Abstract
Background
Tafenoquine (TQ) is an 8-aminoquinoline (8AQ) that has been tested in several Phase II and Phase III clinical studies and is currently in late stage development as an anti-malarial prophylactic agent. NPC-1161B is a promising 8AQ in late preclinical development. It has recently been reported that the 8AQ drug primaquine requires metabolic activation by CYP 2D6 for efficacy in humans and in mice, highlighting the importance of pharmacogenomics in the target population when administering primaquine. A logical follow-up study was to determine whether CYP 2D activation is required for other compounds in the 8AQ structural class.
Methods
In the present study, the anti-malarial activities of NPC-1161B and TQ were assessed against luciferase expressing Plasmodium berghei in CYP 2D knock-out mice in comparison with normal C57BL/6 mice (WT) and with humanized/CYP 2D6 knock-in mice by monitoring luminescence with an in vivo imaging system. These experiments were designed to determine the direct effects of CYP 2D metabolic activation on the anti-malarial efficacy of NPC-1161B and TQ.
Results
NPC-1161B and TQ exhibited no anti-malarial activity in CYP 2D knock-out mice when dosed at their ED100 values (1 mg/kg and 3 mg/kg, respectively) established in WT mice. TQ anti-malarial activity was partially restored in humanized/CYP 2D6 knock-in mice when tested at two times its ED100.
Conclusions
The results reported here strongly suggest that metabolism of NPC-1161B and TQ by the CYP 2D enzyme class is essential for their anti-malarial activity. Furthermore, these results may provide a possible explanation for therapeutic failures for patients who do not respond to 8AQ treatment for relapsing malaria. Because CYP 2D6 is highly polymorphic, variable expression of this enzyme in humans represents a significant pharmacogenomic liability for 8AQs which require CYP 2D metabolic activation for efficacy, particularly for large-scale prophylaxis and eradication campaigns.
Background
The 8-aminoquinoline (8AQ) class of anti-malarial compounds is extremely important in the fight against malaria, as this class of molecules is unique due to the efficacy against relapsing forms of malaria [1,2]. This activity is a result of the anti-hypnozoite activity of the 8AQ class [2,3]. This attribute along with gametocytocidal activity of primaquine (PQ) and other 8AQs make the class attractive for mass administration in efforts towards malaria eradication [4].
Recent advances in understanding the mechanism of action for the 8AQ drug PQ have been reported [5-7]. PQ is metabolized by several different CYP enzymes as well as monoamine oxidases [5-10], however, the anti-malarial activity of PQ is mediated through CYP 2D6-dependent activation to phenolic metabolites [6,7]. These metabolites are capable of redox cycling and generating reactive oxygen species [10-14], which is likely responsible for anti-malarial activity. The activity of PQ was shown to be dependent on CYP 2D6 activation in mouse models and a human clinical trial. The results reported by Pybus et al. clearly demonstrated in a mouse CYP 2D knock-out model that PQ was inactive against Plasmodium berghei and that the anti-malarial activity was partially restored at its ED100 dose in a humanized/CYP 2D6 knock-in mouse strain [6]. Bennett et al. demonstrated an association between poor and intermediate CYP 2D6 metabolizer phenotypes and failure of PQ for radical cure of P. vivax in two human subjects [15].
CYP 2D6 belongs to the cytochrome P450 super family of enzymes that are responsible for a variety of metabolic and biosynthetic processes [16]. Humans have several different hepatic CYP P450 enzymes that metabolize drugs to include CYP 1A1, 1A2, 2A6, 2B6, 2C19, 2C9, 2D6, 2E1, and 3A4. Each CYP isoform has different substrate preferences and rates of metabolism (for a review on the CYP P450 super family see [16]). Of the hepatic CYP P450 enzymes, CYP 2D6 is estimated to make up only four percent of the total human P450 liver content, yet it is estimated to metabolize 20-25% of commonly used drugs. Despite the importance of CYP 2D6 in drug metabolism, the enzyme is highly polymorphic in various human populations with more than 74 alleles reported to date [17]. CYP 2D6 phenotypes can be classified into four groups: poor metabolizers (PMs), intermediate metabolizers (IMs), extensive metabolizers (EMs), and ultra-rapid metabolizers (UMs). The frequency of PMs varies among populations and can have large interethnic differences with estimates in the range of 0-19% in African populations, 0–4.8% in Asian populations, and 1.5-8.9% in Caucasian populations (for a more detailed description of CYP 2D6 genotypes and PM frequencies see [18-20]). These genetic and phenotypic differences contribute to significant differences in the metabolism of CYP 2D6 substrates.
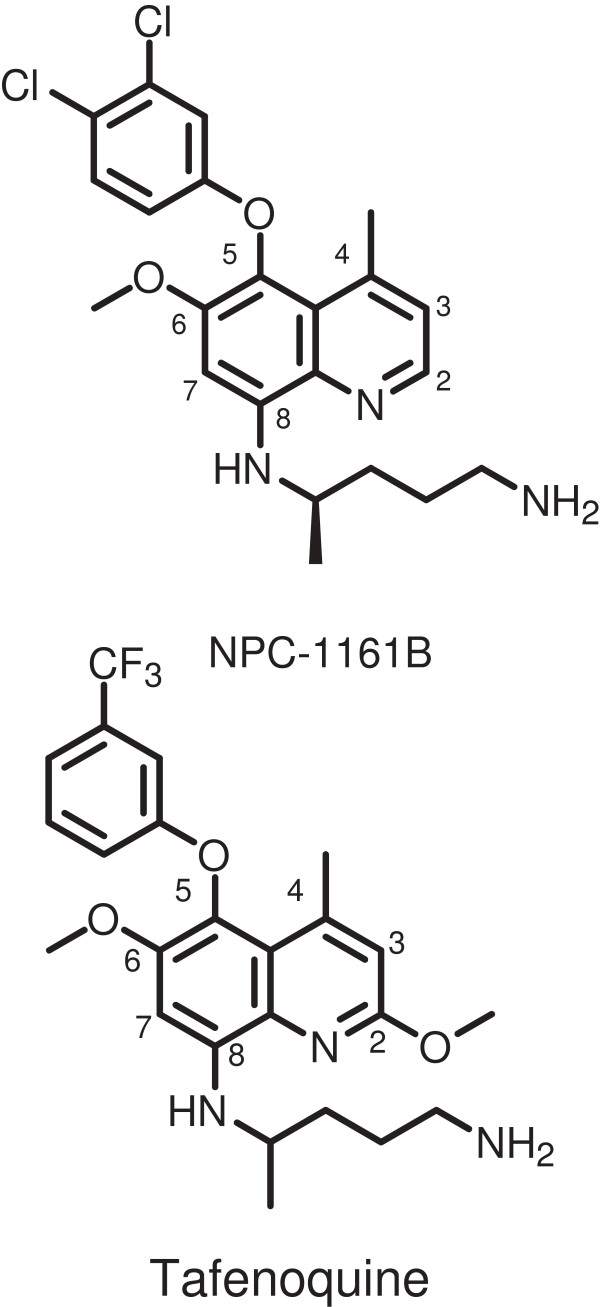
Previous studies with PQ indicated that CYP 2D6 metabolism was required for anti-malarial activity [6] and it remained unclear if other 8AQ compounds similarly require CYP 2D6 activation. For this purpose, the 8AQ compounds NPC-1161B and tafenoquine (TQ) (Figure 1) were chosen and tested using CYP 2D knock-out and humanized/CYP 2D6 knock-in mice. The CYP 2D knock-out mice have a deletion of the mouse CYP 2D gene cluster and do not express a functional CYP 2D enzyme capable of metabolizing CYP 2D6 substrates [21]. The humanized/CYP 2D6 knock-in mice have the deletion of the mouse CYP 2D gene cluster, which is replaced with a human CYP2D6 expression cassette [21]. Both NPC-1161B and TQ are promising 8AQ molecules in development as TQ has been tested in several Phase II and III clinical studies [22-26] and is in late stage development as an anti-malarial prophylactic agent. NPC-1161B is a promising 8AQ in late preclinical development. Both compounds have an O-aryl substituent at the 5 position. In part, these compounds were selected to determine whether this key difference with respect to PQ might overcome/alter CYP 2D dependence for efficacy.
Figure 1.
8-aminoquinoline anti-malarial compounds NPC-1161B and tafenoquine. Shown are the structures of the compounds utilized in this study. The quinoline rings are numbered (2–8) for reference.
Methods
Chemicals used
NPC-1161B was supplied by Dr. Larry Walker and Dr. Dhammika Nanayakkara from the Center for Natural Products Research, University of Mississippi, and TQ was obtained from the Walter Reed Army Institute of Research chemical repository.
Plasmodium berghei sporozoites isolation, inoculation and viability check
Plasmodium berghei sporozoites (luciferase expressing) were obtained from laboratory-reared female Anopheles stephensi and maintained at 18°C for 17 to 22 days after feeding on malaria-infected Swiss CD-1/ICR mice. Salivary glands were extracted from malaria-infected mosquitoes and sporozoites obtained. Briefly, mosquitoes were separated into abdomen and head/thorax. Heads and thoraxes were triturated with a mortar and pestle and suspended in medium RPMI 1640 containing 1% C57BL/6 mouse serum (Rockland Co, Gilbertsville, PA, USA). A total of 50–80 heads with glands were placed into a 0.5 ml Osaki tube on top of glass wool with enough dissection media to cover the heads. The Osaki tube was kept on ice until all mosquitoes had been dissected. Sporozoites isolated from the same batch of mosquitoes were inoculated into C57BL/6, 2D knock-out and 2D knock-out/2D6 knock-in C57BL/6 mice on the same day to control for biological variability in sporozoite preparations. Each mouse was inoculated intravenously in the tail vein with approximately 10,000 sporozoites suspended in 0.1 ml volume on day 0.
To ensure that inoculated sporozoites were viable following the isolation procedure, they were stained with a vital dye containing fluorescein diacetate (50 mg/ml in acetone) and ethidium bromide (20 μg/ml in phosphate-buffered saline; Sigma Chemical Co, St. Louis, MO, USA) and counted in a haemocytometer. The viability of sporozoites ranged from 90 to 100%.
Animals
Male eight- to 12-week old C57BL/6, 2D knock-out and 2D knock-out/2D6 knock-in C57BL/6 mice (Taconic, Hudson, NY, USA) were used. On arrival, the animals were acclimated for seven days (quarantine). The animals were housed in a cage maintained in a room with a temperature range of 64-79°F, 34-68% relative humidity and a 12-hr light/dark cycles. Food and water were provided during quarantine and throughout the study. The animals were fed a standard rodent maintenance diet. All animal studies were performed under IACUC approved protocols. All animal use, care and handling was performed in accordance with the current Guide for the Care and Use of Laboratory Animals (1996).
Test compounds and administration
Compounds tested in these experiments were dosed based on the body weight at the time of preparation of the suspension solution. The suspension solution of oral agents were prepared in 0.5% (w/v) hydroxyethyl cellulose and 0.2% (0.5% HECT, v/v) Tween-80 in distilled water, using homogenizer (PRO Scientific Inc, Monroe, CT, USA) with 10 mm open-slotted generator to homogenize drug powder mixture at 20,000-22,000 rpm for 5 min in ice bath. A once-a-day, three consecutive day-treatment regimen (−1, 0, 1 day) was used in all assessments. Drug suspensions were transferred to a 20-ml bottle, drawn into a 1-ml syringe, and delivered via intragastric feeder (18 gauge) to the designated recipient.
In vivo imaging system spectrum
All in vivo imaging system (IVIS) methods utilized have been described previously [6]. Briefly NPC-1161B and TQ were administered orally on days −1, 0 and 1 with respect to sporozoite inoculation. At 24, 48 and 72 hr post-sporozoite infection, all inoculated mice were tested using the Xenogen IVIS-200 Spectrum (Caliper Life Sciences, Hopkinton, MA, USA) IVIS instrument. Additionally, blood-stage infections were measured by a flow cytometry system (FC500 MPL, Beckman Coulter, Miami, FL, USA). Positive and negative controls were used for the IVIS calibration in each test. D-Luciferin potassium salt, (Xenogen, California and Goldbio, St Louis, MO, USA), the luciferase substrate, was intraperitoneally inoculated into mice at a concentration of 200 mg/kg 15 min before luminescence analysis. Three min post-luciferin administration the mice were anesthetized with isoflurane. The mice were then positioned ventral side up in the IVIS on the 37°C platform. The mice continued to receive isoflurane through nose cone delivery. The camera exposure time was 5 min for the 24, 48 and 72 hr time points with f-stop = 1 and large binning setting. Photons emitted from specific regions were quantified using Living Image® 3.0 software.
Results
NPC-1161B and tafenoquine efficacy in CYP 2D knock-out mice
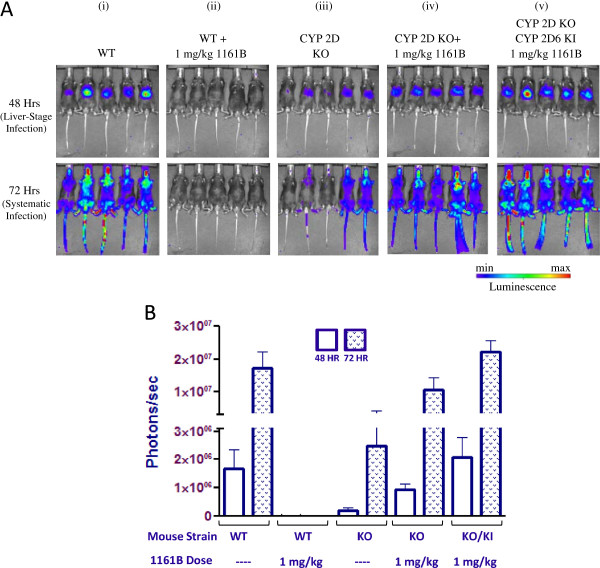
In order to determine if NPC-1161B (Figure 1) requires activation through CYP 2D metabolism, NPC-1161B was tested at its ED100 value (1 mg/kg) in C57BL/6 mice infected with luciferase expressing P. berghei. The IVIS luminescence results for NPC-1161B are shown in Figure 2A and quantitated luminescence values from parasite burden in Figure 2B. The 48-hr IVIS measurements indicate levels of P. berghei liver infection while the 72-hr measurements correspond to systemic P. berghei infection. Of the five wild type (WT) (C57BL/6) mice infected with sporozoites and not treated with NPC-1161B, all five exhibited robust luminescence signal as indicated by the IVIS images at 48 and 72 hr (panel i). The 1161B-treated WT group exhibited no luminescence signal at either 48 or 72 hr, indicating that NPC-1161B prevented P. berghei infection in WT mice (panel ii). Panel iii shows the results from CYP 2D knock-out mice infected with P. berghei as an infection control. Despite lower luminescence signals in this group, five of the five mice showed parasite burden/infection throughout the experiment. When NPC-1161B was tested at its ED100 in mice containing a deletion of the nine mouse CYP 2D genes (nearest mouse orthologue to human CYP 2D6), there was comparable luminescence signal to the WT control P. berghei infected mice at both 48 and 72 hr (panel iv) indicating that NPC-1161B was inactive in the CYP 2D knock-out mice. To investigate the effect of re-introducing a CYP 2D enzyme, NPC-1161B was tested at its ED100 in a humanized mouse strain where the CYP 2D gene cluster has been removed and replaced with the human CYP 2D6 gene and infected with P. berghei sporozoites (panel v). Of the five mice tested, all five mice had similar luminescence signals as compared to the WT control P. berghei-infected mice not treated with any compound. These results indicate that replacing the CYP 2D gene cluster with human CYP 2D6 was not sufficient to fully restore the activity of NPC-1161B.
Figure 2.
Dissemination of malaria parasites in C57BL/6 wild-type (WT), CYP 2D knock-out, and humanized/CYP 2D6 knock-in mice 48 and 72 hr post-inoculation with luciferase expressing Plasmodium berghei in the absence or presence of NPC-1161B. A (i-v). IVIS images of various mouse strains 48 and 72 hr post-inoculation. The mice of each group tested are shown and the corresponding luminescence signal indicated. B. Quantitated luminescence signal for each group tested 48 and 72 hr post-inoculation.
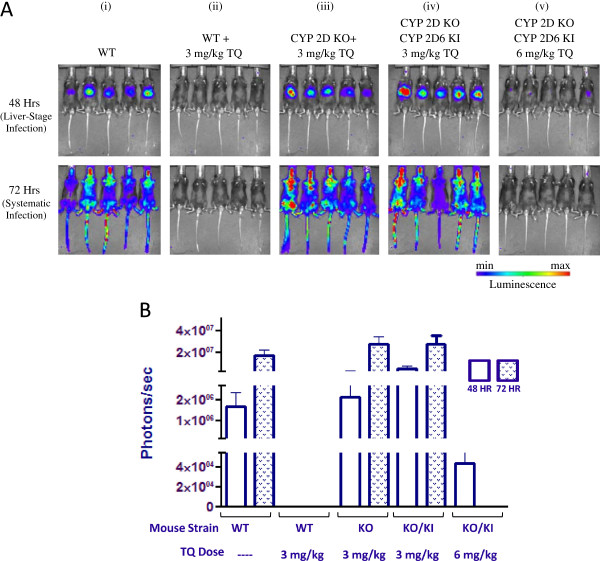
Tafenoquine (Figure 1) was also tested in the CYP 2D knock-out mouse model. IVIS results are shown in Figure 3A and quantitated luminescence values from parasite burden in Figure 3B. Panel i shows the WT mice infected with P. berghei and panel ii shows the P. berghei-infected WT mice treated with TQ at its ED100 (3 mg/kg). There was no luminescence signal at either 48 or 72 hr post -infection in five out of the five mice treated, indicating that TQ prevented P. berghei infection. Panel iii shows P. berghei-infected CYP 2D knock-out mice treated with TQ at its ED100 (3 mg/kg). Each of the five mice showed robust luminescence indicating that TQ was not metabolized to the active form of the molecule required for anti-malarial activity when the CYP 2D gene cluster was removed. When TQ was tested in the humanized/CYP 2D6 knock-in mouse strain at its ED100, five out of the five mice showed robust luminescence (panel iv), indicating that replacing the CYP 2D gene cluster with human CYP 2D6 was not sufficient to fully restore the activity of TQ. To determine if this effect could be compensated for, TQ was tested at two times its ED100 in the humanized/CYP 2D6 knock-in mouse strain. The results in panel v show that there was less luminescence signal in all five mice at 48 hr (as compared to WT-infected controls) and no detectable luminescence signal at 72 hr post-inoculation.
Figure 3.
Dissemination of malaria parasites in C57BL/6 wild-type (WT), CYP 2D knock-out, and humanized/CYP 2D6 knock-in mice 48 and 72 hr post-inoculation with luciferase expressing Plasmodium berghei in the absence or presence of tafenoquine. A (i-v). IVIS images of various mouse strains 48 and 72 hr post-inoculation. The mice of each group tested are shown and the corresponding luminescence signal indicated. B. Quantitated luminescence signal for each group tested 48 and 72 hr post-inoculation.
The results in Figures 2 and 3 correspond to P. berghei infection 48 and 72 hr post-inoculation. In order to determine if the initial IVIS measurements translated to systemic infection, blood stage parasite burden measurements were made out to 31 days post-inoculation and the results shown in Table 1. The mouse strains tested along with the corresponding NPC-1161B/TQ doses given are indicated. The results for NPC-1161B (panel i) are consistent with those shown in Figure 2 in that five out of five WT mice treated with NPC-1161B never developed P. berghei infection. All other NPC-1161B groups were euthanized before day 22 due to blood stage parasite burden.
Table 1.
Cure rates in C57BL/6 wild-type (WT), CYP 2D knock-out, and humanized/CYP 2D6 knock-in mice 31 days post-inoculation with Plasmodium berghei in the absence or presence of NPC-1161B and tafenoquine
| NCP-1161B | |||
|---|---|---|---|
|
i |
Mouse strain |
1161B Dose |
Cure |
| |
WT |
- |
0/5 |
| |
WT |
ED
100
|
5/5 |
| |
2DKO |
- |
0/5 |
| |
2DKO |
ED
100
|
0/5 |
| |
2DKO/2D6KI |
ED
100
|
0/5 |
|
Tafenoquine (TQ) | |||
|
ii |
Mouse strain |
TQ Dose |
Cure |
| |
WT |
- |
0/5 |
| |
WT |
ED
100
|
5/5 |
| |
2DKO |
- |
0/5 |
| |
2DKO |
ED
100
|
0/5 |
| |
2DKO/2D6KI |
ED
100
|
0/5 |
| 2DKO/2D6KI | 2 × ED 100 | 4/5 | |
A cure is indicated if there was no detectable parasite burden in blood measurements after 31 days post-inoculation.
Tafenoquine blood parasite burden results are shown in Table 1 and are consistent with the IVIS results shown in Figure 3. Five out of five WT mice treated with TQ had no detectable parasite burden after 31 days, in comparison to the WT infection control or the CYP 2D knock-out mice treated with TQ. TQ was only active in the humanized/CYP 2D6 knock-in mice at two times its ED100. Four out of five mice were cured in the two times ED100 group. All other TQ groups were euthanized before day 22 due to blood parasite burden. These results along with those above for NPC-1161B illustrate that both TQ and NPC-1161B are active in the presence of the CYP 2D gene cluster. Activity was partially restored when the dose of TQ was adjusted to account for differences between mouse CYP 2D and human CYP 2D6 enzyme activity.
Conclusions
Here direct preclinical evidence is presented that the 8AQ compounds NPC-1161B and TQ require metabolism by the CYP 2D enzyme class for activity. Human CYP 2D6 is involved in the metabolism of 20-25% of commonly used drugs on the market and is the most polymorphic of all CYP P450 enzymes [18,20]. These data demonstrate the critical role that CYP 2D enzymes play in the metabolic activation of NPC-1161B and TQ to the active form(s) of the molecules responsible for anti-malarial activity.
As might be expected, there appear to be differences between the mouse and human CYP 2D enzymes, as the anti-malarial activity of NPC-1161B and TQ were not restored in the CYP 2D knock-out/humanized/CYP 2D6 knock-in mice when tested at their respective ED100 (1 mg/kg and 3 mg/kg, respectively) values. In previous studies, the anti-malarial activity of PQ was partially restored when tested at its ED100 in the CYP 2D knock-out/humanized/CYP 2D6 knock-in mice [6]. While both NPC-1161B and TQ failed to achieve efficacy in CYP 2D knock-out/humanized/CYP 2D6 knock-in mice when tested at their corresponding ED100 values, anti-malarial activity was restored in the CYP 2D knock-out/humanized/CYP 2D6 knock-in experiments when the dose of TQ was doubled from its corresponding ED100 value (3 mg/kg to 6 mg/kg; NPC-1161B was not tested at two times its ED100 due to limited numbers of CYP 2D KO/CYP2D6 KI mice). Additionally, despite NPC-1161B and TQ belonging to the same structural class as PQ, there is little information available on the metabolism of these longer half-life molecules by CYP 2D6. It still remains uncertain if there are any other CYP enzymes capable of metabolising NPC-1161B and TQ, however, under the experimental conditions described above, the CYP 2D family is of primary importance for anti-malarial activity in mice and likely in humans. Further studies are required to understand the mechanism by which CYP 2D6 activates the NPC-1161B and TQ.
The lack of anti-malarial activity for TQ and NPC-1161B when tested in the CYP 2D6 knock-in mice could largely be attributed to intrinsic differences between the two CYP 2D enzymes (human vs. murine) as several reports have indicated differences in substrate metabolism and tissue expression between orthologues [21,27]. Scheer et al. showed using the CYP 2D6 reporter substrate bufuralol, only about a 53% recovery of bufuralol 1′-hydroxylase activity in microsomes generated from livers of the humanized CYP 2D6 knock-in animals versus those from the wild-type [21]. The CYP 2D6 knock-in mice have reduced enzymatic activity as compared to the WT CYP 2D mice that is required for 8AQ activation. Interestingly, this information could be utilized for the dose adjustment of 8AQs in humans that have reduced CYP 2D6 activity, as in some IM phenotypes as is done with other CYP 2D6-dependent drugs such as the anticancer drug tamoxifen [28]. This highlights the potential necessity of pharmacogenomic-guided therapy, with optimal dosing tailored for individual patients based on their CYP 2D6 genotype. It seems that the differences in mouse CYP 2D vs. human CYP 2D6 metabolism required for the anti-malarial activity of the 8AQs is more pronounced for the longer half-life 5-O-aryl analogues (TQ and NPC-1161B) as compared to PQ. TQ and NPC-1161B were not tested at two times their ED100 values in the CYP 2D knock-out mice because it is unlikely that any dose adjustment in mice lacking any functional CYP 2D enzyme(s) would result in restoration of activity as previously reported for PQ [6].
Taking the results presented above in context with literature reports, it is reasonable to conclude several things about NPC-1161B and TQ. 1). The anti-hypnozoite activity of NPC-1161B and TQ is dependent on CYP 2D activation. 2). Both NPC-1161B and TQ will likely fail for either causal prophylaxis and/or treatment indications in patients with CYP 2D6 genotypes resulting in the PM phenotype and may require dose modification in some patients with an IM phenotype. It is interesting to note that at the TQ and NPC-1161B doses tested in the knock-out experiments described above, no blood schizonticidal activity was observed. It is unclear as to how/if the previously reported slow intrinsic blood schizonticidal activity of TQ and NPC-1161B [3,29,30] is linked to CYP 2D6 metabolism. Further work is required in order to determine if CYP 2D6 metabolism is required for the blood schizonticidal activity of TQ and NPC-1161B in humans and whether this blood schizonticidal activity is sufficient to allow TQ and NPC-1161B to act as prophylactic agents in the absence of CYP 2D6 activation. Despite these issues, the requirement for CYP 2D6 activation for activity in the CYP 2D knock-out mouse model sheds light on another controversial issue that has surrounded the 8AQ class for decades: resistance.
There have been numerous reports in the literature of PQ failures that are associated with PQ resistance [1,31,32]. This “resistance” refers to the inability of PQ to clear the hypnozoite form of the Plasmodium parasite. There has been confusion around the idea of PQ resistance as there are many confounding factors associated with the various reports, such as patient population, patient adherence, dosing regimen, and concurrent blood schizonticidal therapy [1,33]. The requirement of CYP 2D6 activation for PQ activity is another factor that needs to be taken into consideration when reporting PQ resistance. Interestingly, the reported PQ failure rates seem to align with CYP 2D6 polymorphic allelic frequencies for the PM genotype [1] as individuals with this genotype will likely fail PQ therapy [15]. This is not a likely coincidence and calls into question the existence of PQ resistance and/or Plasmodium resistance to the 8AQ class in general, particularly since the results reported herein suggest that 8AQs likely have a similar mechanism(s) of anti-malarial activity which is mediated through CYP 2D6 activation. TQ and NPC-1161B do not have the wealth of clinical data that exists for PQ, so the existence of TQ and NPC-1161B treatment failures is more difficult to interpret. Because TQ and NPC-1161B require CYP 2D activation for activity, rates of treatment/prophylactic failures would likely be in line with those noted for PQ use for both compounds when administered to humans. If insurmountable, this would present a major pharmacogenomic liability for the 8AQ class of anti-malarial compounds. New drugs with anti-hypnozoite activity are desperately needed to combat relapsing strains of malaria and future research and development efforts should ensure the complete dissociation between CYP 2D6 metabolism and anti-hypnozoite activity of new potential anti-malarial agents.
Abbreviations
8AQ: 8-aminoquinoline; CYP: Cytochrome P450; PQ: Primaquine; TQ: Tafenoquine; G6PD: Glucose-6-phosphate dehydrogenase; IVIS: in vivo imaging system; WT: Wild type; ED100: 100% efficacious dose.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SRM, JCS, GAR drafted the manuscript, contributed to the experimental design, conducted data analyses, and assisted in study coordination. RJS, JWB, VZ, GD, MH, BS, VM, QL, LW, and LR contributed to the experimental design, experimental coordination, and assisted with drafting the manuscript. DC, QZ, NR, RM, and JC conducted all animal experiments and assisted with data analyses. LAW and NPDN assisted in compound synthesis and study design. BSP conceived of the study, and participated in its design and coordination. All authors read and approved the final manuscript.
Contributor Information
Sean R Marcsisin, Email: sean.r.marcsisin.mil@mail.mil.
Jason C Sousa, Email: jason.c.sousa.ctr@mail.mil.
Gregory A Reichard, Email: gregory.a.reichard2.ctr@mail.mil.
Diana Caridha, Email: diana.caridha2.ctr@mail.mil.
Qiang Zeng, Email: qiang.zeng.ctr@mail.mil.
Norma Roncal, Email: norma.e.roncal.ctr@mail.mil.
Ronan McNulty, Email: ronan.t.mcnulty.mil@mail.mil.
Julio Careagabarja, Email: julio.c.careagabarja.mil@mail.mil.
Richard J Sciotti, Email: richard.j.sciotti.civ@mail.mil.
Jason W Bennett, Email: jason.w.bennett.mil@mail.mil.
Victor E Zottig, Email: victor.e.zottig.mil@mail.mil.
Gregory Deye, Email: gregory.deye@nih.gov.
Qigui Li, Email: qigui.li.civ@mail.mil.
Lisa Read, Email: lisa.t.read.mil@mail.mil.
Mark Hickman, Email: mark.r.hickman.mil@mail.mil.
N P Dhammika Nanayakkara, Email: dhammika@olemiss.edu.
Larry A Walker, Email: lwalker@olemiss.edu.
Bryan Smith, Email: bryan.l.smith5.mil@mail.mil.
Victor Melendez, Email: victor.j.melendez2.mil@mail.mil.
Brandon S Pybus, Email: brandon.s.pybus.mil@mail.mil.
Acknowledgements
We would like to thank David Markman and his team in Entomology Branch, Walter Reed Army Institute of Research, for their assistance with mosquito preparation.
The views, opinions and/or findings contained in this presentation are those of the authors and do not necessarily reflect the views of the US Department of Defense and should not be construed as an official DoD/Army position, policy or decision unless so designated by other documentation. No official endorsement should be made.
References
- Baird JK, Hoffman SL. Primaquine therapy for malaria. Clin Infect Dis. 2004;39:1336–1345. doi: 10.1086/424663. [DOI] [PubMed] [Google Scholar]
- Rieckmann KH, McNamara JV, Kass L, Powell RD. Gametocytocidal and sporontocidal effects of primaquine upon two strains of Plasmodium falciparum. Mil Med. 1969;134:802–819. [PubMed] [Google Scholar]
- Dow GS, Gettayacamin M, Hansukjariya P, Imerbsin R, Komcharoen S, Sattabongkot J, Kyle D, Milhous W, Cozens S, Kenworthy D, Miller A, Veazey J, Ohrt C. Radical curative efficacy of tafenoquine combination regimens in Plasmodium cynomolgi-infected Rhesus monkeys (Macaca mulatta) Malar J. 2011;10:212. doi: 10.1186/1475-2875-10-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maude RJ, Socheat D, Nguon C, Saroth P, Dara P, Li G, Song J, Yeung S, Dondorp AM, Day NP, White NJ, White LJ. Optimising strategies for Plasmodium falciparum malaria elimination in Cambodia: primaquine, mass drug administration and artemisinin resistance. PLoS One. 2012;7:e37166. doi: 10.1371/journal.pone.0037166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Pybus BS, Marcsisin SR, Logan T, Luong TL, Sousa J, Matlock N, Collazo V, Asher C, Carroll D, Olmeda R, Walker LA, Kozar MP, Melendez V. An LC-MS based study of the metabolic profile of primaquine, an 8-aminoquinoline antiparasitic drug, with an in vitro primary human hepatocyte culture model. Eur J Drug Metab Pharmacokinet. 2013. [Epub ahead of print] doi: 10.1007/s13318-013-0139-8. [DOI] [PubMed]
- Pybus BS, Marcsisin SR, Jin X, Deye G, Sousa JC, Li Q, Caridha D, Zeng Q, Reichard GA, Ockenhouse C, Bennett J, Walker LA, Ohrt C, Melendez V. The metabolism of primaquine to its active metabolite is dependent on CYP 2D6. Malar J. 2013;12:212. doi: 10.1186/1475-2875-12-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pybus BS, Sousa JC, Jin X, Ferguson JA, Christian RE, Barnhart R, Vuong C, Sciotti RJ, Reichard GA, Kozar MP, Walker LA, Ohrt C, Melendez V. CYP450 phenotyping and accurate mass identification of metabolites of the 8-aminoquinoline, anti-malarial drug primaquine. Malar J. 2012;11:259. doi: 10.1186/1475-2875-11-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino L, Paixao P, Moreira R, Portela MJ, Do Rosario VE, Iley J. Metabolism of primaquine by liver homogenate fractions. Evidence for monoamine oxidase and cytochrome P450 involvement in the oxidative deamination of primaquine to carboxyprimaquine. Exp Toxicol Pathol. 1999;51:299–303. doi: 10.1016/S0940-2993(99)80010-4. [DOI] [PubMed] [Google Scholar]
- Ganesan S, Tekwani BL, Sahu R, Tripathi LM, Walker LA. Cytochrome P(450)-dependent toxic effects of primaquine on human erythrocytes. Toxicol Appl Pharmacol. 2009;241:14–22. doi: 10.1016/j.taap.2009.07.012. [DOI] [PubMed] [Google Scholar]
- Vasquez-Vivar J, Augusto O. Hydroxylated metabolites of the antimalarial drug primaquine. Oxidation and redox cycling. J Biol Chem. 1992;267:6848–6854. [PubMed] [Google Scholar]
- Augusto O, Schreiber J, Mason RP. Direct ESR detection of a free radical intermediate during the peroxidase-catalyzed oxidation of the antimalarial drug primaquine. Biochem Pharmacol. 1988;37:2791–2797. doi: 10.1016/0006-2952(88)90042-1. [DOI] [PubMed] [Google Scholar]
- Augusto O, Weingrill CL, Schreier S, Amemiya H. Hydroxyl radical formation as a result of the interaction between primaquine and reduced pyridine nucleotides. Catalysis by hemoglobin and microsomes. Arch Biochem Biophys. 1986;244:147–155. doi: 10.1016/0003-9861(86)90103-7. [DOI] [PubMed] [Google Scholar]
- Morais Mda S, Augusto O. Peroxidation of the antimalarial drug primaquine: characterization of a benzidine-like metabolite with methaemoglobin-forming activity. Xenobiotica. 1993;23:133–139. doi: 10.3109/00498259309059369. [DOI] [PubMed] [Google Scholar]
- Vasquez-Vivar J, Augusto O. Oxidative activity of primaquine metabolites on rat erythrocytes in vitro and in vivo. Biochem Pharmacol. 1994;47:309–316. doi: 10.1016/0006-2952(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Bennett JW, Pybus BS, Yadava A, Tosh D, Sousa JC, McCarthy WF, Deye G, Melendez V, Ockenhouse CF. Primaquine failure and cytochrome P-450 2D6 in Plasmodium vivax malaria. N Engl J Med. 2013;369:1381–1382. doi: 10.1056/NEJMc1301936. [DOI] [PubMed] [Google Scholar]
- Anzenbacher P, Anzenbacherova E. Cytochromes P450 and metabolism of xenobiotics. Cell Mol Life Sci. 2001;58:737–747. doi: 10.1007/PL00000897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou SF. Polymorphism of human cytochrome P450 2D6 and its clinical significance: Part I. Clin Pharmacokinet. 2009;48:689–723. doi: 10.2165/11318030-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Bernard S, Neville KA, Nguyen AT, Flockhart DA. Interethnic differences in genetic polymorphisms of CYP2D6 in the U.S. population: clinical implications. Oncologist. 2006;11:126–135. doi: 10.1634/theoncologist.11-2-126. [DOI] [PubMed] [Google Scholar]
- Bogni A, Monshouwer M, Moscone A, Hidestrand M, Ingelman-Sundberg M, Hartung T, Coecke S. Substrate specific metabolism by polymorphic cytochrome P450 2D6 alleles. Toxicol In Vitro. 2005;19:621–629. doi: 10.1016/j.tiv.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Ingelman-Sundberg M. Genetic polymorphisms of cytochrome P450 2D6 (CYP2D6): clinical consequences, evolutionary aspects and functional diversity. Pharmacogenomics J. 2005;5:6–13. doi: 10.1038/sj.tpj.6500285. [DOI] [PubMed] [Google Scholar]
- Scheer N, Kapelyukh Y, McEwan J, Beuger V, Stanley LA, Rode A, Wolf CR. Modeling human cytochrome P450 2D6 metabolism and drug-drug interaction by a novel panel of knockout and humanized mouse lines. Mol Pharmacol. 2012;81:63–72. doi: 10.1124/mol.111.075192. [DOI] [PubMed] [Google Scholar]
- Walsh DS, Wilairatana P, Tang DB, Heppner DG Jr, Brewer TG, Krudsood S, Silachamroon U, Phumratanaprapin W, Siriyanonda D, Looareesuwan S. Randomized trial of 3-dose regimens of tafenoquine (WR238605) versus low-dose primaquine for preventing Plasmodium vivax malaria relapse. Clin Infect Dis. 2004;39:1095–1103. doi: 10.1086/424508. [DOI] [PubMed] [Google Scholar]
- Walsh DS, Looareesuwan S, Wilairatana P, Heppner DG Jr, Tang DB, Brewer TG, Chokejindachai W, Viriyavejakul P, Kyle DE, Milhous WK, Schuster BG, Horton J, Braitman DJ, Brueckner RP. Randomized dose-ranging study of the safety and efficacy of WR 238605 (Tafenoquine) in the prevention of relapse of Plasmodium vivax malaria in Thailand. J Infect Dis. 1999;180:1282–1287. doi: 10.1086/315034. [DOI] [PubMed] [Google Scholar]
- Walsh DS, Eamsila C, Sasiprapha T, Sangkharomya S, Khaewsathien P, Supakalin P, Tang DB, Jarasrumgsichol P, Cherdchu C, Edstein MD, Rieckmann KH, Brewer TG. Efficacy of monthly tafenoquine for prophylaxis of Plasmodium vivax and multidrug-resistant P. falciparum malaria. J Infect Dis. 2004;190:1456–1463. doi: 10.1086/424468. [DOI] [PubMed] [Google Scholar]
- Hale BR, Owusu-Agyei S, Fryauff DJ, Koram KA, Adjuik M, Oduro AR, Prescott WR, Baird JK, Nkrumah F, Ritchie TL, Franke ED, Binka FN, Horton J, Hoffman SL. A randomized, double-blind, placebo-controlled, dose-ranging trial of tafenoquine for weekly prophylaxis against Plasmodium falciparum. Clin Infect Dis. 2003;36:541–549. doi: 10.1086/367542. [DOI] [PubMed] [Google Scholar]
- Elmes NJ, Nasveld PE, Kitchener SJ, Kocisko DA, Edstein MD. The efficacy and tolerability of three different regimens of tafenoquine versus primaquine for post-exposure prophylaxis of Plasmodium vivax malaria in the Southwest Pacific. Trans R Soc Trop Med Hyg. 2008;102:1095–1101. doi: 10.1016/j.trstmh.2008.04.024. [DOI] [PubMed] [Google Scholar]
- Miksys SL, Cheung C, Gonzalez FJ, Tyndale RF. Human CYP2D6 and mouse CYP2Ds: organ distribution in a humanized mouse model. Drug Metab Dispos. 2005;33:1495–1502. doi: 10.1124/dmd.105.005488. [DOI] [PubMed] [Google Scholar]
- Walko CM, McLeod H. Use of CYP2D6 genotyping in practice: tamoxifen dose adjustment. Pharmacogenomics. 2012;13:691–697. doi: 10.2217/pgs.12.27. [DOI] [PubMed] [Google Scholar]
- Pradines B, Mamfoumbi MM, Tall A, Sokhna C, Koeck JL, Fusai T, Mosnier J, Czarnecki E, Spiegel A, Trape JF, Kombila M, Rogier C. In vitro activity of tafenoquine against the asexual blood stages of Plasmodium falciparum isolates from Gabon, Senegal, and Djibouti. Antimicrob Agents Chemother. 2006;50:3225–3226. doi: 10.1128/AAC.00777-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delves M, Plouffe D, Scheurer C, Meister S, Wittlin S, Winzeler EA, Sinden RE, Leroy D. The activities of current antimalarial drugs on the life cycle stages of Plasmodium: a comparative study with human and rodent parasites. PLoS Med. 2012;9:e1001169. doi: 10.1371/journal.pmed.1001169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright AT, Alenazi T, Shokoples S, Tarning J, Paganotti GM, White NJ, Houston S, Winzeler EA, Yanow SK. Genetic analysis of primaquine tolerance in a patient with relapsing vivax malaria. Emerg Infect Dis. 2013;19:802–805. doi: 10.3201/eid1905.121852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langholz Kristensen K, Dragsted UB. Recurrent Plasmodium vivax malaria due to dose-dependent primaquine resistance: a case report. Scand J Infect Dis. 2013;46:63–65. doi: 10.3109/00365548.2013.822093. [DOI] [PubMed] [Google Scholar]
- Fernando D, Rodrigo C, Rajapakse S. Primaquine in vivax malaria: an update and review on management issues. Malar J. 2011;10:351. doi: 10.1186/1475-2875-10-351. [DOI] [PMC free article] [PubMed] [Google Scholar]