Abstract
Background
Swallowing problems following stroke may result in increased risk of aspiration pneumonia, malnutrition, and dehydration.
Objective/hypothesis
Our hypothesis was that three neurostimulation techniques would produce beneficial effects on chronic dysphagia following stroke through a common brain mechanism that would predict behavioral response.
Methods
In 18 dysphagic stroke patients (mean age: 66 ± 3 years, 3 female, time-post-stroke: 63 ± 15 weeks [±SD]), pharyngeal electromyographic responses were recorded after single-pulse transcranial magnetic stimulation (TMS) over the pharyngeal motor cortex, to measure corticobulbar excitability before, immediately, and 30 min, after real and sham applications of neurostimulation. Patients were randomized to a single session of either: pharyngeal electrical stimulation (PES), paired associative stimulation (PAS) or repetitive TMS (rTMS). Penetration-aspiration scores and bolus transfer timings were assessed before and after both real and sham interventions using videofluoroscopy.
Results
Corticobulbar excitability of pharyngeal motor cortex was beneficially modulated by PES, PAS and to a lesser extent by rTMS, with functionally relevant changes in the unaffected hemisphere. Following combining the results of real neurostimulation, an overall increase in corticobulbar excitability in the unaffected hemisphere (P = .005, F1,17 = 10.6, ANOVA) with an associated 15% reduction in aspiration (P = .005, z = −2.79) was observed compared to sham.
Conclusions
In this mechanistic study, an increase in corticobulbar excitability the unaffected projection was correlated with the improvement in swallowing safety (P = .001, rho = −.732), but modality-specific differences were observed. Paradigms providing peripheral input favored change in neurophysiological and behavioral outcome measures in chronic dysphagia patients. Further larger cohort studies of neurostimulation in chronic dysphagic stroke are imperative.
Keywords: Chronic dysphagia, Stroke, Neurostimulation, Plasticity
Abbreviations: cPA, cumulative penetration aspiration; MEP, motor evoked potentials; MT, motor threshold; NIHSS, National Institute of Health Stroke Scale; PAS, paired associative stimulation; PEG, percutaneous endoscopic gastrostomy; PES, pharyngeal electrical stimulation; rTMS, repetitive transcranial magnetic stimulation; MI, motor cortex
Introduction
The presence of oropharyngeal dysphagia is recognized as a major risk factor for pneumonia following stroke and is associated with increased mortality at 30 days and 1 year post-ictus [1]. Dysphagia prolongs hospital length-of-stay [2], and slows recovery; in part secondary to the complications of malnutrition and dehydration. Although many patients recover from dysphagia following stroke [3], [4], a proportion remain chronically dysphagic, dependent on modified diet and/or enteral feeding. In addition to impaired quality-of-life, patients with long-term dysphagia remain at risk of malnutrition, dehydration and aspiration pneumonia. Moreover, while speech and language therapists have employed a variety of labor intensive compensatory maneuvers to treat chronic dysphagia, there is very little evidence of their effectiveness [5].
Recent evidence has given insight into the cortical and sub-cortical brain areas, activated during the highly coordinated sensorimotor activity of swallowing [6]. In dysphagia literature, transcranial magnetic stimulation (TMS) studies have shown that the pharyngeal motor cortex (MI) reorganizes following acute unilateral stroke, and that an increase in cortical excitability in the unaffected hemisphere is associated with the recovery of swallowing function [7]. Consequently, the development of novel rehabilitative approaches to drive beneficial changes in cortical and subcortical activity, hence promoting improved swallowing function, remains a major imperative [8].
Recently, peripheral stimulation of the pharynx (pharyngeal electrical stimulation (PES) [9], [10], [11], and cortical stimulation by repetitive TMS (rTMS) [12], [13], [14], [15], [16], [17], or transcranial direct current stimulation (tDCS) [18], [19], [20], or the combination of both peripheral and central stimulation (paired associative stimulation, PAS) [21], [22], have been used in acute and chronic dysphagic stroke research studies in an attempt to both modulate cortical activity and induce or promote functional improvements in swallowing. Several study protocols have been proposed from different laboratories with varying parameters, intensity frequency and targeted specific muscle groups (i.e. mylohyoid [15], upper esophageal area [13], [17]).
The aim of our study was to compare the effects of a single application of one of three neurostimulation techniques (PES, PAS, rTMS) on swallowing safety and neurophysiological mechanisms in chronic dysphagic stroke. The study was designed to answer the following questions:
-
i.
What are the neurophysiological and behavioral effects of each neurostimulation treatment?
-
ii.
Are the mechanisms of the different neurostimulation modalities comparable?
-
iii.
Are the changes in activation of corticobulbar pathways predictive of the changes in swallowing behavior?
Our hypothesis was that all three interventions would produce beneficial effects on swallowing through a common brain mechanism that would predict behavioral response.
Materials and methods
Recruitment
Patients were recruited via referrals from clinics across the North West of England or self-referral. Ethical approval was gained from Salford and Trafford Local Research Ethics Committee. Written informed consent was obtained from all participants before the experiments. The study adhered to the Good Clinical Practice Guidelines from the International Conference on Harmonization and Code of Ethics of the World Medical Association and was registered at UK Clinical Research Network Study Portfolio (UKCRN) (identification number 2499) and at International Standard Randomized Controlled Trial Number Register ISRCTN (83103698).
Participants
Eligible patients were recruited from direct and indirect referrals over 4 years (September 2006–August 2010). They had a clinical diagnosis of stroke with dysphagia confirmed by a speech and language therapist, which persisted for more than 6 weeks post-ictus. Patients with a history of dementia, cognitive impairment or neurological deficits prior to stroke, pacemaker/cardiac defibrillator in situ, severe concomitant medical conditions (i.e. progressive neurological disorders, chronic respiratory conditions or heart failure), structural oropharyngeal pathology, history of epilepsy, previous head and neck surgery, pregnancy, any intracranial metal or combination of medications acting on CNS were excluded.
Experimental procedures
TMS
Focal TMS was performed using a figure-of-8 shaped magnetic coil (outer diameter, 70 mm) connected to a Magstim BiStim2 magnetic stimulator (Magstim Co, Wales, UK), with maximal output of 2.2 T (see the Supplementary material for full details).
Pharyngeal EMG measurements
Pharyngeal electromyographic responses after single TMS pulses, termed as pharyngeal motor evoked potentials (PMEPs), were recorded via a 3.2 mm diameter intraluminal catheter (Gaeltec Ltd, Isle of Skye), with a built-in pair of bipolar platinum ring electrodes.
Videofluoroscopy (VFS)
The research videofluoroscopic assessment [11], [12], [21] was carried out at the Radiology Department, Salford Royal NHS Trust, UK. The examination was conducted with 6 swallows of 5 ml boluses of liquid barium (60% w/v, EZ-HD®, E-Z-EM Limited, UK) and the images were acquired in lateral view (Siemens Fluorospot®H SIRESKOP SX Unit, Germany).
Experimental interventions
PES
An intraluminal catheter was used for PES, connected to a constant current generator (DS7, Welwyn-Garden City, UK) and a trigger generator (Neurolog system, Digitimer) allowing titration of stimulation intensity against individuals' perception and tolerance thresholds. PES intensity was then set at 75% of the difference between perception and tolerance threshold and delivered at a frequency of 5 Hz for 10 min [9], [10], [11].
Repetitive TMS
Trains of stimuli were delivered to pharyngeal motor cortex (MI) with the TMS coil (Magstim, Wales, UK). The optimal parameters for repetitive TMS were frequency of 5 Hz, intensity 90% of resting thenar Motor Threshold (MT) in train of 250 pulses, in 5 blocks of 50 with 10 s between-blocks pause [12].
PAS
Paired associative stimulation was delivered by pairing a pharyngeal electrical stimulus (0.2 ms pulse) with a single TMS pulse over the pharyngeal MI at MT intensity plus 20% of the stimulator output. The 2 pulses were delivered repeatedly every 20 s through Signal software (Cambridge Electronic Design, Cambridge, UK), with an inter-stimulus interval of 100 ms for 10 min [21], [22].
Sham stimulation
For sham PES, the intraluminal catheter was in situ, but no stimulation was delivered, as used in previous studies [9], [10], [11]. Sham rTMS stimulation was given using a 90° coil tilt, which produced the same noise as active stimulation but no cortical stimulation [23]. This approach was applied reliably in the past, where it was shown that cortical excitability in the pharyngeal motor system was not affected [12], [24]. For sham PAS, the coil was held tangentially to the skull at a 90° angle to the sagittal plane, and no PES was delivered through the pharyngeal catheter in situ [21], [22].
Experimental protocol
Patients were asked to attend the laboratory on two separate occasions. On both occasions, VFS was performed initially to obtain baseline measurements. Following VFS, the catheter was inserted either transnasally (14–17 cm from the nasal flare to pair electrodes) or transorally (13–16 cm) according to patient's preference. This allowed the placement of the electrodes in the mid-pharyngeal area, a position verified by a VFS-still images following insertion (see Supplementary material).
Following identifying the cranial vertex on the scalp [25], the brain sites evoking the largest pharyngeal responses in each hemisphere were identified with mapping procedures using single TMS pulses over MI. Baseline TMS measurements were obtained at MT + 20% from both hemispheric sites.
All participants then received both real and sham applications of one of the three neurostimulation treatments in random order on different days. Randomization was also performed for the neurostimulation paradigms (PES, PAS or rTMS). Cortical excitability (TMS) measurements were repeated immediately and 30 min post-intervention. A follow-up VFS was performed after the 30-minutes TMS measurements on both visits (see also Supplementary material).
Randomization and blindness
A randomization software (minim.exe, Department of Bioengineering, Salford Royal NHS Trust) was used for the process of minimization to evenly distribute patients of different age (above or below 80) and stroke severity (scoring <12 or ≥12 on the National Institute of Health stroke Severity Scale (NIHSS) [26], [27]). One independent researcher delivered the real or sham stimulation, while those researchers analyzing the data remained blinded to the procedure.
Data analysis
Neurophysiological measurements
The peak-to-peak PMEPs amplitude was used as a measure of cortical excitability. Changes in excitability over-time were compared, using generalized linear model repeated measures ANOVA (RmANOVA, SPSSv.19.0), following verification of the assumption of sphericity was not violated (Mauchly's test). Non-parametric paired-wise comparisons were performed with Friedman test [28]. P value of <.05 indicated statistical significance (see Supplementary material).
VFS analysis
Frame-by-frame analysis of the videos took place off-line (see Supplementary material). The safety of all swallows was assessed and scored using the 8-point penetration-aspiration (PA) scale, describing the severity of airway compromise [29]. Abnormal laryngeal protection was verified, if a swallow was scored ≥3 on one or more occasions.
All pairwise comparisons, non-parametric correlations (Spearman's) and Wilcoxon's tests results were corrected with Holms' step-down technique. All data are presented as group mean ± SEM, unless stated otherwise.
Results
Recruitment
From the initial 83 patients enrolled in the study, 4 declined to participate, and only 20 met the inclusion criteria for study participation. One patient had a safe swallow upon initial (baseline) assessment and was therefore excluded, while one additional participant was unable to complete both study days and their data were not used in the analysis. Therefore, 18 patients were left with complete data (see below) (see also Supplementary material).
Patients demographics
Clinical symptoms and demographic data are shown in Table 1. The mean age of the participants was 66 ± 3 (±SD) years and 3 were female. Mean stroke severity as measured with NIHSS score was 7.6 ± 1, while 8 patients had a history of multiple strokes. Seven patients suffered right-sided symptoms, while three exhibited bilateral symptoms. Their symptoms side was recorded as “undetermined.” Fifteen subjects had PEG in situ, four of whom also received modified diet.
Table 1.
Table 1 shows the demographics of the patients consented to participate in the trial. Patient F presented safe swallowing on baseline and was therefore excluded, while patient Q did not complete one of the two study days.
| Groups | ID | Age | Gender | Diet | Weeks post stroke | Previous stroke | NIHSS | Side of symptoms |
|---|---|---|---|---|---|---|---|---|
| PES | B | 51 | M | PEG | 57 | 0 | * | R |
| D | 66 | M | PEG | 159 | 1 | * | U | |
| K | 77 | M | PEG/modified | 28 | 1 | 3 | R | |
| L | 72 | M | PEG | 160 | 1 | 6 | L | |
| O | 65 | F | PEG | 104 | 1 | 6 | U | |
| P | 31 | M | PEG/Modified | 26 | 0 | 11 | L | |
| rTMS | C | 66 | M | PEG | 39 | 0 | 5 | R |
| G | 69 | M | Modified | 12 | 0 | 4 | L | |
| H | 55 | M | PEG | 10 | 0 | 5 | L | |
| I | 64 | M | Modified | 38 | 3 CVAs, 1 TIA | 8 | L | |
| R | 77 | M | PEG | 13 | 1 | 9 | R | |
| T | 73 | M | PEG | 70 | 0 | 3 | L | |
| PAS | A | 83 | M | Modified | 9 | 0 | 4 | L |
| E | 67 | M | Modified | 72 | 0 | 12 | L | |
| J | 76 | F | PEG | 77 | 1 | 6 | R | |
| M | 69 | M | Modified | 8 | 0 | 6 | L | |
| N | 69 | M | PEG/Modified | 15 | 1, 1 TIA | 8 | R | |
| S | 43 | F | Modified | 47 | 0 | 14 | U | |
| Mean ± SD | 66 ± 3 | 63 ± 15 | 7.6 ± 1 |
NIHSS = National Institute of Health Stroke Severity, R = right, L = left, ∗ = no data, U = undetermined, TIA = transient ischemic attack, CVA = cerebrovascular accident.
Patient safety
No adverse events were reported, although two patients reported coughing in response to the catheter placement alone, which subsided within 2 min.
Neurophysiological changes
Mean pharyngeal MT from the affected and unaffected hemispheres were 75 ± 4% and 73 ± 4% respectively. For the subjects with ‘undetermined’ symptom side, the hemispheric site requiring higher intensity to elicit PMEPs was termed as ‘affected.’ No significant differences were observed for baseline cortical excitability in all patients across the two study days (real and sham) (Wilcoxon's test, unaffected hemisphere: z = −.86, P = .386, affected hemisphere: z = −1.47, P = .139), allowing for further statistical analysis.
Modality specific neurophysiological effects following 3 different neurostimulation treatments
The percentage change in cortical excitability of dysphagic stroke patients recruited in each treatment arm is presented in Fig. 1A. Normality assumptions were violated within each group and non-parametric tests were therefore applied. Three separate non-parametric Friedman tests were performed within each arm. The distributions within each arm were found to differ, indicating that at least one set of responses from the unaffected or affected hemisphere was driving the effect (PES: P = .009, Chi-square: 22.2; PAS: P = .04, Chi-square: 18.8, rTMS: P = .01, Chi-square:15.07).
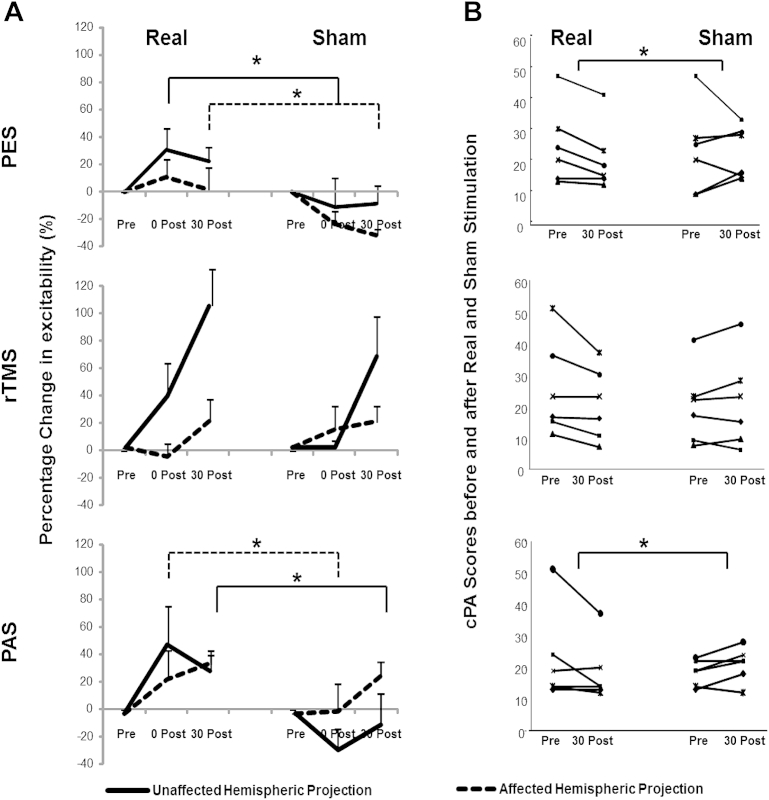
Figure 1.
This figure shows the effects of real and sham neurostimulation techniques on cortical excitability (A) for both affected (dashed line) and unaffected pharyngeal projection (full line) and (B) swallowing safety as measured with cumulative penetration/aspiration scores. The (*) show where a significant difference was observed (for neurophysiological data: full line shows the significant differences in the unaffected, while dashed line shows the significant differences in the affected hemisphere).
Each treatment's effects were therefore examined individually with pairwise comparisons (Wilcoxon's) between real and sham arms.
With PES, we observed significant excitability increase immediately post-treatment in the unaffected hemisphere (real vs. sham P = .043, z = −2.02, d = 3.51, r = 0.86) and in the affected hemisphere 30 min post-intervention (real vs. sham P = .04, z = −2.03, d = 3.36, r = 0.85), indicating bilateral changes in cortical excitability.
With rTMS, cortical excitability in the unaffected hemisphere appeared to visibly increase following real neurostimulation. However, compared to sham, this change was not statistically significant (immediately [P = .08, z = −1.75], 30 min following treatment [P = .08, z = −1.75]). No change in the affected hemisphere was observed.
With PAS, cortical excitability increased 30 min post-intervention in the unaffected (P = .043, z = −2.02, d = 2.28, r = .75) compared to sham. A significant increase was also observed in the affected hemisphere immediately following contra-lateral PAS (P = .027, z = −2.07, d = 1.12, r = .49).
Overall effects of neurostimulation on swallowing neurophysiology
To assess evidence for a common mechanism on swallowing neurophysiology to neurostimulation, the overall effects of the three modalities on cortical function were examined.
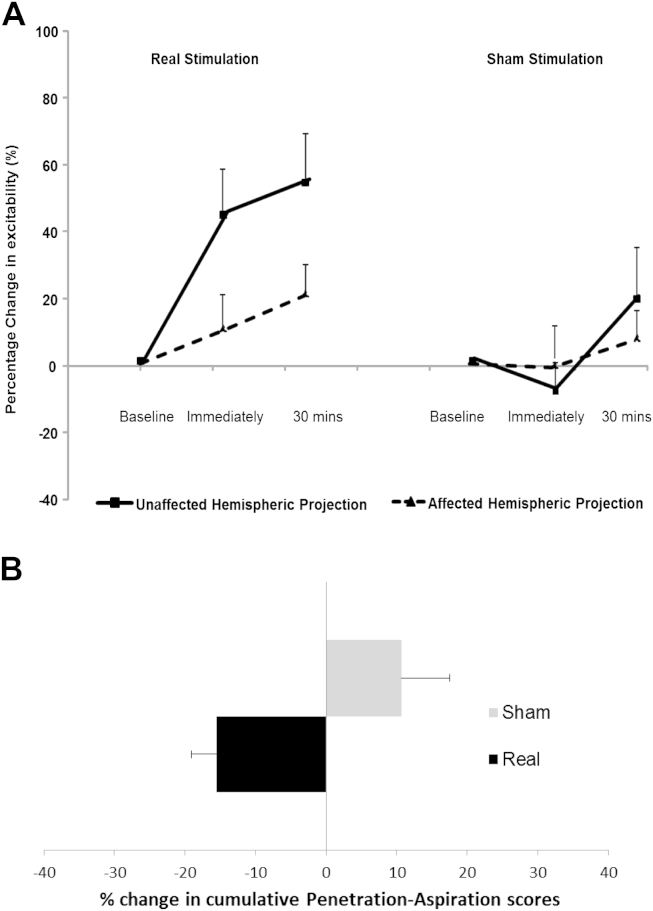
After combining the responses following all real interventions an increase in cortical excitability in the unaffected hemisphere was observed both immediately and 30 min post-treatment (Fig. 2A). A three-way rmANOVA, with factors hemisphere, intervention and time, showed significant interactions between all factors (P = .005, F1,17 = 10.6), a significant interaction of intervention×time (P = .03, F1,17 = 5.6) and of hemisphere×intervention (P = .011, F1,17 = 8.1).
Figure 2.
A) After combining patients' neurophysiological responses of affected (dashed line) and unaffected hemispheric projection (full line) following real and sham neurostimulation, cortical excitability of the unaffected hemisphere was significantly increased compared to sham over time (P < .05). B) Similarly, the group PA scores following real stimulation arms were significantly different to sham arms (P < .05).
Further two-way rmANOVAs on the combined data for each hemisphere examining the effects of real and sham intervention over time showed a significant interaction (P = .005, F1,17 = 10.6) and an effect of intervention (P < .001, F1,17 = 19.9) and time (P = .041, F1,17 = 4.96) for the unaffected hemisphere only. The increase in cortical excitability was seen at both the immediate and 30 min following real treatments (effect size d = 1.49, r = .59 and d = .8, r = .381 respectively).
Modality specific behavioral effects following 3 different neurostimulation treatments
The cumulative PA scores for each subject in each neurostimulation group at baseline and following interventions are shown in Fig. 1B. The cPA scores' distributions across real and sham arms following the different neurostimulation paradigms were significantly different (P = .018, Chi-square: 12.8; Friedman's test). When examining the individual treatments, there was a significant difference between the real and sham cPA scores after PAS (P = .007, d = −.27, r = −.13) and PES (P = .033, d = −.29, r = −.10), but not rTMS.
Overall effects of neurostimulation on swallowing behavior
After combining the groups into real and sham conditions, a reduction in percentage change in cPA scores of −15.5 ± 3.5% was observed, while there was an increase in cPA scores in sham arms by 10.6 ± 6.8. This difference between the two groups was statistically significant (z = −2.794, P = .005, Wilcoxon's, Fig. 2B).
Increased variability was noted in the bolus transport timing results (Table 2), in keeping with literature [30]. Only pharyngeal response time (PRT), reflecting the delay of the laryngeal elevation from the time the bolus reaches the hypopharynx, showed a proportionally significant difference following real neurostimulation treatments compared to the sham arms (z = −2.69, P = .007, d = −.42, r = −.21: negative values here show the increase in PRT in the sham arm group of responses).
Table 2.
Bolus transport timings following real and sham neurostimulation paradigms at baseline and 30 min post.
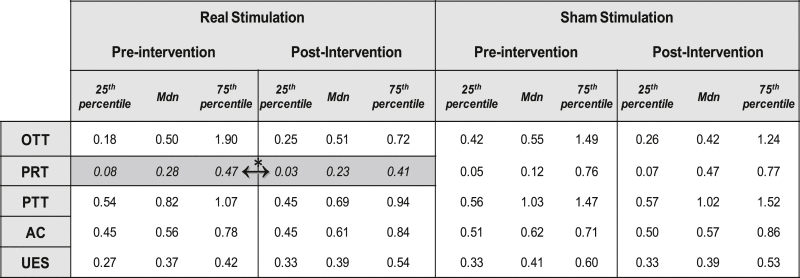 |
Mdn = median, OTT = oral transit time, PRT = pharyngeal response time, PTT = pharyngeal transit time, AC = airway closure time, UES = upper esophageal sphincter opening time.
Relationship between corticobulbar pathways excitability and changes in swallowing behavior
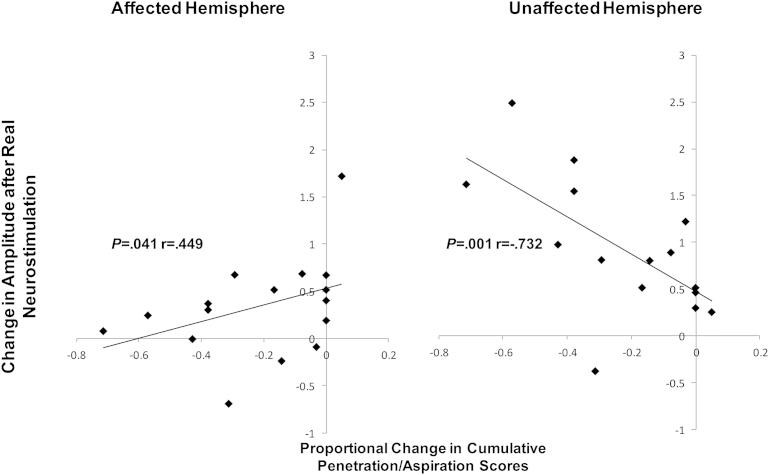
We calculated the proportional change of PA scores and the PMEPs following real and sham stimulation (PES and PAS only) for both time-points (immediately and 30 min) using the ratio ([post–pre]/pre). A strong inverse correlation was observed between the change in cumulative PA scores and the change in cortical excitability of the unaffected hemisphere following real stimulation (Spearman's rho: P = .001, r = −.732), using previously validated methodology [10]. By contrast, there was a weaker positive correlation between the level of cortical excitability of the affected pharyngeal projection and the change in cumulative swallowing scores (P = .041, rho = .449, Fig. 3).
Figure 3.
Increasing penetration-aspiration scores following real neurostimulation paradigms (PAS and PES) was significantly negatively correlated to increasing in cortical excitability of pharyngeal hemispheric projection of the unaffected hemisphere (Spearman's rho: P = .001, r = −.732). By contrast, for the affected hemisphere, a positive correlation was found to the increase in cumulative penetration-aspiration (P = .041, rho = .449).
Discussion
We investigated the effects of 3 neurorehabilitation techniques with distinct properties, namely PES, PAS and rTMS, in a controlled manner by randomizing dysphagic chronic stroke patients to receive both real and sham interventions. Our study showed that a single application of either PES or PAS increases cortical excitability and is associated with reductions in aspiration, while 5 Hz rTMS was less effective. Given the individual characteristics of each intervention, our results merit further discussion.
Neurophysiological and behavioral changes in chronic stroke patients following the different neurostimulation paradigms
Bilateral increases in cortical excitability were evident after the application of 2 of the 3 real neurostimulation paradigms. The greatest increase was observed in the unaffected hemisphere, while an overall reduction in penetration-aspiration scores was found when real stimulation was compared to sham, particularly for both real PAS and PES, accompanied by changes in swallowing transfer timings (pharyngeal response time (PRT)).
Interestingly, the two neurostimulation paradigms, PES and PAS, which mainly employ peripheral stimulation, showed associated increases in cortical excitability of the affected hemisphere, and curiously this took place at different time-points (immediately following PAS and 30 min following PES). Although difficult to explain without neuroimaging studies, we could speculate that these two neurostimulation techniques promote or enhance cortical inter-hemispheric connectivity via different mechanisms in timing or cortical fiber recruitment.
Repetitive TMS did not achieve significant increases in brain excitability when applied to the unaffected pharyngeal projection compared to sham. Intriguingly, while there was a visible increase in brain excitation following real rTMS, there was also an increase with sham rTMS; suggesting some biological effects of sham stimulation on cortical function. Previous studies have advocated the sham rTMS method of coil tilt [23]. but there is evidence suggesting that subclinical stimulation is possible and indeed measurable [31], [32]. Nonetheless, the lack of difference between real and sham rTMS in brain excitation may also explain the lack of behavioral effect. Alternatively, it might be speculated that as PES and PAS produced bilateral cortical effects due to the peripheral component of the two neurostimulation techniques, while the effects of a single application of rTMS may not have been adequate to produce bilateral increases and improve functional outcome. In the literature, the beneficial effects of 5 Hz rTMS on swallowing motor cortex were observed following a repeated application of the technique over a period of weeks suggesting that more persistent doses of rTMS are required for beneficial and long lasting effects on swallowing [16].
A significant correlation between increases in swallowing safety (reduced cumulative penetration-aspiration scores) and increases in unaffected hemisphere excitability was observed, supporting the contention that functional behavioral changes in swallow are mostly driven by improvements in the undamaged circuitry. Hence, our findings continue to support the notion that in (unilateral) stroke, successful neurorehabilitation treatments should both target and promote compensatory changes in the undamaged/unaffected cortex. The unexpected finding that cortical excitability of the affected hemisphere increasing in tandem with an increase in cumulative penetration-aspiration scores, might be of importance for future directions of research. It might be speculated that the long-term neuroplastic changes occurring in the affected hemisphere following stroke might be of maladaptive nature, not facilitating the process of functional recovery, or that priming the undamaged pharyngeal motor system in the chronic phase may assist the affected hemisphere to participate in the process of recovery. It might also be contended that unilateral increases in swallowing activity might be more functionally useful in dysphagia recovery, although the bilateral increases in brain swallowing activity with PES and PAS, both of which were associated with improved swallowing, would be against this notion. Future longitudinal neurophysiological studies in dysphagic stroke patients might provide additional information on how the unaffected hemisphere operationalizes physiological processes several months post-ictus or to what extent the activity of the corticobulbar projections from the affected and adjunct to the lesion areas might be employed for the restitution of adaptive function, in conjunction with the unaffected hemispheric projection.
Since there is limited data on the exact physiologic mechanisms that drive recovery in stroke, it is not surprising that studies focusing on neurostimulation technologies have targeted either affected or unaffected hemisphere without clear rationale. Our contention that excitatory stimulation applied to unaffected cortex is likely to produce greatest benefit was also the rationale by Park et al. [16] who recently targeted that hemisphere with 5 Hz rTMS employing a therapeutic regimen of 2 weeks.
By studying chronic stroke patients, the confounding effect of spontaneous recovery of function post-ictus was also reduced. Our results show that neurostimulation affects neuronal activity even after a single application in chronic stroke patients, who usually present a plateau in their ability to change behaviorally. In the literature, the time-window for functional restoration following stroke is not clear. However, several factors may play a role in any restorative process in the chronic stage, such as the timing and exposure to restorative treatments during the acute stage of recovery [33]. In animals, even weeks and months after a plateau following the acute phase, there is potential for change [34]. However, plastic reorganizational changes in chronic dysphagic stroke patients may involve different mechanisms, implicating the need for longitudinal studies to understand the recovery mechanisms.
Notably, we were only able to study a carefully selected but well-characterized sample of chronic dysphagic stroke patients, so it is not possible to generalize our results to a wider more heterogenous population of chronic dysphagic patients. Although we used specific inclusion and exclusion criteria, there was still some heterogeneity in the dysphagia severity amongst the patients in each group, which may have contributed to the less favorable results following rTMS. Moreover, a cross-over design was employed, with patients serving as their own control. The wash-out period of treatments used in cross-over designs is of importance, however, results from studies in health [10], [11], [12], [21] showed that a single application does not promote effects more than 2 h post-intervention. High variability in biomechanics and transport bolus timings was observed, which may be a result of the small sample sizes per treatment arm. Inter-subject differences in swallowing patterns are more overt in stroke, with the majority of chronic dysphagic patients requiring artificial feeding to retain nutritional levels. Compensatory strategies during swallowing are usually employed, a factor, which by itself may produce additional changes in neurophysiology.
To conclude our preliminary results suggest that even a single application of some types of neurostimulation (PES and PAS) when applied to dysphagic chronic stroke patients can lead to changes in corticobulbar excitability and associated behavioral changes, such changes in penetration-aspiration scores. Further studies are required to elucidate the optimum stimulation paradigms with additional classifiers such as the severity of dysphagia, as well as different location and sizes of lesions, which might reduce the increased heterogeneity in the results. Finally, this study demonstrates the feasibility and acceptability of the interventions in patients with chronic dysphagia following stroke and provides information on likely recruitment rates and effect sizes for the design of a future clinical trial.
Acknowledgments
The authors would like to thank all participants and Lisa Renault, Jackie Johnson, and Daniela Burgess (senior radiographers) who helped conduct the videofluoroscopy examinations. Also we would like to thank the Local Stroke Research Network (North West) for the assistance with patient recruitment.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Funding: Supported by the Wellcome Trust (WT081741MA). EM was recipient of the Greek State Foundation Scholarship and FSF NIHR North West Stroke Research Network. SJ was funded through a grant from Action Medical.
Contributors and authorships: EM (blinded to interventions), SJ (blinded to interventions), SM assisted in the studies. EM and SJ analyzed data and wrote the paper. EM, SM, SJ and PT helped in interpretation of data. SH was the Chief Investigator, wrote the grants to support the studies, helped write the paper and interpret the analysis.
Disclosures: Nothing to disclose.
The study was sponsored by the University of Manchester, UK, which did not have a role in the study design or in the collection, analysis, or interpretation of data.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.brs.2013.09.005.
Supplementary data
References
- 1.Finlayson O., Kapral M., Hall R., Asllani E., Selchen D., Saposnik G. Risk factors, inpatient care, and outcomes of pneumonia after ischemic stroke. Neurology. 2011;77:1338–1345. doi: 10.1212/WNL.0b013e31823152b1. [DOI] [PubMed] [Google Scholar]
- 2.Altman K.W., Yu G.P., Schaefer S.D. Consequence of dysphagia in the hospitalized patient: impact on prognosis and hospital resources. Arch Otolaryngol Head Neck Surg. 2010;136(8):784–789. doi: 10.1001/archoto.2010.129. [DOI] [PubMed] [Google Scholar]
- 3.Smithard D.G., O'Neill P.A., Parks C., Morris J. Complications and outcome after acute stroke. Does dysphagia matter? Stroke. 1996;27(7):1200–1204. doi: 10.1161/01.str.27.7.1200. [DOI] [PubMed] [Google Scholar]
- 4.Mann G., Hankey G.J., Cameron D. Swallowing function after stroke: prognosis and prognostic factors at 6 months. Stroke. 1999;30(4):744–748. doi: 10.1161/01.str.30.4.744. [DOI] [PubMed] [Google Scholar]
- 5.Speyer R., Baijens L., Heijnen M., Zwijnenberg I. Effects of therapy in oropharyngeal dysphagia by speech and language therapists: a systematic review. Dysphagia. 2010;25(1):40–65. doi: 10.1007/s00455-009-9239-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin R.E. Neuroplasticity and swallowing. Dysphagia. 2009 Jun;24(2):218–229. doi: 10.1007/s00455-008-9193-9. [DOI] [PubMed] [Google Scholar]
- 7.Hamdy S., Aziz Q., Rothwell J.C., Power M., Singh K.D., Nicholson D.A. Recovery of swallowing after dysphagic stroke relates to functional reorganization in the intact motor cortex. Gastroenterology. 1998;115(5):1104–1112. doi: 10.1016/s0016-5085(98)70081-2. [DOI] [PubMed] [Google Scholar]
- 8.Michou E., Hamdy S. Cortical input in control of swallowing. Curr Opin Otolaryngol Head Neck Surg. 2009;17(3):166–171. doi: 10.1097/MOO.0b013e32832b255e. [DOI] [PubMed] [Google Scholar]
- 9.Hamdy S., Rothwell J.C., Aziz Q., Singh K.D., Thompson D.G. Long-term reorganization of human motor cortex driven by short-term sensory stimulation. Nat Neurosci. 1998;1:64–68. doi: 10.1038/264. [DOI] [PubMed] [Google Scholar]
- 10.Fraser C., Power M., Hamdy S., Rothwell J., Hobday D., Hollander I. Driving plasticity in human adult motor cortex is associated with improved motor function after brain injury. Neuron. 2002;34(5):831–840. doi: 10.1016/s0896-6273(02)00705-5. [DOI] [PubMed] [Google Scholar]
- 11.Jayasekeran V., Singh S., Tyrrell P., Michou E., Jefferson S., Mistry S. Adjunctive functional pharyngeal electrical stimulation reverses swallowing disability after brain lesions. Gastroenterology. 2010;138(5):1737–1746. doi: 10.1053/j.gastro.2010.01.052. [DOI] [PubMed] [Google Scholar]
- 12.Jefferson S., Mistry S., Michou E., Singh S., Rothwell J.C., Hamdy S. Reversal of a virtual lesion in human pharyngeal motor cortex by high frequency contralesional brain stimulation. Gastroenterology. 2009;137(3):841–849. doi: 10.1053/j.gastro.2009.04.056. [DOI] [PubMed] [Google Scholar]
- 13.Khedr E.M., Abo-Elfetoh N., Rothwell J.C. Treatment of post-stroke dysphagia with repetitive transcranial magnetic stimulation. Acta Neurol Scand. 2009;119(3):155–161. doi: 10.1111/j.1600-0404.2008.01093.x. [DOI] [PubMed] [Google Scholar]
- 14.Kim L., Chun M.H., Kim B.R., Lee S.J. Effect of repetitive transcranial magnetic stimulation on patients with brain injury and Dysphagia. Ann Rehabil Med. 2011;35(6):765–771. doi: 10.5535/arm.2011.35.6.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verin E., Leroi A.M. Poststroke dysphagia rehabilitation by repetitive transcranial magnetic stimulation: a noncontrolled pilot study. Dysphagia. 2009;24(2):204–210. doi: 10.1007/s00455-008-9195-7. [DOI] [PubMed] [Google Scholar]
- 16.Park J.W., Oh J.C., Lee J.W., Yeo J.S., Ryu K.H. The effect of 5 Hz high-frequency rTMS over contralesional pharyngeal motor cortex in post-stroke oropharyngeal dysphagia: a randomized controlled study. Neurogastroenterol Motil. 2013;25(4) doi: 10.1111/nmo.12063. 324–e250. [DOI] [PubMed] [Google Scholar]
- 17.Khedr E.M., Abo-Elfetoh N. Therapeutic role of rTMS on recovery of dysphagia in patients with lateral medullary syndrome and brainstem infarction. J Neurol Neurosurg Psychiatry. 2010;81(5):495–499. doi: 10.1136/jnnp.2009.188482. [DOI] [PubMed] [Google Scholar]
- 18.Jefferson S., Mistry S., Singh S., Rothwell J., Hamdy S. Characterizing the application of transcranial direct current stimulation in human pharyngeal motor cortex. Am J Physiol Gastrointest Liver Physiol. 2009;297(6):G1035–G1040. doi: 10.1152/ajpgi.00294.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang E.J., Baek S.R., Shin J., Lim J.Y., Jang H.J., Kim Y.K. Effects of transcranial direct current stimulation (tDCS) on post-stroke dysphagia. Restor Neurol Neurosci. 2012;30(4):303–311. doi: 10.3233/RNN-2012-110213. [DOI] [PubMed] [Google Scholar]
- 20.Kumar S., Wagner C.W., Frayne C., Zhu L., Selim M., Feng W. Noninvasive brain stimulation may improve stroke-related dysphagia: a pilot study. Stroke. 2011;42(4):1035–1040. doi: 10.1161/STROKEAHA.110.602128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michou E., Mistry S., Jefferson S., Singh S., Rothwell J., Hamdy S. Targeting unlesioned pharyngeal motor cortex improves swallowing in healthy individuals and after dysphagic stroke. Gastroenterology. 2012;142(1):29–38. doi: 10.1053/j.gastro.2011.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh S., Mistry S., Jefferson S., Davies K., Rothwell J., Williams S. A magnetic resonance spectroscopy study of brain glutamate in a model of plasticity in human pharyngeal motor cortex. Gastroenterology. 2009;136(2):417–424. doi: 10.1053/j.gastro.2008.10.087. [DOI] [PubMed] [Google Scholar]
- 23.Loo C.K., Taylor J.L., Gandevia S.C., McDarmont B.N., Mitchell P.B., Sachdev P.S. Transcranial magnetic stimulation (TMS) in controlled treatment studies: are some “sham” forms active? Biol Psychiatry. 2000;47(4):325–331. doi: 10.1016/s0006-3223(99)00285-1. [DOI] [PubMed] [Google Scholar]
- 24.Gow D., Rothwell J., Hobson A., Thompson D., Hamdy S. Induction of long-term plasticity in human swallowing motor cortex following repetitive cortical stimulation. Clin Neurophysiol. 2004;115(5):1044–1051. doi: 10.1016/j.clinph.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Klem G.H., Lüders H.O., Jasper H.H., Elger C. The ten-twenty electrode system of the International Federation. The International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Suppl. 1999;52:3–6. [PubMed] [Google Scholar]
- 26.Alshekhlee A., Ranawat N., Syed T.U., Conway D., Ahmad S.A., Zaidat O.O. National Institutes of Health Stroke Scale assists in predicting the need for percutaneous endoscopic gastrostomy tube placement in acute ischemic stroke. J Stroke Cerebrovasc Dis. 2010;19:347–352. doi: 10.1016/j.jstrokecerebrovasdis.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 27.Okubo P.C., Fábio S.R., Domenis D.R., Takayanagui O.M. Using the National Institute of Health Stroke Scale to predict dysphagia in acute ischemic stroke. Cerebrovasc Dis. 2012;33(6):501–507. doi: 10.1159/000336240. [DOI] [PubMed] [Google Scholar]
- 28.Conover W.J. 3rd ed. John Wiley & Sons; New York: 1999. Practical nonparametric statistics. [Google Scholar]
- 29.Rosenbek J.C., Robbins J.A., Roecker E.B., Coyle J.L., Wood J.L. A penetration-aspiration scale. Dysphagia. 1996;11:93–98. doi: 10.1007/BF00417897. [DOI] [PubMed] [Google Scholar]
- 30.Molfenter S.M., Steele C.M. Temporal variability in the deglutition literature. Dysphagia. 2012;27(2):162–177. doi: 10.1007/s00455-012-9397-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wassermann E.M., Lisanby S.H. Therapeutic application of repetitive transcranial magnetic stimulation: a review. Clin Neurophysiol. 2001;112:1367–1377. doi: 10.1016/s1388-2457(01)00585-5. [DOI] [PubMed] [Google Scholar]
- 32.Strafella A.P., Ko J.H., Monchi O. Therapeutic application of transcranial magnetic stimulation in Parkinson's disease: the contribution of expectation. Neuroimage. 2006;31(4):1666–1672. doi: 10.1016/j.neuroimage.2006.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murphy T.H., Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci. 2009;10:861–872. doi: 10.1038/nrn2735. [DOI] [PubMed] [Google Scholar]
- 34.Zhao L.R., Berra H.H., Duan W.M., Singhal S., Mehta J., Apkarian A.V. Beneficial effects of hematopoietic growth factor therapy in chronic ischemic stroke in rats. Stroke. 2007;38(10):2804–2811. doi: 10.1161/STROKEAHA.107.486217. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.