Abstract
Objective
To test 3 theories of hypercortisolemia in depression – hypothalamic overdrive, impaired glucocorticoid feedback, or autonomous cortisol production.
Methods
We applied an overnight low cortisol feedback strategy by administering metyrapone to hypercortisolemic depressed inpatients and control subjects.
Results
Under metyrapone, the increases of plasma ACTH concentrations and of basal and pulsatile ACTH secretion were not exaggerated in hypercortisolemic depressed patients compared with control subjects. ACTH approximate entropy (ApEn) did not differ at baseline or under metyrapone. Thus, neither hypothalamic overdrive nor irregular ACTH secretion was seen. We did not detect impaired cortisol feedback: the ACTH response was not reduced, and ApEn measures that are sensitive to feedback changes were comparable in both groups. Metyrapone disrupted cortisol secretory regularity in depressed and control subjects. On the baseline day, basal cortisol secretion was significantly increased and was highly irregular (high ApEn), and ACTH-cortisol cross-ApEn was markedly elevated in high-cortisol patients.
Conclusions
Classical feed forward overdrive and impaired feedback theories of hypercortisolemia in depression were not supported. Depressive hypercortisolemia may result from alternative pathophysiological mechanisms involving irregular basal hypersecretion of cortisol, associated with adrenal enlargement, possibly through splanchnic sympathetic activation of the adrenal cortex.
Keywords: Adrenocorticotropic hormone, approximate entropy, cortisol, deconvolution, depressive disorder, major, feedback, physiological, metyrapone
Introduction
The mechanisms of hypothalamo-pituitary-adrenal (HPA) axis activation in depression remain unsettled. Three leading general theories are forward drive from an activated limbic-hypothalamic system (1–5); defective negative glucocorticoid feedback (6–8); and augmented basal secretion of cortisol (9–11) associated with reversible enlargement of the adrenal gland (12). Increased HPA activity is seen most often in those depressed patients marked by melancholic or psychotic or mixed bipolar features (13,14). Many studies of HPA axis function in DSM-III–IV-defined major depression are limited by clinical and consequent neuroendocrine heterogeneity.
The present study was designed to evaluate these 3 general theories in depressed patients who were classified by hypercortisolemic status. We first acquired baseline measures, then we challenged the HPA axis with low glucocorticoid feedback, which is the opposite of the strategy involved in the dexamethasone suppression test or DST (15). For this purpose metyrapone, which inhibits cortisol synthesis in the adrenal cortex, was administered by multiple dosing during the time period predicted from classical studies to be most likely to demonstrate inappropriate hypersecretion of cortisol, viz., the period from midnight to the circadian acrophase (16). With intensive 10-minute sampling at 2 levels of the HPA axis (ACTH and cortisol), and with the use of advanced statistical procedures (deconvolution and approximate entropy (ApEn and cross-ApEn) analyses) (11,17), we could evaluate the 3 general theories simultaneously. The principles and key terms in deconvolution and approximate entropy analyses are given in the Appendix.
The primary hypothesis was that the magnitude and the regularity (ApEn) of ACTH secretion under low glucocorticoid feedback will differ between hypercortisolemic depressed and control subjects. If the baseline hypercortisolemia reflects excessive central drive of hypothalamic secretagogues on pituitary corticotrope cells in depression, then the magnitude of increase in ACTH secretion under low feedback will be greater than in control subjects. ApEn analysis will identify any potential dysregulation of ACTH secretory patterns that could indicate underlying dysfunction of limbic-hypothalamic circuits in hypercortisolemic depression, which has been suggested from post mortem studies of the hypothalamic paraventricular nucleus (18,19).
On the other hand, if baseline hypercortisolemia reflects impaired glucocorticoid feedback, then the prediction is that the magnitude of increase in ACTH secretion under low feedback will be less than in control subjects. The analyses of reverse cortisol → ACTH cross-ApEn will be informative for the defective feedback theory, as will the changes in ACTH ApEn and in reverse cortisol → ACTH cross-ApEn between the normal feedback day and the low feedback day. Finally, the deconvolution and ApEn analyses of cortisol secretory patterns will allow the identification of any possible independent adrenocortical disturbance, such as increased basal, non-pulsatile cortisol secretion that is not linked to ACTH secretion (9,17).
In an initial report, we compared ACTH and cortisol secretory dynamics in depressed and healthy subjects under baseline conditions (unmodified feedback) over 24 hours (11). The major findings were elevated basal and pulsatile ACTH secretion, increased total cortisol secretion, and increased cortisol secretory-burst mass in high-cortisol depressed patients. Direct estimation of basal cortisol secretion was not possible with this early-generation deconvolution method (17). We also reported that the entropic regularity of ACTH secretion was undisturbed in hypercortisolemic depressed patients, whereas cortisol secretion was increased in a highly irregular fashion (high ApEn), accompanied by high ACTH → cortisol cross-ApEn (impaired feed-forward coupling). The present investigation extends that report into a second day of sampling, wherein low glucocorticoid feedback was induced by metyrapone administration.
Aims of the Study
To simultaneously test the 3 major theories of hypercortisolemia in depression – limbic-hypothalamic-pituitary overdrive, impaired cortisol feedback, and autonomous cortisol secretion. The magnitude and dynamics of the ACTH and cortisol responses to low feedback induced by metyrapone will be informative for all 3 theories.
Methods
Subjects
A complete description of the baseline study involving 17 control and 12 depressed patients was given in (11). The screening, evaluation, and inclusion/exclusion procedures have been fully described (11). The protocols were approved by the Institutional Review Board of Duke University Medical Center, and subjects provided written, informed consent. Fourteen control subjects and 12 depressed patients commenced the metyrapone study. Eleven control subjects (5 males) and all 12 depressed patients (7 males) completed the metyrapone protocol from 0000h through 1000h. The hypercortisolemic depressed cohort (N=7; 4 males) was defined operationally by a 24-h mean plasma cortisol concentration exceeding the normal (N = 17) mean value plus one SD, viz., > 8.0 μg/dL. Actual 24-h mean cortisol values ranged from 8.8–11 μg/dL in hypercortisolemic and from 3.4–5.1 μg/dL in non-hypercortisolemic patients (N=5; 3 males). Patients had stopped all psychotropic medications for 5 days or more. None had been exposed to long-acting antidepressant agents (e.g. fluoxetine) within 6 months. All depressed patients met Research Diagnostic Criteria (RDC) for major depressive episode (20). Of the 7 hypercortisolemic patients, 6 met RDC and DSM-IV criteria for psychotic features. All 7 presented with RDC-defined endogenous and incapacitating depression (11). Of the 5 low-cortisol depressed patients, none was psychotic, 2 displayed DSM-IV atypical features, and only 1 met the RDC definition of incapacitated depression. All 3 cohorts had similar body-mass indices (grand mean ± SD 26 ± 4.5 kg/m2). Low-cortisol patients were younger (29 ± 7.7 yr) than high-cortisol patients (46 ± 8.2 yr) and control subjects (46 ± 8.7 yr). The mean scores of both patient groups on the Carroll Depression Scale (CDS) (21) were in the severe range at 29 and 31, respectively (11). The CDS (22) is a validated, self-rated version of the 17-item Hamilton Depression Scale (23).
Inpatient studies
Depressed inpatients were studied between 4 and 11 days after hospitalization. All patients were hospitalized for clinical need. Subjects were transferred from psychiatric inpatient status to the Duke University Clinical Research Unit for a 2-day study, with blood sampling every 10 min from 0000h through 2400h on each day. On the baseline day (Day 1), 24-h ACTH and cortisol data (0000h through 2400h) have been reported for 17 controls and 12 depressed patients (11). Immediately following Day 1, the Day 2 metyrapone protocol commenced, with metyrapone 1 g administered every 2 hours orally beginning at 0000h, whereas on Day 1 placebo was given orally every 2 hours. Beyond 1000h on Day 2 there were frequent significant side effects of metyrapone (nausea and vomiting). To limit confounding from these side effects, Day 2 data were analyzed only during the period 0000h through 1000h. Four controls and one depressed patient had mild and transient gastrointestinal side effects before 1000h. For analyses of change, the identical segments of each patient’s Day 1 data (0000h to 1000h) were extracted. None of the 10-h placebo or metyrapone segments has been analyzed previously.
Assays
Plasma ACTH and cortisol were measured as described previously (11). Median within-assay coefficients of variation were 8% and 5%, and sensitivities 5 ng/L and 1.4 μg/dL, respectively. Cross reactivity of the cortisol assay with 11-desoxycortisol was 6.3%. Based on expected plasma 11-desoxycortisol concentrations of 15–20 μg/dL at the circadian peak under repeated dosing with metyrapone (24), our reported Day 2 plasma cortisol concentrations could thus be overstated by up to 1.25 μg/dL.
Analyses of secretory dynamics
Matching ten-hour ACTH and cortisol time series on both Day 1 baseline and Day 2 low feedback were subjected to deconvolution and approximate entropy analyses. The deconvolution method is described in (17), except that only a single secretory-burst waveform was allowed. Technical aspects of deconvolution and ApEn analyses are given in the Appendix.
Statistical analysis
Because each subject had matching 10-h placebo and metyrapone sessions, ANCOVA was used to adjust for intraindividual correlations between Day 1 and Day 2 data. The covariate was the subject’s parameter value on Day 1. The model structure comprised hierarchical mixed-effect two-way ANCOVA with 2 treatment levels (placebo/metyrapone) and 3 specification parameters (diagnostic subject groups: healthy control, nonhypercortisolemic and hypercortisolemic depressed) (25). Natural logarithmic transformation was utilized to limit heterogeneity of variance. The equal-slopes assumption of the ANCOVA structure was verified by a generalized F-ratio test, followed by restricted maximum-likelihood estimation of parameters. Rejection of pre-specified hypotheses was based on a multiple-comparison experiment-wise Type 1 error rate of < 0.05 using Tukey’s honestly significantly different (HSD) post hoc test (25). Group comparisons of hypercortisolemic patients with control subjects employed an unpaired two-tailed Student’s t-test and for robustness the rank-sum (Kruskal-Wallis) test.
Statistical power
The primary analyses involve comparisons of hypercortisolemic depressed patients with control subjects. For these, power estimates were made to detect hypothesized differences at P < 0.05. Statistical power was > 80 – 99% to detect a 30% difference in pulsatile ACTH secretion and a 15% difference in ApEn or cross-ApEn assuming evaluation of a total of 18 subjects (normal plus high-cortisol depressed) over 10 hours via an unpaired Student’s two-tailed t-test using the pooled variance: (data available on request). Secondary comparisons between non- and hypercortisolemic patients or between control subjects and non-hypercortisolemic patients were not powered. Any comparisons involving these contrasts are shown for trend signaling only.
Results
Consistent with the group definitions, mean baseline 10h plasma cortisol values differed significantly by group (P<0.001 by 1-way ANOVA). The group means of the baseline 10h mean plasma cortisol values were 7.63 (SD 1.80) ug/dL in controls, 11.39 (SD 1.35) ug/dL in high cortisol depressed patients, and 4.41 (SD 1.87 ug/dL in low cortisol depressed patients. All post hoc pairwise comparisons were highly significant (P<0.002).
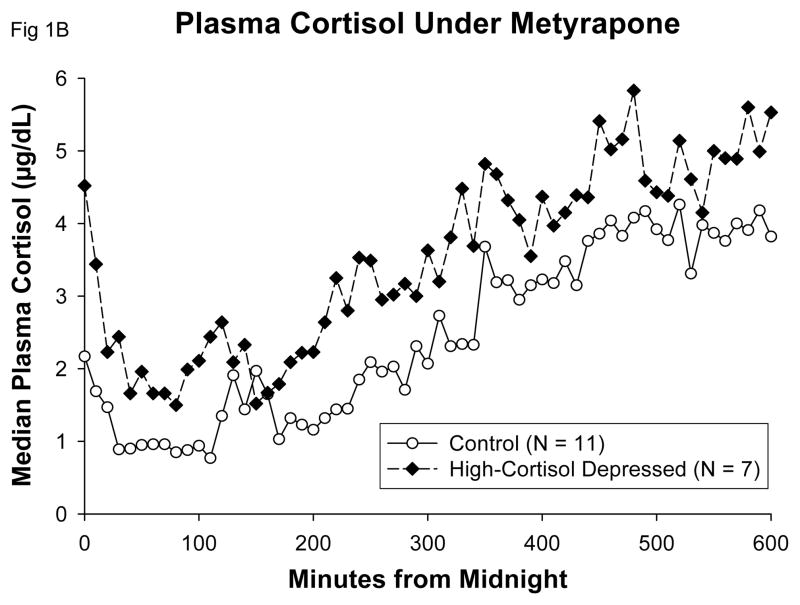
Metyrapone reduced mean 10-h plasma cortisol concentrations by 4.7 μg/dL (66%) in control subjects, by 7.3 μg/dL (66%) in high-cortisol depressed patients, and by 2.2 μg/dL (54%) in low-cortisol depressed patients: Table 1. Absolute ranges of mean 10-h overnight plasma cortisol concentrations (μg/dL) were 1.5–5.5 (control) and 2.4–4.6 (high-cortisol depressed). Mean peak plasma cortisol concentrations likewise were reduced by 71%, 68% and 62%, respectively. Peak plasma cortisol levels on Day 2 occurred near the end of the 10-h period, ranging up to 8.9 ug/dL in control subjects, and up to 8.6 ug/dL in hypercortisolemic depressed patients (Table 1). These breakthrough plasma cortisol levels match what we have previously reported in control subjects (26) and they agree with those reported by Ur et al 1992 (5) at 0900 h after repeated overnight dosing of metyrapone; they reflect the intense stimulation of the adrenal gland by very high circulating ACTH levels at that time (Table 1 and Figure 1).
Table 1.
Median and peak ACTH and cortisol concentrations during normal and low-cortisol feedback
| Group
|
ACTH (ng/L)
|
Cortisol (μg/dL)
|
||
|---|---|---|---|---|
| Median | Peak | Median | Peak | |
| Control (N = 11) | ||||
| baseline | 22 (16–34)A | 46 (31–94)A | 7.1 (5.3–11)A | 18 (14–22)A |
| metyrapone | 153 (81–373)B | 388 (216–1195)B | 2.4 (1.5–5.5)B | 5.2 (2.7–8.9)B |
| increment or decrement | 129 (55–351) | 322 (143–1148) | −4.9 (−7.0 to −1.6) | −12.7 (−16.8 to −8.4) |
| Depression High Cortisol (N = 7) | ||||
| baseline | 30 (18–32)A | 48 (42–57)A | 11 (9.7–14)A | 20 (18–24)A |
| metyrapone | 143 (78–267)B | 390 (234–868)B | 3.7 (2.4–4.6)B | 6.3 (4.4–8.6)B |
| increment or decrement | 115 (49–235) | 335 (187–811) | −7.0 (−10.8 to −6.2) | −14.0 (−16.6 to −11.1) |
| Depression Low Cortisol (N = 5) | ||||
| baseline | 18 (16–37)A | 36 (26–91)A | 4.1 (1.9–6.7)A | 11 (5.8–15)A |
| metyrapone | 129 (25–217)B | 383 (66–701)B | 1.9 (0.89–2.9)B | 4.2 (2.3–6.1)B |
| increment or decrement | 111 (10–180) | 347 (40–610) | −2.8 (−4.2 to −1.0) | −6.5 (−8.6 to −3.5) |
| ANCOVA P value: | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
Data are the median (range) for the indicated groups over the interval 0000 – 1000 h.
Values after metyrapone were significantly different from the matching baseline values. The overall P value for the latter contrast is indicated on the bottom line of the table.
Data were first natural-log transformed before ANCOVA. Accordingly, given the skewed distributions, the median and range of values are presented.
The mean concentrations and peak concentrations were calculated as single values in each person over the 10 hours. From the set of mean and peak values, the median and absolute range were taken, representing the values printed in the table.
ANOVA confirms lower cortisol values in each group on the metyrapone day compared to the baseline day. On the metyrapone day the cortisol values were similar among the 3 subject groups. The decrements were significantly less for cortisol in the low cortisol group.
P values for median vs peak (paired column comparisons) were all P<0.01, and hence are not marked separately.
Means with unique (unshared) alphabetic superscripts differ between paired baseline and metyrapone rows within each of the three separate groups. Thus, A differs from B.
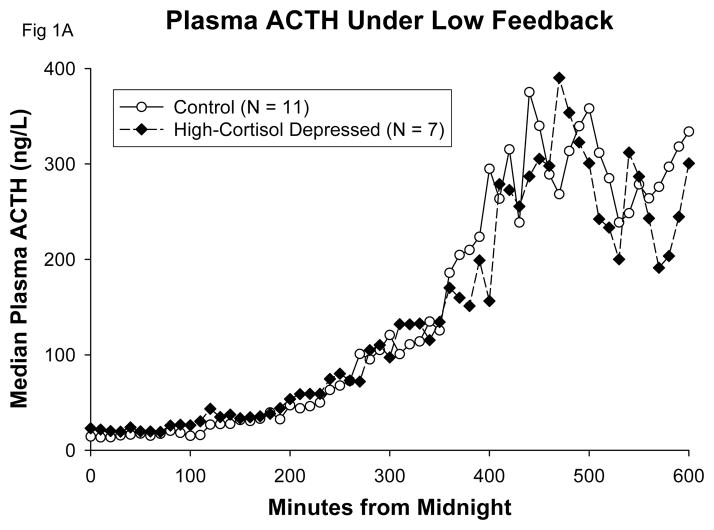
Figure 1.
Median plots in control subjects and high-cortisol depressed patients, showing comparable ACTH A and cortisol levels B at 0200h–0230h when both groups exceeded their Day 1 mean ACTH concentrations under metyrapone.
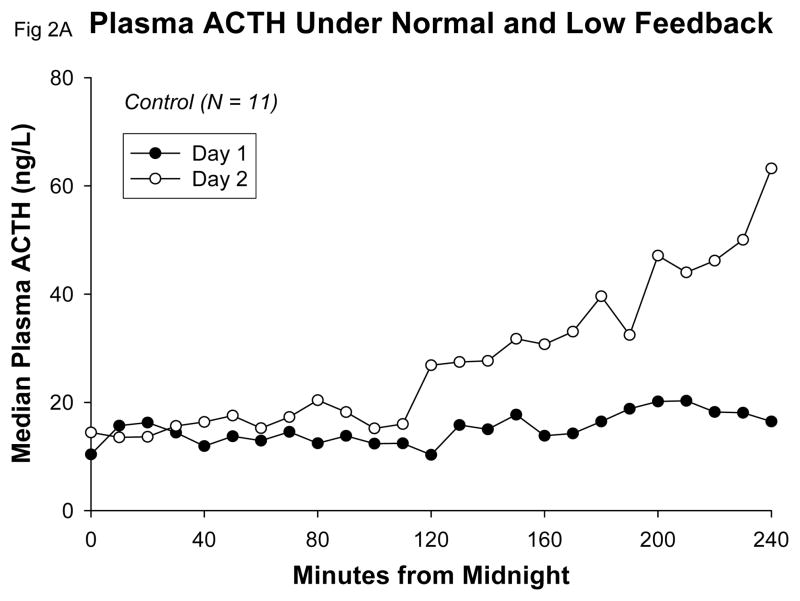
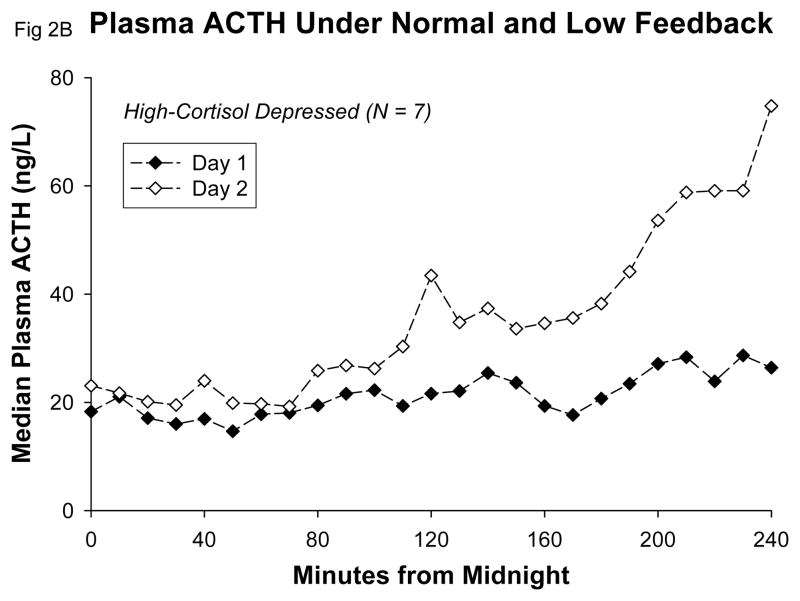
Beginning early in the sampling period, plasma ACTH concentrations on Day 2 increased markedly over Day 1 values in all groups (Table 1). A detailed view of the early increase of plasma ACTH that commenced between 0200h and 0400h, following the second dose of metyrapone, is given in Figure 2. There were no significant differences among groups in mean 10-h or peak plasma ACTH concentrations (Table 1). There was no difference between groups in the change of mean ACTH or in change of peak ACTH concentrations from Day 1 to Day 2. In particular, there was no trend for the high-cortisol depressives to display either exaggerated or reduced mean ACTH-concentration increases in comparison with control subjects under low-cortisol feedback with metyrapone. There was no significant correlation between mean 10-h plasma ACTH levels on Day 1 and mean 10-h plasma ACTH levels on Day 2, either in control subjects (r = −0.20; p = 0.54) or in high-cortisol depressed patients (r = 0.44; p = 0.32).
Figure 2.
Detail showing early increase of median ACTH concentrations under metyrapone in control subjects A and high-cortisol depressed patients B.
The rise of plasma ACTH concentrations under low feedback occurred early in all groups, and we saw no evidence of abnormal timing of this effect in the hypercortisolemic depressed patients. Under metyrapone, plasma ACTH exceeded the mean 24-h Day 1 concentration at (means) 0215h in controls, 0211h in high-cortisol depressed patients, and 0200h in low-cortisol depressed patients. Likewise, plasma ACTH concentrations on Day 2 exceeded the 24-hr Day 1 maximum levels at (means) 0357h in control subjects, 0327h in high-cortisol depressed patients, and at 0436h in the low cortisol depressed patients (one-way ANOVA not significant, P=0.21). Peak plasma ACTH concentrations occurred at a mean time of 0802h on Day 2 in control subjects, at 0800h in high-cortisol depressed patients, and at 0856h in low-cortisol depressed patients. None of these group differences was statistically significant by parametric or nonparametric ANOVA. There were no significant correlations of mean or peak Day 2 plasma ACTH with mean or peak Day 2 plasma cortisol within the entire sample or in any subgroup.
In order to exclude potential confounding by negative feedback from breakthrough cortisol levels late in the sampling period, we examined closely the ACTH responses early in the night. The lack of difference between hypercortisolemic patients and normal controls is displayed in Figure 1. Between 0200h and 0230h, when both groups first exceeded their Day 1 mean 24-h plasma ACTH values, the corresponding median plasma cortisol concentrations were indistinguishable, and in the range of 1–2 ug/dL (Figure 1B).
The maximum ACTH values on Day 2 occurred at (means) 0800h in high-cortisol depressed patients and at 0802h in control subjects. These maxima thus occurred 2 hours before sampling ended. The ambient plasma cortisol levels (3-point average beginning at the time of ACTH maximum) were 4.2 ug/dL (SD 1.6) in control subjects and 4.9 ug/dL (SD 1.6) in the hypercortisolemic depressed patients. This difference was not statistically significant (P=0.35). It cannot be determined whether the decline of plasma ACTH levels after 0800h on Day 2 (Figure 1A) resulted from the negative feedback of gradually rising cortisol levels or from the underlying circadian programming of the HPA axis. On Day 2, the ACTH peak time relative to Day 1 was delayed by (means) 55 minutes in control subjects and by 56 minutes in hypercortisolemic depressed patients.
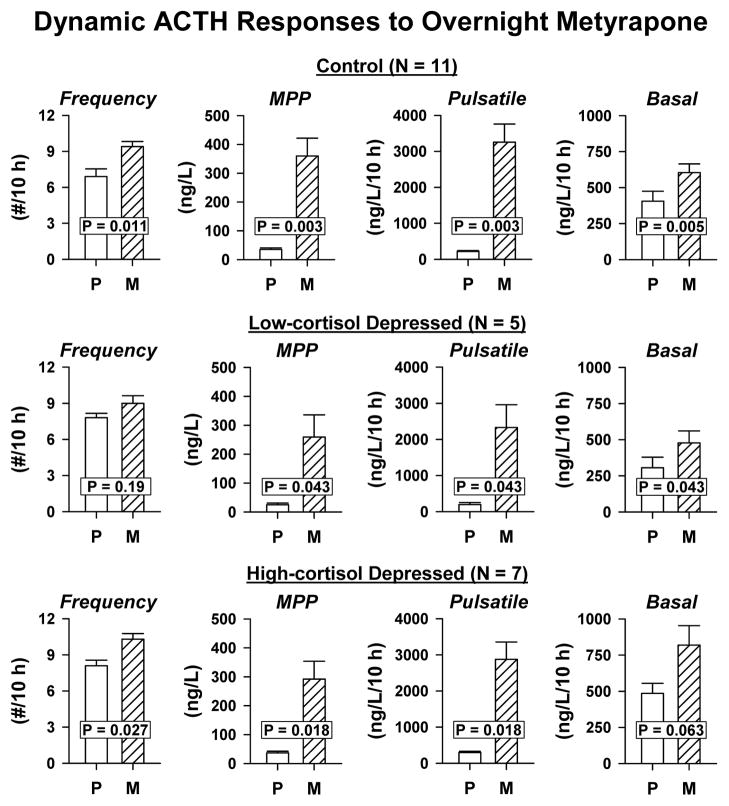
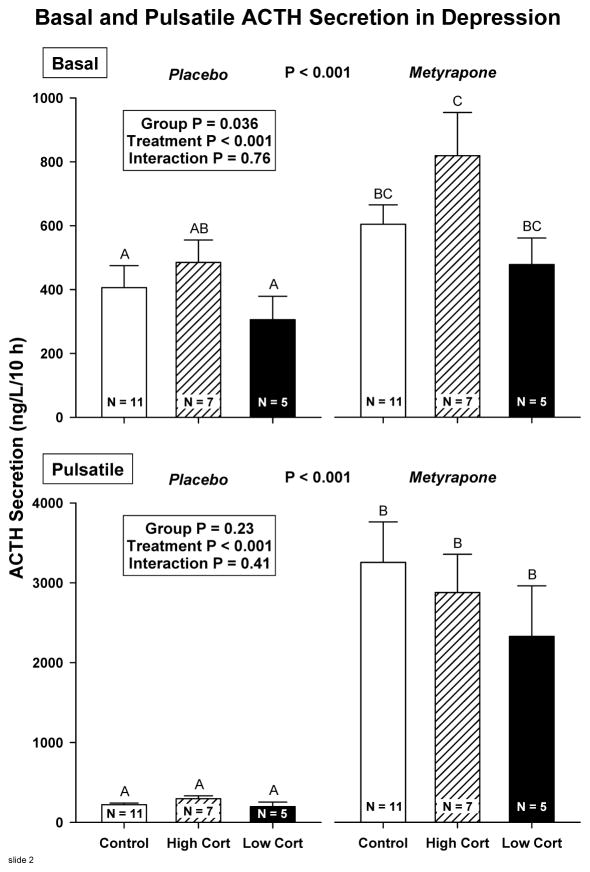
Deconvolution analysis of 10-h ACTH data on Day 1 (placebo) and Day 2 (metyrapone) showed that metyrapone treatment stimulated ACTH secretory-burst size (mass per pulse, MPP), pulsatile ACTH secretion and basal ACTH secretion similarly in all 3 groups: Figure 3. ACTH secretory-burst frequency rose significantly in control (P = 0.011) and high-cortisol depressed (P = 0.027) patients. In contrast, by ANCOVA of main effects, basal (nonpulsatile) ACTH secretion was higher in high-cortisol than low-cortisol depressives (group effect P = 0.036, Tukey’s test P = 0.047): Figure 4. Pulsatile ACTH secretion rose similarly in all 3 groups (P = 0.23 by cohort). Unpaired subgroup comparisons were therefore performed on ACTH secretory dynamics in the high-cortisol depressed and control groups. The high-cortisol patients maintained higher basal cortisol secretion on the placebo day (0.031 ≤ P ≤ 0.052), and narrower cortisol secretory bursts after metyrapone (0.042 ≤ P ≤ 0.051). They also displayed trends to higher pulsatile ACTH (0.077 ≤ P ≤ 0.089) and cortisol (0.077 ≤ P ≤ 0.082) secretion on the placebo day: Table 2.
Figure 3.
Bar graphs representing paired (within-subject) comparisons of ACTH secretory changes during metyrapone (M) compared with placebo (P) exposure in the subjects presented in Figure 1. MPP denotes mass per pulse.
Figure 4.
Estimates of basal (top) and pulsatile (bottom) ACTH secretion in healthy subjects (N =11), high-cortisol depressed (N=7) and low-cortisol depressed (N=5) patients. Data are the mean ± SEM. The overall ANCOVA model yielded P<0.001. Group, treatment and interaction effects are stated in the box. Bars with different (unshared) alphabetic superscripts differ significantly (P<0.05) by Tukey’s honestly significantly different post hoc multiple-comparison test. Thus, A differs from BC but not from AB.
Table 2.
Significant and trend-level comparisons between control (N = 11) and high-cortisol depressed (N = 7) subjects.
| Measurement
|
Day1
|
Control Group (N = 11)
|
Depressed High Cortisol (N = 7)
|
P values
|
|||
|---|---|---|---|---|---|---|---|
| Mean ± SEM
|
Median (range)
|
Mean ± SEM
|
Median (range)
|
Unpaired t -test
|
Kruskal-Wallis
|
||
| Pulsatile ACTH sec | 1 | 221 ± 21 | 187 (140–334) | 296 ± 36 | 265 (170–421) | 0.089 | 0.077 |
| Basal cortisol secr | 1 | 17 ± 4.7 | 13 (0.68–51) | 33 ± 6.7 | 28 (15–59) | 0.031 | 0.052 |
| Pulsatile cortisol secr | 1 | 66 ± 6.6 | 63 (37–115) | 79 ± 5.0 | 77 (61–96) | 0.082 | 0.077 |
| Cortisol burst mode | 2 | 27 ± 4.2 | 23 (11–58) | 16 ± 4.4 | 9.6 (6.2–37) | 0.042 | 0.051 |
| Cortisol ApEn | 1 | 1.2458 ± 0.0217 | 1.2343 (1.1283–1.3492) | 1.3986 ± 0.0381 | 1.4249 (1.2448–1.5316) | 0.005 | 0.004 |
| ACTH-cortisol X-ApEn | 1 | 1.6875 ± 0.0274 | 1.7022 (1.5058–1.8162) | 1.8155 ± 0.0292 | 1.8361 (1.6967–1.9043) | 0.006 | 0.013 |
Day 1 = Placebo; Day 2 = Metyrapone
Unpaired parametric and nonparametric P values
Trend was defined as 0.05 < P < 0.10.
The mode is the time delay from secretory-burst onset to maximum.
Units are ng/L/10 hr for pulsatile ACTH secretion, μg/dL/10 hr for basal and pulsatile cortisol secretion, and min for cortisol burst mode. ApEn and cross-ApEn are unitless.
In relation to 10-h cortisol secretion on Day 1, depressives with high-cortisol status tended to maintain numerically higher pulsatile and basal cortisol secretion rates than normal individuals (0.031 < P ≤ 0.082) Table 2. They also displayed elevated basal and pulsatile Day 1 10-h cortisol secretion, and shorter cortisol secretory bursts than the low-cortisol depressed patients (0.004 ≤ P ≤ 0.083) (data available on request). Except for cortisol burst mode, there were no trends for differences in ACTH or cortisol secretory dynamics on Day 2 between the high-cortisol depressed patients and the two other groups (Table 2).
Notable distinctions between the present Day 1 (placebo day) 10-h and earlier 24-h Day 1 analyses (11) were (1) no reduction of ACTH half-life in depressed compared with control subjects, and (2) similar mean basal, pulsatile, and total ACTH secretion (data available on request). The differences may reflect the more refined, new generation deconvolution methods, as well as the smaller sample, 10-h vs 24-h sampling, analysis restricted to the circadian ACTH/cortisol surge, and use of ANCOVA vs ANOVA. Confirmatory findings included (1) comparable measures of pulsatile and total ACTH secretion in low- and high-cortisol depressives, and (2) higher basal ACTH secretion in high- than low-cortisol depressives. A new finding was abbreviated cortisol secretory bursts (reduced cortisol-burst mode) in the high-cortisol cohort compared with the low-cortisol depressed group (data available upon request).
ApEn and cross-ApEn at the hypothalamo-pituitary level
ApEn analysis was applied to quantify irregularity of ACTH secretion. As shown in Table 3, there were no differences in ApEn of ACTH profiles among control and depressed patients with low- or high-cortisol concentrations on the placebo day (group effects, P = 0.94). These 10-h ApEn results for ACTH secretion are consistent with the 24-h Day 1 results (11). Moreover, metyrapone reduced ACTH ApEn comparably in each subgroup (P < 0.001), consistent with hypocortisolemia-associated feedback reduction in this axis (27).
Table 3.
ApEn and Cross-ApEn: Summary Data for 0000h to 1000h Time Series.
| Control Group (N = 11)
|
Depressed High Cortisol (N = 7)
|
Depressed Low Cortisol (N = 5)
|
P values
|
||
|---|---|---|---|---|---|
| ANOVA | Kruskal-Wallis | ||||
| Day 1 (Placebo) | |||||
| ACTH ApEn | 1.3546 ± 0.0293 (1.3532) | 1.3714 ± 0.0455 (1.3720) | 1.3796 ± 0.0397 (1.3821) | 0.89 | 0.80 |
| Cortisol ApEn | 1.2458 ± 0.0217A (1.2343) | 1.3986 ± 0.0381B (1.4249) | 1.2046 ± 0.0553A (1.2093) | 0.004 | 0.008 |
| ACTH-Cortisol X-ApEn | 1.6875 ± 0.0274AB (1.7022) | 1.8155 ± 0.0292A (1.8361) | 1.6051 ± 0.0664B (1.6678) | 0.007 | 0.011 |
| Cortisol-ACTH X-ApEn | 1.6895 ± 0.0544 (1.6552) | 1.8161 ± 0.0367 (1.8126) | 1.6066 ± 0.0543 (1.6393) | 0.074 | 0.069 |
| Day 2 (Metyrapone) | |||||
| ACTH ApEn | 1.0199 ± 0.0772 (0.9760) | 1.0405 ± 0.0771 (1.0040) | 1.0055 ± 0.0858 (0.9985) | 0.95 | 0.95 |
| Cortisol ApEn | 1.3902 ± 0.0292 (1.3665) | 1.3607 ± 0.0285 (1.3613) | 1.3233 ± 0.0525 (1.2823) | 0.43 | 0.59 |
| ACTH-Cortisol X-ApEn | 1.6625 ± 0.0651 (1.7143) | 1.7467 ± 0.0857 (1.8686) | 1.4546 ± 0.1128 (1.5193) | 0.096 | 0.23 |
| Cortisol-ACTH X-ApEn | 1.6619 ± 0.0520 (1.6152) | 1.6617 ± 0.0490 (1.6352) | 1.5263 ± 0.0843 (1.4848) | 0.24 | 0.27 |
Data are mean ± SEM (median) for 10h period 0000h to 1000h on each day.
ApEn and cross-ApEn (X-ApEn) are unitless.
Differing alphabetical superscripts denote statistical differences according to post hoc Tukey’s HSD test.
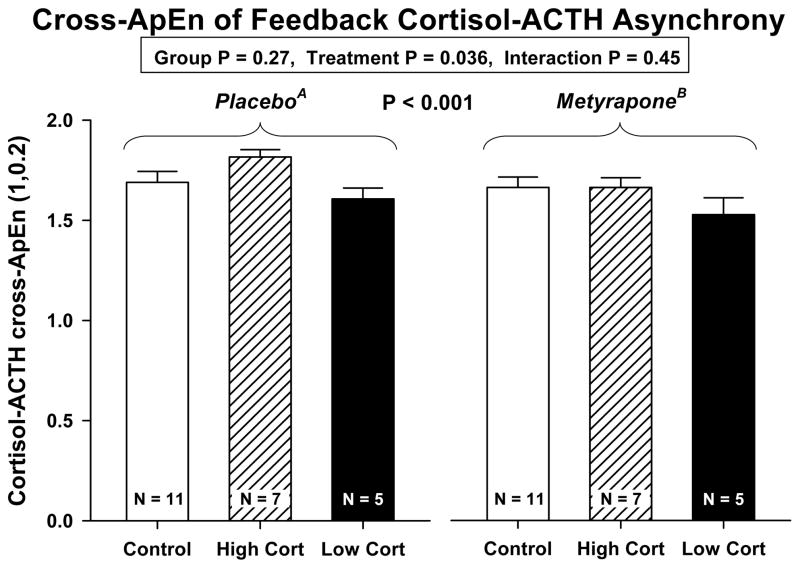
Cross-ApEn analysis (a measure of joint asynchrony) of cortisol-ACTH coordination at the pituitary level in the feedback direction revealed no difference between groups on either Day 1 or Day 2 (Table 3, and Figure 5). Metyrapone treatment significantly reduced cortisol-ACTH asynchrony (increased synchrony) in the 3 study cohorts at P = 0.036 (overall P < 0.001, covariate P = 0.001). However, there were no group differences by ANCOVA (interaction P = 0.45, group P = 0.27): Figure 5. When high- and low-cortisol depressives were compared, cortisol-ACTH cross-ApEn on Day 1 was significantly higher in the former group (0.012 ≤ P ≤ 0.021). There was no difference between high-cortisol depressed and control data (P>0.10).
Figure 5.
Cross-ApEn of paired cortisol-ACTH (feedback asynchrony) concentration profiles. ANCOVA yielded overall P<0.001 for combined effects of treatment and group. Subanalysis showed a drug treatment effect of P=0.053 (see Figure 4 format). ACTH-cortisol (feedforward) cross-ApEn data are given in Table 3.
ApEn and cross-ApEn at the adrenal level
Day 1 Cortisol ApEn
At the level of the adrenal cortex, the 10-h Day 1 ApEn for cortisol secretion revealed significant irregularity [high ApEn] in the high-cortisol depressed group compared to normal controls: Table 3. Cortisol ApEn on Day 1 also was significantly elevated in exploratory comparisons of high-cortisol versus low-cortisol depressed patients (0.019 ≤ P ≤ 0.029). This result is consistent with the previously reported 24-h Day 1 results for cortisol ApEn (11).
Day 2 Cortisol ApEn
Under metyrapone, cortisol ApEn increased significantly in control subjects (P<0.01) but not in either depressed subgroup (Table 3). Differences between day 2 and day 1 ApEn (mean ± SEM) were: control +0.144 ± 0.045 (P < 0.001 vs all others), 7 high-cortisol depressed −0.331 ± 0.101 and low-cortisol-depressed −0.374 ± 0.110. Note that the difference between mean (group) ApEn is not the same mathematically as the mean of the paired (day 2 – day 1) ApEn differences. On Day 2 there was no significant difference of cortisol ApEn among groups: Table 3.
ACTH-cortisol Cross-ApEn
ACTH-cortisol feedforward cross-ApEn was more asynchronous in the high-cortisol depressed than the other two cohorts (0.006 ≤ P ≤ 0.037): Tables 2 and 3. All statistically significant adrenal-level ApEn and cross-ApEn contrasts were restricted to Day 1, indicating that the administration of the adrenal enzyme inhibitor metyrapone abolished the baseline group differences in adrenal secretory regularity and synchrony. Moreover, all statistically significant ApEn and cross-ApEn contrasts between hypercortisolemic depressed patients and normal control subjects were restricted to the adrenal-level cortisol entropy measures. We found no evidence of erratic or chaotic ACTH secretion, even when cortisol feedback was reduced.
A potential limitation of this study is the small number of hypercortisolemic depressed patients. However, the data give no reason to infer that the key finding of no difference in ACTH secretory responses to low feedback would change with a larger sample. Based on the observed data, power analysis indicates that a sample size in excess of 300 per group would be needed to establish a difference between control subjects and hypercortisolemic depressed patients in Day 2 maximum plasma ACTH concentrations with alpha 5% and beta 10%. For Day 2 mean plasma ACTH concentrations, the corresponding sample required would be >180 per group. These and additional power analyses based on the observed data are available upon request. These follow-up power analyses are based on unpaired t-tests using the observed data, with alpha set at the conventional 0.05. Because beta indicates the probability of declaring no difference when a true difference exists, we set beta at the more stringent level of 0.10 rather than the familiar 0.20.
Discussion
The data do not support either the central overdrive theory or the impaired glucocorticoid feedback theory of HPA axis activation in hypercortisolemic depressed patients. There is support in the data for dysregulated function at the level of the adrenal cortex in such patients.
With respect to the central overdrive theory, hypercortisolemic depressed patients and normal control subjects achieved similarly low plasma cortisol levels under metyrapone, especially early in the night (Table 1, Figure 1B). In response to this reduced cortisol signal, there was no evidence of exaggerated ACTH responses (Figure 1A and Table 1). The three study cohorts exhibited similar augmentation of both basal (nonpulsatile) and pulsatile ACTH secretion in response to low feedback (Figure 3). The former increase was about 1.7-fold and the latter 10-fold matching values of the baseline day. Thus, amplification of pulsatile ACTH secretion predominates over elevation of basal ACTH secretion in a low-cortisol milieu, confirming our earlier 24-h data in normal subjects (26). Enhanced pulsatile ACTH secretion was due in turn to a 1.3-fold rise in ACTH secretory-burst frequency and an 8-fold rise in secretory-burst size (mass of ACTH (ng) released per burst per unit (L) distribution volume). These results establish that both basal and pulsatile ACTH secretion are strongly modulated by glucocorticoid feedback in both healthy and depressed individuals. We conclude that normal adults and hypercortisolemic depressed patients maintain highly comparable dynamics of ACTH secretion when assessed under a low-cortisol clamp at good (> 90% to 99%) statistical power. A potential objection is that differences were not observed between groups in the secretory response of ACTH to low feedback because of a ceiling effect on maximal Day 2 ACTH secretion. The data in the first half of the night, well before maximal plasma ACTH levels were achieved, do not support that potential objection (Figure 1). In addition, the temporal profile of ACTH increases on Day 2 was almost identical in controls and in hypercortisolemic depressed patients: both groups passed their respective Day 1 mean ACTH level and maximal ACTH level, and achieved peak ACTH levels on Day 2 at similar times (Results).
Likewise, the ACTH ApEn results did not suggest irregular or chaotic stimulation of the pituitary corticotrope cells by hypothalamic secretagogues on Day 1 or on Day 2, in keeping with our previously reported 24-h Day 1 data (11). Posener and colleagues (10) reported decreased baseline ACTH ApEn in male depressed patients, based, however, on hourly rather than 10-minute sampling. Young and colleagues (28) reported increased baseline ACTH ApEn based on 10-minute sampling in female patients with mild depression severity who were not shown to be hypercortisolemic. In the present study, metyrapone treatment decreased ACTH ApEn, signifying more coordinated central drive of corticotrope cell secretion and/or greater hypothalamopituitary system isolation with low feedback (27,29). However, the groups did not differ in this respect (Table 3 and Results). These 10-h ApEn results for ACTH secretion under both normal and reduced glucocorticoid feedback give no support to the hypothesis of chaotic dysregulation of anterior pituitary corticotrope cells by overactive limbic-hypothalamic circuits in hypercortisolemic depressed patients.
We did observe in the deconvolution analyses that basal (nonpulsatile) 10-h ACTH secretion trended higher in high-cortisol than low-cortisol depressed patients under metyrapone, and in comparison with normal subjects on the combined baseline and metyrapone days (Figure 4). The latter outcome supports an earlier report in female depressed patients of mixed cortisolemic status (9), and it is in agreement with our previously reported 24-h data (11). Since basal ACTH secretion is not known to depend upon arginine vasopressin (AVP) or CRH pulses, greater basal secretion could reflect diminished inhibition of constitutive ACTH synthesis and release by endogenous inhibitors, such as somatostatin, cortisol, annexin-1, nitric oxide and antiinflammatory cytokines and certain prostaglandins (30). Elevated basal ACTH secretion also would be congruent with the higher cortisol → ACTH cross-ApEn observed here, and with reports of increased anterior pituitary volume in depression (31).
A potential limitation of this study is the small number of hypercortisolemic depressed patients. However, the power analyses based on the observed data do not suggest that our key conclusions about ACTH secretory dynamics would change with larger samples within the logistically feasible range (Results). Moreover, for mean and peak Day 2 plasma ACTH levels, the direction of difference is controls > hypercortisolemic depressed (Table 1). Thus, despite the small number of cases, the ACTH data under low feedback are not at all consistent with the theory of HPA axis overdrive by an activated limbic system in hypercortisolemic major depression. Specifically, as seen in Figure 1A, we found no evidence of the excessive and phase advanced nocturnal ACTH secretion predicted by classic studies in depression (16). In this regard, it should be recalled that the ACTH response to low feedback under metyrapone in humans is in part mediated through prefrontal and/or limbic system override of a hypothalamic GABA-ergic mechanism that inhibits the release of corticotropin releasing factor (CRF) (32). Other trends in the data might well become statistically significant with a larger sample. Examples are the control – high-cortisol depression contrasts for pulsatile ACTH secretion and for pulsatile cortisol secretion on Day 1 (Table 2). However, confirmation of these trends would not necessarily change the conclusion that we found no increase of central drive on the HPA axis in hypercortisolemic depression. Contemporary modeling of HPA-axis physiology emphasizes continuous dynamic feedforward and feedback regulatory loops interacting with glucocorticoid receptors, with or without the necessity for phasic hypothalamic activity as the source of pulsatile hormone secretion (33–35).
Another possible limitation is confounding by recent discontinuation of antidepressant drug intake by the depressed patients. Direct study of this issue, however, has found that discontinuation of fluoxetine, sertraline or paroxetine was associated with no significant change of 24-h urinary free cortisol excretion or of evening plasma cortisol concentrations (36).
Previous studies of low feedback using metyrapone in depression have yielded inconsistent results. Several early studies used a single dose overnight protocol (37). These are not readily interpreted by current standards, in light of the 30-minute plasma half-life of metyrapone (24). When metyrapone has been administered in the morning (0800h–0900h) no abnormal response of ACTH or beta-endorphin was reported in 2 studies (6,38) but these results are not consistent with the report of Ur et al (5). When metyrapone has been commenced in the late afternoon or early evening the results again are inconsistent, with findings of either no difference in ACTH response (39,40), or an exaggerated beta-endorphin response in depression (41).
With overnight metyrapone dosing, results again have been inconsistent. In a 24-hour multiple dosing protocol commencing at 0900h, Ur et al. (5) gave metyrapone 750 mg every 4 hours. They first found early and sustained exaggerated ACTH responses to metyrapone in depressed patients whose DST results were abnormal – as early as 1200h. As noted above, this early increase of ACTH in daytime hours was not confirmed by others (6,38). Ur and colleagues (5) reported a maximum effect on ACTH after 24 hours, at which time (0900h) plasma cortisol had broken through the metyrapone inhibition, with levels of 6–7 ug/dL. The same breakthrough of cortisol at the time of the circadian acrophase was seen in our data in depression (Results) and in normal subjects (26). However, Young et al (42) reported no such cortisol breakthrough in a 24h metyrapone protocol that commenced at 1600h. In the same study, no ACTH response to metyrapone was detected until 12 hours after metyrapone was commenced, i.e. at 0400h. Thus, the overnight study of Young and colleagues is in disagreement with our data, because we did see significant ACTH increases beginning at 0200h. The reasons for these conflicting reports are not clear. We can only point out that the patient samples were likely heterogeneous and that most investigators did not address the condition of baseline hypercortisolemia in their data analyses.
Our observations and those of Ur et al (5) are consistent with the preclinical findings of Plotsky and Sawchenko (43), who described massive activation of the HPA axis during what they termed pharmacological adrenalectomy by metyrapone in rats, once the animals passed through the circadian acrophase. Our results diverge from those of Ur et al (5) in finding no difference in ACTH response between depressed patients and control subjects. We characterized baseline HPA axis hyperactivity by mean 24-h plasma cortisol concentration, whereas Ur et al (5) used DST results for that purpose. These are not identical benchmarks of the level of HPA axis activity (13), and the study of Ur et al (5) did not control for the known confound of plasma dexamethasone pharmacokinetics (44).
Our data and the follow-up power analyses also fail to support the theory of defective glucocorticoid feedback on the HPA axis in hypercortisolemic depression. Under that theory, the increase of ACTH secretion during reduced glucocorticoid feedback on Day 2 would be smaller in the patients than in control subjects. However, we saw no significant difference for mean Day 2 plasma ACTH or for Day 2 maximum ACTH concentrations. We also found no difference in the ambient plasma cortisol levels at the time of the plasma ACTH peak on Day 2 (Results). This finding gives no support to a hypothesized feedback deficiency in the hypercortisolemic depressed patients. The follow-up power analysis of this variable indicated that samples of at least 80 subjects per group would be needed to establish a statistically significant difference. The functional and heuristic relevance of any such finding would be dubious.
Further confirmation of no difference in glucocorticoid feedback comes from the ApEn analyses: Under low feedback, ACTH ApEn fell significantly and comparably in all groups (Table 3). Likewise, the reverse cross-ApEn results (cortisol feedback on regularity of ACTH secretion) did not discriminate hypercortisolemic patients from control subjects either on Day 1 or on Day 2, and the reverse cross-ApEn response to reduced feedback did not differ between these groups (Table 3 and Figure 5).
Overall, our data are most consistent with the third general theory, which proposes pathology at the level of the adrenal cortex in hypercortisolemic depression. The most powerful evidence in our data is the increase of basal cortisol secretion, which we could identify with the new deconvolution methodology, as well as the increased cortisol ApEn (Table 2 and Table 3). Exploratory comparisons between the two subgroups of depressed patients on the control day suggested that hypercortisolemic patients had shorter (reduced mode of) 10-h cortisol secretory bursts, increased basal cortisol secretion, more irregular cortisol patterns, and greater ACTH-cortisol and cortisol-ACTH asynchrony. These differences were not observed during metyrapone administration. On the metyrapone day, however, all groups had achieved markedly non-physiological plasma ACTH concentrations for a sustained duration (Figure 1 and Table 1). This factor, in combination with the severe degree of 11β-hydroxylase enzyme inhibition in the adrenal cortex makes a valid Day 1 – Day 2 comparison of cortisol secretory dynamics impossible. Thus, only the Day 1 data are physiologically valid, so these are determinative in suggesting adrenal involvement in the pathophysiology. Although based only on hourly sampling, Posener et al (2004) (10) also reported increased 24-h cortisol ApEn in depressed males. By way of precedence, cortisol ApEn is elevated in ACTH-dependent hypercortisolism, cortisol-secreting adrenal adenoma and untreated congenital adrenal hyperplasia (17,45,46). In fact, enlarged adrenal size has been reported in depression (12,47). There is now general agreement that adrenocortical sensitivity to physiological stimulation with ACTH is not increased in depression, even when adrenal gland enlargement has been established (11,48,49).
Adrenocortical enlargement occurs in subacute or chronic stress and is generally considered reversible, which is known to occur in depression (12). Increased basal (non-pulsatile) cortisol secretion is an expected consequence of adrenocortical enlargement. The primary candidate mechanism of adrenocortical enlargement in stress is limbic-hypothalamic driven autonomic activation, acting via the splanchnic nerve (50). Thus, our interpretive formulation may still be viewed as consistent with limbic-hypothalamic overactivity in hypercortisolemic depression, but with the mechanism being through hypothalamic-splanchnic sympathetic nervous system activation rather than through the classically proposed ACTH mechanism. A number of additional non-ACTH factors are known to influence adrenocortical steroidogenesis; these include cytokines, adipokines, endothelial paracrine factors, and neuropeptides (51). However the effects of these autonomic and humoral factors on adrenal secretory dynamics are largely unexplored, and none has been definitely linked to hypercortisolemic depression.
Differences in CBG concentrations would not explain elevations in ApEn, cross-ApEn and basal cortisol secretion in hypercortisolemic depression. Although not measured here, CBG concentrations are not reported to be decreased in depressed patients, even those with HPA axis activation (52,53). Moreover, ApEn is a scale-independent measure of regularity, which is not influenced by absolute hormone concentrations in the time series (27). Therefore, high cortisol ApEn and bidirectional cross-ApEn estimates may have heuristic value in future physiological studies of hypercortisolemic depression.
Qualifications and limitations include the caveats that measures of ACTH and cortisol secretion in patients hospitalized for major depression (as studied here) may differ from those in outpatients or others with less severe illness. This project was designed to evaluate specific theories about hypercortisolemic depression, not to develop biomarkers of major depression as it is treated broadly in the community. Whereas low-feedback provocation of the HPA axis was studied here, psychosocial or physical stress protocols need further study because these will produce less extreme and more ecologically relevant activation of the HPA axis than what is observed under metyrapone in our overnight protocol. Our strategy of stratifying depressed patients using the biomarker of hypercortisolemia reduced the heterogeneity encountered when patients are classified by current syndromal clinical criteria. In addition, we do not presently know whether the abnormalities seen in the high-cortisol depressed patients will resolve after clinical recovery. As for the low-cortisol depressed patients, we have reported their data for trend signaling only, and no firm conclusions can be advanced based on this very small sample.
In summary, the data obtained with this strategically timed low feedback protocol did not support either the theory of limbic-hypothalamic-pituitary overdrive or the theory of defective glucocorticoid feedback as the mechanism of hypercortisolemia in depression. The results point to elevated and irregular secretion of cortisol, especially basal cortisol secretion, associated with elevated basal ACTH secretion and/or non-ACTH mechanisms.
Significant Outcomes
There was no evidence of excessive or irregular ACTH secretion in hypercortisolemic depressed patients at baseline or in the low feedback condition. The classic theory of HPA axis overdrive by activated limbic-hypothalamic circuits was not supported.
The data also did not support a more recent theory that hypercortisolemia in depressed patients is the result of impaired glucocorticoid receptor mediated feedback.
At baseline, basal cortisol secretion was increased and was markedly irregular (high cortisol ApEn), with impaired linkage between ACTH and cortisol secretory profiles (high ACTH-cortisol cross ApEn). Hypercortisolemia in severe depression appears to be driven largely by increased basal cortisol secretion that is dissociated from ACTH stimulation.
Limitations
The sample size is small. Nevertheless, power analyses based on the observed data suggest that the negative conclusions regarding the two major theories of hypercortisolism would not change even if the sample were greatly enlarged.
The conclusions apply only to depressed patients with demonstrated hypercortisolemia. As not all depressed patients are hypercortisolemic, the conclusions do not necessarily apply to cases that are not hypercortisolemic.
We did not succeed in obtaining usable data over 24 hours because of drug (metyrapone) side effects. Nevertheless, the period from midnight to 1000h did yield valid data, and this period captures both the circadian nadir as well as the circadian peak of HPA axis activity.
Acknowledgments
We thank Uwe Meya, M.D. from Ciba-Geigy (now Novartis) in Basel, Switzerland, who assisted us in obtaining a supply of metyrapone for these experiments. We thank Jill Smith, Donna Scott and Sam Rudolph for support of manuscript preparation; Ashley Bryant for data analysis and graphics; Duke research coordinators Cammie Hellegers and Gina Harris; and the Duke research nursing staff for implementing the protocol. Supported in part via the Center for Translational Science Activities (CTSA) Grant Number 1 UL 1 RR024150 to Mayo Clinic from the National Center for Research Resources (Rockville, MD), R01 DK073148 and DK050456 (Metabolic Studies Core of the Minnesota Obesity Center) from the National Institutes of Health (Bethesda, MD), HD 2RO1 MH-39593 (Neuroendocrine Regulation in Depression), P30 MH-40159 (Clinical Research Center for the Study of Depression in Late Life) and MO1-RR-30 to Duke University Medical Center.
APPENDIX
Deconvolution analysis is a statistical method of resolving the contributions of hormone secretion and hormone clearance to the observed plasma hormone concentration over time. Deconvolution analysis permits one to estimate hormone secretion, without otherwise confounding variations in the plasma half-life among different individuals and/or different conditions of study (17). Thus, the ACTH secretory response is considered a “purer” signal than plasma ACTH levels.
For ACTH, the main outcomes of deconvolution analysis are ACTH secretory-burst mass (ng/L) and number (per 10 h), pulsatile and nonpulsatile (basal) ACTH secretion (ng/L/10 h), ACTH half-life (min) and secretory-burst shape (mode of time delay to maximal secretion in min). Sensitivity and specificity of pulse detection in this study exceeded 92.5% (54). Corresponding outcomes also were computed for cortisol. For inferences concerning the level of activity in the HPA axis, basal and pulsatile secretion rates of ACTH and cortisol are more informative than serial plasma ACTH and cortisol changes over time.
ApEn quantifies the relative orderliness or subpattern consistency in a time series, with higher ApEn corresponding to less regularity [greater randomness]. ApEn is a unitless, scale-independent measure of regularity (27). Regularity values change whenever negative or positive feedback changes, and thus ApEn provides a measure of altered feedback state (27). In the corticotropic axis, feedback withdrawal is predicted to decrease ACTH ApEn, as well as forward and reverse cross-ApEn (55). Cross-ApEn by analogy quantifies the relative synchrony (joint regularity) of subpatterns within paired time series, wherein higher cross-ApEn denotes less synchrony [greater asynchrony]. The terms forward and reverse cross-ApEn refer to ACTH-cortisol forward drive and cortisol-ACTH feedback restraint, respectively, as defined earlier (55).
In addition, the approximate entropy (ApEn) statistic is uniquely suited as a sensitive and highly specific marker of feedback alterations or of adaptations in pathology (56). ApEn evaluates the consistency or reproducibility of short subpatterns in time series. We have shown directly in computer simulations (57) and in seven types of clinical experiments (27) that ApEn provides > 90% sensitivity and specificity for detecting network-like effects of pathophysiology. ApEn in two other studies detected significant loss of ACTH and/or cortisol regularity in depressed individuals (10,11,28).
The mathematical basis for ApEn is geometric reproducibility of patterns and subpatterns in the time series (58). ApEn will detect subtle differences in pattern synchrony between two hormone profiles, but independently of magnitude per se. The latter point is particularly important when dealing with endocrine systems, wherein stability of synchrony must be estimated independently of absolute magnitude of the time-series values. This independence is achieved by normalizing the cross-ApEn and ApEn threshold used for testing similarities in pattern (and detecting differences in pattern) against the individual series standard deviation (SD). Thus, ApEn is a normalized statistic. Changes in the time latency of ACTH-cortisol coupling are not themselves detected in this approach, but might be detectable using a new dose-response downregulation model which we have yet to apply to these data.
ApEn is not the same as a within-individual standard deviation in (e.g.) cortisol levels across the day. ApEn uses a fraction of the within-individual SD to normalize for amplitude differences. The typical well validated fraction is an ApEn threshold of 0.2 times the SD of that series. Thereby, ApEn is sensitive to pattern differences, even when SD’s of the series do not differ.
Footnotes
Conflict of Interest
No part of this work has been previously published or is under consideration for publication elsewhere. The authors have no competing financial interests in relation to this work. In the past 3 years, Dr. Bernard Carroll has received compensation from AstraZeneca, MultiHealth Systems (licensee for the Carroll Depression Scales), South Carolina Psychiatric Association, Springer Publishing Company and Brentwood Biomedical Research Institute, Los Angeles. Dr. Frederick Cassidy has had a grant from Pfizer.
Reference List
- 1.RUBIN RT, MANDELL AJ. Adrenal cortical activity in pathological emotional states: a review. Am J Psychiatry. 1966;123(4):387–400. doi: 10.1176/ajp.123.4.387. [DOI] [PubMed] [Google Scholar]
- 2.CARROLL BJ, MARTIN FI, DAVIES B. Resistance to suppression by dexamethasone of plasma 11-O.H.C.S. levels in severe depressive illness. Br Med J. 1968;3(613):285–287. doi: 10.1136/bmj.3.5613.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.SACHAR EJ, HELLMAN L, FUKUSHIMA DK, GALLAGHER TF. Cortisol production in depressive illness. A clinical and biochemical clarification. Arch Gen Psychiatry. 1970;23(4):289–298. doi: 10.1001/archpsyc.1970.01750040001001. [DOI] [PubMed] [Google Scholar]
- 4.NEMEROFF CB, WIDERLOV E, BISSETTE G, et al. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984;226(4680):1342–1344. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- 5.UR E, DINAN TG, O’KEANE V, et al. Effect of metyrapone on the pituitary-adrenal axis in depression: relation to dexamethasone suppressor status. Neuroendocrinol. 1992;56(4):533–538. doi: 10.1159/000126271. [DOI] [PubMed] [Google Scholar]
- 6.RUPPRECHT R, KORNHUBER J, WODARZ N, et al. Disturbed glucocorticoid receptor autoregulation and corticotropin response to dexamethasone in depressives pretreated with metyrapone. Biol Psychiatry. 1991;29(11):1099–1109. doi: 10.1016/0006-3223(91)90252-h. [DOI] [PubMed] [Google Scholar]
- 7.HOLSBOER F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23(5):477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- 8.PARIANTE CM. The glucocorticoid receptor: part of the solution or part of the problem? J Psychopharmacol. 2006;20(4 Suppl):79–84. doi: 10.1177/1359786806066063. [DOI] [PubMed] [Google Scholar]
- 9.YOUNG EA, CARLSON NE, BROWN MB. Twenty-four-hour ACTH and cortisol pulsatility in depressed women. Neuropsychopharmacology. 2001;25(2):267–276. doi: 10.1016/S0893-133X(00)00236-0. [DOI] [PubMed] [Google Scholar]
- 10.POSENER JA, CHARLES D, VELDHUIS JD, PROVINCE MA, WILLIAMS GH, SCHATZBERG AF. Process irregularity of cortisol and adrenocorticotropin secretion in men with major depressive disorder. Psychoneuroendocrinol. 2004;29(9):1129–1137. doi: 10.1016/j.psyneuen.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 11.CARROLL BJ, CASSIDY F, NAFTOLOWITZ D, et al. Pathophysiology of hypercortisolism in depression. Acta Psychiatr Scand. 2007;115(Suppl 433):90–103. doi: 10.1111/j.1600-0447.2007.00967.x. [DOI] [PubMed] [Google Scholar]
- 12.RUBIN RT, PHILLIPS JJ, SADOW TF, MCCRACKEN JT. Adrenal gland volume in major depression. Increase during the depressive episode and decrease with successful treatment. Arch Gen Psychiatry. 1995;52(3):213–218. doi: 10.1001/archpsyc.1995.03950150045009. [DOI] [PubMed] [Google Scholar]
- 13.CARROLL BJ. The dexamethasone suppression test for melancholia. Br J Psychiatry. 1982;140:292–304. doi: 10.1192/bjp.140.3.292. [DOI] [PubMed] [Google Scholar]
- 14.NELSON JC, DAVIS JM. DST studies in psychotic depression: a meta-analysis. Am J Psychiatry. 1997;154(11):1497–1503. doi: 10.1176/ajp.154.11.1497. [DOI] [PubMed] [Google Scholar]
- 15.CARROLL BJ, CURTIS GC, MENDELS J. Neuroendocrine regulation in depression. I. Limbic system-adrenocortical dysfunction. Arch Gen Psychiatry. 1976;33(9):1039–1044. doi: 10.1001/archpsyc.1976.01770090029002. [DOI] [PubMed] [Google Scholar]
- 16.SACHAR EJ, HELLMAN L, ROFFWARG HP, HALPERN FS, FUKUSHIMA DK, GALLAGHER TF. Disrupted 24-hour patterns of cortisol secretion in psychotic depression. Arch Gen Psychiatry. 1973;28(1):19–24. doi: 10.1001/archpsyc.1973.01750310011002. [DOI] [PubMed] [Google Scholar]
- 17.VELDHUIS JD, KEENAN DM, PINCUS SM. Motivations and methods for analyzing pulsatile hormone secretion. Endocr Rev. 2008;29(7):823–864. doi: 10.1210/er.2008-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.RAADSHEER FC, HOOGENDIJK WJ, STAM FC, TILDERS FJ, SWAAB DF. Increased numbers of corticotropin-releasing hormone expressing neurons in the hypothalamic paraventricular nucleus of depressed patients. Neuroendocrinol. 1994;60(4):436–444. doi: 10.1159/000126778. [DOI] [PubMed] [Google Scholar]
- 19.SWAAB DF, BAO AM, LUCASSEN PJ. The stress system in the human brain in depression and neurodegeneration. Ageing Res Rev. 2005;4(2):141–194. doi: 10.1016/j.arr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 20.SPITZER RL, ENDICOTT J, ROBINS E. Research Diagnostic Criteria (RDC) for a selected group of functional disorders. New York State Psychiatric Institute; New York: 1977. [Google Scholar]
- 21.CARROLL BJ. Carroll Depression Scales Technical Manual. Multi-Health Systems, Inc; Toronto: 1998. [Google Scholar]
- 22.CARROLL BJ, FEINBERG M, SMOUSE PE, RAWSON SG, GREDEN JF. The Carroll rating scale for depression. I. Development, reliability and validation. Br J Psychiatry. 1981;138:194–200. doi: 10.1192/bjp.138.3.194. [DOI] [PubMed] [Google Scholar]
- 23.HAMILTON M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.JUBIZ W, MATSUKURA S, MEIKLE AW, HARADA G, WEST CD, TYLER FH. Plasma metyrapone, adrenocorticotropic hormone, cortisol, and deoxycortisol levels. Sequential changes during oral and intravenous metyrapone administration. Arch Intern Med. 1970;125(3):468–471. [PubMed] [Google Scholar]
- 25.ZAR JH. Biostatistical analysis. Prentice Hall; Upper Saddle River, NJ: 1996. [Google Scholar]
- 26.VELDHUIS JD, IRANMANESH A, NAFTOLOWITZ D, TATHAM N, CASSIDY F, CARROLL BJ. Corticotropin secretory dynamics in humans under low glucocorticoid feedback. J Clin Endocrinol Metab. 2001;86(11):5554–5563. doi: 10.1210/jcem.86.11.8046. [DOI] [PubMed] [Google Scholar]
- 27.VELDHUIS JD, STRAUME M, IRANMANESH A, et al. Secretory process regularity monitors neuroendocrine feedback and feedforward signaling strength in humans. Am J Physiol. 2001;280(3):R721–R729. doi: 10.1152/ajpregu.2001.280.3.R721. [DOI] [PubMed] [Google Scholar]
- 28.YOUNG EA, VELDHUIS JD. Disordered adrenocorticotropin secretion in women with major depression. J Clin Endocrinol Metab. 2006;91(5):1924–1928. doi: 10.1210/jc.2005-2397. [DOI] [PubMed] [Google Scholar]
- 29.PINCUS SM. Greater signal regularity may indicate increased system isolation. Math Biosci. 1994;122:161–181. doi: 10.1016/0025-5564(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 30.PETERSENN S, SCHULTE HM. Differentiation and regulation of the corticotropic pituitary cell. Eur J Clin Invest. 2000;30 (Suppl 3):10–13. doi: 10.1046/j.1365-2362.2000.0300s3010.x. [DOI] [PubMed] [Google Scholar]
- 31.KRISHNAN KR, DORAISWAMY PM, LURIE SN, et al. Pituitary size in depression. J Clin Endocrinol Metab. 1991;72(2):256–259. doi: 10.1210/jcem-72-2-256. [DOI] [PubMed] [Google Scholar]
- 32.ARVAT E, MACCAGNO B, RAMUNNI J, et al. The inhibitory effect of alprazolam, a benzodiazepine, overrides the stimulatory effect of metyrapone-induced lack of negative cortisol feedback on corticotroph secretion in humans. J Clin Endocrinol Metab. 1999;84(8):2611–2615. doi: 10.1210/jcem.84.8.5911. [DOI] [PubMed] [Google Scholar]
- 33.KEENAN DM, LICINIO J, VELDHUIS JD. A feedback-controlled ensemble model of the stress-responsive hypothalamo-pituitary-adrenal axis. Proc Natl Acad Sci USA. 2001;98(7):4028–4033. doi: 10.1073/pnas.051624198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.WALKER JJ, TERRY JR, LIGHTMAN SL. Origin of ultradian pulsatility in the hypothalamic-pituitary-adrenal axis. Proc Biol Sci. 2010;277(1688):1627–1633. doi: 10.1098/rspb.2009.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.LIGHTMAN SL, CONWAY-CAMPBELL BL. The crucial role of pulsatile activity of the HPA axis for continuous dynamic equilibration. Nat Rev Neurosci. 2010;11(10):710–718. doi: 10.1038/nrn2914. [DOI] [PubMed] [Google Scholar]
- 36.MICHELSON D, AMSTERDAM J, APTER J, et al. Hormonal markers of stress response following interruption of selective serotonin reuptake inhibitor treatment. Psychoneuroendocrinology. 2000;25(2):169–177. doi: 10.1016/s0306-4530(99)00046-3. [DOI] [PubMed] [Google Scholar]
- 37.MORPHY MA, FAVA GA, PERINI GI, MOLNAR G, ZIELEZNY M, LISANSKY J. The dexamethasone suppression and metyrapone tests in depression. Psychiatry Res. 1985;15(2):153–158. doi: 10.1016/0165-1781(85)90051-4. [DOI] [PubMed] [Google Scholar]
- 38.YOUNG EA, LOPEZ JF, MURPHY-WEINBERG V, WATSON SJ, AKIL H. Normal pituitary response to metyrapone in the morning in depressed patients: implications for circadian regulation of corticotropin-releasing hormone secretion. Biol Psychiatry. 1997;41(12):1149–1155. doi: 10.1016/s0006-3223(96)00291-0. [DOI] [PubMed] [Google Scholar]
- 39.LISANSKY J, PEAKE GT, STRASSMAN RJ, et al. Augmented pituitary corticotropin response to a threshold dosage of human corticotropin-releasing hormone in depressives pretreated with metyrapone. Arch Gen Psychiatry. 1989;46 (7):641–649. doi: 10.1001/archpsyc.1989.01810070067011. [DOI] [PubMed] [Google Scholar]
- 40.YOUNG EA, AKIL H, HASKETT RF, WATSON SJ. Evidence against changes in corticotroph CRF receptors in depressed patients. Biol Psychiatry. 1995;37 (6):355–363. doi: 10.1016/0006-3223(94)00153-T. [DOI] [PubMed] [Google Scholar]
- 41.YOUNG EA, HASKETT RF, GRUNHAUS L, et al. Increased evening activation of the hypothalamic-pituitary-adrenal axis in depressed patients. Arch Gen Psychiatry. 1994;51(9):701–707. doi: 10.1001/archpsyc.1994.03950090033005. [DOI] [PubMed] [Google Scholar]
- 42.YOUNG EA, RIBEIRO SC, YE W. Sex differences in ACTH pulsatility following metyrapone blockade in patients with major depression. Psychoneuroendocrinology. 2007;32(5):503–507. doi: 10.1016/j.psyneuen.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.PLOTSKY PM, SAWCHENKO PE. Hypophysial-portal plasma levels, median eminence content, and immunohistochemical staining of corticotropin-releasing factor, arginine vasopressin, and oxytocin after pharmacological adrenalectomy. Endocrinol. 1987;120(4):1361–1369. doi: 10.1210/endo-120-4-1361. [DOI] [PubMed] [Google Scholar]
- 44.RITCHIE JC, BELKIN BM, KRISHNAN KR, NEMEROFF CB, CARROLL BJ. Plasma dexamethasone concentrations and the dexamethason suppression test. Biol Psychiatry. 1990;27(2):159–173. doi: 10.1016/0006-3223(90)90646-j. [DOI] [PubMed] [Google Scholar]
- 45.VAN DEN BERG G, PINCUS SM, VELDHUIS JD, FROLICH M, ROELFSEMA F. Greater disorderliness of ACTH and cortisol release accompanies pituitary-dependent Cushing’s Disease. Eur J Endocrinol. 1997;136:394–400. doi: 10.1530/eje.0.1360394. [DOI] [PubMed] [Google Scholar]
- 46.ROELFSEMA F, PINCUS SM, VELDHUIS JD. Patients with Cushing’s disease secrete adrenocorticotropin and cortisol jointly more asynchronously than healthy subjects. J Clin Endocrinol Metab. 1998;83(2):688–692. doi: 10.1210/jcem.83.2.4570. [DOI] [PubMed] [Google Scholar]
- 47.NEMEROFF CB, KRISHNAN KR, REED D, LEDER R, BEAM C, DUNNICK NR. Adrenal gland enlargement in major depression. A computed tomographic study. Arch Gen Psychiatry. 1992;49(5):384–387. doi: 10.1001/archpsyc.1992.01820050048008. [DOI] [PubMed] [Google Scholar]
- 48.RUBIN RT, PHILLIPS JJ, MCCRACKEN JT, SADOW TF. Adrenal gland volume in major depression: relationship to basal and stimulated pituitary-adrenal cortical axis function. Biol Psychiatry. 1996;40(2):89–97. doi: 10.1016/0006-3223(95)00358-4. [DOI] [PubMed] [Google Scholar]
- 49.KRISHNAN KR, RITCHIE JC, SAUNDERS WB, NEMEROFF CB, CARROLL BJ. Adrenocortical sensitivity to low-dose ACTH administration in depressed patients. Biol Psychiatry. 1990;27(8):930–933. doi: 10.1016/0006-3223(90)90476-i. [DOI] [PubMed] [Google Scholar]
- 50.BORNSTEIN SR, CHROUSOS GP. Adrenocorticotropin (ACTH)- and non-ACTH-mediated regulation of the adrenal cortex: neural and immune inputs. J Clin Endocrinol Metab. 1999;84(5):1729–1736. doi: 10.1210/jcem.84.5.5631. [DOI] [PubMed] [Google Scholar]
- 51.BORNSTEIN SR, ENGELAND WC, EHRHART-BORNSTEIN M, HERMAN JP. Dissociation of ACTH and glucocorticoids. Trends Endocrinol Metab. 2008;19 (5):175–180. doi: 10.1016/j.tem.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 52.SCHLECHTE JA, SHERMAN B, PFOHL B. A comparison of adrenal cortical function in patients with depressive illness and Cushing’s disease. Horm Res. 1986;23(1):1–8. doi: 10.1159/000180281. [DOI] [PubMed] [Google Scholar]
- 53.DEUSCHLE M, SCHWEIGER U, STANDHARDT H, WEBER B, HEUSER I. Corticosteroid-binding globulin is not decreased in depressed patients. Psychoneuroendocrinology. 1996;21(8):645–649. doi: 10.1016/s0306-4530(96)00033-9. [DOI] [PubMed] [Google Scholar]
- 54.LIU PY, KEENAN DM, KOK P, PADMANABHAN V, O’BYRNE KT, VELDHUIS JD. Sensitivity and specificity of pulse detection using a new deconvolution method. Am J Physiol Endo Metab. 2009;297(2):E538–E544. doi: 10.1152/ajpendo.00071.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.LIU PY, PINCUS SM, KEENAN DM, ROELFSEMA F, VELDHUIS JD. Analysis of bidirectional pattern synchrony of concentration-secretion pairs: implementation in the human testicular and adrenal axes. Am J Physiol. 2005;288(2):R440–R446. doi: 10.1152/ajpregu.00414.2004. [DOI] [PubMed] [Google Scholar]
- 56.LIU PY, PINCUS SM, KEENAN DM, ROELFSEMA F, VELDHUIS JD. Joint synchrony of reciprocal hormonal signaling in human paradigms of both ACTH excess and cortisol depletion. Am J Physiol Endocrinol Metab. 2005;289(1):160–165. doi: 10.1152/ajpendo.00007.2005. [DOI] [PubMed] [Google Scholar]
- 57.VELDHUIS JD, JOHNSON ML, VELDHUIS OL, STRAUME M, PINCUS S. Impact of pulsatility on the ensemble orderliness (approximate entropy) of neurohormone secretion. Am J Physiol. 2001;281:R1975–R1985. doi: 10.1152/ajpregu.2001.281.6.R1975. [DOI] [PubMed] [Google Scholar]
- 58.PINCUS SM, SINGER BH. Randomness and degrees of irregularity. Proc Natl Acad Sci USA. 1996;93:2083–2088. doi: 10.1073/pnas.93.5.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]