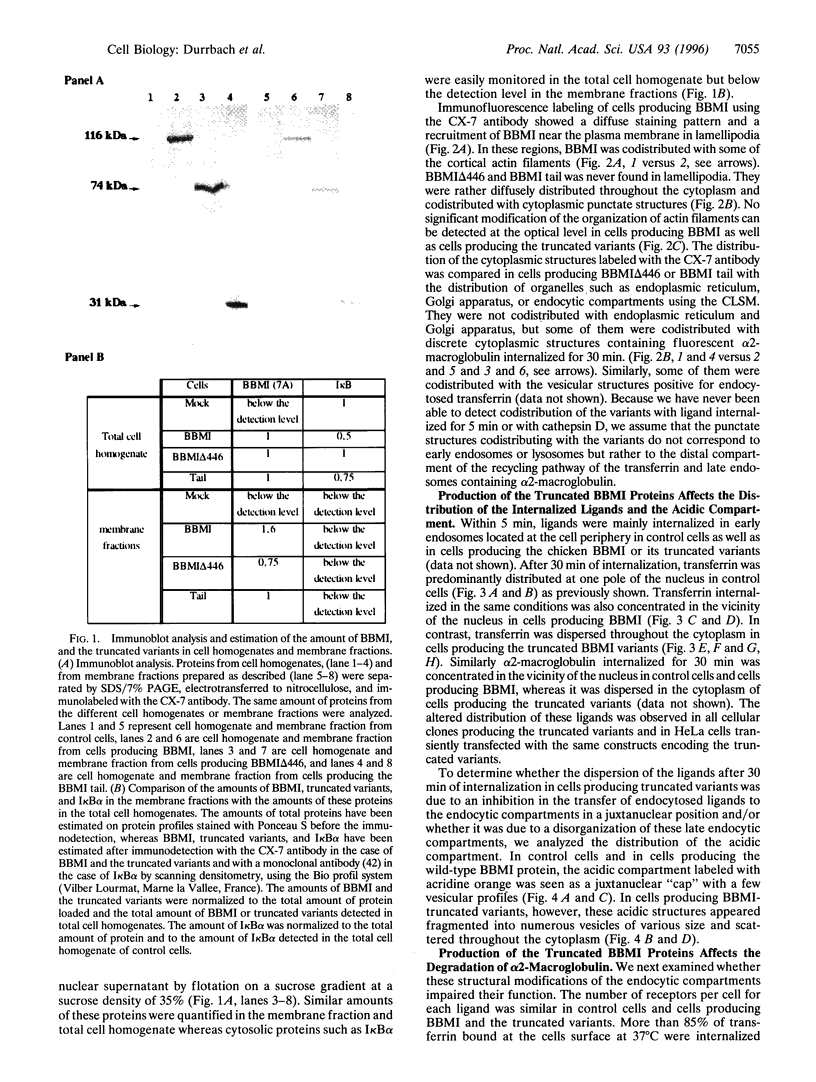
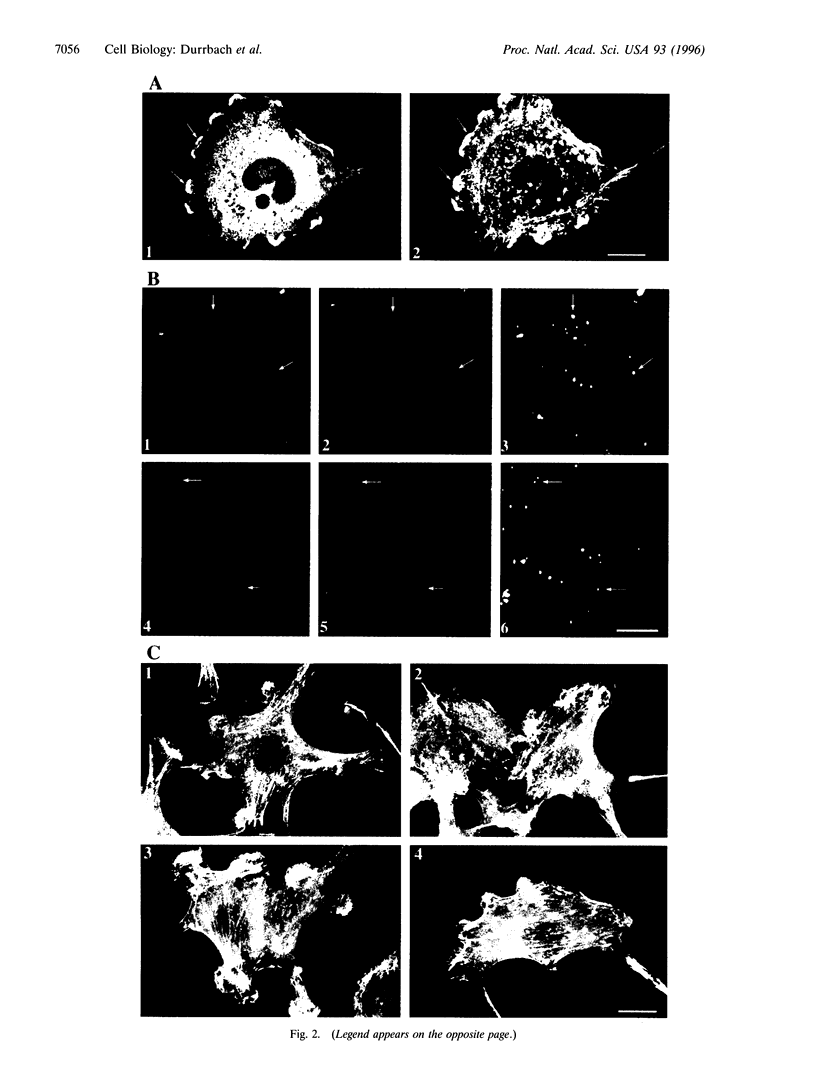
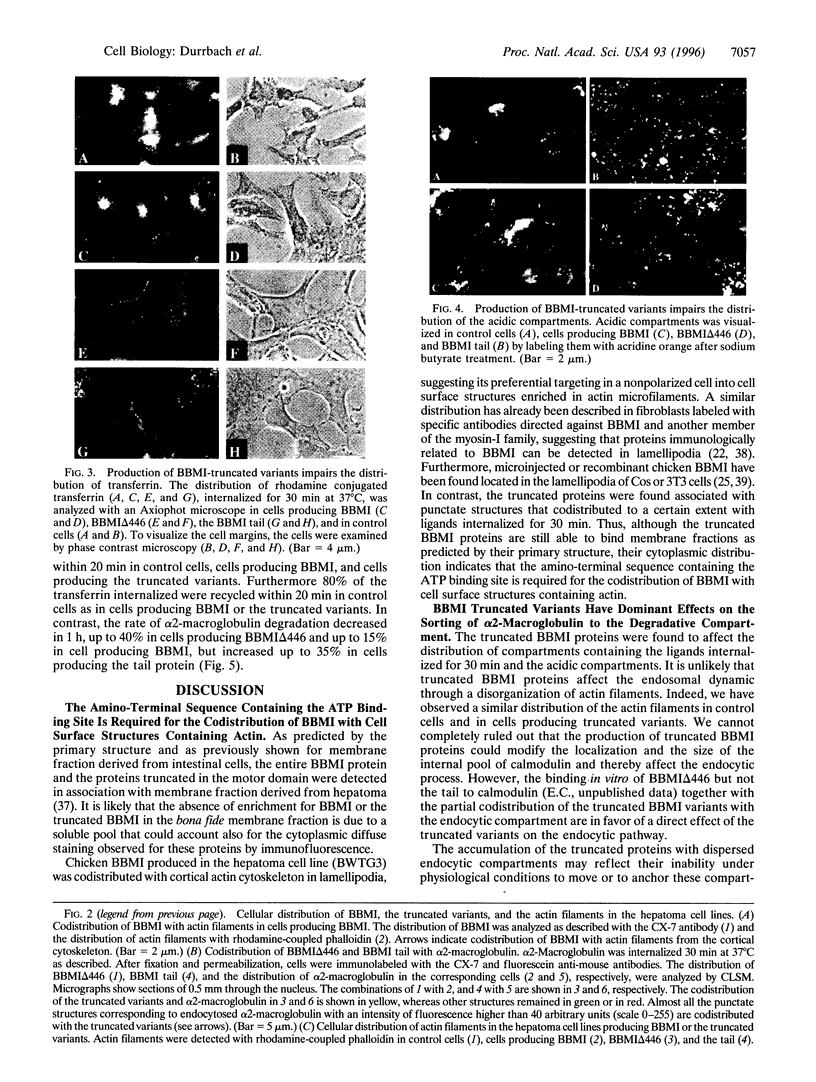
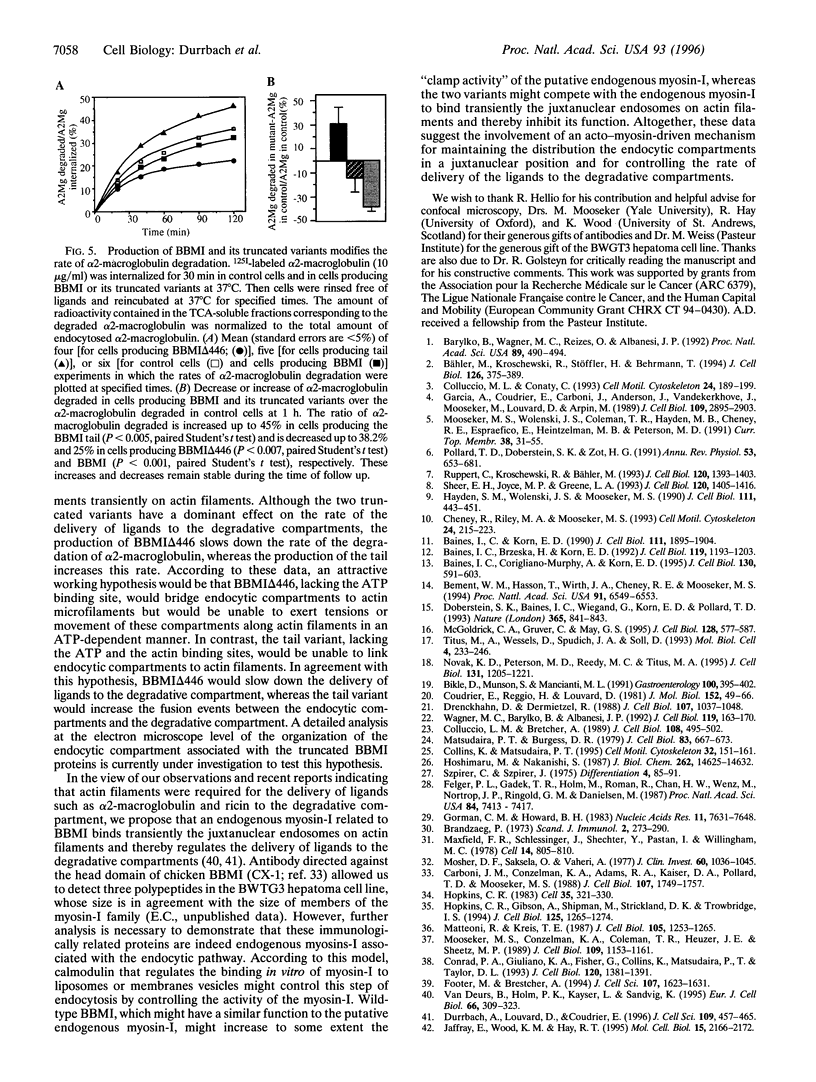
Abstract
Myosins I, a ubiquitous monomeric class of myosins that exhibits actin-based motor properties, are associated with plasma and/or vesicular membranes and have been suggested as players for trafficking events between cell surface and intracellular membranous structures. To investigate the function of myosins 1, we have transfected a mouse hepatoma cell line (BWTG3) with cDNAs encoding the chicken brush border myosin-I (BBMI) and two variants truncated in the motor domain. One variant is deleted of the first 446 amino acids and thereby lacks the ATP binding site, whereas the other is deleted of the entire motor domain and lacks the ATP and actin binding sites. We have observed (i) that significant amounts of the truncated variants are recovered with membrane fractions after cell fractionation, (ii) that they codistribute with a compartment containing alpha2-macroglobulin internalized for 30 min as determined by fluorescent microscopy, (iii) that the production of BBMI-truncated variants impairs the distribution of the acidic compartment and ligands internalized for 30 min, and (iv) that the production of the truncated variant containing the actin binding site decreases the rate of alpha2-macroglobulin degradation whereas the production of the variant lacking the ATP binding site and the actin binding site increases the rate of a2-macroglobulin degradation. These observations indicate that the two truncated variants have a dominant negative effect on the distribution and the function of the endocytic compartments. We propose that an unidentified myosin-I might contribute to the distribution of endocytic compartments in a juxtanuclear position and/or to the regulation of the delivery of ligands to the degradative compartment in BWTG3 cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baines I. C., Brzeska H., Korn E. D. Differential localization of Acanthamoeba myosin I isoforms. J Cell Biol. 1992 Dec;119(5):1193–1203. doi: 10.1083/jcb.119.5.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines I. C., Corigliano-Murphy A., Korn E. D. Quantification and localization of phosphorylated myosin I isoforms in Acanthamoeba castellanii. J Cell Biol. 1995 Aug;130(3):591–603. doi: 10.1083/jcb.130.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines I. C., Korn E. D. Localization of myosin IC and myosin II in Acanthamoeba castellanii by indirect immunofluorescence and immunogold electron microscopy. J Cell Biol. 1990 Nov;111(5 Pt 1):1895–1904. doi: 10.1083/jcb.111.5.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barylko B., Wagner M. C., Reizes O., Albanesi J. P. Purification and characterization of a mammalian myosin I. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):490–494. doi: 10.1073/pnas.89.2.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bement W. M., Hasson T., Wirth J. A., Cheney R. E., Mooseker M. S. Identification and overlapping expression of multiple unconventional myosin genes in vertebrate cell types. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6549–6553. doi: 10.1073/pnas.91.14.6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle D. D., Munson S., Mancianti M. L. Limited tissue distribution of the intestinal brush border myosin I protein. Gastroenterology. 1991 Feb;100(2):395–402. doi: 10.1016/0016-5085(91)90208-3. [DOI] [PubMed] [Google Scholar]
- Bähler M., Kroschewski R., Stöffler H. E., Behrmann T. Rat myr 4 defines a novel subclass of myosin I: identification, distribution, localization, and mapping of calmodulin-binding sites with differential calcium sensitivity. J Cell Biol. 1994 Jul;126(2):375–389. doi: 10.1083/jcb.126.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carboni J. M., Conzelman K. A., Adams R. A., Kaiser D. A., Pollard T. D., Mooseker M. S. Structural and immunological characterization of the myosin-like 110-kD subunit of the intestinal microvillar 110K-calmodulin complex: evidence for discrete myosin head and calmodulin-binding domains. J Cell Biol. 1988 Nov;107(5):1749–1757. doi: 10.1083/jcb.107.5.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney R. E., Riley M. A., Mooseker M. S. Phylogenetic analysis of the myosin superfamily. Cell Motil Cytoskeleton. 1993;24(4):215–223. doi: 10.1002/cm.970240402. [DOI] [PubMed] [Google Scholar]
- Conrad P. A., Giuliano K. A., Fisher G., Collins K., Matsudaira P. T., Taylor D. L. Relative distribution of actin, myosin I, and myosin II during the wound healing response of fibroblasts. J Cell Biol. 1993 Mar;120(6):1381–1391. doi: 10.1083/jcb.120.6.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coudrier E., Reggio H., Louvard D. Immunolocalization of the 110,000 molecular weight cytoskeletal protein of intestinal microvilli. J Mol Biol. 1981 Oct 15;152(1):49–66. doi: 10.1016/0022-2836(81)90095-4. [DOI] [PubMed] [Google Scholar]
- Doberstein S. K., Baines I. C., Wiegand G., Korn E. D., Pollard T. D. Inhibition of contractile vacuole function in vivo by antibodies against myosin-I. Nature. 1993 Oct 28;365(6449):841–843. doi: 10.1038/365841a0. [DOI] [PubMed] [Google Scholar]
- Drenckhahn D., Dermietzel R. Organization of the actin filament cytoskeleton in the intestinal brush border: a quantitative and qualitative immunoelectron microscope study. J Cell Biol. 1988 Sep;107(3):1037–1048. doi: 10.1083/jcb.107.3.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrbach A., Louvard D., Coudrier E. Actin filaments facilitate two steps of endocytosis. J Cell Sci. 1996 Feb;109(Pt 2):457–465. doi: 10.1242/jcs.109.2.457. [DOI] [PubMed] [Google Scholar]
- Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Footer M., Bretscher A. Brush border myosin-I microinjected into cultured cells is targeted to actin-containing surface structures. J Cell Sci. 1994 Jun;107(Pt 6):1623–1631. doi: 10.1242/jcs.107.6.1623. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Howard B. H., Reeves R. Expression of recombinant plasmids in mammalian cells is enhanced by sodium butyrate. Nucleic Acids Res. 1983 Nov 11;11(21):7631–7648. doi: 10.1093/nar/11.21.7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins C. R., Gibson A., Shipman M., Strickland D. K., Trowbridge I. S. In migrating fibroblasts, recycling receptors are concentrated in narrow tubules in the pericentriolar area, and then routed to the plasma membrane of the leading lamella. J Cell Biol. 1994 Jun;125(6):1265–1274. doi: 10.1083/jcb.125.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins C. R. Intracellular routing of transferrin and transferrin receptors in epidermoid carcinoma A431 cells. Cell. 1983 Nov;35(1):321–330. doi: 10.1016/0092-8674(83)90235-0. [DOI] [PubMed] [Google Scholar]
- Hoshimaru M., Nakanishi S. Identification of a new type of mammalian myosin heavy chain by molecular cloning. Overlap of its mRNA with preprotachykinin B mRNA. J Biol Chem. 1987 Oct 25;262(30):14625–14632. [PubMed] [Google Scholar]
- Jaffray E., Wood K. M., Hay R. T. Domain organization of I kappa B alpha and sites of interaction with NF-kappa B p65. Mol Cell Biol. 1995 Apr;15(4):2166–2172. doi: 10.1128/mcb.15.4.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira P. T., Burgess D. R. Identification and organization of the components in the isolated microvillus cytoskeleton. J Cell Biol. 1979 Dec;83(3):667–673. doi: 10.1083/jcb.83.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteoni R., Kreis T. E. Translocation and clustering of endosomes and lysosomes depends on microtubules. J Cell Biol. 1987 Sep;105(3):1253–1265. doi: 10.1083/jcb.105.3.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGoldrick C. A., Gruver C., May G. S. myoA of Aspergillus nidulans encodes an essential myosin I required for secretion and polarized growth. J Cell Biol. 1995 Feb;128(4):577–587. doi: 10.1083/jcb.128.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooseker M. S., Conzelman K. A., Coleman T. R., Heuser J. E., Sheetz M. P. Characterization of intestinal microvillar membrane disks: detergent-resistant membrane sheets enriched in associated brush border myosin I (110K-calmodulin). J Cell Biol. 1989 Sep;109(3):1153–1161. doi: 10.1083/jcb.109.3.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher D. F., Saksela O., Vaheri A. Synthesis and secretion of alpha-2-macroglobulin by cultured adherent lung cells. Comparison with cell strains derived from other tissues. J Clin Invest. 1977 Nov;60(5):1036–1045. doi: 10.1172/JCI108854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak K. D., Peterson M. D., Reedy M. C., Titus M. A. Dictyostelium myosin I double mutants exhibit conditional defects in pinocytosis. J Cell Biol. 1995 Dec;131(5):1205–1221. doi: 10.1083/jcb.131.5.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard T. D., Doberstein S. K., Zot H. G. Myosin-I. Annu Rev Physiol. 1991;53:653–681. doi: 10.1146/annurev.ph.53.030191.003253. [DOI] [PubMed] [Google Scholar]
- Ruppert C., Kroschewski R., Bähler M. Identification, characterization and cloning of myr 1, a mammalian myosin-I. J Cell Biol. 1993 Mar;120(6):1393–1403. doi: 10.1083/jcb.120.6.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr E. H., Joyce M. P., Greene L. A. Mammalian myosin I alpha, I beta, and I gamma: new widely expressed genes of the myosin I family. J Cell Biol. 1993 Mar;120(6):1405–1416. doi: 10.1083/jcb.120.6.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpirer C., Szpirer J. A mouse hepatoma cell line which secretes several serum proteins including albumin and alpha-foetoprotein. Differentiation. 1975 Oct 16;4(2):85–91. doi: 10.1111/j.1432-0436.1975.tb01446.x. [DOI] [PubMed] [Google Scholar]
- Titus M. A., Wessels D., Spudich J. A., Soll D. The unconventional myosin encoded by the myoA gene plays a role in Dictyostelium motility. Mol Biol Cell. 1993 Feb;4(2):233–246. doi: 10.1091/mbc.4.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M. C., Barylko B., Albanesi J. P. Tissue distribution and subcellular localization of mammalian myosin I. J Cell Biol. 1992 Oct;119(1):163–170. doi: 10.1083/jcb.119.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deurs B., Holm P. K., Kayser L., Sandvig K. Delivery to lysosomes in the human carcinoma cell line HEp-2 involves an actin filament-facilitated fusion between mature endosomes and preexisting lysosomes. Eur J Cell Biol. 1995 Apr;66(4):309–323. [PubMed] [Google Scholar]