Abstract
Background
Shiga toxin-producing Escherichia coli (STEC) is recognized as an important human diarrheal pathogen. Swine plays an important role as a carrier of this pathogen. In this study we determined the prevalence and characteristics of STEC from healthy swine collected between May 2011 and August 2012 from 3 cities/provinces in China.
Results
A total of 1003 samples, including 326 fecal, 351 small intestinal contents and 326 colon contents samples, was analyzed. Two hundred and fifty five samples were stx-positive by PCR and 93 STEC isolates were recovered from 62 stx-positive samples. Twelve O serogroups and 19 O:H serotypes including 6 serotypes (O100:H20/[H20], O143:H38/[H38], O87:H10, O172:H30/[H30], O159:H16, O9:H30/[H30]) rarely found in swine and ruminants were identified. All 93 STEC isolates harbored stx2 only, all of which were stx2e subtype including 1 isolate being a new variant of stx2e. 53.76%, 15.05% and 2.15% STEC isolates carried astA, hlyA and ehxA respectively. Four STEC isolates harbored the high-pathogenicity island. Of the 15 adherence-associated genes tested, 13 (eae, efa1, iha, lpfAO113, lpfAO157/OI-154, lpfAO157/OI-141, toxB, saa, F4, F5, F6, F17 or F41) were all absent while 2 (paa and F18) were present in 7 and 4 STEC isolates respectively. The majority of the isolates were resistant to tetracycline (79.57%), nalidixic acid (78.49%), trimethoprim-sulfamethoxazole (73.12%) and kanamycin (55.91%). The STEC isolates were divided into 63 pulsed-field gel electrophoresis patterns and 21 sequence types (STs). Isolates of the same STs generally showed the same or similar drug resistance patterns. A higher proportion of STEC isolates from Chongqing showed multidrug resistance with one ST (ST3628) resistant to 14 antimicrobials.
Conclusions
Our results indicate that swine is a significant reservoir of STEC strains in China. Based on comparison by serotypes and sequence types with human strains and presence of virulence genes, the swine STEC may have a low potential to cause human disease.
Keywords: Shiga toxin-producing Escherichia coli (STEC), Shiga toxin, Multilocus sequence typing, Adhesin genes, Putative virulence genes, Antibiotic resistance, Pulsed-field gel electrophoresis, Swine
Background
Escherichia coli that produces one or more types of cytotoxins known as Shiga toxin (Stx) or Verocytotoxin (VT) is referred to as Shiga toxin-producing E. coli (STEC) or Verocytoxion-producing E. coli (VTEC) [1]. STEC is a well-known pathogen as a cause of diarrhea, hemorrhagic colitis (HC) and hemolytic uremic syndrome (HUS) [2]. Most cases of HC and HUS have been attributed to STEC O157:H7, but the importance of non-O157 STEC is increasingly recognized [3].
STEC possesses a number of virulence factors. Besides the stx genes, human pathogenic STEC strains often carry the eae gene, one of the genes located on LEE pathogenicity island encoding the adherence factor intimin [4] and the astA gene encoding a heat-stable enterotoxin EAST1 [5]. STEC strains may also be hemolytic due to the presence of the α-hemolysin or the enterohemolysin or both. The α-hemolysin gene hlyA is located on the chromosome [6] while the enterohemolysin (ehxA) is harbored by a plasmid [7]. Many adherence-related factors were found in STEC [8-13]. EHEC factor for adherence (efa1) was shown to be essential for the adherence of the bacteria to cultured epithelial cells [11]. The IrgA homologue adhesin (iha) is a STEC adherence-conferring molecule conferring the adherence phenotype upon a nonadherent laboratory E. coli strain [13]. lpfAO113, lpfAO157/OI-154 and lpfAO157/OI-141 are adhesion genes in LEE-negative STEC strains [9,14]. Many STEC strains contain the heterologous 60-MDa virulence plasmid, which encodes a potential adhesin ToxB [10]. Other novel adhesion factors reported include autoagglutinating adhesin (saa) [12] and porcine attaching and effacing (A/E) associated protein (paa) [8]. Most STEC strains isolated from diarrheal pigs can produce one or more of the fimbriae, F4, F5, F6, F17, F18 and F41. Different types of fimbriae were reported to be associated with STEC diarrhea in animals of different age groups [15-18]. The Yersinia high-pathogenicity island (HPI) carrying fyuA (encoding the pesticin receptor) and irp (encoding the siderophore yersiniabactin) is also present in certain non-O157 STEC lineages and was previously reported only in stx2e carrying human isolates [19].
Domestic ruminants, especially cattle, are the major reservoirs of STEC. Other animals like sheep, goats have been confirmed as important natural reservoirs in some countries [2,20-22]. Swine also play an important role as a carrier of this pathogen. STEC strains that produce Stx2e can cause edema disease in pigs [23] and can also been isolated from human stools at low frequency. STEC carried by healthy pigs may pose a potential risk to humans [24-27]. Relatively little is known about the prevalence and characteristics of STEC in pigs in China. In this study, we isolated and characterized STEC from different pig slaughter houses and pig farms from 3 geographical regions, Beijing city, Chongqing city and Guizhou province in China.
Results
Prevalence of STEC in swine samples
Out of 1003 swine samples collected in this study, 25.42% (255/1003) were stx-positive by PCR. A total of 93 STEC isolates was obtained from 62 samples, giving a culture positive rate of 24.31% (62/255) of all stx-positive samples. Different stx-positive rates in small intestine contents (10.83%), colon contents (47.24%) and feces (19.33%) samples were observed. The colon contents samples gave the highest stx-positive rate (P < 0.05) and also the highest culture positive rate (18.09%) (P < 0.05) (Table 1).
Table 1.
Prevalence of STEC in swine samples
| Sample location (city/province) | No. of samples | Type of samples (N, %) | stx positive samples (N, %) | Samples with STEC isolates (N, %) | STEC isolates (N, %) |
|---|---|---|---|---|---|
| Beijing |
523 |
SC (248, 24.73) |
SC (30, 8.55) |
SC (3, 0.85) |
SC (7, 1.99) |
| CC (275, 27.42) |
CC (139, 42.64) |
CC (36, 11.04) |
CC (57, 17.48) |
||
| Chongqing |
326 |
F (326, 32.50) |
F (63, 19.33) |
F (17, 5.21) |
F (23, 7.06) |
| Guizhou |
154 |
SC (103, 10.27) |
SC (8, 2.28) |
SC (4, 1.14) |
SC (4, 1.14) |
| CC (51, 5.08) |
CC (15, 4.60) |
CC (2, 0.61) |
CC (2, 0.61) |
||
| Total |
1003 |
SC (351, 35.00) |
SC (38, 10.83) |
SC (7, 1.99) |
SC (11, 3.13) |
| CC (326, 32.50) |
CC (154, 47.24) |
CC (38, 11.66) |
CC (59, 18.09) |
||
| F (326, 32.50) | F (63, 19.33) | F (17, 5.21) | F (23, 7.06) |
Sample codes: F, fecal samples; CC, colon contents samples; SC, small intestine contents samples. The number (N) and rate (%) are showed in the parentheses.
Only a single isolate was recovered from 44 stx-positive samples each. But 2 isolates per sample were recovered from 15 samples, 3 isolates per sample from 3 samples, 4 isolates per sample from 2 samples and 5 isolates per sample from 1 sample.
Serogroups and serotypes
The 93 STEC isolates were typed into 19 serotypes, comprising 12 O serogroups and 15 H types. Forty-four isolates were O antigen untypable and 21 isolates were non motile which were designated as [H]. Nineteen serotypes were found including O2:H32/[H32], O9:H30/[H30], O20:H30/[H30], O20:H26, O76:H25, O86:H11, O87:H10, O100:H20/[H20], O114:[H30], O116:H11, O143:H38/[H38], O159:H16, O172:H30/[H30], ONT:H7, ONT:H17, ONT:H19/[H19], ONT:H21/[H21], ONT:H30/[H30], ONT:[H33].
The predominant serotypes were O20:H30/[H30], ONT:H30/[H30], O2:H32/[H32], O100:H20/[H20], O9:H30/[H30], ONT:H19/[H19], O143:H38/[H38], O172:H30/[H30] which consisted of 22 (23.66%), 22 (23.66%), 11 (11.83%), 8 (8.60%), 4 (4.30%), 4 (4.30%), 3 (3.23%) and 3 (3.23%) isolates respectively. Five serotypes (O20:H26, O86:H11, ONT:H7, ONT:H17, ONT:H21/[H21]) contained 2 isolates each and 6 serotypes (O76:H25, O87:H10, O114:[H30], O116:H11, O159:H16, ONT:[H33]) contained only 1 isolate each (Table 2).
Table 2.
Serotypes, virulence factors and sequence types (STs) of swine STEC isolates
| ST | No. of isolates | Serotype a | stx 2e b | hlyA | ehxA | astA | irp2 | fyuA | paa | F18 |
|---|---|---|---|---|---|---|---|---|---|---|
| ST10 |
2 |
O2:H32/[H32](1CC, 1SC) |
+ |
- |
- |
- |
- |
- |
- |
- |
| ST88 |
4 |
ONT:H19/[H19](1SC, 3CC) |
+ |
- |
- |
+ |
+ |
+ |
- |
- |
| ST206 |
3 |
O143:H38/[H38](3CC) |
+ |
- |
- |
- |
- |
- |
- |
- |
| ST361 |
1 |
O20:H30 (1CC) |
+ |
- |
- |
+ |
- |
- |
- |
- |
| 1 |
ONT:H30 (1CC) |
+ |
- |
- |
+ |
- |
- |
- |
- |
|
| ST501 |
2 |
O86:H11 (2CC) |
+ |
+ |
- |
+ |
- |
- |
- |
+ |
| ST540 |
1 |
ONT:H30 (1SC) |
+ |
- |
- |
- |
- |
- |
- |
- |
| 3 |
ONT:[H30] ( 1SC, 2CC) |
+ |
- |
- |
- |
- |
- |
- |
- |
|
| 1 |
O114:[H30] (1CC) |
+ |
- |
- |
- |
- |
- |
- |
- |
|
| ST641 |
1 |
O87:H10 (1SC) |
+ |
+ |
- |
- |
- |
- |
- |
+ |
| ST694 |
1 |
ONT:[H33] (1CC) |
+ |
- |
- |
+ |
- |
- |
- |
- |
| ST710 |
2 |
O20:H26 (2 F) |
+ |
- |
- |
+ |
- |
- |
- |
- |
| 17 |
O20:H30/[H30](4 F, 13CC) |
+ |
- |
- |
+ |
- |
- |
- |
- |
|
| 1 |
O20:[H30] (1 F) |
+ |
- |
+ |
+ |
- |
- |
+ |
- |
|
| 3 |
O20:[H30](1 F, 2CC) |
+ |
- |
- |
+ |
- |
- |
- |
- |
|
| 3 |
O172:H30/[H30](3CC) |
+ |
- |
- |
+ |
- |
- |
- |
- |
|
| ST953 |
2 |
ONT:H17 (2CC) |
+ |
- |
- |
- |
- |
- |
+ |
- |
| ST993 |
10 |
ONT:H30 (10CC) |
+ |
- |
- |
- |
- |
- |
- |
- |
| 2 |
ONT:H30 (2CC) |
+ |
- |
- |
+ |
- |
- |
- |
- |
|
| 3 |
ONT:H30/[H30](2 F, 1CC) |
+ |
- |
- |
- |
- |
- |
- |
- |
|
| ST1294 |
1 |
ONT:H30 (1CC) |
+ |
- |
- |
- |
- |
- |
- |
- |
| ST1494 |
2 |
ONT:H21/[H21](2CC) |
+ |
- |
- |
+ |
- |
- |
- |
- |
| ST2514 |
1 |
O100:H20 (1 F) |
+ |
- |
- |
+ |
- |
- |
- |
- |
| 1 |
O100:H20 (1SC) |
+ |
- |
- |
+ |
- |
- |
+ |
- |
|
| 5 |
O100:H20/[H20](1 F,4CC) |
+ |
- |
- |
- |
- |
- |
- |
- |
|
| 1 |
O100:[H20] (1CC) |
+ |
- |
+ |
- |
- |
- |
+ |
- |
|
| ST3628 |
9 |
O2:H32/[H32](9 F) |
+ |
+ |
- |
- |
- |
- |
- |
- |
| ST3629 |
4 |
O9:H30/[H30](4CC) |
+ |
- |
- |
+ |
- |
- |
- |
- |
| 1 |
ONT:H30 (1CC) |
+ |
- |
- |
+ |
- |
- |
- |
- |
|
| ST3630 |
1 |
O159:H16 (1CC) |
- |
- |
- |
+ |
- |
- |
+ |
- |
| ST3633 |
1 |
O76:H25 (1 F) |
+ |
+ |
- |
- |
- |
- |
- |
- |
| ST3631 |
1 |
ONT:H7 (1SC) |
+ |
- |
- |
+ |
- |
- |
+ |
- |
| ST3634 |
1 |
ONT:H7 (1SC) |
+ |
- |
- |
+ |
- |
- |
- |
- |
| ST3870 |
1 |
O116:H11(1 F) |
+ |
+ |
- |
+ |
- |
- |
- |
+ |
| Total | 93 | 93 | 93 | 14 | 2 | 50 | 4 | 4 | 7 | 4 |
aThe numbers and sources are showed in the parentheses. F, fecal samples; CC, colon contents samples; SC, small intestine contents samples. ONT, Not typeable with available O antisera. The H types of non-motility isolates are determined by fliC sequencing and indicated in the square brackets.
bNinety-two STEC isolates were subtyped by primer-specific PCR except one isolate of O159:H16.
Sorbitol fermentation and hemolysis
Out of the 93 STEC isolates, 53 (56.99%) were sorbitol-positive, covering all three types of samples and three regions. Twelve serotypes including O2:H32/[H32], O9:H30/[H30], O20:H26, O76:H25, O86:H11, O87:H10, O114:[H30], O116:H11, ONT:H17, ONT:H19/[H19], ONT:H21/[H21], ONT:[H33] were sorbitol-positive while 6 serotypes (O20:H30/[H30], O100:H20/[H20], O143:H38/[H38], O159:H16, O172:H30/[H30], ONT:H7) were sorbitol negative. All except 1 ONT:H30/[H30] isolate was sorbitol-positive.
Fourteen isolates displayed apparent β-hemolytic activity on sheep blood agar including 9 of the 11 O2:H32/[H32] isolates and 2 of the 11 O86:H11 isolates, and the single O76:H25, O87:H10 and O116:H11 isolates, the majority of which (11 isolates) were recovered from swine feces in Chongqing city. The 2 hemolytic O86:H11 isolates were isolated from colon contents in a slaughter house in Beijing city and the single O87:H10 isolate was isolated from a small intestine content in a slaughter house in Guizhou province.
Shiga toxin genes, adhesin genes and putative virulence genes
The 93 STEC isolates were tested positive for stx2 only. All except 1 isolate was stx2e subtype by PCR subtyping. The exception was an O159:H16 isolate which was found to carry a new variant of stx2e by sequencing. The new variant differs from the closest stx2e (GenBank: AM904726) by 4.51% at nucleotide level.
Three virulence-related genes (astA, ehxA and hlyA) and 2 markers for HPI (irp2 and fyuA) were screened. 53.76% (50/93) STEC isolates carried astA, 15.05% (14/93) isolates contained hemolysin gene hlyA and only 2.15% (2/93) isolates contained enterohemolysin gene ehxA. All hlyA positive STEC isolates showed hemolytic activity on standard sheep blood agar. Hemolysis was not observed in the 2 ehxA-positve STEC isolates. The irp2 and fyuA genes were identified in 4 STEC isolates, all of which were ONT:H19/[H19] serotypes (Table 2).
Among the 15 adherence-associated genes, 13 (eae, efa1, iha, lpfAO113, lpfAO157/OI-154, lpfAO157/OI-141, toxB, saa, F4, F5, F6, F17 or F41) were not detected in the 93 STEC isolates. paa was present in 7 STEC isolates. Two O86:H11 isolates, 1 O87:H10 isolate and 1 O116:H11 isolate carried F18. Eighty-two STEC isolates did not carry any of the adherence-associated genes tested (Table 2).
Antibiotic resistance in the swine STEC isolates
Antimicrobial resistance was determined against 23 antibiotics. The highest prevalence was tetracycline resistance with a rate of 79.57%. Most isolates were resistant to nalidixic acid and trimethoprim-sulfamethoxazole, followed by resistance to kanamycin with a rate of 78.49%, 73.12% and 55.91% respectively. Resistance rate to streptomycin, chloramphenicol, ampicillin and piperacillin was 48.39%, 37.63%, 25.81% and 20.43%, respectively. Lower resistance was observed for cephalothin, nitrofurantoin, ciprofloxacin, ceftriaxone, aztreonam, cefotaxime, cefuroxime, gentamicin, norfloxacin, levofloxacin, ampicillin-sulbactam with a rate ranging from 2.15% to 17.20%. All isolates were susceptible to imipenem and meropenem (Additional file 1: Table S1).
Four isolates (4.3%) were susceptible to all 23 antimicrobial agents tested. Thirteen isolates (13.98%) were only resistant to 1 antimicrobial substance, while 76 isolates (81.72%) exhibited resistance to 2 or more antimicrobials tested. The STEC isolated from pig farms in Chongqing city showed resistance to a larger number of antimicrobial agents, and at a significantly higher rate than those isolated from slaughter houses in Beijing city (P < 0.05) (Figure 1 and Additional file 1: Table S1). An O116:H11 isolate exhibited multi-drug resistant phenotype against 19 of all 23 antimicrobial agents (excluding imipenem, meropenem, gentamicin and levofloxacin).
Figure 1.
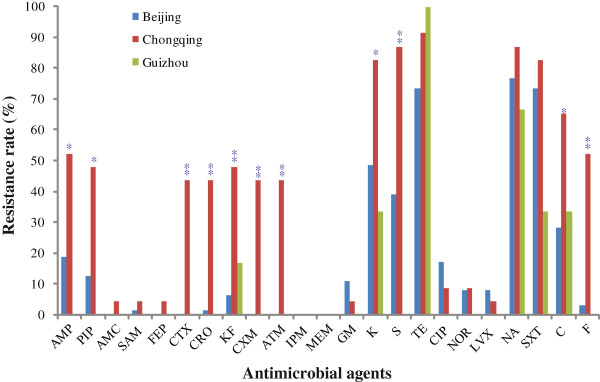
Antimicrobial resistance profiles of the STEC isolates. Three regions sampled are Beijing city (in blue), Chongqing city (in red), Guizhou province (in green). Statistical test was only performed between Chongqing and Beijing. A *and** were placed above the histogram for Chonqing samples if P < 0.05 and P < 0.001 respectively. Antibiotics abbreviations are: AMP, Ampicillin; PIP, Piperacillin; AMC, Amoxicillin-clavulanic acid; SAM, Ampicillin-sulbactam; FEP, Cefepime; CTX, Cefotaxime; CRO, Ceftriaxone; KF, Cephalothin; CXM, Cefuroxime; ATM, Aztreonam; IPM, Imipenem; MEM, Meropenem; GM, Gentamicin; K, Kanamycin; S, Streptomycin; TE, Tetracycline; CIP, Ciprofloxacin; NOR, Norfloxacin; LVX, Levofloxacin; NA, Nalidixic acid; SXT, Trimethoprim-sulfamethoxazole; C, Chloramphenicol; F, Nitrofurantoin.
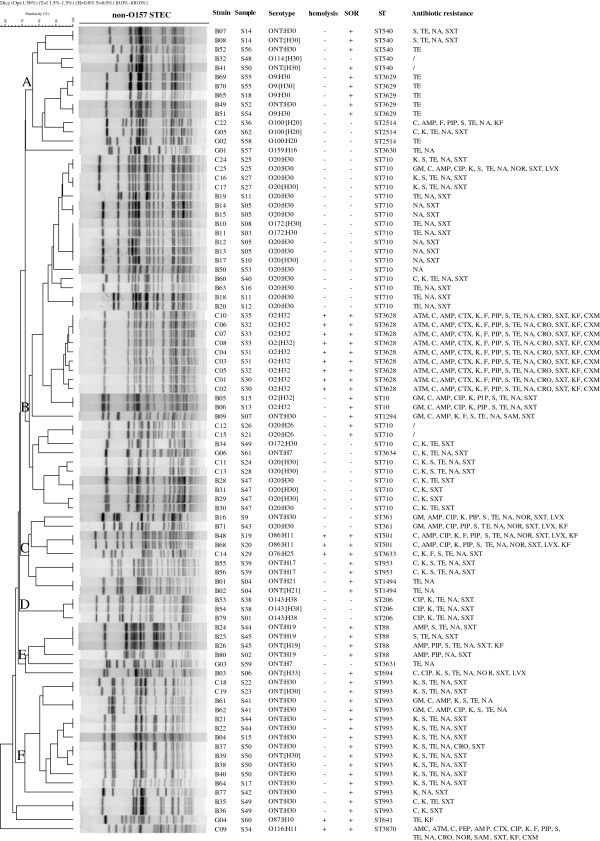
Pulsed-field gel electrophoresis (PFGE)
All 93 STEC isolates were analyzed by PFGE but only 88 isolates produced clear bands to give a PFGE profile which were divided into 63 PFGE patterns (EZKX01001 to EZKX01063). The most prevalent serotype O20:H30/[H30] with 22 isolates were typed into 16 PFGE patterns and the 11 O2:H32/[H32] isolates were typed into 8 PFGE patterns. An UPGMA dendrogram was constructed (Figure 2). The 88 STEC isolates could be divided into six clusters, A to F, at a similarity of 75% or greater. Cluster A contains all 4 O9:H30/[H30] and all 3 O100:H20/[H20] isolates. Cluster B contained the majority of O20:H30 isolates which were grouped into 3 subclusters. All the 11 of O2:H32/[H32] isolates also fell into cluster B as one subcluster. Cluster C was heterogenous containing 6 serotypes. Clusters D to F contained mostly one serotype: O143:H38/[H38], ONT:H19/[H19], ONT:H30/[H30] respectively. Although isolates were largely grouped together by serotypes, identical PFGE profiles were also found among isolates of different serotypes (O20:H30/[H30] and O172:H30/[H30]) which were not from the same sample but from the same sampling point.
Figure 2.
Dendrogram of PFGE profiles of 88 STEC isolates from pigs in farms and slaughter houses. The 6 PFGE clusters were marked on the node as A to F. Non-typeable with available O antisera was marked ONT and non-motile isolates were labeled with the H types in square brackets. Displayed on the right hand side are strain name, sample name, serotype, hemolysis, sorbitol fermentation (SOR), sequence type (ST) and antibiotic resistance. Abbreviations for antibiotics are: AMP, Ampicillin; PIP, Piperacillin; AMC, Amoxicillin-clavulanic acid; SAM, Ampicillin-sulbactam; FEP, Cefepime; CTX, Cefotaxime; CRO, Ceftriaxone; KF, Cephalothin; CXM, Cefuroxime; ATM, Aztreonam; IPM, Imipenem; MEM, Meropenem; GM, Gentamicin; K, Kanamycin; S, Streptomycin; TE, Tetracycline; CIP, Ciprofloxacin; NOR, Norfloxacin; LVX, Levofloxacin; NA, Nalidixic acid; SXT, Trimethoprim-sulfamethoxazole; C, Chloramphenicol; F, Nitrofurantoin. Place of isolates were contained in the first letter of strain names: B means Beijing city, C means Chongqing city and G means Guizhou province.
Multi-locus sequence typing (MLST)
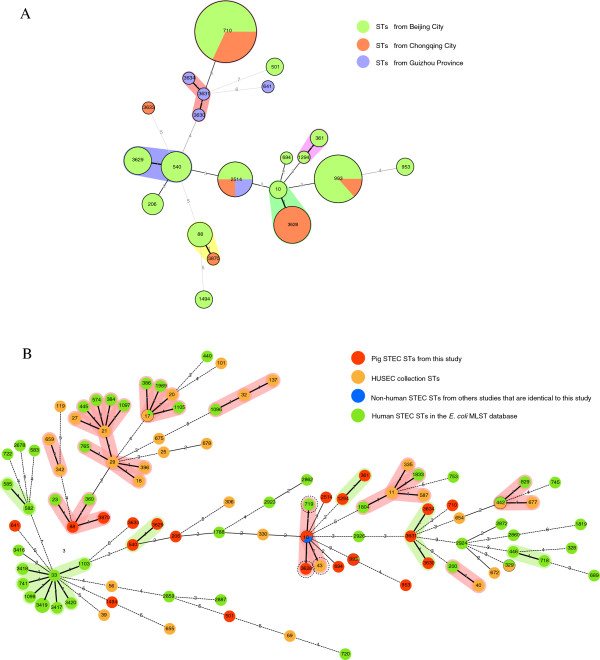
The 93 STEC isolates were typed into 21 sequence types (STs) with 7 novel STs (Table 2). Four new STs (ST3628, ST3629, ST3633 and ST3634) were resulted from a novel allele in fumC (allele 470), gyrB (allele 351), icd (allele 396) and recA (allele 267) respectively. Three new STs (ST3630, ST3631 and ST3870) were due to new combinations of previously known alleles. The predominant STs were ST710 and ST993 containing 25 (26.88%) and 15 (16.13%) isolates respectively. Six STs contained 3 or more isolates with ST3628, ST2514, ST540, ST3629, ST88 and ST206 comprising 9 (9.68%), 8 (8.60%), 6 (6.45%), 5 (5.38%), 4 (4.30%) and 3 (3.23%) isolates respectively. Five STs (ST10, ST361, ST1494, ST953 and ST501) contained 2 isolates each. Eight STs (ST641, ST691, ST1294, ST3630, ST3631, ST3633, ST3634 and ST3870) had only 1 isolate each. STEC isolates from Beijing, Chongqing and Guizhou were typed into 14, 6 and 5 STs respectively. ST2514 were recovered from all 3 regions and ST710 and ST993 were recovered from 2 regions, while other STs was only found in one region.
A minimum spanning tree was constructed (Figure 3A). Most STs differed from each other by 2 or more alleles while three pairs of STs (ST10 and ST3628, ST540 and ST3629, and ST88 and ST3870) and one set of 3 STs (ST3630, ST3631 and ST3634) differed from each other by only 1 allele. There is good concordance between STs and serotype. One ST consisted of solely or predominantly one serotype. However ST710, the most frequent ST, contained 3 serotypes, O20:H30/[H30], O172:H30/[H30] and O20:H26 with the first serotype being predominant. PFGE and MLST were also largely consistent in the clustering of the isolates (Figure 2). ST540 and ST3629 with 1 SNP difference in icd allele were grouped together with ST2514 in PFGE cluster A. All ST710 isolates were grouped into 2 subclusters within PFGE cluster B which were separated by ST3628, ST10 and ST1294. ST10 and ST3628 isolates were grouped together which differed by 1 SNP difference in gyrB. PFGE clusters D and F were inclusive of all ST206 isolates and ST993 isolates respectively. However, the 5 STs (ST361, ST501, ST953, ST1494 and ST3633) within PFGE cluster C and the 3 STs (ST88, ST3631 and ST694) within PFGE cluster E were not closely related to each other by MLST (Figure 3A). On the other hand, ST88 was not grouped together with ST3870 by PFGE, which differed by 1 SNP difference in gyrB. The sole ST3870 isolate C09 also differed from the 4 ST88 isolates by serotype, hemolysis and antibiotic resistance profile.
Figure 3.
Genetic relationships of STEC isolates based on MLST. A) Genetic relationships of STEC sequence types (STs) from this study. Each circle represents a given ST with size proportional to the number of isolates. The colors for the slices of the pie represent places of isolates: Beijing city in green, Chongqing city in red and Guizhou province in purple. The numbers on connecting lines show the number of allelic difference between two STs. The number in a circle is the ST number. B) Minimal spanning tree of STs from this study, STs from the HUSEC collection and other human STEC STs. Ninety-three pig STEC isolates (in red) were compared to STs of HUSEC collection (in orange), human STEC STs (in green) and STs from other source that are identical to STs in our study (in blue) in E. coli MLST database. Each circle represents a given ST with the pie proportional to number of isolates in a given ST from different sources. The numbers on connecting lines show the number of allelic difference between two STs. The number in a circle is the ST number.
Isolates of the same STs generally showed the same or similar drug resistance patterns (Figure 2). All ST3628 isolates showed the same multi-drug resistance to 14 antibiotics. Similarly, isolates of ST206, ST953and ST1494 showed respective identical resistance profiles. All ST3629 isolates were resistant to tetracycline. However there existed variations of drug resistance within an ST. ST710 showed the most variability with resistance to 1 to 11 drugs. ST2514 which was isolated from all 3 regions also showed varied resistance profiles.
Discussion
Different prevalence of STEC in pigs were reported previously [24,25,27-29]. Kaufmann et al. [24] compared the STEC shedding rate in pigs at slaughter, which varied widely and ranged from 2.1% to 70% depending on the health conditions of the pigs and the detection method used. As shown in this study the anatomic sites sampled also affected the rate of isolation and consequently affected the prevalence in the population reported. Fecal samples were commonly used [24-26]. In our study we sampled the small intestinal content, the colon content and the feces. The prevalence of STEC in the colon (47.24%) was almost 2.5 times higher than in feces (19.33%) (P < 0.05) and 4.4 times higher than in the small intestine (10.83%) (P < 0.05). STEC strains are thought to mostly colonize the colons of humans [30] and it is likely to be the same for pigs.
In this study, 93 isolates were recovered from 62 of the 255 stx-positive samples, giving a culture positve rate of 24.31%, this result is similar to that of Botteldoorn et al.[28], in which STEC isolates were obtained from 31% of the stx PCR-positive pig samples. Failure to isolate STEC from the stx-positive samples may due to the perturbation of high levels of background microflora, the loss of Stx prophages during subculture, the presence of other bacteria carrying stx or low levels of STEC in the samples.
In the present study, 12 serogroups and 19 serotypes were identified. The majority of these serotypes have been isolated from swine, sheep, cattle, food, and water in other countries [24,31-36]. The most prevalent serotype is O20:H30/[H30], which was also reported in cattle and sheep in different countries [31,32]. Six serotypes (O100:H20/[H20], O143:H38/[H38], O87:H10, O172:H30/[H30], O159:H16, O9:H30/[H30]) were rarely found in STEC isolates isolated from swine and other ruminants, implying that these serotypes may be restricted to the swine populations in these regions and their environments. Serotypes O86:H11, O20:NM, O100:NM, O9:NM, O172:NM and O114:NM have previously been described among STEC isolated from human patients [37-42]. Serotype O157:H7, which is common serotype causing human disease in some countries, was not detected.
A possible reason for no isolation of O157:H7 might be the method used. Isolation of O157 STEC often requires more targeted methods, such as the use of O157 immunomagnetic beads to capture the bacteria from enrichment broth and then culture on selective media [43]. We previously used immunomagnatic separation to successfully isolate O157 STEC from pigs, although that was in an outbreak setting and was in a different geographic region [44]. In this study we used CHROMagar™ ECC only and didn’t specifically target O157 STEC. CHROMagar™ ECC has been used by others for isolation of STEC from pigs [45]. However, that study did not isolate O157 STEC either. Therefore, the CHROMagar™ ECC may not be an ideal media for O157 STEC isolation.
We used sorbitol-MacConkey agar as a quick method to pick potential O157 colonies since sorbitol fermentation is a traditional feature for differentiating O157:H7 which is sorbitol-negative although there are sorbitol-positive O157 STEC [46]. In this study, a fair proportion (43%) of non-O157 STEC is actually sorbitol-negative. Therefore sorbitol fermentation is not a good indicator for O157:H7.
We analyzed multiple colonies from 21 samples to determine diversity within a sample (Figure 2). Two samples contained isolates with identical properties, suggesting they are the same strain, while the majority of the samples contain isolates belonging to the same sequence type but differing by one or more of the phenotypic or genetic properties tested, indicating that they are variants of the same clone. The most common variations are non-expression of the H antigen, variation of antibiotic resistance and/or variation in PFGE patterns. However 4 samples contained 2 different STs. Samples S15, S41, S49 and S50 all contain the prevalent ST993 and an additional ST, being ST10, ST88, ST710 and ST540 respectively, suggesting 2 different clones infecting the same pig.
Many studies have underlined the potential key role of the Stx2 subtypes in the severity of disease. Although Stx2e is not a potent subtype [47], strains harboring Stx2e have been isolated from patients with diarrhea [48]. Intimin contributes to the development of A/E lesions and is a key virulence for some STEC serotypes [49], while ehxA can be found in many STEC serotypes, such as O157:H7 and O26:H11 that are associated with diarrheal disease and HUS [7,50]. However, Sonntag et al. reported that the stx2e-positive E. coli isolated from healthy pigs rarely contains genes for intimin and enterohemolysin [19]. The prevalence of ehxA is very low in our samples at 2.15%, consistent with the findings of Sonntag et al. [19].
Other virulence factors may contribute to the pathogenicity of STEC. Although the role of EAST1 toxin in virulence to pigs has not been clearly determined, several studies have shown that astA gene is widely present among STEC isolates from both diarrheal and healthy pigs [15,24,26]. astA gene was also the most prevalent virulent gene (53.76%) among the 20 virulence genes tested in our study.
HPI was originally identified in Yersinia and now found in a range of pathogens [51], including the HUS-associated E. coli HUSEC041 [52] and the 2011 German HUS outbreak strain O104:H4 [53]. HPI had previously been detected in Stx2e- producing STEC strains from humans only [19]. In this study we found 4 stx2e STEC isolates, all ONT:H19/[H19], harbored the 2 HPI genes fyuA and irp although the frequency is low at 4.3%.
Fimbrial adhesins play an important role in colonization of the pig intestine and STEC strains may express up to 5 antigenically distinct fimbrial adhesins, F4, F5, F6, F18 and F41 [18]. Different types of fimbriae can be associated with STEC diarrhea in animals of different ages [15-18]. In this study, only 4 isolates contained a fimbrial adhesin (F18). None of the other fimbrial adhesins (F4, F5, F6, F17 and F41) was detected. Of the nonfimbrial adhesin-encoding genes, paa was found in 7 isolates (7.5%), but efa1, toxB, lpfAO157/OI-154, lpfAO157/OI-141, lpfAO113 and saa were not detected in any of the 93 STEC isolates. Eighty-two STEC isolates did not carry any of the adherence-associated genes tested.
Coombes et al. [54] reported that non-LEE encoded T3SS effector (nle) genes of non-O157 STEC strains are correlated with outbreak and HUS potential in humans. It will be interesting to examine our STEC isolates for the presence of the nle genes in future studies.
Many non-O157 STEC isolated from humans and animals have shown resistance to multiple antimicrobials [26,55,56], including resistance to trimethoprim-sulfamethoxazole and β-lactams [56,57]. STEC isolates from swine feces in the United States show high resistance rates (>38%) to tetracycline, sulfamethoxazole and kanamycin but susceptible to nalidixic acid (resistance rate 0.5%) [26]. In our study, we found that only 1 of the 12 categories of antimicrobial resistance types (carbapenems) and 2 of the 23 antimicrobial agents (imipenem and meropenem) were active against all the STEC isolates. The high prevalence (>50%) of resistance to tetracycline, trimethoprim-sulfamethoxazole, nalidixic acid and kanamycin is similar to that of other studies in China [55,58]. In a study [55] of STEC from diseased pigs in Guangdong province, China, the majority of the isolates (95%) were resistant to more than 3 antimicrobials and the resistance rates to chloramphenicol (89%) and streptomycin (83%) were far higher than that of our study (37.63% and 48.39%, respectively). We also found that isolates from Chongqing showed a higher rate than those from the other 2 cities in this study. It should be noted that all samples collected from Chongqing were fecal samples while those from Beijing and Guizhou were small intestinal contents and colon contents samples, which may affect resistance profiles if different E. coli strains have a preference for the anatomic sites. However, it is more likely that the difference reflected the presence of resistant E. coli strains in different regions. Chongqing was dominated by the multidrug resistant ST3628. The differences in drug resistance rates between cities may be related to the differences in the prevalence of drug resistant STs.
Comparison with STs observed in human infections gives an indication of the potential risk for human infection of the swine STEC. We constructed an MST containing our STs, the 32 STs of the HUSEC collection and 52 human STEC STs from the E. coli MLST database (Figure 3B). None of the 21 STs in this study was identical to any of the 32 STs of HUSEC collection [52]. We only found one ST, ST993, which was observed in human infections. When comparison was made at clonal complex level, some of our STs fell into the same clonal complex as the human STs (Figure 3B). ST10 clonal complex contained 2 of our STs (ST10 and ST3628), 1 HUSEC ST (ST43) and 1 human STEC ST (ST719) from the MLST database. However, Hauser et al. found that 8 of the 35 STEC STs they isolated from foods shared the same STs with HUSEC strains and were similar in their virulence gene composition [59]. Since the STECs from foods and HUSEC collection were from the same geographical region, it is likely some of the HUSEC STECs were from local sources and not globally distributed. Our STECs from pigs may cause local human infections but there is no surveillance of human STECs in the regions where we sampled the swine STECs.
Conclusions
In conclusion, the prevalence of STEC in healthy pigs is high (25.42%) by PCR screening although only 6.18% of the swine samples yielded an STEC isolate by microbiological culture. The vast majority of isolates belonged to a limited number of serogroups and serotypes, with O20:H30/[H30] being the predominant serotype. The majority of the STEC serotypes found in this study were also reported in other countries. All 93 STEC isolates carried the pig associated stx2e subtype. Only a small proportion of the STEC isolates harbored hlyA, ehxA and adhersin genes. Based on comparison by serotypes and sequence types with human strains and presence of virulence genes, the STEC isolated from pigs may have a low potential to cause human disease. However, further investigations are needed to assess their public health significance in causing human disease in China.
Methods
Sample collection
A total of 1003 samples was collected from May 2011 to August 2012, of which 326 were fecal samples collected in pig farms in Chongqing city, 351 were small intestinal contents and 326 were colon contents collected in pig slaughter houses in Beijing city and Guizhou province. Samples were transported as soon as possible to the laboratory in the National Institute for Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention in ice-bags cold conditions for the isolation of STEC.
Isolation of STEC
One gram of each sample was enriched in 5 ml of modified Tryptone Soya Broth (mTSB) supplemented with novobiocin (10 mg/liter) (Oxoid, UK) and incubated at 37°C for 18 to 24 h with shaking at 200 rpm. Briefly, 150 μl of the lysis buffer (100 mM NaCl, 10 mM Tris–HCl [pH 8.3], 1 mM EDTA [pH 9.0], 1% Triton X-100) were added to the centrifuged enrichment sample, boiled for 10 min and centrifuged. The supernatant was used as template to test for the presence of stx1 and stx2 by TaqMan duplex real time PCR assay developed by Bai et al. [60]. One loopful of the stx-positive enrichment culture was directly streaked onto CHROMagar™ ECC plate (CHROMagar, Microbiology, Paris, France). After overnight incubation at 37°C, 10 blue or colorless, round moist presumptive colonies on each plate were initially picked randomly to test for the presence of stx1 and stx2 by conventional duplex PCR assay (primers listed in Table 3) and another 10 colonies were picked if the initial 10 were negative for any of the stx genes. The stx-positive colonies were plated onto Luria-Bertani (LB) plates and incubated overnight for further identification. One to 5 stx-positive isolates from each sample were collected for further investigation.
Table 3.
PCR primers used for the detection of STEC virulence or adherence genes
| Targets | Primer | Oligonucleotide sequence (5′-3′) | Amplicon size (bp) | Reference |
|---|---|---|---|---|
|
stx1 |
stx
1
-F |
AAATCGCCATTCGTTGACTACTTCT |
370 |
[61] |
| |
stx
1
-R |
TGCCATTCTGGCAACTCGCGATGCA |
||
|
stx2 |
stx
2
-F |
CAGTCGTCACTCACTGGTTTCATCA |
283 |
[61] |
| |
stx
2
-R |
GGATATTCTCCCCACTCTGACACC |
||
|
stx2e |
stx
2e
-F |
CGGAGTATCGGGGAGAGGC |
411 |
[62] |
| |
stx
2e
-R |
CTTCCTGACACCTTCACAGTAAAGGT |
||
| SLT-II |
GK1 |
ATGAAGTGTATATTATTTAAATGG |
1241 |
[63] |
| |
GK4 |
TCAGTCATTATTAAACTGCAC |
||
|
ehxA |
ehxA-F |
GGTGCAGCAGAAAAAGTTGTAG |
1551 |
[64] |
| |
ehxA-R |
TCTCGCCTGATAGTGTTTGGTA |
||
|
hlyA |
hlyA1-F |
GACAAAGCACGAAAGATG |
2930 |
[6] |
| |
hlyA2-R |
CAACTGCAATAAAGAAGC |
||
|
astA |
EAST11a |
CCATCAACACAGTATATCCGA |
111 |
[65] |
| |
EAST11b |
GGTCGCGAGTGACGGCTTTGT |
||
|
irp2 |
irp2-F |
AAGGATTCGCTGTTACCGGAC |
280 |
[66] |
| |
irp2-R |
TCGTCGGGCAGCGTTTCTTCT |
||
|
fyuA |
fyuA-F |
TGATTAACCCCGCGACGGGAA |
880 |
[66] |
| |
fyuA-R |
CGCAGTAGGCACGATGTTGTA |
||
|
eae |
eae-F |
ACGTTGCAGCATGGGTAACTC |
815 |
[36] |
| |
eae-R |
GATCGGCAACAGTTTCACCTG |
||
|
paa |
M155-F1 |
ATGAGGAAACATAATGGCAGG |
350 |
[67] |
| |
M155-R1 |
TCTGGTCAGGTCGTCAATAC |
||
|
iha |
iha-F |
CAGTTCAGTTTCGCATTCACC |
1305 |
[68] |
| |
iha-R |
GTATGGCTCTGATGCGATG |
||
|
saa |
saa-F |
CGTGATGAACAGGCTATTGC |
119 |
[14] |
| |
saa-R |
ATGGACATGCCTGTGGCAAC |
||
|
toxB |
toxB-F |
ATACCTACCTGCTCTGGATTGA |
602 |
[69] |
| |
toxB-R |
TTCTTACCTGATCTGATGCAGC |
||
|
efa1 |
efa1-F |
GAGACTGCCAGAGAAAG |
479 |
[11] |
| |
efa1-R |
GGTATTGTTGCATGTTCAG |
||
|
lpfAO157/OI-154 |
lpfAO157/OI-154-F |
GCAGGTCACCTACAGGCGGC |
525 |
[14] |
| |
lpfAO157/OI-154-R |
CTGCGAGTCGGCGTTAGCTG |
||
|
lpfAO157/OI-141 |
lpfAO157/OI-141-F |
CTGCGCATTGCCGTAAC |
412 |
[70] |
| |
lpfAO157/OI-141-R |
ATTTACAGGCGAGATCGTG |
||
|
lpfAO113 |
lpfAO113-F |
ATGAAGCGTAATATTATAG |
573 |
[9] |
| |
lpfAO113-R |
TTATTTCTTATATTCGAC |
||
| F4(K88) |
F4-F |
GCTGCATCTGCTGCATCTGGTATGG |
792 |
[15] |
| |
F4-R |
CCACTGAGTGCTGGTAGTTACAGCC |
||
| F5(K99) |
F5-F |
TGCGACTACCAATGCTTCTG |
450 |
[15] |
| |
F5-R |
TATCCACCATTAGACGGAGC |
||
| F6(P987) |
F6-F |
TCTGCTCTTAAAGCTACTGG |
333 |
[15] |
| |
F6-R |
AACTCCACCGTTTGTATCAG |
||
| F17 |
F17-F |
GGGCTGACAGAGGAGGTGGGGC |
411 |
[15] |
| |
F17-R |
CCCGGCGACAACTTCATCACCGG |
||
| F18 |
F18-F |
GTGAAAAGACTAGTGTTTATTTC |
510 |
[15] |
| |
F18-R |
CTTGTAAGTAACCGCGTAAGC |
||
| F41 |
F41-F |
GAGGGACTTTCATCTTTTAG |
431 |
[15] |
| F41-R | AGTCCATTCCATTTATAGGC |
Biochemical tests and serotyping of STEC isolates
All stx-containing isolates were confirmed to be E. coli by using API 20E biochemical test strips (bioMérieux, Lyon, France). Sorbitol fermentation characteristic was examined by using sorbitol-MacConkey agar (SMAC) (Oxoid, UK).
The hemolytic activity was tested by using sheep blood agar (Oxoid, UK). The presence of transparent zones around the colonies was interpreted as positive hemolytic activity [71].
The determination of O antigens was firstly carried out by testing for specific E. coli O groups of interest, targeting group specific genes within the O-antigen gene cluster described by DebRoy et al.[72]. The entire coding sequence of the fliC gene was amplified by PCR with the primers fliC-F (5′-ATGGCACAAGTCATTAATACCCAAC-3′) and fliC-R (5′-CTAACCCTGCAGCAGAGACA-3′) reported by Fields et al. [73], and then sequenced to determine the H type of each isolate. In vitro motility was determined by inoculation of each isolate in the center of motility agar plates (0.3% LB agar) at 37°C for up to 48 h [74]. Bacterial motility was assessed by examining the swimming ring. The O:H serotype was confirmed by the O antisera and the H antisera obtained from the Statens Serum Institut (Copenhagen, Denmark).
stx subtyping
E. coli isolates were cultured in LB broth at 37º C for 18–24 h. DNA was extracted using Wizard Genomic DNA Purification kits (Promega, USA). The presence of Shiga toxin genes were assessed in all isolates by PCR using primers targeting the stx1 and stx2 genes (Table 3) as described by Brian et al.[61]. The stx2 subtypes were determined by the PCR-based subtyping method devised by Scheutz et al. [62]. The complete stx2 gene from a selected set of STEC isolates was amplified using primers GK1 and GK2 from Gunzer et al. [63] and sequenced to verify the PCR-based subtyping results. The neighbor-joining cluster analysis was employed to assign new subtypes or variants as mentioned by Scheutz et al. [62].
Identification of virulence and adherence factors
All STEC isolates were tested by PCR to investigate the presence of astA, hemolysis related genes (ehxA and hlyA), HPI genes (fyuA and irp) and adhesion-related genes (eae, paa, efa1, toxB, lpfAO157/OI-154, lpfAO157/OI-141, lpfAO113, saa, F4, F5, F6, F17, F18 and F41) using the primers listed in Table 3.
Antimicrobial susceptibility testing
Antimicrobial resistance was determined by the disc diffusion method [75]. Twelve antimicrobial groups covering 23 antimicrobial agents including penicillins (ampicillin and piperacillin), β-lactam/β-lactamase inhibitor combinations (amoxicillin-clavulanic acid and ampicillin-sulbactam), cephems (parenteral) (cephalosporins I, II, III, and IV, cefepime, cefotaxime, ceftriaxone, cephalothin and cefuroxime), monobactams (aztreonam), carbapenems (imipenem and meropenem), aminoglycosides (gentamicin, kanamycin and streptomycin), tetracyclines (tetracycline), fluoroquinolones (ciprofloxacin, norfloxacin and levofloxacin), quinolones (nalidixic acid), folate pathway inhibitors (trimethoprim-sulfamethoxazole), phenicols (chloramphenicol) and nitrofurans (nitrofurantoinz) were tested. Results were interpreted using the Clinical and Laboratory Standards Institute (CLSI, 2012) breakpoints, when available. E. coli ATCCR 25922 was used as quality control.
PFGE and MLST
STEC isolates were digested with XbaI and separated by PFGE using the non-O157 STEC PulseNet protocol (http://www.pulsenetinternational.org). Gel images were converted to Tiff files and then analyzed using BioNumerics software (Applied Maths, Sint-Martens-Latem, Belgium).
MLST was performed according to the recommendations of the E. coli MLST website (http://mlst.ucc.ie/mlst/dbs/Ecoli) using 7 housekeeping genes (adk, fumC, gyrB, icd, mdh, purA and recA). Alleles and sequence types (STs) were determined following the website instructions [76]. MLST data for the HUS-associated enterohemorrhagic E. coli (HUSEC) collection were obtained from http://www.ehec.org[52]. All human STEC STs from the E. coli MLST databases were downloaded for comparison. A minimum spanning tree based on these STs was generated with BioNumerics software.
Four novel alleles, fumC470, gyrB351, icd396 and recA267 were submitted to E. coli MLST website. The sequences obtained in this study have been deposited in GenBank: KC924398 (icd396), KC924399 (gyrB351), KC924400 (fumC470), KC924401 (recA267) and KC339670 (a new variant of stx2e).
Statistical analysis
Statistical tests were performed using SAS, Version 9.1 (SAS Institute Inc., Cary, NC., USA). Statistically significant differences were calculated using a χ2 test where appropriate. P values of <0.05 were considered statistically significant.
Ethics statement
Samples of pig feces, small intestinal contents and colon contents of finished pig were acquired with the oral consent from the pig owners. This study was reviewed and approved by the ethics committee of the National Institute for Communicable Disease Control and Prevention, China CDC, according to the medical research regulations of the National Health and Family Planning Commission of People’s Republic of China (permit number 2011-10-4).
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
QM carried out the sample collection, isolation of STEC, biochemical tests and serotyping of STEC isolates, identification of virulence and adherence factors, antimicrobial susceptibility testing, MLST, stx subtyping, data analysis and drafting of the manuscript. YX and RL carried out study design, overseeing the study, and editing of the manuscript. The rest of the authors contributed sample collection, strains isolation, biochemical tests and serotyping of STEC isolates, MLST, or PFGE. All authors read and approved the final manuscript.
Supplementary Material
Antibiotic resistances of swine STEC isolates.
Contributor Information
Qiong Meng, Email: joanmeng47@163.com.
Xiangning Bai, Email: baixiangning@icdc.cn.
Ailan Zhao, Email: zhaoailan@icdc.cn.
Ruiting Lan, Email: r.lan@unsw.edu.au.
Huamao Du, Email: duhmao@swu.cn.
Tao Wang, Email: wangtao309@tom.com.
Changyou Shi, Email: changyou523-@163.com.
Xuejiao Yuan, Email: yuanxuejiao@icdc.cn.
Xuemei Bai, Email: baixuemei@icdc.cn.
Shaobo Ji, Email: jishaobo@icdc.cn.
Dong Jin, Email: jindong@icdc.cn.
Bo Yu, Email: 583440158@qq.com.
Yan Wang, Email: wangyan@icdc.cn.
Hui Sun, Email: sunhui@icdc.cn.
Kai Liu, Email: good_reward@126.com.
Jianguo Xu, Email: xujianguo@icdc.cn.
Yanwen Xiong, Email: xiongyanwen@icdc.cn.
Acknowledgements
This work was supported by grants from the National Basic Research Program of China (2011CB504901), the National Natural Science Foundation of China (81290340 and 81290345), the China Mega-Project for Infectious Disease (2013ZX10004-001 and 2012ZX10004-215), and the State Key Laboratory for Infectious Disease Prevention and Control (2012SKLID305).
We appreciate Dr. Flemming Scheutz for helping us in stx subtyping and Dr. Mark Achtman for the support of MLST submission.
References
- Nataro JP, Kaper JB. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11(1):142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin PM, Tauxe RV. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol Rev. 1991;13:60–98. doi: 10.1093/oxfordjournals.epirev.a036079. [DOI] [PubMed] [Google Scholar]
- Bettelheim KA. The non-O157 shiga-toxigenic (verocytotoxigenic) Escherichia coli; under-rated pathogens. Crit Rev Microbiol. 2007;33(1):67–87. doi: 10.1080/10408410601172172. [DOI] [PubMed] [Google Scholar]
- Paton JC, Paton AW. Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clin Microbiol Rev. 1998;11(3):450–479. doi: 10.1128/cmr.11.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarino SJ, Fasano A, Watson J, Martin BM, Levine MM, Guandalini S, Guerry P. Enteroaggregative Escherichia coli heat-stable enterotoxin 1 represents another subfamily of E. coli heat-stable toxin. Proc Natl Acad Sci USA. 1993;90(7):3093–3097. doi: 10.1073/pnas.90.7.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd EF, Hartl DL. Chromosomal regions specific to pathogenic isolates of Escherichia coli have a phylogenetically clustered distribution. J Bacteriol. 1998;180(5):1159–1165. doi: 10.1128/jb.180.5.1159-1165.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cookson AL, Bennett J, Thomson-Carter F, Attwood GT. Molecular subtyping and genetic analysis of the enterohemolysin gene (ehxA) from Shiga toxin-producing Escherichia coli and atypical enteropathogenic E. coli. Appl Environ Microbiol. 2007;73(20):6360–6369. doi: 10.1128/AEM.00316-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batisson I, Guimond MP, Girard F, An H, Zhu C, Oswald E, Fairbrother JM, Jacques M, Harel J. Characterization of the novel factor paa involved in the early steps of the adhesion mechanism of attaching and effacing Escherichia coli. Infect Immun. 2003;71(8):4516–4525. doi: 10.1128/IAI.71.8.4516-4525.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doughty S, Sloan J, Bennett-Wood V, Robertson M, Robins-Browne RM, Hartland EL. Identification of a novel fimbrial gene cluster related to long polar fimbriae in locus of enterocyte effacement-negative strains of enterohemorrhagic Escherichia coli. Infect Immun. 2002;70(12):6761–6769. doi: 10.1128/IAI.70.12.6761-6769.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TJ, Nolan LK. Pathogenomics of the virulence plasmids of Escherichia coli. Microbiol Mol Biol Rev. 2009;73(4):750–774. doi: 10.1128/MMBR.00015-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls L, Grant TH, Robins-Browne RM. Identification of a novel genetic locus that is required for in vitro adhesion of a clinical isolate of enterohaemorrhagic Escherichia coli to epithelial cells. Mol Microbiol. 2000;35(2):275–288. doi: 10.1046/j.1365-2958.2000.01690.x. [DOI] [PubMed] [Google Scholar]
- Paton AW, Srimanote P, Woodrow MC, Paton JC. Characterization of Saa, a novel autoagglutinating adhesin produced by locus of enterocyte effacement-negative Shiga-toxigenic Escherichia coli strains that are virulent for humans. Infect Immun. 2001;69(11):6999–7009. doi: 10.1128/IAI.69.11.6999-7009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr PI, Bilge SS, Vary JC Jr, Jelacic S, Habeeb RL, Ward TR, Baylor MR, Besser TE. Iha: a novel Escherichia coli O157:H7 adherence-conferring molecule encoded on a recently acquired chromosomal island of conserved structure. Infect Immun. 2000;68(3):1400–1407. doi: 10.1128/IAI.68.3.1400-1407.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toma C, Martinez Espinosa E, Song T, Miliwebsky E, Chinen I, Iyoda S, Iwanaga M, Rivas M. Distribution of putative adhesins in different seropathotypes of Shiga toxin-producing Escherichia coli. J Clin Microbiol. 2004;42(11):4937–4946. doi: 10.1128/JCM.42.11.4937-4946.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu-Khac H, Holoda E, Pilipcinec E, Blanco M, Blanco JE, Dahbi G, Mora A, Lopez C, Gonzalez EA, Blanco J. Serotypes, virulence genes, intimin types and PFGE profiles of Escherichia coli isolated from piglets with diarrhoea in Slovakia. Vet J. 2007;174(1):176–187. doi: 10.1016/j.tvjl.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Toledo A, Gomez D, Cruz C, Carreon R, Lopez J, Giono S, Castro AM. Prevalence of virulence genes in Escherichia coli strains isolated from piglets in the suckling and weaning period in Mexico. J Med Microbiol. 2012;61(Pt 1):148–156. doi: 10.1099/jmm.0.031302-0. [DOI] [PubMed] [Google Scholar]
- Smeds A, Pertovaara M, Timonen T, Pohjanvirta T, Pelkonen S, Palva A. Mapping the binding domain of the F18 fimbrial adhesin. Infect Immun. 2003;71(4):2163–2172. doi: 10.1128/IAI.71.4.2163-2182.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy B, Fekete PZ. Enterotoxigenic Escherichia coli (ETEC) in farm animals. Vet Res. 1999;30(2–3):259–284. [PubMed] [Google Scholar]
- Sonntag AK, Bielaszewska M, Mellmann A, Dierksen N, Schierack P, Wieler LH, Schmidt MA, Karch H. Shiga toxin 2e-producing Escherichia coli isolates from humans and pigs differ in their virulence profiles and interactions with intestinal epithelial cells. Appl Environ Microbiol. 2005;71(12):8855–8863. doi: 10.1128/AEM.71.12.8855-8863.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast DM, Lendrum L, Pearce R, Ball C, McLernon J, O’Grady D, Scott L, Fanning S, Egan J, Gutierrez M. Verocytotoxigenic Escherichia coli O157 in beef and sheep abattoirs in Ireland and characterisation of isolates by Pulsed-Field Gel Electrophoresis and Multi-Locus Variable Number of Tandem Repeat Analysis. Int J Food Microbiol. 2011;144(3):519–527. doi: 10.1016/j.ijfoodmicro.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Karmali MA, Gannon V, Sargeant JM. Verocytotoxin-producing Escherichia coli (VTEC) Vet Microbiol. 2010;140(3–4):360–370. doi: 10.1016/j.vetmic.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Meng Q, Xiong Y, Lan R, Ye C, Wang T, Qi T, Wang Y, Wang H, Bai X, Bai X. et al. SNP genotyping of enterohemorrhagic Escherichia coli O157:H7 isolates from China and genomic identity of the 1999 Xuzhou outbreak. Infect Genet Evol. 2013;16C:275–281. doi: 10.1016/j.meegid.2013.02.018. [DOI] [PubMed] [Google Scholar]
- Weinstein DL, Jackson MP, Samuel JE, Holmes RK, O’Brien AD. Cloning and sequencing of a Shiga-like toxin type II variant from Escherichia coli strain responsible for edema disease of swine. J Bacteriol. 1988;170(9):4223–4230. doi: 10.1128/jb.170.9.4223-4230.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann M, Zweifel C, Blanco M, Blanco JE, Blanco J, Beutin L, Stephan R. Escherichia coli O157 and non-O157 Shiga toxin-producing Escherichia coli in fecal samples of finished pigs at slaughter in Switzerland. J Food Prot. 2006;69(2):260–266. doi: 10.4315/0362-028x-69.2.260. [DOI] [PubMed] [Google Scholar]
- Fratamico PM, Bagi LK, Bush EJ, Solow BT. Prevalence and characterization of shiga toxin-producing Escherichia coli in swine feces recovered in the national animal health monitoring system’s swine 2000 study. Appl Environ Microbiol. 2004;70(12):7173–7178. doi: 10.1128/AEM.70.12.7173-7178.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratamico PM, Bhagwat AA, Injaian L, Fedorka-Cray PJ. Characterization of Shiga toxin-producing Escherichia coli strains isolated from swine feces. Foodborne Pathog Dis. 2008;5(6):827–838. doi: 10.1089/fpd.2008.0147. [DOI] [PubMed] [Google Scholar]
- Rios M, Prado V, Trucksis M, Arellano C, Borie C, Alexandre M, Fica A, Levine MM. Clonal diversity of Chilean isolates of enterohemorrhagic Escherichia coli from patients with hemolytic-uremic syndrome, asymptomatic subjects, animal reservoirs, and food products. J Clin Microbiol. 1999;37(3):778–781. doi: 10.1128/jcm.37.3.778-781.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botteldoorn N, Heyndrickx M, Rijpens N, Herman L. Detection and characterization of verotoxigenic Escherichia coli by a VTEC/EHEC multiplex PCR in porcine faeces and pig carcass swabs. Res Microbiol. 2003;154(2):97–104. doi: 10.1016/S0923-2508(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Cardeti GF, Tagliabue S, Losio N, Caprioli A, Pacciarini ML. Detection and characterization of Shiga toxin-producing E. coli (STEC) in different samples from various animal species: One year of experience. University of Liège, Belgium: Proceedings of the Conference of Pathogenicity and Virulence of VTEC: 8–10 November 1999; 1999. [Google Scholar]
- Valdivieso-Garcia A, MacLeod DL, Clarke RC, Gyles CL, Lingwood C, Boyd B, Durette A. Comparative cytotoxicity of purified Shiga-like toxin-IIe on porcine and bovine aortic endothelial and human colonic adenocarcinoma cells. J Med Microbiol. 1996;45(5):331–337. doi: 10.1099/00222615-45-5-331. [DOI] [PubMed] [Google Scholar]
- Houser BA, Donaldson SC, Padte R, Sawant AA, DebRoy C, Jayarao BM. Assessment of phenotypic and genotypic diversity of Escherichia coli shed by healthy lactating dairy cattle. Foodborne Pathog Dis. 2008;5(1):41–51. doi: 10.1089/fpd.2007.0036. [DOI] [PubMed] [Google Scholar]
- Grant MA, Mogler MA, Harris DL. Comparison of enrichment procedures for shiga toxin-producing Escherichia coli in wastes from commercial swine farms. J Food Prot. 2009;72(9):1982–1986. doi: 10.4315/0362-028x-72.9.1982. [DOI] [PubMed] [Google Scholar]
- Sanchez S, Garcia-Sanchez A, Martinez R, Blanco J, Blanco JE, Blanco M, Dahbi G, Mora A, Hermoso de Mendoza J, Alonso JM. et al. Detection and characterisation of Shiga toxin-producing Escherichia coli other than Escherichia coli O157:H7 in wild ruminants. Vet J. 2009;180(3):384–388. doi: 10.1016/j.tvjl.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Beutin L, Miko A, Krause G, Pries K, Haby S, Steege K, Albrecht N. Identification of human-pathogenic strains of Shiga toxin-producing Escherichia coli from food by a combination of serotyping and molecular typing of Shiga toxin genes. Appl Environ Microbiol. 2007;73(15):4769–4775. doi: 10.1128/AEM.00873-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lienemann T, Pitkanen T, Antikainen J, Molsa E, Miettinen I, Haukka K, Vaara M, Siitonen A. Shiga toxin-producing Escherichia coli O100:H(−): stx2e in drinking water contaminated by waste water in Finland. Curr Microbiol. 2011;62(4):1239–1244. doi: 10.1007/s00284-010-9832-x. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Shimada J, Nakazawa M, Morozumi T, Pohjanvirta T, Pelkonen S, Yamamoto K. Prevalence and characteristics of shiga toxin-producing Escherichia coli from healthy cattle in Japan. Appl Environ Microbiol. 2001;67(1):484–489. doi: 10.1128/AEM.67.1.484-489.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JR, Congeni BL, Cleary TG, Stone RT, Wanger A, Murray BE, Mathewson JJ, Pickering LK. Escherichia coli O114:nonmotile as a pathogen in an outbreak of severe diarrhea associated with a day care center. J Infect Dis. 1989;160(2):243–247. doi: 10.1093/infdis/160.2.243. [DOI] [PubMed] [Google Scholar]
- Blanco JE, Blanco M, Alonso MP, Mora A, Dahbi G, Coira MA, Blanco J. Serotypes, virulence genes, and intimin types of Shiga toxin (verotoxin)-producing Escherichia coli isolates from human patients: prevalence in Lugo, Spain, from 1992 through 1999. J Clin Microbiol. 2004;42(1):311–319. doi: 10.1128/JCM.42.1.311-319.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth D, Grif K, Fisher I, Fruth A, Tschape H, Scheutz F, Dierich MP, Wurzner R. Emerging Shiga toxin-producing Escherichia coli serotypes in Europe: O100:H– and O127:H40. Curr Microbiol. 2006;53(5):428–429. doi: 10.1007/s00284-006-0209-0. [DOI] [PubMed] [Google Scholar]
- Kappeli U, Hachler H, Giezendanner N, Beutin L, Stephan R. Human infections with non-O157 Shiga toxin-producing Escherichia coli, Switzerland, 2000–2009. Emerg Infect Dis. 2011;17(2):180–185. doi: 10.3201/eid1702.100909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornu G, Proesmans W, Dediste A, Jacobs F, Van De Walle J, Mertens A, Ramet J, Lauwers S. Hemolytic uremic syndrome in Belgium: incidence and association with verocytotoxin-producing Escherichia coli infection. Clin Microbiol Infect. 1999;5(1):16–22. doi: 10.1111/j.1469-0691.1999.tb00093.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Diaz C, Marino M, Cloralt R, Pequeneze M, Perez-Schael I. Age-specific prevalence of Escherichia coli with localized and aggregative adherence in Venezuelan infants with acute diarrhea. J Clin Microbiol. 1997;35(5):1103–1107. doi: 10.1128/jcm.35.5.1103-1107.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman PA, Wright DJ, Siddons CA. A comparison of immunomagnetic separation and direct culture for the isolation of verocytotoxin-producing Escherichia coli O157 from bovine faeces. J Med Microbiol. 1994;40(6):424–427. doi: 10.1099/00222615-40-6-424. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Wang P, Lan R, Ye C, Wang H, Ren J, Jing H, Wang Y, Zhou Z, Bai X. et al. A novel Escherichia coli O157:H7 clone causing a major hemolytic uremic syndrome outbreak in China. PLoS One. 2012;7(4):e36144. doi: 10.1371/journal.pone.0036144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho WS, Tan LK, Ooi PT, Yeo CC, Thong KL. Prevalence and characterization of verotoxigenic-Escherichia coli isolates from pigs in Malaysia. BMC Vet Res. 2013;9:109. doi: 10.1186/1746-6148-9-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch H, Bielaszewska M. Sorbitol-fermenting Shiga toxin-producing Escherichia coli O157:H(−) strains: epidemiology, phenotypic and molecular characteristics, and microbiological diagnosis. J Clin Microbiol. 2001;39(6):2043–2049. doi: 10.1128/JCM.39.6.2043-2049.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller CA, Pellino CA, Flagler MJ, Strasser JE, Weiss AA. Shiga toxin subtypes display dramatic differences in potency. Infect Immun. 2011;79(3):1329–1337. doi: 10.1128/IAI.01182-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich AW, Bielaszewska M, Zhang WL, Pulz M, Kuczius T, Ammon A, Karch H. Escherichia coli harboring Shiga toxin 2 gene variants: frequency and association with clinical symptoms. J Infect Dis. 2002;185(1):74–84. doi: 10.1086/338115. [DOI] [PubMed] [Google Scholar]
- Jerse AE, Kaper JB. The eae gene of enteropathogenic Escherichia coli encodes a 94-kilodalton membrane protein, the expression of which is influenced by the EAF plasmid. Infect Immun. 1991;59(12):4302–4309. doi: 10.1128/iai.59.12.4302-4309.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WL, Bielaszewska M, Liesegang A, Tschape H, Schmidt H, Bitzan M, Karch H. Molecular characteristics and epidemiological significance of Shiga toxin-producing Escherichia coli O26 strains. J Clin Microbiol. 2000;38(6):2134–2140. doi: 10.1128/jcm.38.6.2134-2140.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert S, Rakin A, Heesemann J. The Yersinia high-pathogenicity island (HPI): evolutionary and functional aspects. Int J Med Microbiol. 2004;294(2–3):83–94. doi: 10.1016/j.ijmm.2004.06.026. [DOI] [PubMed] [Google Scholar]
- Mellmann A, Bielaszewska M, Kock R, Friedrich AW, Fruth A, Middendorf B, Harmsen D, Schmidt MA, Karch H. Analysis of collection of hemolytic uremic syndrome-associated enterohemorrhagic Escherichia coli. Emerg Infect Dis. 2008;14(8):1287–1290. doi: 10.3201/eid1408.071082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielaszewska M, Mellmann A, Zhang W, Kock R, Fruth A, Bauwens A, Peters G, Karch H. Characterisation of the Escherichia coli strain associated with an outbreak of haemolytic uraemic syndrome in Germany, 2011: a microbiological study. Lancet Infect Dis. 2011;11(9):671–676. doi: 10.1016/S1473-3099(11)70165-7. [DOI] [PubMed] [Google Scholar]
- Coombes BK, Wickham ME, Mascarenhas M, Gruenheid S, Finlay BB, Karmali MA. Molecular analysis as an aid to assess the public health risk of non-O157 Shiga toxin-producing Escherichia coli strains. Appl Environ Microbiol. 2008;74(7):2153–2160. doi: 10.1128/AEM.02566-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XM, Liao XP, Liu SG, Zhang WJ, Jiang HX, Zhang MJ, Zhu HQ, Sun Y, Sun J, Li AX. et al. Serotypes, virulence genes, and antimicrobial susceptibility of Escherichia coli isolates from pigs. Foodborne Pathog Dis. 2011;8(6):687–692. doi: 10.1089/fpd.2010.0739. [DOI] [PubMed] [Google Scholar]
- Stephan R, Schumacher S. Resistance patterns of non-O157 Shiga toxin-producing Escherichia coli (STEC) strains isolated from animals, food and asymptomatic human carriers in Switzerland. Lett Appl Microbiol. 2001;32(2):114–117. doi: 10.1046/j.1472-765x.2001.00867.x. [DOI] [PubMed] [Google Scholar]
- Uemura R, Sueyoshi M, Nagayoshi M, Nagatomo H. Antimicrobial susceptibilities of Shiga toxin-producing Escherichia coli isolates from pigs with edema disease in Japan. Microbiol Immunol. 2003;47(1):57–61. doi: 10.1111/j.1348-0421.2003.tb02786.x. [DOI] [PubMed] [Google Scholar]
- Zhao S, White DG, Ge B, Ayers S, Friedman S, English L, Wagner D, Gaines S, Meng J. Identification and characterization of integron-mediated antibiotic resistance among Shiga toxin-producing Escherichia coli isolates. Appl Environ Microbiol. 2001;67(4):1558–1564. doi: 10.1128/AEM.67.4.1558-1564.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser E, Mellmann A, Semmler T, Stoeber H, Wieler LH, Karch H, Kuebler N, Fruth A, Harmsen D, Weniger T. et al. Phylogenetic and molecular analysis of food-borne shiga toxin-producing Escherichia coli. Appl Environ Microbiol. 2013;79(8):2731–2740. doi: 10.1128/AEM.03552-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai X, Zhao A, Lan R, Xin Y, Xie H, Meng Q, Jin D, Yu B, Sun H, Lu S. et al. Shiga toxin-producing Escherichia coli in yaks (Bos grunniens) from the Qinghai-Tibetan plateau, China. PLoS One. 2013;8(5):e65537. doi: 10.1371/journal.pone.0065537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brian MJ, Frosolono M, Murray BE, Miranda A, Lopez EL, Gomez HF, Cleary TG. Polymerase chain reaction for diagnosis of enterohemorrhagic Escherichia coli infection and hemolytic-uremic syndrome. J Clin Microbiol. 1992;30(7):1801–1806. doi: 10.1128/jcm.30.7.1801-1806.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheutz F, Teel LD, Beutin L, Pierard D, Buvens G, Karch H, Mellmann A, Caprioli A, Tozzoli R, Morabito S. et al. Multicenter evaluation of a sequence-based protocol for subtyping Shiga toxins and standardizing Stx nomenclature. J Clin Microbiol. 2012;50(9):2951–2963. doi: 10.1128/JCM.00860-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunzer F, Bohm H, Russmann H, Bitzan M, Aleksic S, Karch H. Molecular detection of sorbitol-fermenting Escherichia coli O157 in patients with hemolytic-uremic syndrome. J Clin Microbiol. 1992;30(7):1807–1810. doi: 10.1128/jcm.30.7.1807-1810.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey J, Blanco JE, Blanco M, Mora A, Dahbi G, Alonso JM, Hermoso M, Hermoso J, Alonso MP, Usera MA. et al. Serotypes, phage types and virulence genes of shiga-producing Escherichia coli isolated from sheep in Spain. Vet Microbiol. 2003;94(1):47–56. doi: 10.1016/S0378-1135(03)00064-6. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Echeverria P. Detection of the enteroaggregative Escherichia coli heat-stable enterotoxin 1 gene sequences in enterotoxigenic E. coli strains pathogenic for humans. Infect Immun. 1996;64(4):1441–1445. doi: 10.1128/iai.64.4.1441-1445.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch H, Schubert S, Zhang D, Zhang W, Schmidt H, Olschlager T, Hacker J. A genomic island, termed high-pathogenicity island, is present in certain non-O157 Shiga toxin-producing Escherichia coli clonal lineages. Infect Immun. 1999;67(11):5994–6001. doi: 10.1128/iai.67.11.5994-6001.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifel C, Schumacher S, Beutin L, Blanco J, Stephan R. Virulence profiles of Shiga toxin 2e-producing Escherichia coli isolated from healthy pig at slaughter. Vet Microbiol. 2006;117(2–4):328–332. doi: 10.1016/j.vetmic.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Schmidt H, Zhang WL, Hemmrich U, Jelacic S, Brunder W, Tarr PI, Dobrindt U, Hacker J, Karch H. Identification and characterization of a novel genomic island integrated at selC in locus of enterocyte effacement-negative, Shiga toxin-producing Escherichia coli. Infect Immun. 2001;69(11):6863–6873. doi: 10.1128/IAI.69.11.6863-6873.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr CL, Large TM, Moeller CL, Lacher DW, Tarr PI, Acheson DW, Whittam TS. Molecular characterization of a serotype O121:H19 clone, a distinct Shiga toxin-producing clone of pathogenic Escherichia coli. Infect Immun. 2002;70(12):6853–6859. doi: 10.1128/IAI.70.12.6853-6859.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szalo IM, Goffaux F, Pirson V, Pierard D, Ball H, Mainil J. Presence in bovine enteropathogenic (EPEC) and enterohaemorrhagic (EHEC) Escherichia coli of genes encoding for putative adhesins of human EHEC strains. Res Microbiol. 2002;153(10):653–658. doi: 10.1016/S0923-2508(02)01379-7. [DOI] [PubMed] [Google Scholar]
- Frydendahl K. Prevalence of serogroups and virulence genes in Escherichia coli associated with postweaning diarrhoea and edema disease in pigs and a comparison of diagnostic approaches. Vet Microbiol. 2002;85(2):169–182. doi: 10.1016/S0378-1135(01)00504-1. [DOI] [PubMed] [Google Scholar]
- DebRoy C, Roberts E, Fratamico PM. Detection of O antigens in Escherichia coli. Anim Health Res Rev. 2011;12(2):169–185. doi: 10.1017/S1466252311000193. [DOI] [PubMed] [Google Scholar]
- Fields PI, Blom K, Hughes HJ, Helsel LO, Feng P, Swaminathan B. Molecular characterization of the gene encoding H antigen in Escherichia coli and development of a PCR-restriction fragment length polymorphism test for identification of E. coli O157:H7 and O157:NM. J Clin Microbiol. 1997;35(5):1066–1070. doi: 10.1128/jcm.35.5.1066-1070.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine F, Stewart EJ, Lindner AB, Taddei F. Mutations in two global regulators lower individual mortality in Escherichia coli. Mol Microbiol. 2008;67(1):2–14. doi: 10.1111/j.1365-2958.2007.05988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Second Informational ament. Wayne, Pennsylvania: Clinical and Laboratory Standards Institute; 2012. [Google Scholar]
- Wirth T, Falush D, Lan R, Colles F, Mensa P, Wieler LH, Karch H, Reeves PR, Maiden MC, Ochman H. et al. Sex and virulence in Escherichia coli: an evolutionary perspective. Mol Microbiol. 2006;60(5):1136–1151. doi: 10.1111/j.1365-2958.2006.05172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Antibiotic resistances of swine STEC isolates.