Abstract
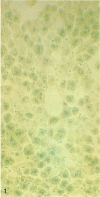
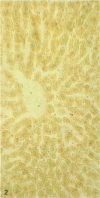
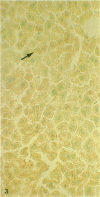
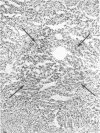
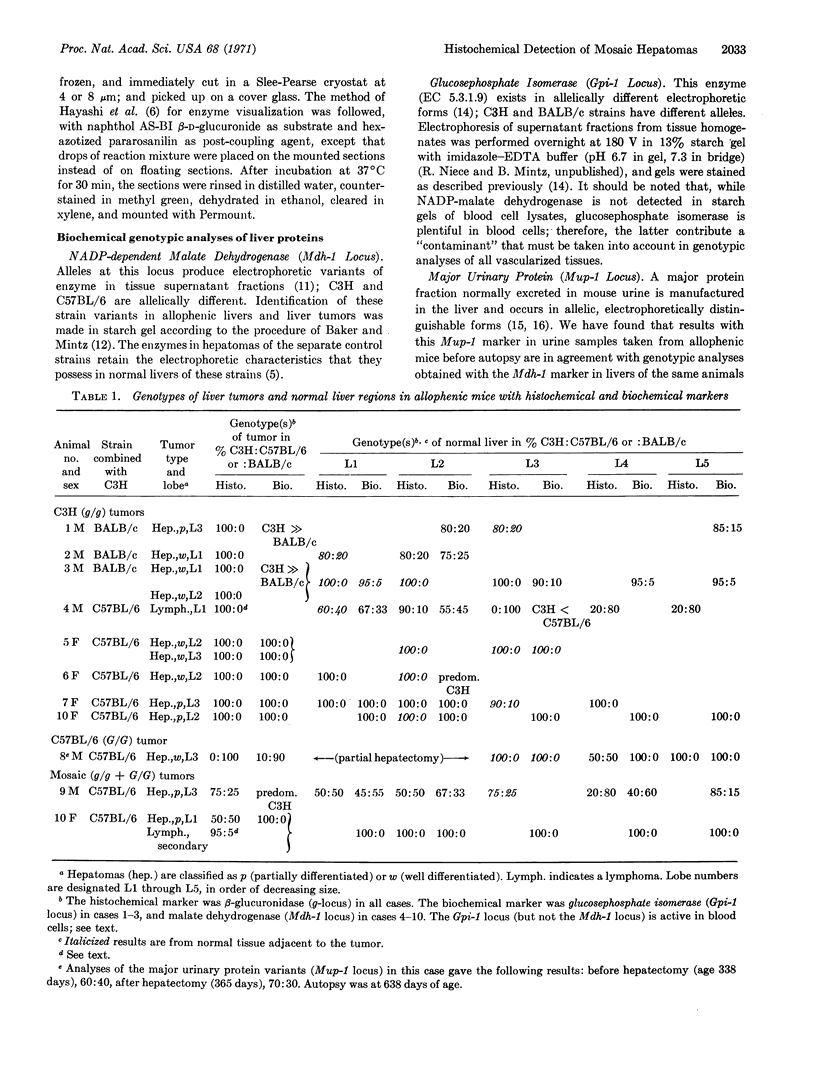
A histochemical procedure for β-glucuronidase has been used to make visible the cellular genotypes of liver tumors and of surrounding normal liver clones in allophenic mice. The animals had lifelong genetic mosaicism for cells with the allele for low β-glucuronidase activity (g/g genotype, C3H strain) and cells with the allele for high activity (G/G genotype, C57BL/6 or BALB/c strain). The former strain is also hepatoma-susceptible; both the latter are nonsusceptible. Of 12 “spontaneous” hepatomas examined, nine were entirely of susceptible-strain hepatic cells and one was of the nonsusceptible strain; the pure-strain tumors usually arose in a liver environment containing clones of each genotype. The cells therefore behave largely autonomously with respect to gene control of tumor susceptibility. However, two tumors with malignant cells of both genotypes were formed, which suggests some measure of intercellular transmission of tumor information. Alternatively, transformation might have occurred in two or more cells concurrently. Mosaic tumors in either case imply that even a hepatoma of one inbred strain, whether in a single-genotype animal or an allophenic mouse, may comprise diverse clones of transformed cells. Possibly many or all hepatomas may therefore be genetically complex entities.
Keywords: gene control of neoplasia, hepatoma susceptibility
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badran A. F., Leonard E. P., Provenza D. V. Histochemical demonstration of beta-glucuronidase activity during sequential molar development in the Swiss albino mouse. Histochemie. 1970;21(1):27–32. doi: 10.1007/BF00304803. [DOI] [PubMed] [Google Scholar]
- Baker W. W., Mintz B. Subunit structure and gene control of mouse NADP-malate dehydrogenase. Biochem Genet. 1969 Jan;2(4):351–360. doi: 10.1007/BF01458495. [DOI] [PubMed] [Google Scholar]
- Carter N. D., Parr C. W. Isoenzymes of phosphoglucose isomerase in mice. Nature. 1967 Nov 4;216(5114):511–511. doi: 10.1038/216511a0. [DOI] [PubMed] [Google Scholar]
- Finlayson J. S., Asofsky R., Potter M., Runner C. C. Major urinary protein complex of normal mice: origin. Science. 1965 Aug 27;149(3687):981–982. doi: 10.1126/science.149.3687.981. [DOI] [PubMed] [Google Scholar]
- Finlayson J. S., Mushinski J. F., Hudson D. M., Potter M. Components of the major urinary protein complex in inbred mice: separation and peptide mapping. Biochem Genet. 1968 Sep;2(2):127–140. doi: 10.1007/BF01458712. [DOI] [PubMed] [Google Scholar]
- HAYASHI M., NAKAJIMA Y., FISHMAN W. H. THE CYTOLOGIC DEMONSTRATION OF BETA-GLUCURONIDASE EMPLOYING NAPHTHOL AS-BI GLUCURONIDE AND HEXAZONIUM PARAROSANILIN; A PRELIMINARY REPORT. J Histochem Cytochem. 1964 Apr;12:293–297. doi: 10.1177/12.4.293. [DOI] [PubMed] [Google Scholar]
- Henderson N. S. Isozymes and genetic control of NADP-malate dehydrogenase in mice. Arch Biochem Biophys. 1966 Oct;117(1):28–33. doi: 10.1016/0003-9861(66)90121-4. [DOI] [PubMed] [Google Scholar]
- Mintz B. Gene control of mammalian pigmentary differentiation. I. Clonal origin of melanocytes. Proc Natl Acad Sci U S A. 1967 Jul;58(1):344–351. doi: 10.1073/pnas.58.1.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz B., Palm J. Gene control of hematopoiesis. I. Erythrocyte mosaicism and permanent immunological tolerance in allophenic mice. J Exp Med. 1969 May 1;129(5):1013–1027. doi: 10.1084/jem.129.5.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz B., Silvers W. K. "Intrinsic" immunological tolerance in allophenic mice. Science. 1967 Dec 15;158(3807):1484–1486. doi: 10.1126/science.158.3807.1484. [DOI] [PubMed] [Google Scholar]
- Mintz B., Slemmer G. Gene control of neoplasia. I. Genotypic mosaicism in normal and preneoplastic mammary glands of allophenic mice. J Natl Cancer Inst. 1969 Jul;43(1):87–109. [PubMed] [Google Scholar]
- PAIGEN K. The effect of mutation on the intracellular location of beta-glucuronidase. Exp Cell Res. 1961 Nov;25:286–301. doi: 10.1016/0014-4827(61)90280-4. [DOI] [PubMed] [Google Scholar]
- SABATINI D. D., BENSCH K., BARRNETT R. J. Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J Cell Biol. 1963 Apr;17:19–58. doi: 10.1083/jcb.17.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]