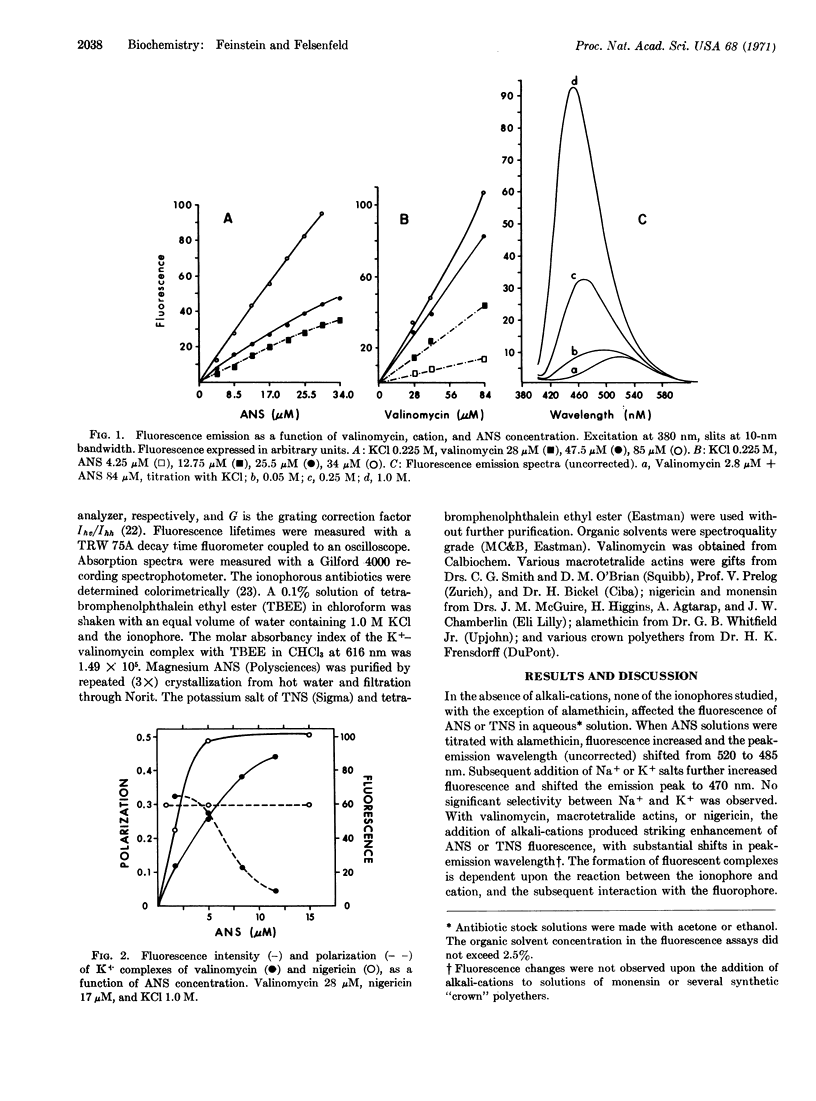
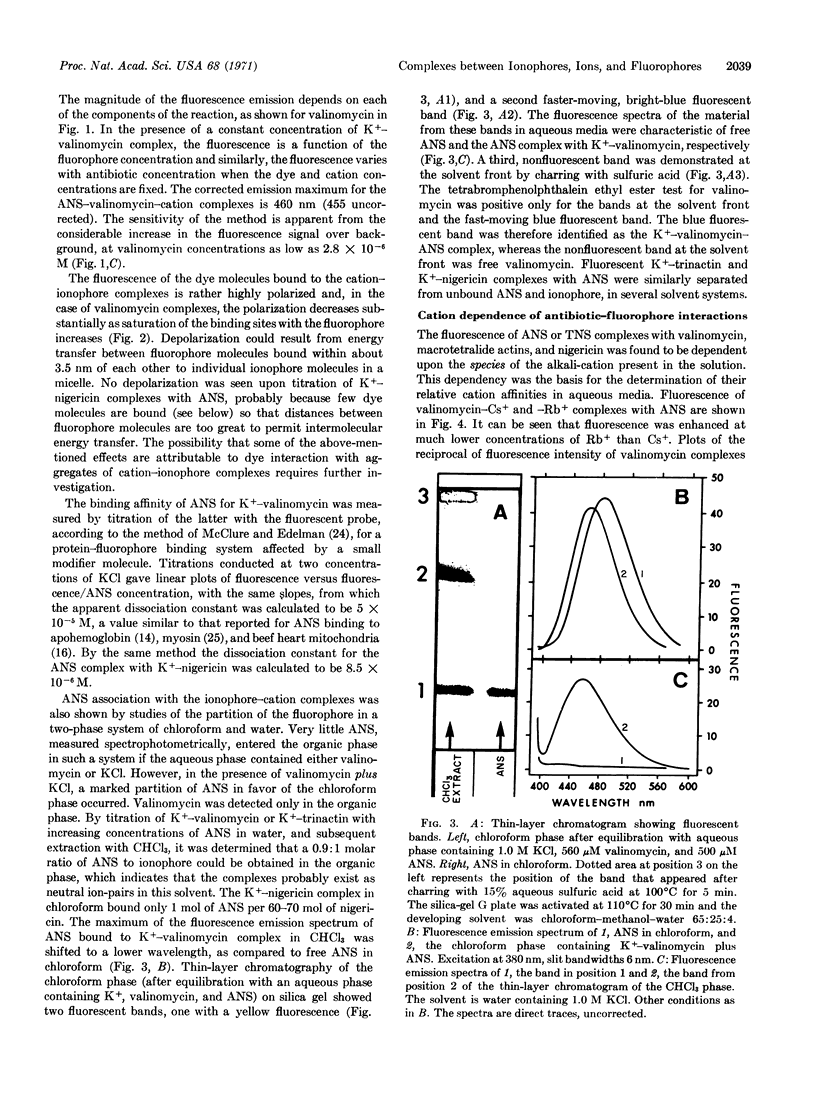
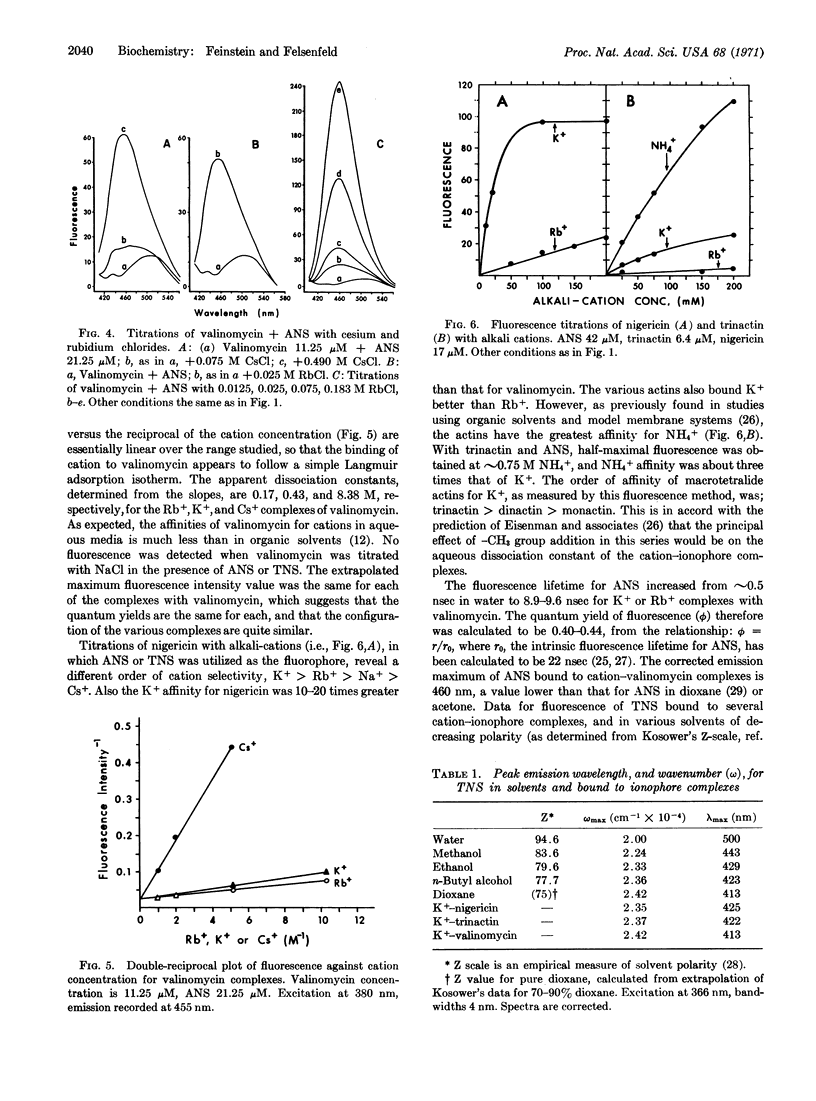
Abstract
The binding of alkali cations by the ionophorous antibiotics valinomycin, nigericin, alamethicin, and the macrotetralide actins has been shown to occur, in aqueous media, by the use of the fluorescent probes 1-anilino-8-naphthalene sulfonate and 2-p-toluidinyl-6-naphthalene sulfonate. The interaction of the ionophore-cation complexes with the fluorescent dyes produced enhanced fluorescence emission, increased lifetime and polarization, and a significant blue-shift of the emission maxima of the fluorescence spectrum. At constant antibiotic and fluorophore concentrations in water, the intensity of the fluorescence emission was found to be a function of the cation concentration. This permitted relative cation affinities to be determined for alamethicin (Na+ ≅ K+), valinomycin (Rb+ > K+ > Cs+), nigericin (K+ > Rb+ > Na+ > Cs+) and trinactin (NH4+ > K+ > Rb+ > Cs+).
Keywords: alkali cations, alamethicin, nigericin, trinactin, valinomycin, relative affinities
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azzi A., Chance B., Radda G. K., Lee C. P. A fluorescence probe of energy-dependent structure changes in fragmented membranes. Proc Natl Acad Sci U S A. 1969 Feb;62(2):612–619. doi: 10.1073/pnas.62.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN R. F., BOWMAN R. L. FLUORESCENCE POLARIZATION: MEASUREMENT WITH ULTRAVIOLET-POLARIZING FILTERS IN A SPECTROPHOTOFLUOROMETER. Science. 1965 Feb 12;147(3659):729–732. doi: 10.1126/science.147.3659.729. [DOI] [PubMed] [Google Scholar]
- Chen R. F. Reduction of light scatter in fluorometry by the use of horizontally polarized excitation. Anal Biochem. 1966 Mar;14(3):497–499. doi: 10.1016/0003-2697(66)90295-8. [DOI] [PubMed] [Google Scholar]
- Cheung H. C., Morales M. F. Studies of myosin conformation by fluorescent techniques. Biochemistry. 1969 May;8(5):2177–2182. doi: 10.1021/bi00833a059. [DOI] [PubMed] [Google Scholar]
- Feinstein M. B., Spero L., Felsenfield H. Interaction of a fluorescent probe with erythrocyte membrane and lipids: Effects of local anesthetics and calcium. FEBS Lett. 1970 Feb 16;6(3):245–248. doi: 10.1016/0014-5793(70)80069-2. [DOI] [PubMed] [Google Scholar]
- Hundley L., Coburn T., Garwin E., Stryer L. Nanosecond fluorimeter. Rev Sci Instrum. 1967 Apr;38(4):488–492. doi: 10.1063/1.1720743. [DOI] [PubMed] [Google Scholar]
- Kilbourn B. T., Dunitz J. D., Pioda L. A., Simon W. Structure of the K+ complex with nonactin, a macrotetrolide antibiotic possessing highly specific K+ transport properties. J Mol Biol. 1967 Dec 28;30(3):559–563. doi: 10.1016/0022-2836(67)90370-1. [DOI] [PubMed] [Google Scholar]
- Lutz W. K., Wipf H. K., Simon W. Alkalikationen-Spezifität und Träger-Eigenschaften der Antibiotica Nigericin und Monensin. Helv Chim Acta. 1970;53(7):1741–1746. doi: 10.1002/hlca.19700530723. [DOI] [PubMed] [Google Scholar]
- McClure W. O., Edelman G. M. Fluorescent probes for conformational states of proteins. I. Mechanism of fluorescence of 2-p-toluidinylnaphthalene-6-sulfonate, a hydrophobic probe. Biochemistry. 1966 Jun;5(6):1908–1919. doi: 10.1021/bi00870a018. [DOI] [PubMed] [Google Scholar]
- McClure W. O., Edelman G. M. Fluorescent probes for conformational states of proteins. II. The binding of 2-p-toluidinylnaphthalene-6-sulfonate to alpha-chymotrypsin. Biochemistry. 1967 Feb;6(2):559–566. doi: 10.1021/bi00854a025. [DOI] [PubMed] [Google Scholar]
- Mueller P., Rudin D. O. Action potentials induced in biomolecular lipid membranes. Nature. 1968 Feb 24;217(5130):713–719. doi: 10.1038/217713a0. [DOI] [PubMed] [Google Scholar]
- Mueller P., Rudin D. O. Development of K+-Na+ discrimination in experimental bimolecular lipid membranes by macrocyclic antibiotics. Biochem Biophys Res Commun. 1967 Feb 21;26(4):398–404. doi: 10.1016/0006-291x(67)90559-1. [DOI] [PubMed] [Google Scholar]
- Pressman B. C., Harris E. J., Jagger W. S., Johnson J. H. Antibiotic-mediated transport of alkali ions across lipid barriers. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1949–1956. doi: 10.1073/pnas.58.5.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressman B. C. Ionophorous antibiotics as models for biological transport. Fed Proc. 1968 Nov-Dec;27(6):1283–1288. [PubMed] [Google Scholar]
- Prestegard J. H., Chan S. I. Proton magnetic resonance studies of the cation-binding properties of nonactin. II. Comparison of the sodium ion, potassium ion, and cesium ion complexes. J Am Chem Soc. 1970 Jul 15;92(14):4440–4446. doi: 10.1021/ja00717a049. [DOI] [PubMed] [Google Scholar]
- Rubalcava B., Martínez de Muñoz D., Gitler C. Interaction of fluorescent probes with membranes. I. Effect of ions on erythrocyte membranes. Biochemistry. 1969 Jul;8(7):2742–2747. doi: 10.1021/bi00835a008. [DOI] [PubMed] [Google Scholar]
- Seliskar C. J., Brand L. Solvent dependence of the luminescence of N-arylaminonaphthalenesulfonates. Science. 1971 Feb 26;171(3973):799–800. doi: 10.1126/science.171.3973.799. [DOI] [PubMed] [Google Scholar]
- Shemyakin M. M., Ovchinnikov Y. A., Ivanov V. T., Antonov V. K., Shkrob A. M., Mikhaleva I. I., Evstratov A. V., Malenkov G. G. The physicochemical basis of the functioning of biological membranes: conformational specificity of the interaction of cyclodepsipeptides with membranes and of their complexation with alkali metal ions. Biochem Biophys Res Commun. 1967 Dec 29;29(6):834–841. doi: 10.1016/0006-291x(67)90295-1. [DOI] [PubMed] [Google Scholar]
- Stryer L. The interaction of a naphthalene dye with apomyoglobin and apohemoglobin. A fluorescent probe of non-polar binding sites. J Mol Biol. 1965 Sep;13(2):482–495. doi: 10.1016/s0022-2836(65)80111-5. [DOI] [PubMed] [Google Scholar]
- Turner D. C., Brand L. Quantitative estimation of protein binding site polarity. Fluorescence of N-arylaminonaphthalenesulfonates. Biochemistry. 1968 Oct;7(10):3381–3390. doi: 10.1021/bi00850a011. [DOI] [PubMed] [Google Scholar]
- Vanderkooi J., Martonosi A. Sarcoplasmic reticulum. 8. Use of 8-anilino-1-naphthalene sulfonate as conformational probe on biological membranes. Arch Biochem Biophys. 1969 Aug;133(1):153–163. doi: 10.1016/0003-9861(69)90499-8. [DOI] [PubMed] [Google Scholar]
- Wipf H. K., Pioda L. A., Stefanac Z., Simon W. Komplexe von Enniatinen und anderen Antibiotica mit Alkalimentall-Ionen. Helv Chim Acta. 1968 Mar 11;51(2):377–381. doi: 10.1002/hlca.19680510218. [DOI] [PubMed] [Google Scholar]



