Abstract
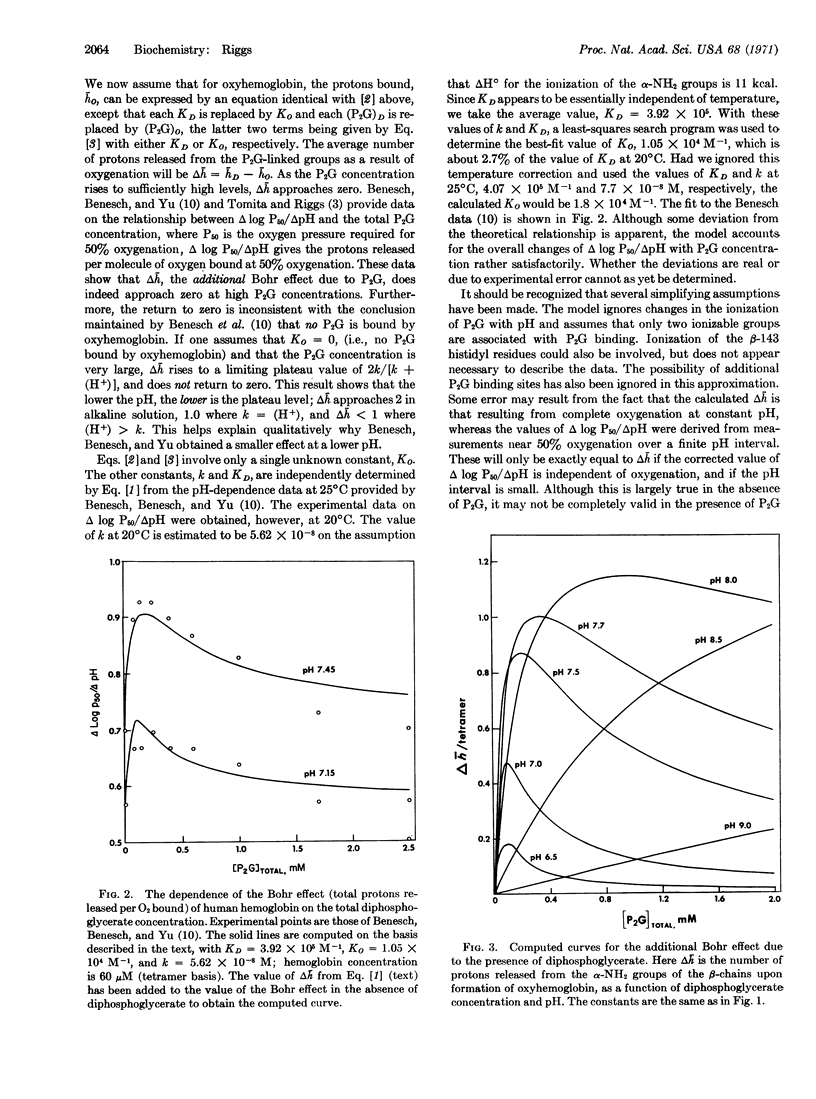
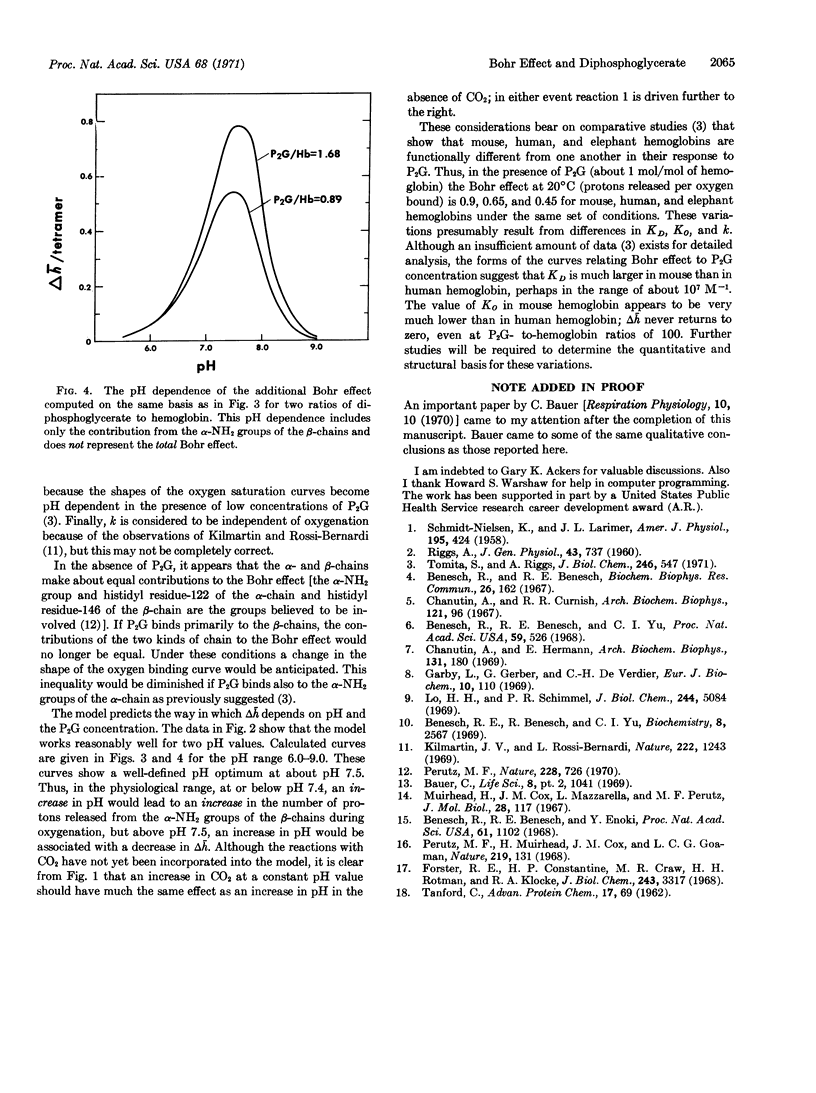
The number of protons released from several mammalian hemoglobins as a consequence of oxygenation is greater in the presence of low concentrations of 2,3-diphosphoglycerate than in its absence. A mechanism for this enhancement of proton release is proposed. The basis of this mechanism is that 2,3-diphosphoglycerate binds primarily between the protonated α-NH2 terminal groups of the two β chains in deoxyhemoglobin. This binding will shift the ionization equilibria in favor of the protonation of the deoxyhemoglobin. Partial release of 2,3-diphosphoglycerate upon oxygenation of the hemoglobin is then accompanied by a release of protons. The apparent enthalpy of diphosphoglycerate binding appears to be close to zero. The previously reported temperature dependence appears to be due entirely to the associated protonation reaction. If only a single diphosphoglycerate binding site is assumed, the intrinsic association constant is estimated to be 3.9 × 105 M-1 for deoxyhemoglobin and 1.05 × 104 M-1 for oxyhemoglobin at 20°C in 0.1 M NaCl.
Keywords: computer, association constants, oxygen, carbon dioxide
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer C. Antagonistic influence of Co2 and 2,3 diphosphoglycerate on the Bohr effect of human haemoglobin. Life Sci. 1969 Oct 15;8(20):1041–1046. doi: 10.1016/0024-3205(69)90455-x. [DOI] [PubMed] [Google Scholar]
- Benesch R. E., Benesch R., Yu C. I. The oxygenation of hemoglobin in the presence of 2,3-diphosphoglycerate. Effect of temperature, pH, ionic strength, and hemoglobin concentration. Biochemistry. 1969 Jun;8(6):2567–2571. doi: 10.1021/bi00834a046. [DOI] [PubMed] [Google Scholar]
- Benesch R., Benesch R. E., Enoki Y. The interaction of hemoglobin and its subunits with 2,3-diphosphoglycerate. Proc Natl Acad Sci U S A. 1968 Nov;61(3):1102–1106. doi: 10.1073/pnas.61.3.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benesch R., Benesch R. E. The effect of organic phosphates from the human erythrocyte on the allosteric properties of hemoglobin. Biochem Biophys Res Commun. 1967 Jan 23;26(2):162–167. doi: 10.1016/0006-291x(67)90228-8. [DOI] [PubMed] [Google Scholar]
- Benesch R., Benesch R. E., Yu C. I. Reciprocal binding of oxygen and diphosphoglycerate by human hemoglobin. Proc Natl Acad Sci U S A. 1968 Feb;59(2):526–532. doi: 10.1073/pnas.59.2.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanutin A., Curnish R. R. Effect of organic and inorganic phosphates on the oxygen equilibrium of human erythrocytes. Arch Biochem Biophys. 1967 Jul;121(1):96–102. doi: 10.1016/0003-9861(67)90013-6. [DOI] [PubMed] [Google Scholar]
- Chanutin A., Hermann E. The interaction of organic and inorganic phosphates with hemoglobin. Arch Biochem Biophys. 1969 Apr;131(1):180–184. doi: 10.1016/0003-9861(69)90119-2. [DOI] [PubMed] [Google Scholar]
- Forster R. E., Constantine H. P., Craw M. R., Rotman H. H., Klocke R. A. Reaction of CO2 with human hemoglobin solution. J Biol Chem. 1968 Jun 25;243(12):3317–3326. [PubMed] [Google Scholar]
- Garby L., Gerber G., De Verdier C. H. Binding of 2,3-diphosphoglycerate and adenosine triphosphate to human haemoglobin A. Eur J Biochem. 1969 Aug;10(1):110–115. doi: 10.1111/j.1432-1033.1969.tb00662.x. [DOI] [PubMed] [Google Scholar]
- Kilmartin J. V., Rossi-Bernardi L. Inhibition of CO2 combination and reduction of the Bohr effect in haemoglobin chemically modified at its alpha-amino groups. Nature. 1969 Jun 28;222(5200):1243–1246. doi: 10.1038/2221243a0. [DOI] [PubMed] [Google Scholar]
- Lo H. H., Schimmel P. R. Interaction of human hemoglobin with adenine nucleotides. J Biol Chem. 1969 Sep 25;244(18):5084–5086. [PubMed] [Google Scholar]
- Muirhead H., Cox J. M., Mazzarella L., Perutz M. F. Structure and function of haemoglobin. 3. A three-dimensional fourier synthesis of human deoxyhaemoglobin at 5.5 Angstrom resolution. J Mol Biol. 1967 Aug 28;28(1):117–156. doi: 10.1016/s0022-2836(67)80082-2. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Muirhead H., Cox J. M., Goaman L. C. Three-dimensional Fourier synthesis of horse oxyhaemoglobin at 2.8 A resolution: the atomic model. Nature. 1968 Jul 13;219(5150):131–139. doi: 10.1038/219131a0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Stereochemistry of cooperative effects in haemoglobin. Nature. 1970 Nov 21;228(5273):726–739. doi: 10.1038/228726a0. [DOI] [PubMed] [Google Scholar]
- SCHMIDT-NEILSEN K., LARIMER J. L. Oxygen dissociation curves of mammalian blood in relation to body size. Am J Physiol. 1958 Nov;195(2):424–428. doi: 10.1152/ajplegacy.1958.195.2.424. [DOI] [PubMed] [Google Scholar]
- Tomita S., Riggs A. Studies of the interaction of 2,3-diphosphoglycerate and carbon dioxide with hemoglobins from mouse, man, and elephant. J Biol Chem. 1971 Feb 10;246(3):547–554. [PubMed] [Google Scholar]


