Summary
The cells of the innate immune system mobilize a coordinated immune response towards invading microbes and after disturbances in tissue homeostasis. These immune responses typically lead to infection control and tissue repair. Exaggerated or uncontrolled immune responses, however, can also induce acute of chronic inflammatory pathologies that are characteristic for many common diseases such as sepsis, arthritis, atherosclerosis or Alzheimer’s disease. In recent years the concerted efforts of many scientists have uncovered numerous mechanisms by which immune cells detect foreign or changed self-substances that appear in infections or during tissue damage. These substances stimulate signaling receptors, which leads to cellular activation and the induction of effector mechanisms. Here, we review the role of inflammasomes, a family of signaling molecules that form multi-molecular signaling platforms and activate inflammatory caspases and IL-1β cytokines.
Keywords: Monocytes/Macrophages < Cell Lineages and Subsets, Neutrophils < Cell Lineages and Subsets, Toll-like Receptors/Pattern Recognition Receptors < Molecules, Inflammation < Processes, Adapter proteins < Molecules, Lipopolysaccharide < Molecules
Introduction
Innate immune cells are able to mount an immediate response towards infectious microorganisms or sterile tissue insults by virtue of a set of germline-encoded receptor signaling molecules. In recent years, rapid progress has been made in our understanding of the molecular mechanisms of immune activation. Several families of pattern recognition receptors have been discovered that are engaged in surveillance of the extracellular and intracellular space for the presence of microbial products or endogenously derived danger signals. These families include the Toll-like receptor (TLR) and C-type lectin receptor (CLR) transmembrane proteins and the cytoplasmic nucleotide-binding domain, leucine-rich repeat containing receptors (NLRs) or Rig-I like receptors (RLRs) (see reviews: (1–3)). While most receptors evolved to modulate the transcriptional response of cells after activation, members of the NLR protein family can form multimolecular protein complexes termed inflammasomes. These inflammasomes activate a proteolytic cascade culminating in the maturation and release of pro-cytokines of the IL-1β family of cytokines amongst other proteins.
Inflammasomes are typically formed by a ‘receptor’ protein, the adaptor molecule apoptosis-related speck-like protein (ASC) and the effector molecule pro-caspase-1. Whether the receptor part of the inflammasome actually interacts directly with a ligand has not yet been firmly established. Indeed, for some inflammasomes there is indication that other proteins can function in binding the activating ligand. The main function of inflammasomes appears to be in controlling the activation state of caspase-1 and thereby the activity of the main substrates, the members of the pro-IL-1β family of cytokines.
The proximal ‘receptor’ part of inflammasomes is categorized by the domain structure of the proteins. The NLRP proteins (formerly termed NALPs) contain a pyrin (PYD) domain, while the NLRC proteins carry a caspase recruitment domain (CARD). Other members include the MHC class II transactivator (CIITA) and baculoviral IAP repeat-containing protein 1 (NAIP) (Fig. 1). Another protein that can form an inflammasome together with ASC is the absent in melanoma 2 (AIM2) protein, which lacks the typical NACHT domain of the NLR inflammasome proteins. Here, we review the biology and function of inflammasomes and their relevance in disease.
Figure 1. Inflammasomes.
Upon their activation inflammasomes form multi-molecular protein complexes that typically consist of an NLR protein, or AIM2, the adapter molecule ASC and the effector pro-caspase-1. The different inflammasomes differ in their domain organization and composition as depicted in the figure. The NLRP1 inflammasome contains both a CARD and a PYD domain and can recruit several caspases, while the NLRC4 inflammasome can also contain NAIP5.
NLRP1
NLRP1 (NAC, DEFCAP, CARD7, NALP1) is the first NLR protein that was shown to form large structures akin to the apoptosome and since these were caspase-1 and IL-1β activating platforms, the term ‘inflammasome’ was coined (4). The domain structure for human NLRP1 (containing the NLRP-specific PYD-, nucleotide-binding NACHT- and LRR-domains followed by a FIIND- and a CARD-domain) differs from the mouse Nlrp1, as the latter lacks the PYD-domain at the N-terminus. Moreover, the mouse genome contains three paralogue genes of human NLRP1, and there are five different strain-specific Nlrp1b alleles in inbred mice (5). Human CARD8 (CARDINAL, TUCAN) is highly similar to the C-terminus of mouse Nlrp1b, and since it is not found in the mouse genome, it was hypothesized that Nlrp1 might play a dual role in mice (6).
Microbial sensing
NLRP1 was identified as the cytosolic sensor for lethal toxin (LT) from Bacillus anthracis and for muramyl dipeptide (MDP) peptidoglycan from bacterial cell walls. B. anthracis, the causative agent of anthrax disease, secretes three proteins: the cell-binding and pore-forming protective antigen (PA), and two enzymatic proteins, edema (EF) and lethal factor (LF). LF is delivered to the endosomal compartment by binding to a PA heptamer/octamer, where acidification causes the receptors to form small pores and denaturation of LF, which, in turn, is secreted through the pore into the cytosol (7). LT infection of mouse macrophages isolated from an Nlrp1b-sensitive mouse strain have been shown to engage the Nlrp1 inflammasome leading to processing of pro-caspase-1 and -11, as well as pro-IL-18 (8). LT-induced lysosomal degradation and cathepsin B release with subsequent cell death, was also Nlrp1b-dependent, probably due to processing of the protein Bid (9). Interestingly, human fibroblasts reconstituted with Nlrp1b and pro-caspase-1 were susceptible to LT-infection. In contrast, the Nlrp1 CARD-domain alone was not rendering cells susceptible, however, together with some parts of the FIIND-domain (similar to CARD8 in humans) it was able to catalytically process pro-caspase-1 without the requirement for ASC (10).
Whilst MDP was shown to directly activate NLRP1 in cell-free systems (11), it is also known to be a ligand for NLRC2 (NOD2) (another member of the NLR family), in vivo (12). It was recently observed, that the direct interplay between these two receptors is responsible for caspase-1-dependent IL-1β release upon B. anthracis infection and bacterial MDP (13). NLRC2 can directly interact with caspase-1 via its two N-terminal CARD-domains and can also induce NF-κB via RIP2. NF-κB-activation initiates the transcription of pro-IL-1β, which in turn is processed by the NLRC2-NLRP1 (but not the NLRP3) inflammasome.
Genetic disorders
Genetic variations in the NLRP1 genomic region have been associated with high risk to generalized vitiligo, a multifactorial disease characterized by loss of skin pigmentation. Genetic analysis of chromosome 17 in patients with autoimmune and autoinflammatory diseases (generalized vitiligo, autoimmune thyroid disease, rheumatoid arthritis, lupus and Addison’s disease) identified several single-nucleotide polymorphisms (SNPs) in the NLRP1 gene (14). One SNP encoded an L155H non-synonymous amino acid change in an otherwise highly conserved region of NLRP1 and this was strongly associated with generalized vitiligo alone or together with other autoinflammatory diseases. Sequence variants in the promoter region of NLRP1 were also identified, however, their exact role remains to be elucidated.
Mechanism of activation
NLRP1 was the first protein found to form inflammasomes that processed caspase-1 and -5 in an ASC-dependent manner (4). The minimal protein constituents for NLRP1 inflammasome formation were later characterized in cell-free reconstitution assays (11). NLRP1 directly interacted with pro-caspase-1 via CARD/CARD-domain interaction, and MDP-induced NLRP1 activation was sufficient for processing caspase-1 while the addition of ASC enhanced caspase-1 activity. NLRP1 activation follows a two-step mechanism upon MDP-treatment. MDP, as an effective ligand, could bind to the LRR-domain of NLRP1 to induce a conformational change in the protein, that allows for the binding of NTPs and subsequent oligomerization of NLRP1 via its NACHT-domain. Electron microscopic images of MDP- and ATP-activated NLRP1 showed a mixture of small and large particles, where alignment and averaging of the larger oligomer structures showed five- and seven-fold symmetries, not unlike structures found in the APAF-1 apoptosome. NLRP1 was also shown to interact with caspase-2 and -9 in an apoptosome and contribute to apoptosis; however, the exact mechanism remains unknown (15). Furthermore, the mechanism by which NLRP1 is activated is yet to be described.
Regulation
NLRP1 is not widely expressed, with the highest levels seen in T cells and Langerhans cells and at lower levels in stomach epithelial cells, gut and lungs (16). Interestingly, the anti- apoptotic BCL-2 and -XL directly bind to the LRR-domain of NLRP1 and block caspase-1-activation (17). Furthermore, its short loop-domain is sufficient to inhibit NLRP1 ATP-binding and oligomerization (18). A viral NLR homolog, Orf63 (isolated from Kaposi’s sarcoma-associated herpesvirus), was shown to block NLRP1-mediated caspase-1 and IL-1β processing and interact with NLRP3 and NLRC2 (19).
NLRP3
Danger signal and microbial sensing
The NLRP3 inflammasome can be activated by a plethora of agonists, endogenous and exogenous, sterile or pathogen-derived. However, judging from the diverse nature of these activators, it is highly unlikely that these danger- and pathogen-associated molecular patterns (DAMPs and PAMPs) all directly activate or interact with NLRP3, and NLRP3 is rather considered as a sensor for changes in the cellular environment.
Potent danger signals in the extracellular matrix and tissue environment can be a result of cell death and elevated levels of metabolites, aggregate or crystalline material. These molecules can be physiological constituents of functioning cells, that when displaced from their physiological locations or when aggregate can be inflammatory (20).
In vitro studies have shown that LPS-primed macrophages respond to extracellular ATP by turning on the NLRP3 inflammasome (21) via the purinergic receptor P2X7 (22). Conceptually, ATP can appear in extracellular space as a result of cell death following tissue damage and thus, NLRP3 activation may serve as a sensing mechanism for tissue damage. ATP may be derived from cytosolic contents that are released following cell disintegration. Additionally, ATP could be actively secreted by mitochondria expelled into the extracellular environment. Even though sensing of ATP in the extracellular environment occurs via the P2X7 receptor, ATP is also involved in the direct activation of NLRP3. There is evidence in vitro and in reconstitution assays, that ATP binds to the NACHT-domain of NLRP3 and is hydrolyzed by the ATPase activity of NLRP3. This activity appears to be necessary for the activation of NLRP3 (23).
Uric acid is a normal catabolic product of purine metabolism and it has higher solubility inside cells than outside cells, since the ion composition in these environments dramatically differs. During cell death, cytosolic uric acid is released into the extracellular environment where it can precipitate to form monosodium urate (MSU) crystals that activate NLRP3. Thus, NLRP3 can also sense cell death by recognizing the phase transition of uric acid. Uric acid crystals can also trigger the classic crystal-induced disease, gout.
Patients displaying elevated levels of uric acid in their blood (hyperuricemia), produce MSU crystals that result in an acute and painful inflammatory response in joints that can lead to chronic arthritis (24). Similarly, calcium pyrophosphate (CPPD) can form spontaneous crystal deposits in joints, leading to the acute arthritis known as pseudogout (25). Release of IL-1β upon stimulation with MSU and CPPD crystals was shown to be dependent on the components of the NLRP3 inflammasome in mouse macrophages and human monocytes and this could be blocked by colchicine, a drug used for treatment of patients with gout (26).
Alzheimer’s disease is an inflammatory disease associated with amyloid β deposits in so-called senile plaques. These plaques attract resident macrophage-like microglia cells and astrocytes (27). Phagocytosis of the fibrillar form of these aggregates can activate the NLRP3 inflammasome in microglia cells and the subsequent release of pro-inflammatory cytokines and neurotoxic factors could contribute to disease pathogenesis (28). IL-1β is a known pro-inflammatory cytokine involved in the pathogenesis of type 2 diabetes mellitus (T2DM) (29) and atherosclerosis and recently, some of the mechanisms that govern IL-1β production in these diseases have been described. Obesity is the most common underlying condition for many diseases in the Western world and hyperlipidemia-induced insulin resistance and pancreatic islet beta-cell dysfunction are the characteristic features of T2DM. IL-1β was shown to induce cell death of beta cells (30) and hyperglycemia-induced IL-1β production by the beta cells further aggravates this cytotoxic effect (31). Islet amyloid polypeptide (IAPP) is a peptide hormone that is secreted in low amounts together with insulin in the pancreas and probably has a role in glucose metabolism and gut function. Increased insulin secretion results in increased quantities of this peptide that can lead to the formation of oligomers and amyloid deposits and result in apoptosis of the beta cells (32). Recently, IAPP was also found to engage the NLRP3 inflammasome and produce IL-1β in bone-marrow derived macrophages (BMDMs) that were primed by products of heightened glucose metabolism (33). Hence, increased production of insulin and amylin during peripheral insulin resistance states in T2DM could lead to the generation of NLRP3-dependent beta cell toxic factors. These factors could in turn, favor the development of insulin-dependent diabetes, a feature commonly observed in patients that have T2DM for a prolonged time. Intriguingly, it is also possible that by treating patients with drugs that increase insulin production and thereby potentially also amylin secretion, the rate of amylin deposition may be iatrogenically increased.
It was recently shown that fatty substances are also directly involved in NLRP3 activation. Saturated, but not unsaturated fatty acids, can induce IL-1β production in a Nlrp3- and Asc-dependent manner (34). The activation of NLRP3 by palmitate and LPS inhibited the AMP-activated protein kinase pathway, which led to impaired autophagocytosis and elevated mitochondrial ROS generation, and together, IL-1β and TNF mediated insulin signaling and resistance in vitro and in vivo. Obesity and T2DM are two of several risk factors for atherosclerosis. This disease is characterized by thickening of the artery walls due to the accumulation of fatty metabolites and cholesterol. The subsequent invasion of macrophages elicits a chronic inflammatory response to the cholesterol crystals and since they cannot be cleared, the unstable atherosclerotic plaques can rupture and cause thrombosis. The appearance of cholesterol crystals in the plaques were thought to be a late sign of atherosclerotic lesions (35), however, utilizing a novel microscopic technique, it was revealed that crystal depositions could be found in the lesions as early as two weeks following a high cholesterol diet in mice prone to atherosclerosis (ApoE−/ −) (36). In vitro experiments showed that cholesterol crystals and oxidized LDL activated NLRP3 in a manner dependent on phagolysosomal rupture. LDL receptor knock-out mice reconstituted with bone marrow from mice lacking genes of the components of NLRP3 inflammasome also showed a reduced phenotype of cholesterol crystal-induced inflammation and atherosclerosis. Overall, there is a growing body of evidence suggesting that the severe inflammatory response to metabolic disorders resulting from crystal and aggregate formation is mediated by the NLRP3 inflammasome.
As in the case of endogenous crystal formation, exogenous inorganic fibrils and crystals from environmental hazards can engage the NLRP3 inflammasome in severe fibrosing disorders, like asbestosis and silicosis. In vitro studies in human monocytes and mouse macrophages showed that once silica crystals and asbestos engage NLRP3, the subsequent processing of pro-IL-1β is dependent on the endocytic pathway and ROS production (37). Additionally, in an in vivo silicosis model, Nlrp3- and Asc-deficient mice showed decreased pulmonary pathology to inhaled crystalline silica (38). It was also shown that lysosomal destabilization might be a common trigger for NLRP3 activation as in the case of silica, aluminum salts and other lysosomal damaging agents (39).
Pathogens that can gain access to the cytosol and their associated PAMPs can also activate NLRP3. Several bacteria expressing a type III secretion system (T3SS) were shown to activate NLRC4 (see below), however, certain bacteria are able to engage NLRP3 directly and multiple NLRs in a redundant or cooperative fashion. The exact mechanism of how these various pathogens act is not yet clear, however, destabilization of the ion-milieu of various cellular compartments by pore-forming bacterial toxins seems to be a common theme. Studies in a human monocyte-like cell line and mouse macrophages primed with S. aureus-secreted lipoproteins showed recombinant hemolysins (exotoxins produced from Staphylococcus aureus) to produce pro-inflammatory cytokines (IL-1β and IL-18) in an NLRP3-, ASC- and caspase-1-dependent manner (40,41). Mycobacterium tuberculosis causes tuberculosis that affects about one-third of the world population and kills 1.7 million people every year (42). M. tuberculosis has the ability to induce the exocytosis of lysosomes by its microbial toxins and secretion systems, which corresponded to increased release of IL-1β and IL-18 in an NLRP3 inflammasome dependent manner (43). In an elegant RNA interference screen, multiple NLRs were identified to be involved in recognizing various elements of M. tuberculosis infection and NLRP3 was found to be the main constituent for IL-1β-secretion (44). Granuloma formation in the lungs is an important mechanism for arresting chronic M. tuberculosis growth. While TLRs and NLRs are required for the initial response against the bacteria, antigen-presenting T-cells of the adaptive immune system initiate secondary invasion of multiple cell types that results in granuloma formation. Interestingly, in mouse models, Asc but not Nlrp3 or caspase-1, was indispensible for containing bacteria in granulomas and promoting survival (45). For Salmonella typhimurium infection, both NLRC4 and NLRP3 were implicated in IL-1β production at specific ASC-containing cytosolic loci, but while NLRC4 responds to flagellin, NLRP3 probably senses the bacteria in its quiescent state during the systemic phase of the infection (46). Listeria monocytogenes was found to activate the inflammasomes in a similar fashion (47). Recent findings have indicated that not only NLRP3, but also AIM2 and other NLRs are involved in IL-1β production during L. monocytogenes-infection (48). Clostridium difficile can invade the intestines after antibiotic treatment and cause nosocomial diarrhea and colitis and although its associated toxins (TcdA and TcdB) are not pore-forming molecules, they show partial phenotypes in mouse macrophages deficient for various NLRs. Furthermore, toxin-induced cell death was fully dependent on Asc (49). Fungal pathogens can also induce IL-1β secretion, which is indispensible for host survival. PAMPs from several species are detected by TLRs and CLRs, which turn on the canonical or Syk-mediated NF-κB-signaling pathway for the transcription of pro-IL-1β and Candida ablicans activates NLRP3-mediated IL-1β-cleavage via ROS production and K+ efflux (50,51).
The NLRP3 inflammasome has also been implicated in the recognition of viral RNA and DNA. Nlrp3-deficient mice showed reduced survival in influenza A infection, and Nlrp3 inflammasome components were important for pro-inflammatory cytokine production in mouse models (52). However, a recent publication indicated that influenza A RNA is sensed by TLR7 to initiate transcription of pro-IL-1β and NLRP3, and the ion-channel activity of the viral M2 protein, via acidification of the Golgi apparatus, is responsible for activation of the NLRP3 inflammasome (53). In light of these findings, the endosomal and cytosolic RNA and DNA receptors are more likely to play a direct role in recognition of viruses by the innate immune system.
Recently it was also shown that certain antibiotics (polymyxin B, gramicidin, tyrothricin and neomycin) could trigger Nlrp3-dependent IL-1β-secretion in mouse BMDMs and this was dependent on K+ efflux, but independent of the P2X7 receptor (54).
Genetic disorders
Diseases associated with IL-1β production are collectively called inflammasomopathies. These can be intrinsic, where the changes happen on the proteins that are part of the inflammasome itself, or alternatively, where the change affects proteins upstream or downstream of the IL-1β activation platform (55). NLRP3 was previously named cryopyrin when it was found to be associated with cold-induced fevers in patients and to contain a pyrin domain, and now a range of genetic disorders in the NLRP3 gene are called cryopyrin-associated periodic syndromes (CAPS) or cryopyrinopathies. These dominant or de novo mutations exhibit three illnesses with a broad spectrum of varying severity: familial cold autoinflammatory syndrome (FCAS), Muckle-Wells syndrome (MWS) and chronic infantile neurologic cutaneous and articular (CINCA) syndrome or neonatal-onset multisystem inflammatory disease (NOMID). FCAS is the mildest form of the disease and presents with cold-induced fevers and rash. MWS patients show symptoms of fevers, rashes and hives, hearing loss and arthritis. The most severe form, CINCA/NOMID has a neonatal or early onset with fevers, skin lesions, chronic meningitis, joint inflammations, hearing loss and overgrowth of the epiphysis in the long bones (56).
The identified mutations that occur in protein-coding regions are mostly in the NACHT-domain and a few appear in the LRR-domain of NLRP3 (57). These mutations cluster around several loci on the NLRP3 gene, one of which is next to the conserved ATPase motif, but there isn’t any clear correlation between sequence location of the mutation and disease severity. Interestingly, ATP was no longer required for inflammasome activation in monocytes isolated from CAPS patients (58) or in mutant Nlrp3 knock-in mice (59,60); however, when both gain-of-function (R260W or A439V) and ATPase (Walker A) mutations were present, IL-1β release was completely abrogated in reconstituted human monocytes (23). Based on crystal-structure homology models, it is hypothesized that the mutation clusters are located at a solvent-exposed surface of the NACHT-domain and destabilize the potential inactive closed conformation of the protein, thus, keeping it constitutively in its active form. Similarly, mutations in the hinge-region between the NACHT- and LRR-domain, or in the LRR-region itself (e.g. G755A/R), may render the protein more prone to change to an open conformation (61).
As inflammasomopathies are primarily linked to increased IL-1β production, therapies that target IL-1 receptor or IL-1β itself became the primary approach. Anakinra, an IL-1 receptor antagonist was shown to be effective for reducing symptoms even in the more severe disease phenotypes of NOMID patients (62). Recently, two anti-IL-1β therapies have been evaluated (rilonacept (63) and canakinumab (64)), and their efficiency and better pharmacology holds great promise for future therapies in CAPS patients. However, there are still several open questions in the understanding of disease propagation and treatment procedures (65).
Mechanisms of action
In the last decade following the discovery of NLRP3, a wealth of information has been generated towards understanding the mechanisms involved in activation and regulation of the NLRP3 inflammasome. Even though the exact mechanisms are still unknown, there are a few hypotheses that illuminate parts of the processes. In general, at least two signals are required for a successful activation of the NLRP3 inflammasome. In resting macrophages the protein levels of NLRP3 are too low to support the activation of an inflammasome. Signal one is acting to transcriptionally induce NLRP3 to sufficient levels for its activation and this signal can be provided by innate immune receptors, cytokine receptors or other factors that act in ‘priming’ of macrophages (66,67). At the same time the substrate for caspase-1, that is, IL-1β is also transcriptionally induced awaiting its cleavage after inflammasome activation. The second step of activation is much less clear. Crystalline substances can induce lysosomal damage leading to the translocation of the proteolytically active lysosomal content into the cytosol. Conceivably, this could lead to the cleavage of an as of yet unknown substrate involved in the activation of the NLRP3 inflammasome (28,36,39). It was also proposed that NADPH oxidase driven reactive oxygen species (ROS) production is involved in crystal mediated NLRP3 activation. Since crystal uptake can lead to both lysosomal leakage and ROS, a separation of these processes is not possible and both effects may in fact be necessary for the activation of the NLRP3 inflammasome as suggested by studies using pharmacological inhibitors (reviewed in (68,69)).
Recently, the source of ROS involved in the activation of the NLRP3 inflammasome was challenged as it was shown to be mitochondria rather than a NADPH oxidase (70–72). ROS appear to be important for IL-1b production, but it has yet to be established whether they play a direct, or indirect role in the activation of the NLRP3 inflammasome. It has been shown that ROS act upstream of the induction of cytokines (73) and thus, it would appear possible that ROS influence the required priming step of the NLRP3 inflammasome. Moreover, caspase-1 can be covalently modified and thereby functionally inactivated under conditions of enhanced oxidative challenge which seemingly would argue that ROS decrease the production of active IL-1b cytokines (74). A potential explanation for this apparent paradox was recently provided by the description that ROS activity may in fact be required to initiate an antioxidative response, which may be important to counteract caspase-1 inhibition (75,76).
It seems that the activation of the NLRP3 inflammasome is very complex and potentially requires multiple signals that appear in a coordinated fashion for full activation. Given the dramatic inflammatory consequences of inflammasome activation for the host, it is not surprising that this process is well controlled and complex in nature.
Regulation
NLRP3 is tightly regulated at the transcriptional level and also in a tissue-specific manner. Most tissues do not express NLRP3 at the RNA level, except peripheral blood leukocytes, among which, monocytes express the highest levels, and granulocytes and T-cells show detectable expression levels (77). In a more comprehensive screen, organ- and tissue-specific mRNA levels of various pattern recognition receptors were compared in human and mouse, and NLRP3 expression was also found in the brain (from microglia), lungs and testis (78).
The induction of NLRP3 is also regulated by CARD8 as it inhibits NF-κB induction by binding to the regulatory subunit of the IκB kinase complex, NEMO (6) and it also regulates caspase-1 activation (79). A heterozygous polymorphism (C10X) in CARD8 is a common variation (34%) in Caucasian populations, and the resulting truncated protein appears to be important for disease phenotype in an otherwise non-symptomatic NLRP3 polymorphism (Q705K); however, its role in inflammatory bowel disease is highly debated (80). A separate analysis showed that functional CARD8 protein is present, even in people with homozygous C10X mutation due to a splice variant where the nucleotide change does not result in truncated protein (81).
NLRP10 (PYNOD) is an NLR protein that is missing the LRR-domain and is widely expressed in many tissues and organs (82). It was shown to inhibit pro-caspase-1 and pro-IL-1β cleavage via its NACHT-domain in reconstitution assays, and its PYD-domain inhibited NLRP3- and ASC-mediated IL-1β-release and ASC speck-formation in vivo (83). Additionally, NLRP7 (PYPAF3) is also a widely expressed NF-κB inducible protein, which was able to inhibit NLRP3-mediated IL-1β secretion (84).
Recently, NLRC5 has been shown to be indispensible for IL-1β and IL-18 processing in RNA interference experiments and NLRC5 forms inflammasomes either with itself or cooperatively with NLRP3 (85).
NLRC4
NLRC4 (also known as IPAF, CARD12, and CLAN) was originally described in 2001 (86–88). It is the only known member of the NLRC family to form an inflammasome.
Microbial sensing
NLRC4 has been implicated in the sensing of a number of bacterial pathogens via flagellin recognition. The flagellin protein is a major component of bacterial flagella. Salmonella typhimurium was one of the first bacterial species demonstrated to activate an NLRC4-dependent inflammasome response (21,89). Interestingly, only caspase-1−/− and not Nlrc4−/− mice, showed enhanced susceptibility to oral infection by S. typhimurium (90). This seems to indicate redundant pathways for the detection of S. typhimurium. Purified S. typhimurium flagellin has since been shown to induce NLRC4-dependent, TLR5-independent inflammasome activation when delivered directly to the cytosol (91,92). Furthermore, S. typhimurium mutants lacking fliC and fliB, the genes that encode flagellin monomers, are unable to induce caspase-1 autoproteolysis, IL-1β secretion, and pyroptosis; thus, flagellin is essential for S. typhimurium-induced inflammasome activation via NLRC4.
Pseudomonas aeruginosa also activates caspase-1 processing, IL-1β and IL-18 secretion, and cell death in a NLRC4- and flagellin-dependent manner (93,94). However, unlike with S. typhimurium, mice lacking functional Nlrc4 were significantly more susceptible to P. aeruginosa peritonitis, as measured by increased IL-1β and IL-18 secretion and bacterial loads in the nose and spleen (95).
Legionella pneumophila, the cause of Legionnaire’s disease, has also been shown to be sensed by the NLRC4 inflammasome, and the resulting caspase-1 cleavage occurs in a flagellin-dependent manner (96–98). NLRC4-dependent caspase-1 activation is implicated in restriction of L. pneumophila growth. BMDMs from Nlrc4−/− and caspase-1−/− mice failed to arrest L. pneumophila replication by allowing Legionella-containing vacuole to avoid fusion with lysosomes (98). Subsequent experiments showed that caspase-7 is actually required for restriction of intracellular replication in mice (99). Similarly, L. pneumophila with mutated or removed fla, the gene responsible for encoding flagellin, was able to replicate in normally restrictive mouse B6 macrophages. Unlike in mice, L. pneumophila is able to replicate in human macrophages and lung epithelial cells, leading to a severe pneumonia known as Legionnaire’s disease. However, NLRC4 does still play a role in inhibiting bacterial growth in humans, as siRNA silencing of NLRC4 in human THP-1 macrophages allows enhanced bacterial growth, while overexpression of the gene restricts growth (100). As in mouse macrophages, flagellin-deficient L. pneumophila replicate more efficiently in human macrophages and epithelial cells. It appears that NLRC4 plays the same role in restricting L. pneumophila in both mice and humans; however, the human cells allow a much higher baseline replication rate than mouse cells.
L. pneumophila is unique among these three bacterial species in that Naip5, as well as Nlrc4, are required to form an immune response in mice. Naip5 (also known as Birc1) was originally described in 2003 as the component required for resistance to L. pneumophila, and L. pneumophila replication in A/J mice was pinpointed to variations in the Naip5 gene at the Lgn locus (101,102). When L. pneumophila flagellin was demonstrated to activate the inflammasome in a Nlrc4-dependent manner, flagellin recognition was also shown to be Naip5-dependent (96,97,103). NAIP, the closest Naip5 homolog in humans, was also shown to be essential for L. pneumophila flagellin recognition (100). Naip5 was not strictly necessary for flagellin-induced Nlrc4 activation in S. typhimurium or P. aeruginosa infection (104). This differential requirement for Naip5 will be further examined below.
The intracellular pathogen Listeria monocytogenes is also sensed by the NLRC4 inflammasome. L. monocytogenes pathogenesis includes escape from the macrophage phagosome into the cytosol, where it replicates (105). Once in the cytosol, L. monocytogenes activates caspase-1 processing and IL-1β secretion through NLRC4 in a flagellin-dependent manner, and detection of flagellin resulted in bacterial clearance (47). Naip5-deficiency does not appear to greatly inhibit the detection of L. monocytogenes (94). Although the NLRC4 inflammasome was activated upon artificial delivery of purified flagellin to the cytosol and in L. monocytogenes infection, a functional type III (S. typhimurium, P. aeruginosa) or type IV (L. pneumophila) secretion system was also necessary for flagellin-mediated caspase-1 cleavage to other bacteria (91,92,94,96–98). The common consensus is that these secretion systems are responsible for mistakenly secreting small amounts of flagellin into the cytosol to be recognized by NLRC4, and this was later demonstrated to occur in the S. typhimurium T3SS (106). Legionella parisensis and Legionella tusconensis do not induce pyroptosis or IL-1β secretion in mouse macrophages, however, purified flagellin from these two species does induce pyroptosis and IL-1β secretion when delivered directly to the cytosol (107). These two species lack type IV secretion system-dependent characteristics, so it appears that lack of functional secretion prevents flagellin delivery to the cytosol and subsequent NLRC4 inflammasome activation. Thus, it appears that NLRC4 detection of flagellin can either indicate an intracellular pathogen is in the cytosol, or that an active type III or IV secretion system is present. Some bacterial species are able to activate NLRC4 in a flagellin-independent manner due to the direct detection of the T3SS, such as Shigella flexneri, the bacteria responsible for bacillary dysentery. It had been shown previously that S. flexneri infection of caspase-1−/− mice does not result in inflammation and resolution of bacterial infection, due primarily to a failure to secret IL-18 (108). THP-1 cells that overexpressed NLRC4 showed an increase in IL-1β secretion in response to S. flexneri (89), but it was not until 2007 that the work of Suzuki and colleagues demonstrated that NLRC4-dependent recognition of S. flexneri was flagellin-independent but T3SS-dependent (109). Interestingly, they found that knockout of caspase-1 or Nlrc4 greatly increased autophagy in S. flexneri-infected macrophages. Another group found caspase-1 promoted pyroptosis to be dependent on the T3SS-effector protein IpaB (110).
In addition to the previously described flagellin-dependent pathway, S. typhimurium and P. aeruginosa can also be detected via an NLRC4-dependent but flagellin-independent pathway. Although S. typhimurium activates NLRC4 primarily via flagellin, a flagellin-deficient strain of S. typhimurium was able to induce small amounts of T3SS-dependent IL-1β secretion at a high multiplicity of infection (MOI) (91). Similarly, WT mouse macrophages infected with a flagellin-deficient mutant of P. aeruginosa secreted IL-1β in a T3SS-dependent manner, however, not to the extent of that seen in macrophages infected with a flagellin-expressing strain (95). This T3SS-dependent, flagellin-independent activation of NLRC4 was later observed to be the result of NLRC4 detection of the T3SS basal body rod protein (111). Although the rod proteins sensed by NLRC4 are slightly different for S. flexneri (Mxil), S. typhimurium (PrgJ) and P. aeruginosa (PscI), they share a sequence motif that is required for detection by NLRC4, and this motif is related to the flagellin motif also sensed by NLRC4. In a subsequent review, the authors hypothesize that these rod proteins may reach the cytosol through accidental translocation or sloughing off during effector translocation (112). Thus, it is possible that L. pneumophila can only be recognized by the flagellin-dependent pathway because it lacks a T3SS.
Mechanism of action
Like NLRP1 and NLRP3, NLRC4 self-oligomerizes via homotypic interactions of its NACHT domain (113). It contains a CARD domain and is able to associate with pro-caspase-1 directly through CARD-CARD interactions (86–88), and it can also associate directly with ASC in the same manner (87).
The adaptor molecule ASC is required for NLRP3 activation of caspase-1 because it lacks a CARD domain, but NLRC4 is able to interact with pro-caspase-1 directly, casting doubt on the usefulness of ASC. In response to Nlrc4 stimulation, Asc−/− mouse macrophages generally cannot initiate caspase-1 autoproteolysis and they secret significantly less IL-1β and IL-18, however, they remain capable of undergoing pyroptosis at similar levels to Asc-expressing macrophages (93,95,109,114). Interestingly, L. pneumophila infection appears to inhibit Asc expression, thus inhibiting caspase-1 processing and allowing bacterial replication (115). A recent paper demonstrated that Nlrc4-dependent cell death and cytokine processing are actually mediated by different inflammasomes (116). In the Asc-containing inflammasome, a large Asc speck is formed, which then leads to the autoproteolysis of caspase-1 and IL-1β/-18 secretion. In the absence of Asc, no large inflammasome structure is formed and caspase-1 fails to undergo autoproteolysis; however, caspase-1 is still activated and can induce pyroptosis. This appears to answer the question of the role of ASC in NLRC4-mediated inflammasome activation.
As previously mentioned, Naip5 has been shown to play an essential role in Nlrc4-dependent recognition of L. pneumophila. NAIP and NLRC4 are grouped in the same clade within the NLR protein family based on their functional similarities (117). Naip5 and Nlrc4 do physically interact with each other (103). Naip5 was shown to be essential for the recognition of the 35 amino acids at the C-terminus of both L. pneumophila and S. typhimurium flagellin (104), and the same sequence is also necessary to activate Nlrc4. S. typhimurium- and P. aeruginosa-induced cell death is partially Naip5 dependent, but strictly Nlrc4 dependent. When L. pneumophila is forced to express the flagellin of S. typhimurium, detection of this strain is still dependent on Naip5 (118). This seems to indicate that it is the bacteria, not the flagellin, that results in differential Naip5 requirements for sensing. However, PrgJ activates Nlrc4 in a Naip5-independent manner. Lightfield and colleagues proposed a specificity model in which Naip5 is responsible for recognizing flagellin, while the Naip5-independent Nlrc4 activation is due to direct recognition of the T3SS.
Regulation
NLRC4 is expressed predominately in hematopoietic tissues (86), however, it is also expressed in the colon epithelia (119). The leucine-rich-repeat (LRR) domain appears to auto-repress NLRC4 activation in the absence of a ligand since NLRC4 protein lacking an LRR domain is constitutively active (86). The chaperone molecules SGT1 and HSP90 associate with NLRC4 and are essential for IL-1β processing (120). It had previously been reported that increased levels of extracellular K+ ions do not inhibit NLRC4 inflammasome formation (121). A recent study showed that high extracellular K+ levels inhibited activation of the NLRC4 inflammasome to non-flagellated P. aeruginosa and S. typhimurium (122), however, the K+ levels were higher than those required to inhibit the NLRP3 inflammasome and that might actually inhibit other processes, for example bacterial uptake. Interestingly, mouse CD4+ effector and memory T cells suppress Nlrp1 and Nlrp3 signaling, but do not suppress the Nlrc4 inflammasome (123). As mentioned earlier, inhibition of caspase-1 has been shown to upregulate autophagy in macrophages infected with S. flexneri, while induction of autophagy lead to pyroptosis resistance (124). A recent review by Bortoluci and Medzhitov (125) proposes a mechanism in which inflammasomes and pyroptosis are used to clear pathogenic microorganisms, while autophagy is used for less dangerous microbes.
The NLRC4 inflammasome is also negatively regulated by multiple bacterial components. The Yersinia T3SS is partially recognized by NLRC4, but the effector protein YopK has been shown to interact with the T3SS translocon to inhibit its recognition (126). The P. aeruginosa PA103 strain does not stimulate caspase-1 cleavage or IL-1β secretion due to the phospholipase activity of the effector molecule ExoU (95).
Genetic disorders
NLRC4 and NAIP have been implicated in a few genetic disorders. NLRC4 and NAIP are two of the five genes upregulated in acute-phase Kawasaki disease, which is characterized by an increased activation of the immune system and upregulation of chemokines and cytokines (127). The disease is associated with vasculitis, damage to the coronary artery, and even heart attack and death. There is also evidence of a role for NLRC4 in atopic dermatitis, since a rare haplotype of the NLRC4 gene is more frequent in atopic dermatitis patients (128). Spinal muscular atrophy has been linked to a failure in regulating motor neuron apoptosis, and the gene for neuronal apoptosis inhibitory protein (NAIP) is truncated in these patients (129).
NLRC4 deficiency has also been shown to play a role in inflammation-induced colonic tumorigenesis (119). Caspase-1−/− mice demonstrate increased tumor formation in response to injury, and it was determined that caspase-1 was essential for regulation of colonic epithelial cell growth and death. NLRC4 is upregulated in colonic epithelial cells, and Nlrc4−/− mice also showed increases in colonic tumor formation and tumor load. Thus, it appears that NLRC4-mediated caspase-1 activation is essential for regulating colonic epithelial cell response to injury.
AIM2
Microbial sensing
It has been known for some time that introduction of double-stranded DNA (dsDNA) into the cytosol can cause cell death in a sequence-independent manner (130). Single-stranded DNA does not activate the immune system when delivered into the cytosol (131). Subsequent studies have shown that delivery of viral, bacterial, and mammalian dsDNA into the cell leads to ASC-dependent cleavage of caspase-1 and IL-1β secretion (132). The ASC-dependent nature demonstrated that there must be a receptor upstream that detects dsDNA, but no known NLRs were able to do so. Subsequently, four different groups identified AIM2 (absent in melanoma 2, PYHIN4) as the dsDNA receptor that was able to form an ASC-dependent inflammasome (133–136).
AIM2 has been demonstrated to play an essential role in the recognition of certain viruses by detecting viral DNA in the cytosol (135,137). Aim2-deficient mouse macrophages were impaired in their ability to recognize vaccinia virus and murine cytomegalovirus (mCMV) (136,137). Additionally, Aim2−/− mice infected with mCMV secreted significantly lower levels of IL-18 and had fewer IFN-γ-producing NK cells when compared to Aim2+/+ mice, thus leading to increased viral loads in the spleen. However, thioglycollate-elicited macrophages from Aim2−/− mice were not deficient in their response to herpes simplex virus type 1 (HSV-1) (137) and adenovirus recognition was dependent on the NLRP3 inflammasome (132), indicating that AIM2 is not able to recognize all DNA viruses. The inability of AIM2 to recognize these DNA viruses is most likely due to their infection and replication cycles, which seem to insulate viral DNA from the cytosol, and thus, from AIM2 entirely (138,139).
The highly infectious intracellular bacteria Francisella tularensis, the causative agent of tularemia, is also sensed by the AIM2 inflammasome (137,140). F. tularensis enters cells, primarily macrophages, through phagocytosis, but then quickly escapes the phagosome/endosome and replicates within the cytosol (141). It had been shown previously that caspase-1−/− and Asc−/− mice are significantly more susceptible to F. tularensis infection, however bacterial recognition was not Nlrc4-dependent (142). Following infection with F. tularensis, Aim2−/− mouse macrophages were deficient in caspase-1 cleavage, IL-1β secretion, and cell death, but not type I IFN secretion, and Aim2 and Asc were shown to colocalize with F. tularensis DNA in WT macrophages. Furthermore, Aim2−/− mice had higher mortality rates and bacterial loads following F. tularensis infection, demonstrating that Aim2 is essential for sensing F. tularensis infection (143,144). The F. tularensis gene mviN was shown to be important for limiting Aim2 inflammasome activation, as mviN mutant strains of F. tularensis induced increased IL-1β secretion and cell death, possibly due to increased lysis, in an Aim2-dependent manner (145).
As mentioned previously, L. monocytogenes are intracellular bacteria that are sensed at least partially, by NLRC4 and NLRP3. Studies using shRNA knockdown of Aim2 in mouse macrophages showed a decrease in IL-1β secretion and cell death as compared to a controlled shRNA upon challenge with L. monocytogenes (146–148), and similar decreases were shown in Aim2−/− mouse macrophages. AIM2-dependent inflammasome activation increased when macrophages were infected with a L. monocytogenes strain engineered to undergo higher levels of bacteriolysis (147). Also, L. monocytogenes DNA was shown to colocalize with ASC specs (148) and induce IL-18 in an AIM2-dependent manner (149). Wu and colleagues demonstrated that AIM2 is essential for detecting L. monocytogenes that do not express flagellin in pre-primed macrophages, however, NLRC4 is essential for detecting L. monocytogenes in unprimed cells (150). Therefore, it was speculated that NLRC4 recognition of L. monocytogenes flagellin is upstream of NLRP3 and AIM2 signaling.
Mechanism of action
AIM2 activates an inflammasome complex by first binding to DNA via its HIN-200 domain (134–136). Nine members of the HIN-200 (hematopoietic IFN-inducible nuclear proteins with 200-amino acids) family have been described: four in humans (IFI16, IFIX, MNDA, AIM2) and five in mice (p202, p203, p204, Mndal, Aim2) (151). All members contain DNA-binding HIN-200 domains, but only AIM2 and p202 locate to the cytosol (133,135,152). AIM2 binds preferentially to dsDNA over ssDNA or RNA, and this binding can be diminished by a F165A point mutation in the HIN-200 domain. Recognition of dsDNA is non-specific, since bacterial, viral, or self-DNA can activate AIM2. Interestingly, the high-mobility group box (HMGB) proteins, which have been shown to bind to all nucleic acids, may also be necessary for AIM2 to detect DNA (153). AIM2 appears to self-oligomerize through the binding of AIM2 molecules to multiple sites on the dsDNA molecule. Once AIM2 binds dsDNA, it associates with ASC via PYD-PYD homotypic interactions, which then catalyzes the cleavage of caspase-1. Although all members of the HIN-200 family, except p202, contain a PYD domain, only the PYD domain of AIM2 can interact with the PYD domain of ASC.
Regulation
Human AIM2 is expressed constitutively in the small intestine, spleen, and peripheral blood leukocytes (154). AIM2 is also an interferon-inducible gene, as demonstrated by the fact that growth of HL60 cells in the presence of IFN-γ induces AIM2 expression. Treatment of human THP-1 cells with IFN-β can also upregulate AIM2 expression (134). Induction of Aim2 can also affect Aim2 inflammasome responses in vitro, as it is upregulated in mouse BMDMs in a type I IFN-dependent manner following infection with F. tularensis, and failure to upregulate Aim2 expression resulted in decreased IL-1β secretion and cell death (144). Similarly, type I IFN signaling is important to form an inflammasome response in mouse macrophages infected with the cytosolic bacteria F. tularensis subspecies novicida and L. monocytogenes, however, is not important for infection with vacuole-located S. typhimurium (155), providing additional evidence for the importance of type I IFN in AIM2 inflammasome formation.
Other members of the HIN-200 family may also regulate AIM2. The p202 protein was able to bind dsDNA in the cytosol and siRNA knockdown of p202 induced caspase-1 cleavage in BMDM challenged with poly(dA):(dT) (133). Furthermore, p202 can heterodimerize with Aim2 (152) and inhibit Aim2 from clustering with Asc, so it appears that p202 can negatively regulate Aim2 responses by sequestering DNA or preventing Aim2-Asc oligomerization. IFI16 is the only human HIN-200 protein shown to heterodimerize with AIM2 (156), however it has not been shown to antagonize AIM2 inflammasome activation, most likely since it is predominately found in the nucleus.
Genetic disorders
Since AIM2 has been shown to recognize self-DNA in the cytosol, it has been postulated that it plays a role in systemic lupus erythematous (SLE). The chromosomal location of the HIN-200 genes falls in a lupus susceptibility locus for both humans and mice (157). Additionally, mRNA transcripts for IL-1β were elevated in many SLE patients when compared to controls, indicating possible AIM2 inflammasome activation (158). Conversely, a recent study showed the gene for p202, Ifi202, was upregulated in Aim2−/− mouse macrophages, and lupus-susceptible mice expressed reduced levels of Aim2, but elevated levels of p202, when compared to healthy controls (159). It was speculated that this imbalance could inhibit Aim2 inflammasome-mediated cell death, allowing cells with cytosolic DNA to survive longer and secrete more type I IFNs, leading to lupus. The same group subsequently discovered that increased expression of p202 as a result of Aim2-deficiency suppressed the expression of the Fcgr2b (FcγRIIB receptor) gene, of which deficient expression is linked to lupus susceptibility (160). The results of these studies seem to indicate a role for Aim2 deficiency in susceptibility to lupus through its effect on p202 expression. However, humans lack the p202 protein, so it is unclear how these results translate to human SLE.
AIM2 has also been linked to psoriasis. Keratinocytes of psoriatic lesions have increased levels of both dsDNA and AIM2, and AIM2 can form an inflammasome and release IL-1β in response to cytosolic DNA in cultured keratinocytes (161). The antimicrobial peptide LL-37 can reduce AIM2-dependent inflammasome activation in keratinocytes by binding to dsDNA. Thus, AIM2 appears to play a role in some autoinflammatory diseases.
Other Inflammasomes
Although the four inflammasomes previously discussed are by far the most studied ones, other NLRP proteins have recently been implicated in inflammasome formation. Here, we review the NLRP6 and NLRP12 inflammasomes.
NLRP6
It had been demonstrated that NLRP6 (also known as PYPAF5, NALP6) and ASC colocalize and induce caspase-1 dependent IL-1β secretion when co-expressed in COS-7L cells (162). Recently, three different groups have elucidated the role of Nlrp6 in a mouse model of colitis. Nlrp6−/− mice given dextran sodium sulfate (DSS) in their drinking water had exacerbated colitis when compared to WT mice (163), and this lead to increased tumorigenesis in the colon (164,165). Interestingly, Nlrp6−/− mice have an altered gut microbiota, which together with the exacerbated colitis phenotype, can be transferred to cohoused WT mice and this appears to be due to the lack of inflammasome-induced IL-18 secretion by Nlrp6−/− mice (163). Nlrp6 also appears to regulate epithelial cell proliferation and repair (164,165). Two independent studies have shown conflicting results as to whether Nlrp6 expression in the nonhematopoietic or hematopoietic compartment of the intestine is responsible for protection; one group found that the decreased colonic epithelial IL-18 production in Nlrp6−/− mice was responsible for enhanced colitogenic microbiota in DSS-induced colitis (163), while the other group assessed that Nlrp6 in hematopoietic cells is essential for protection against colitis-associated tumorigenesis (164). Nevertheless, NLRP6 appears to be essential for preventing recurring colitis, and it is speculated that NLRP6-deficiency may contribute to IBD.
NLRP12
Like NLRP6, NLRP12 (PYPAF7, Monarch-1, NALP12) has also been shown to colocalize with ASC and induce caspase-1 dependent IL-1β secretion when co-expressed in COS-7L cells (166). However, NLRP12 was also shown to inhibit non-canonical activation of NF-κB in human monocytes (167). When two non-ambiguous mutations in the NLRP12 gene were found in three patients with CAPS symptoms but without NLRP3 mutation, it was observed that both mutations decreased NF-κB inhibition in vitro (168). It was later demonstrated that PBMCs from two of these patients with a nonsense mutation in the NACHT-domain spontaneously secreted dramatically more IL-1β than control cells, implying that this mutation results in constitutive NLRP12 inflammasome activation (169). These patients initially responded well to anakinra, with clinical improvement and lowering of IL-1β to near normal levels, however, they developed resistance in less than a year. A missense mutation (D294E) in the NACHT-domain of NLRP12 was found in multiple members of one family presenting with sensitivity to cold exposure and this mutation accelerated the kinetics of PAMP-induced IL-1β secretion in vitro (170). Two unrelated patients with genetically unexplained CAPS symptoms were found with a different missense mutation (R352C) in the NACHT-domain and this mutation had no effect on NF-κB inhibition, but increased ASC speck formation and caspase-1 cleavage (171). Thus, the term NLRP12-associated disorders (NLRP12AD) was proposed for these diseases.
Conclusion
We have experienced a dramatic increase in our knowledge of how inflammasomes are formed and regulated, how they influence the production of key pro-inflammatory cytokines and what roles they play in infection control and tissue inflammation. While the gain in information has been rapid and has opened great promises for potential therapeutic manipulation of inflammatory conditions, many open questions remain. Most importantly, the exact molecular mechanisms of activation for most inflammasomes are still unclear. We will, undoubtedly, learn about many more activators of inflammasomes and their involvement in disease pathology and it is expected that this research will generate exciting novel future therapies needed for a variety of inflammatory pathologies.
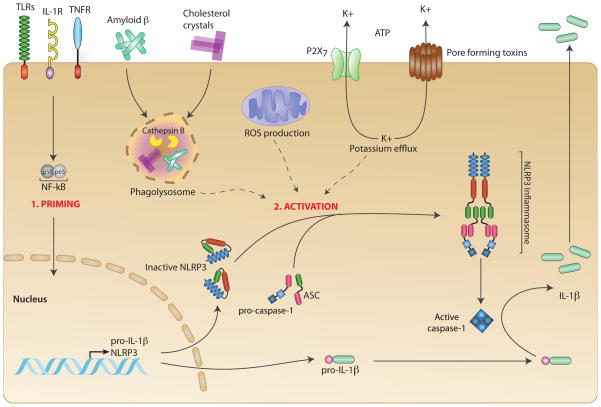
Figure 2. The NLRP3 inflammasome.
Successful NLRP3 inflammasome formation requires multiple steps. In a priming step, transcriptionally active signaling receptors, such as PRRs or cytokine receptors, first induce NF-κB-dependent induction of NLRP3 itself as well as the caspase-1 substrates of the pro-IL-1β family. NLRP3 is thought to exist in a signaling incompetent conformation, which changes upon activity of a second signal, leading to the assembly of a multimolecular inflammasome complex together with the proteins ASC and caspase-1. Multiple signals which are potentially provided in combination, such as lysosome-derived molecules, reactive oxygen species (ROS) and potassium efflux can trigger the formation of an active inflammasome, which, in turn, leads to the cleavage and release of bioactive IL-1β cytokines.
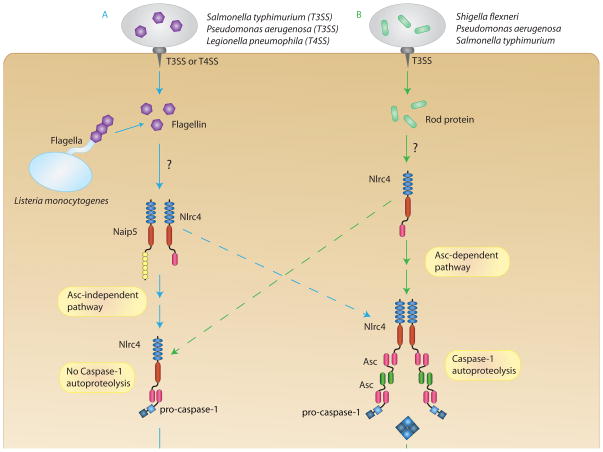
Figure 3. Bacterial recognition by the Nlrc4 inflammasome.
The Nlrc4 inflammasome is able to recognize Gram-negative bacteria in two distinct ways. (A) Cytosolic flagellin can activate Nlrc4 in a Naip5-dependent manner. Flagellin from cytosolic L. monocytogenes can activate Nlrc4 directly, however, flagellin-dependent activation by S. typhimurium, P. aeruginosa, and L. pneumophila requires a type III or type IV secretion system to secrete flagellin into the cytosol. (B) Nlrc4 can recognize the rod protein from the type III secretion system of S. flexneri, P. aeruginosa, and S. typhimurium directly in a Naip5-independent manner. Once activated by either ligand, Nlrc4 can recruit pro-caspase-1 directly through CARD-CARD interactions (Asc-independent pathway) or recruit Asc and pro-caspase-1 (Asc-dependent pathway). In the Asc-independent pathway, pro-caspase-1 does not undergo autoproteolysis, but it is adequately activated to promote cell death. In the Asc-dependent pathway, Asc is able to form a large speck through CARD-CARD and PYD-PYD interactions, and pro-caspase-1 can undergo autoproteolysis. Fully cleaved caspase-1 can then process IL-1β and IL-18 for secretion.
Table 1. Inflammasome activators and associated diseases.
Inflammasomes, their stimuli and potentially associated diseases are listed.
| Inflammasome | Stimulus | Diseases |
|---|---|---|
| NLRP1 | Lethal toxin from B. anthracis muramyl dipeptide (MDP) |
vitiligo |
| NLRP3 | bacterial pore-forming toxins ion channels crystaline and fibrous material protein aggregates |
cryopyrinopathies (CAPS): FCAS MWS CINCA/NOMID |
| NLRC4 | flagellin bacterial type III secretion system |
Kawasaki disease, atopic dermatitis, IBD? |
| AIM2 | double-stranded DNA | Psoriasis, SLE? |
| NLRP6 | unknown | IBD? |
| NLRP12 | unknown | NLRP12AD |
References
- 1.Kawai T, Akira S. Toll-like Receptors and Their Crosstalk with Other Innate Receptors in Infection and Immunity. Immunity. 2011 May 27;34(5):637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010 May 1;11(5):373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 3.Chen GY, Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010 Dec 1;10(12):826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002 Aug 1;10(2):417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 5.Boyden ED, Dietrich WF. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nature Genetics. 2006 Jan 22;38(2):240–244. doi: 10.1038/ng1724. [DOI] [PubMed] [Google Scholar]
- 6.Bouchier-Hayes L. CARDINAL, a Novel Caspase Recruitment Domain Protein, Is an Inhibitor of Multiple NF-kappa B Activation Pathways. Journal of Biological Chemistry. 2001 Sep 10;276(47):44069–44077. doi: 10.1074/jbc.M107373200. [DOI] [PubMed] [Google Scholar]
- 7.Abrami L, Reig N, van der Goot FG. Anthrax toxin: the long and winding road that leads to the kill. Trends Microbiol. 2005 Feb;13(2):72–78. doi: 10.1016/j.tim.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Nour AM, Yeung YG, Santambrogio L, Boyden ED, Stanley ER, Brojatsch J. Anthrax Lethal Toxin Triggers the Formation of a Membrane-Associated Inflammasome Complex in Murine Macrophages. Infect Immun. 2009 Feb 16;77(3):1262–1271. doi: 10.1128/IAI.01032-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Averette KM, Pratt MR, Yang Y, Bassilian S, Whitelegge JP, Loo JA, et al. Anthrax lethal toxin induced lysosomal membrane permeabilization and cytosolic cathepsin release is Nlrp1b/Nalp1b-dependent. PLoS ONE. 2009;4(11):e7913. doi: 10.1371/journal.pone.0007913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liao KC, Mogridge J. Expression of Nlrp1b Inflammasome Components in Human Fibroblasts Confers Susceptibility to Anthrax Lethal Toxin. Infect Immun. 2009 Sep 19;77(10):4455–4462. doi: 10.1128/IAI.00276-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faustin B, Lartigue L, Bruey J, Luciano F, Sergienko E, Bailly-Maitre B, et al. Reconstituted NALP1 Inflammasome Reveals Two-Step Mechanism of Caspase-1 Activation. Mol Cell. 2007 Mar;25(5):713–724. doi: 10.1016/j.molcel.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 12.Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003 Mar 14;278(11):8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 13.Hsu L, Ali SR, McGillivray S, Tseng P, Mariathasan S, Humke EW, et al. A NOD2-NALP1 complex mediates caspase-1-dependent IL-1beta secretion in response to Bacillus anthracis infection and muramyl dipeptide. Proc Natl Acad Sci USA. 2008 Jun 3;105(22):7803–7808. doi: 10.1073/pnas.0802726105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin Y, Mailloux CM, Gowan K, Riccardi SL, LaBerge G, Bennett DC, et al. NALP1 in vitiligo-associated multiple autoimmune disease. N Engl J Med. 2007 Mar 22;356(12):1216–1225. doi: 10.1056/NEJMoa061592. [DOI] [PubMed] [Google Scholar]
- 15.Hlaing T, Guo RF, Dilley KA, Loussia JM, Morrish TA, Shi MM, et al. Molecular cloning and characterization of DEFCAP-L and -S, two isoforms of a novel member of the mammalian Ced-4 family of apoptosis proteins. J Biol Chem. 2001 Mar 23;276(12):9230–9238. doi: 10.1074/jbc.M009853200. [DOI] [PubMed] [Google Scholar]
- 16.Kummer JA, Broekhuizen R, Everett H, Agostini L, Kuijk L, Martinon F, et al. Inflammasome components NALP 1 and 3 show distinct but separate expression profiles in human tissues suggesting a site-specific role in the inflammatory response. Journal of Histochemistry and Cytochemistry. 2007 May 1;55(5):443–452. doi: 10.1369/jhc.6A7101.2006. [DOI] [PubMed] [Google Scholar]
- 17.Bruey J, Bruey-Sedano N, Luciano F, Zhai D, Balpai R, Xu C, et al. Bcl-2 and Bcl-XL Regulate Proinflammatory Caspase-1 Activation by Interaction with NALP1. Cell. 2007 Apr;129(1):45–56. doi: 10.1016/j.cell.2007.01.045. [DOI] [PubMed] [Google Scholar]
- 18.Faustin B, Chen Y, Zhai D, Le Negrate G, Lartigue L, Satterthwait A, et al. Mechanism of Bcl-2 and Bcl-X-L inhibition of NLRP1 inflammasome: Loop domain-dependent suppression of ATP binding and oligomerization. Proc Natl Acad Sci USA. 2009;106(10):3935–3940. doi: 10.1073/pnas.0809414106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gregory SM, Davis BK, West JA, Taxman DJ, Matsuzawa SI, Reed JC, et al. Discovery of a Viral NLR Homolog that Inhibits the Inflammasome. Science. 2011 Jan 20;331(6015):330–334. doi: 10.1126/science.1199478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palm NW, Medzhitov R. Pattern recognition receptors and control of adaptive immunity. Immunol Rev. 2009 Jan;227(1):221–233. doi: 10.1111/j.1600-065X.2008.00731.x. [DOI] [PubMed] [Google Scholar]
- 21.Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006 Jan 11;440(7081):228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 22.Ferrari D, Chiozzi P, Falzoni S, Dal Susino M, Melchiorri L, Baricordi OR, et al. Extracellular ATP triggers IL-1 beta release by activating the purinergic P2Z receptor of human macrophages. J Immunol. 1997 Aug 1;159(3):1451–1458. [PubMed] [Google Scholar]
- 23.Duncan JA, Bergstralh DT, Wang Y, Willingham SB, Ye Z, Zimmermann AG, et al. Cryopyrin/NALP3 binds ATP/dATP, is an ATPase, and requires ATP binding to mediate inflammatory signaling. Proc Natl Acad Sci USA. 2007 May 8;104(19):8041–8046. doi: 10.1073/pnas.0611496104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nuki G, Simkin PA. A concise history of gout and hyperuricemia and their treatment. Arthritis Res Ther. 2006;8( Suppl 1):S1. doi: 10.1186/ar1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beck C, Morbach H, Richl P, Stenzel M, Girschick HJ. How can calcium pyrophosphate crystals induce inflammation in hypophosphatasia or chronic inflammatory joint diseases? Rheumatol Int. 2008 Sep 28;29(3):229–238. doi: 10.1007/s00296-008-0710-9. [DOI] [PubMed] [Google Scholar]
- 26.Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006 Jan 11;440(7081):237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 27.Weiner HL, Frenkel D. Immunology and immunotherapy of Alzheimer’s disease. Nat Rev Immunol. 2006 May;6(5):404–416. doi: 10.1038/nri1843. [DOI] [PubMed] [Google Scholar]
- 28.Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-β. Nat Immunol. 2008 Jul 11;9(8):857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spranger J, Kroke A, Möhlig M, Hoffmann K, Bergmann MM, Ristow M, et al. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes. 2003 Mar;52(3):812–817. doi: 10.2337/diabetes.52.3.812. [DOI] [PubMed] [Google Scholar]
- 30.Bendtzen K, Mandrup-Poulsen T, Nerup J, Nielsen JH, Dinarello CA, Svenson M. Cytotoxicity of human pI 7 interleukin-1 for pancreatic islets of Langerhans. Science. 1986 Jun 20;232(4757):1545–1547. doi: 10.1126/science.3086977. [DOI] [PubMed] [Google Scholar]
- 31.Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, et al. Glucose-induced β cell production of IL-1β contributes to glucotoxicity in human pancreatic islets. J Clin Invest. 2002 Sep 15;110(6):851–860. doi: 10.1172/JCI15318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lorenzo A, Razzaboni B, Weir GC, Yankner BA. Pancreatic islet cell toxicity of amylin associated with type-2 diabetes mellitus. Nature. 1994 Apr 21;368(6473):756–760. doi: 10.1038/368756a0. [DOI] [PubMed] [Google Scholar]
- 33.Masters SL, Dunne A, Subramanian SL, Hull RL, Tannahill GM, Sharp FA, et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat Immunol. 2010 Oct 1;11(10):897–904. doi: 10.1038/ni.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011 Apr 10; doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.SMALL D. George Lyman Duff Memorial Lecture - Progression and Regression of Atherosclerotic Lesions - Insights From Lipid Physical Biochemistry. Arteriosclerosis. 1988;8(2):103–129. doi: 10.1161/01.atv.8.2.103. [DOI] [PubMed] [Google Scholar]
- 36.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010 Apr 29;464(7293):1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dostert C, Pétrilli V, van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008 May 2;320(5876):674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cassel SL, Eisenbarth SC, Iyer SS, Sadler JJ, Colegio OR, Tephly LA, et al. The Nalp3 inflammasome is essential for the development of silicosis. Proc Natl Acad Sci USA. 2008;105(26):9035–9040. doi: 10.1073/pnas.0803933105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008 Aug;9(8):847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Craven RR, Gao X, Allen IC, Gris D, Bubeck Wardenburg J, McElvania-TeKippe E, et al. Staphylococcus aureus alpha-hemolysin activates the NLRP3-inflammasome in human and mouse monocytic cells. PLoS ONE. 2009;4(10):e7446. doi: 10.1371/journal.pone.0007446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muñoz-Planillo R, Franchi L, Miller LS, Nunez G. A Critical Role for Hemolysins and Bacterial Lipoproteins in Staphylococcus aureus-Induced Activation of the Nlrp3 Inflammasome. The Journal of Immunology. 2009;183(6):3942–3948. doi: 10.4049/jimmunol.0900729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.CDC. Tuberculosis. Centers for Disease Control and Prevention; 2009. [Google Scholar]
- 43.Koo IC, Wang C, Raghavan S, Morisaki JH, Cox JS, Brown EJ. ESX-1-dependent cytolysis in lysosome secretion and inflammasome activation during mycobacterial infection. Cellular Microbiology. 2008 Sep;10(9):1866–1878. doi: 10.1111/j.1462-5822.2008.01177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mishra BB, Moura-Alves P, Sonawane A, Hacohen N, Griffiths G, Moita LF, et al. Mycobacterium tuberculosis protein ESAT-6 is a potent activator of the NLRP3/ASC inflammasome. Cellular Microbiology. 2010 Feb 9;12(8):1046–1063. doi: 10.1111/j.1462-5822.2010.01450.x. [DOI] [PubMed] [Google Scholar]
- 45.Tekippe EM, Allen IC, Hulseberg PD, Sullivan JT, McCann JR, Sandor M, et al. Granuloma Formation and Host Defense in Chronic Mycobacterium tuberculosis Infection Requires PYCARD/ASC but Not NLRP3 or Caspase-1. PLoS ONE. 2010 Aug 20;5(8):e12320. doi: 10.1371/journal.pone.0012320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Broz P, Newton K, Lamkanfi M, Mariathasan S, Dixit VM, Monack DM. Redundant roles for inflammasome receptors NLRP3 and NLRC4 in host defense against Salmonella. The Journal of experimental medicine. 2010;207(8):1745–1755. doi: 10.1084/jem.20100257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Warren SE, Mao DP, Rodriguez AE, Miao EA, Aderem A. Multiple Nod-like receptors activate caspase 1 during Listeria monocytogenes infection. J Immunol. 2008 Jun 1;180(11):7558–7564. doi: 10.4049/jimmunol.180.11.7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meixenberger K, Pache F, Eitel J, Schmeck B, Hippenstiel S, Slevogt H, et al. Listeria monocytogenes-infected human peripheral blood mononuclear cells produce IL-1beta, depending on listeriolysin O and NLRP3. The Journal of Immunology. 2010 Jan 15;184(2):922–930. doi: 10.4049/jimmunol.0901346. [DOI] [PubMed] [Google Scholar]
- 49.Ng J, Hirota SA, Gross O, Li Y, Ulke Lemee A, Potentier MS, et al. Clostridium difficile Toxin–Induced Inflammation and Intestinal Injury Are Mediated by the Inflammasome. Gastroenterology. 2010 Aug;139(2):542–552.e3. doi: 10.1053/j.gastro.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 50.Gross O, Poeck H, Bscheider M, Dostert C, Hannesschläger N, Endres S, et al. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009 Apr 1;459(7245):433–436. doi: 10.1038/nature07965. [DOI] [PubMed] [Google Scholar]
- 51.Hise AG, Tomalka J, Ganesan S, Patel K, Hall BA, Brown GD, et al. An Essential Role for the NLRP3 Inflammasome in Host Defense against the Human Fungal Pathogen Candida albicans. Cell Host Microbe. 2009 May;5(5):487–497. doi: 10.1016/j.chom.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Allen IC, Scull MA, Moore CB, Holl EK, McElvania-TeKippe E, Taxman DJ, et al. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009 Apr 17;30(4):556–565. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ichinohe T, Pang IK, Iwasaki A. Influenza virus activates inflammasomes via its intracellular M2 ion channel. Nat Immunol. 2010 Apr 11;11(5):404–410. doi: 10.1038/ni.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Allam R, Darisipudi MN, Rupanagudi KV, Lichtnekert J, Tschopp J, Anders HJ. Cutting Edge: Cyclic Polypeptide and Aminoglycoside Antibiotics Trigger IL-1 Secretion by Activating the NLRP3 Inflammasome. The Journal of Immunology. 2011 Feb 15;186(5):2714–2718. doi: 10.4049/jimmunol.1002657. [DOI] [PubMed] [Google Scholar]
- 55.Masters SL, Simon A, Aksentijevich I, Kastner DL. Horror Autoinflammaticus: The Molecular Pathophysiology of Autoinflammatory Disease *. Annu Rev Immunol. 2009 Apr;27(1):621–668. doi: 10.1146/annurev.immunol.25.022106.141627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Feldmann J, Prieur A, Quartier P, Berquin P, Certain S, Cortis E, et al. Chronic Infantile Neurological Cutaneous and Articular Syndrome Is Caused by Mutations in CIAS1, a Gene Highly Expressed in Polymorphonuclear Cells and Chondrocytes. The American Journal of Human Genetics. 2002 Jul;71(1):198–203. doi: 10.1086/341357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle|[ndash]|Wells syndrome. Nature Genetics. 2001 Nov 1;29(3):301–305. doi: 10.1038/ng756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gattorno M, Tassi S, Carta S, Delfino L, Ferlito F, Pelagatti MA, et al. Pattern of interleukin-1β secretion in response to lipopolysaccharide and ATP before and after interleukin-1 blockade in patients with CIAS1 mutations. Arthritis Rheum. 2007;56(9):3138–3148. doi: 10.1002/art.22842. [DOI] [PubMed] [Google Scholar]
- 59.Brydges SD, Mueller JL, McGeough MD, Pena CA, Misaghi A, Gandhi C, et al. Inflammasome-Mediated Disease Animal Models Reveal Roles for Innate but Not Adaptive Immunity. Immunity. 2009 Jun 19;30(6):875–887. doi: 10.1016/j.immuni.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meng G, Zhang F, Fuss I, Kitani A, Strober W. A Mutation in the Nlrp3 Gene Causing Inflammasome Hyperactivation Potentiates Th17 Cell-Dominant Immune Responses. Immunity. 2009 Jun 19;30(6):860–874. doi: 10.1016/j.immuni.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aksentijevich I, Putnam DC, Remmers EF, Mueller JL, Le J, Kolodner RD, et al. The clinical continuum of cryopyrinopathies: Novel CIAS1 mutations in North American patients and a new cryopyrin model. Arthritis Rheum. 2007;56(4):1273–1285. doi: 10.1002/art.22491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goldbach-Mansky R, Dailey NJ, Canna SW, Gelabert A, Jones J, Rubin BI, et al. Neonatal-Onset Multisystem Inflammatory Disease Responsive to Interleukin-1β Inhibition. N Engl J Med. 2006 Aug 10;355(6):581–592. doi: 10.1056/NEJMoa055137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hoffman HM, Yasothan U, Kirkpatrick P. Rilonacept. Nature reviews Drug discovery. 2008 May;7(5):385–386. [Google Scholar]
- 64.Lachmann HJ, Kone-Paut I, Kuemmerle-Deschner JB, Leslie KS, Hachulla E, Quartier P, et al. Use of canakinumab in the cryopyrin-associated periodic syndrome. N Engl J Med. 2009 Jun 4;360(23):2416–2425. doi: 10.1056/NEJMoa0810787. [DOI] [PubMed] [Google Scholar]
- 65.Goldbach-Mansky R. Current Status of Understanding the Pathogenesis and Management of Patients With NOMID/CINCA. Curr Rheumatol Rep. 2011 May 3; doi: 10.1007/s11926-011-0165-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009 Jul 15;183(2):787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Franchi L, Eigenbrod T, Nunez G. Cutting Edge: TNF-alpha Mediates Sensitization to ATP and Silica via the NLRP3 Inflammasome in the Absence of Microbial Stimulation. The Journal of Immunology. 2009;183(2):792–796. doi: 10.4049/jimmunol.0900173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tschopp J, Schroder K. NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 2010 Mar 1;10(3):210–215. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- 69.Morishige T, Yoshioka Y, Inakura H, Tanabe A, Yao X, Narimatsu S, et al. The effect of surface modification of amorphous silica particles on NLRP3 inflammasome mediated IL-1beta production, ROS production and endosomal rupture. Biomaterials. 2010 Sep;31(26):6833–6842. doi: 10.1016/j.biomaterials.2010.05.036. [DOI] [PubMed] [Google Scholar]
- 70.Sorbara MT, Girardin SE. Mitochondrial ROS fuel the inflammasome. Cell Res. 2011 Feb 1;21(4):558–560. doi: 10.1038/cr.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nakahira K, Haspel JA, Rathinam VAK, Lee S, Dolinay T, Lam HC, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2010 Dec 12;12(3):222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2010 Dec 1; doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 73.Bulua AC, Simon A, Maddipati R, Pelletier M, Park H, Kim K, et al. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS) Journal of Experimental Medicine. 2011 Mar 14;208(3):519–533. doi: 10.1084/jem.20102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meissner F, Molawi K, Zychlinsky A. Superoxide dismutase 1 regulates caspase-1 and endotoxic shock. Nat Immunol. 2008 Aug 1;9(8):866–872. doi: 10.1038/ni.1633. [DOI] [PubMed] [Google Scholar]
- 75.Tassi S, Carta S, Delfino L, Caorsi R, Martini A, Gattorno M, et al. Altered redox state of monocytes from cryopyrin-associated periodic syndromes causes accelerated IL-1beta secretion. Proc Natl Acad Sci USA. 2010 May 25;107(21):9789–9794. doi: 10.1073/pnas.1000779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Carta S, Tassi S, Pettinati I, Delfino L, Dinarello CA, Rubartelli A. The rate of IL-1{beta} secretion in different myeloid cells varies with the extent of redox response to Toll-like receptor triggering. Journal of Biological Chemistry. 2011 May 31; doi: 10.1074/jbc.M110.203398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Manji GA, Wang L, Geddes BJ, Brown M, Merriam S, Al-Garawi A, et al. PYPAF1, a PYRIN-containing Apaf1-like protein that assembles with ASC and regulates activation of NF-kappa B. J Biol Chem. 2002 Mar 29;277(13):11570–11575. doi: 10.1074/jbc.M112208200. [DOI] [PubMed] [Google Scholar]
- 78.Lech M, Avila-Ferrufino A, Skuginna V, Susanti HE, Anders HJ. Quantitative expression of RIG-like helicase, NOD-like receptor and inflammasome-related mRNAs in humans and mice. International Immunology. 2010 Aug 26;22(9):717–728. doi: 10.1093/intimm/dxq058. [DOI] [PubMed] [Google Scholar]
- 79.Razmara M, Srinivasula SM, Wang L, Poyet J, Geddes BJ, DiStefano PS, et al. CARD-8 protein, a new CARD family member that regulates caspase-1 activation and apoptosis. J Biol Chem. 2002 Apr 19;277(16):13952–13958. doi: 10.1074/jbc.M107811200. [DOI] [PubMed] [Google Scholar]
- 80.Verma D, Lerm M, Blomgran Julinder R, Eriksson P, Söderkvist P, Särndahl E. Gene polymorphisms in the NALP3 inflammasome are associated with interleukin-1 production and severe inflammation: Relation to common inflammatory diseases? Arthritis Rheum. 2008;58(3):888–894. doi: 10.1002/art.23286. [DOI] [PubMed] [Google Scholar]
- 81.Bagnall RD, Roberts RG, Mirza MM, Torigoe T, Prescott NJ, Mathew CG. Novel isoforms of the CARD8 (TUCAN) gene evade a nonsense mutation. European journal of human genetics : EJHG. 2008 Jan 23;16(5):619–625. doi: 10.1038/sj.ejhg.5201996. [DOI] [PubMed] [Google Scholar]
- 82.Wang Y, Hasegawa M, Imamura R, Kinoshita T, Kondo C, Konaka K, et al. PYNOD, a novel Apaf-1/CED4-like protein is an inhibitor of ASC and caspase-1. International Immunology. 2004 Jun 1;16(6):777–786. doi: 10.1093/intimm/dxh081. [DOI] [PubMed] [Google Scholar]
- 83.Imamura R, Wang Y, Kinoshita T, Suzuki M, Noda T, Sagara J, et al. Anti-inflammatory activity of PYNOD and its mechanism in humans and mice. The Journal of Immunology. 2010 May 15;184(10):5874–5884. doi: 10.4049/jimmunol.0900779. [DOI] [PubMed] [Google Scholar]
- 84.Kinoshita T, Wang Y, Hasegawa M, Imamura R, Suda T. PYPAF3, a PYRIN-containing APAF-1-like protein, is a feedback regulator of caspase-1-dependent interleukin-1beta secretion. J Biol Chem. 2005 Jun 10;280(23):21720–21725. doi: 10.1074/jbc.M410057200. [DOI] [PubMed] [Google Scholar]
- 85.Davis BK, Roberts RA, Huang MT, Willingham SB, Conti BJ, Brickey WJ, et al. Cutting Edge: NLRC5-Dependent Activation of the Inflammasome. The Journal of Immunology. 2011 Jan 19;186(3):1333–1337. doi: 10.4049/jimmunol.1003111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Poyet JL, Srinivasula SM, Tnani M, Razmara M, Fernandes-Alnemri T, Alnemri ES. Identification of Ipaf, a human caspase-1-activating protein related to Apaf-1. J Biol Chem. 2001 Jul 27;276(30):28309–28313. doi: 10.1074/jbc.C100250200. [DOI] [PubMed] [Google Scholar]
- 87.Geddes BJ, Wang L, Huang WJ, Lavellee M, Manji GA, Brown M, et al. Human CARD12 is a novel CED4/Apaf-1 family member that induces apoptosis. Biochem Biophys Res Commun. 2001 Jun 1;284(1):77–82. doi: 10.1006/bbrc.2001.4928. [DOI] [PubMed] [Google Scholar]
- 88.Damiano JS, Stehlik C, Pio F, Godzik A, Reed JC. CLAN, a novel human CED-4-like gene. Genomics. 2001 Jul;75(1–3):77–83. doi: 10.1006/geno.2001.6579. [DOI] [PubMed] [Google Scholar]
- 89.Damiano JS, Newman RM, Reed JC. Multiple roles of CLAN (caspase-associated recruitment domain, leucine-rich repeat, and NAIP CIIA HET-E, and TP1-containing protein) in the mammalian innate immune response. J Immunol. 2004 Nov 15;173(10):6338–6345. doi: 10.4049/jimmunol.173.10.6338. [DOI] [PubMed] [Google Scholar]
- 90.Lara-Tejero M, Sutterwala FS, Ogura Y, Grant EP, Bertin J, Coyle AJ, et al. Role of the caspase-1 inflammasome in Salmonella typhimurium pathogenesis. The Journal of experimental medicine. 2006 Jun 12;203(6):1407–1412. doi: 10.1084/jem.20060206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Miao EA, Alpuche-Aranda CM, Dors M, Clark AE, Bader MW, Miller SI, et al. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1β via Ipaf. Nat Immunol. 2006 Apr 30;7(6):569–575. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- 92.Franchi L, Amer A, Body-Malapel M, Kanneganti T, Özören N, Jagirdar R, et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1β in salmonella-infected macrophages. Nat Immunol. 2006 Apr 30;7(6):576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- 93.Franchi L, Stoolman J, Kanneganti T, Verma A, Ramphal R, Nunez G. Critical role for Ipaf in Pseudomonas aeruginosa-induced caspase-1 activation. Eur J Immunol. 2007 Nov;37(11):3030–3039. doi: 10.1002/eji.200737532. [DOI] [PubMed] [Google Scholar]
- 94.Miao EA, Ernst RK, Dors M, Mao DP, Aderem A. Pseudomonas aeruginosa activates caspase 1 through Ipaf. Proc Natl Acad Sci USA. 2008 Feb 19;105(7):2562–2567. doi: 10.1073/pnas.0712183105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sutterwala FS, Mijares LA, Li L, Ogura Y, Kazmierczak BI, Flavell RA. Immune recognition of Pseudomonas aeruginosa mediated by the IPAF/NLRC4 inflammasome. The Journal of experimental medicine. 2007 Dec 17;204(13):3235–3245. doi: 10.1084/jem.20071239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ren T, Zamboni DS, Roy CR, Dietrich WF, Vance RE. Flagellin-Deficient Legionella Mutants Evade Caspase-1- and Naip5-Mediated Macrophage Immunity. PLoS Pathog. 2006;2(3):e18. doi: 10.1371/journal.ppat.0020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Molofsky AB, Byrne BG, Whitfield NN, Madigan CA, Fuse ET, Tateda K, et al. Cytosolic recognition of flagellin by mouse macrophages restricts Legionella pneumophila infection. The Journal of experimental medicine. 2006 Apr 17;203(4):1093–1104. doi: 10.1084/jem.20051659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Amer A, Franchi L, Kanneganti TD, Body-Malapel M, Ozoren N, Brady G, et al. Regulation of Legionella Phagosome Maturation and Infection through Flagellin and Host Ipaf. Journal of Biological Chemistry. 2006 Sep 12;281(46):35217–35223. doi: 10.1074/jbc.M604933200. [DOI] [PubMed] [Google Scholar]
- 99.Akhter A, Gavrilin MA, Frantz L, Washington S, Ditty C, Limoli D, et al. Caspase-7 Activation by the Nlrc4/Ipaf Inflammasome Restricts Legionella pneumophila Infection. PLoS Pathog. 2009 Apr 3;5(4):e1000361. doi: 10.1371/journal.ppat.1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vinzing M, Eitel J, Lippmann J, Hocke AC, Zahlten J, Slevogt H, et al. NAIP and Ipaf control Legionella pneumophila replication in human cells. J Immunol. 2008 May 15;180(10):6808–6815. doi: 10.4049/jimmunol.180.10.6808. [DOI] [PubMed] [Google Scholar]
- 101.Diez E, Lee S, Gauthier S, Yaraghi Z, Tremblay M, Vidal S, et al. Birc1e is the gene within the Lgn1 locus associated with resistance to Legionella pneumophila. Nature Genetics. 2003 Jan;33(1):55–60. doi: 10.1038/ng1065. [DOI] [PubMed] [Google Scholar]
- 102.Wright EK, Goodart SA, Growney JD, Hadinoto V, Endrizzi MG, Long EM, et al. Naip5 affects host susceptibility to the intracellular pathogen Legionella pneumophila. Curr Biol. 2003 Jan 8;13(1):27–36. doi: 10.1016/s0960-9822(02)01359-3. [DOI] [PubMed] [Google Scholar]
- 103.Zamboni DS, Kobayashi KS, Kohlsdorf T, Ogura Y, Long EM, Vance RE, et al. The Birc1e cytosolic pattern-recognition receptor contributes to the detection and control of Legionella pneumophila infection. Nat Immunol. 2006 Jan 29;7(3):318–325. doi: 10.1038/ni1305. [DOI] [PubMed] [Google Scholar]
- 104.Lightfield KL, Persson J, Brubaker SW, Witte CE, Moltke von J, Dunipace EA, et al. Critical function for Naip5 in inflammasome activation by a conserved carboxy-terminal domain of flagellin. Nat Immunol. 2008 Aug 24;9(10):1171–1178. doi: 10.1038/ni.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pizarro-Cerdá J, Cossart P. [Google Scholar]
- 106.Sun YH, Rolan HG, Tsolis RM. Injection of Flagellin into the Host Cell Cytosol by Salmonella enterica Serotype Typhimurium. Journal of Biological Chemistry. 2007 Oct 1;282(47):33897–33901. doi: 10.1074/jbc.C700181200. [DOI] [PubMed] [Google Scholar]
- 107.Whitfield NN, Byrne BG, Swanson MS. Mouse Macrophages Are Permissive to Motile Legionella Species That Fail To Trigger Pyroptosis. Infect Immun. 2009 Dec 22;78(1):423–432. doi: 10.1128/IAI.00070-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sansonetti PJ, Phalipon A, Arondel J, Thirumalai K, Banerjee S, Akira S, et al. Caspase-1 activation of IL-1beta and IL-18 are essential for Shigella flexneri-induced inflammation. Immunity. 2000 May;12(5):581–590. doi: 10.1016/s1074-7613(00)80209-5. [DOI] [PubMed] [Google Scholar]
- 109.Suzuki T, Franchi L, Toma C, Ashida H, Ogawa M, Yoshikawa Y, et al. Differential Regulation of Caspase-1 Activation, Pyroptosis, and Autophagy via Ipaf and ASC in Shigella-Infected Macrophages. PLoS Pathog. 2007;3(8):e111. doi: 10.1371/journal.ppat.0030111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schroeder GN, Jann NJ, Hilbi H. Intracellular type III secretion by cytoplasmic Shigella flexneri promotes caspase-1-dependent macrophage cell death. Microbiology. 2007 Sep 1;153(9):2862–2876. doi: 10.1099/mic.0.2007/007427-0. [DOI] [PubMed] [Google Scholar]
- 111.Miao EA, Mao DP, Yudkovsky N, Bonneau R, Lorang CG, Warren SE, et al. From the Cover: Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proc Natl Acad Sci USA. 2010 Feb 16;107(7):3076–3080. doi: 10.1073/pnas.0913087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Miao EA, Warren SE. Innate Immune Detection of Bacterial Virulence Factors Via the NLRC4 Inflammasome. J Clin Immunol. 2010 Mar 27;30(4):502–506. doi: 10.1007/s10875-010-9386-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schroder K, Tschopp J. The Inflammasomes. Cell. 2010 Mar 19;140(6):821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 114.Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004 Jul 8;430(6996):213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 115.Abdelaziz DH, Gavrilin MA, Akhter A, Caution K, Kotrange S, Khweek AA, et al. Apoptosis-associated speck-like protein (ASC) controls Legionella pneumophila infection in human monocytes. Journal of Biological Chemistry. 2011 Feb 4;286(5):3203–3208. doi: 10.1074/jbc.M110.197681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Broz P, Moltke von J, Jones JW, Vance RE, Monack DM. Differential Requirement for Caspase-1 Autoproteolysis in Pathogen-Induced Cell Death and Cytokine Processing. Cell Host Microbe. 2010 Dec 16;8(6):471–483. doi: 10.1016/j.chom.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Guarda G, So A. Regulation of inflammasome activity. Immunology. 2010 Feb 5;130(3):329–336. doi: 10.1111/j.1365-2567.2010.03283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lightfield KL, Persson J, Trinidad NJ, Brubaker SW, Kofoed EM, Sauer JD, et al. Differential Requirements for NAIP5 in Activation of the NLRC4 Inflammasome. Infect Immun. 2011 Mar 18;79(4):1606–1614. doi: 10.1128/IAI.01187-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hu B, Elinav E, Huber S, Booth CJ, Strowig T, Jin C, et al. Inflammation-induced tumorigenesis in the colon is regulated by caspase-1 and NLRC4. Proc Natl Acad Sci USA. 2010 Dec 14;107(50):21635–21640. doi: 10.1073/pnas.1016814108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mayor A, Martinon F, De Smedt T, Pétrilli V, Tschopp J. A crucial function of SGT1 and HSP90 in inflammasome activity links mammalian and plant innate immune responses. Nat Immunol. 2007 May 1;8(5):497–503. doi: 10.1038/ni1459. [DOI] [PubMed] [Google Scholar]
- 121.Pétrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007 Jun 29;14(9):1583–1589. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- 122.Arlehamn CSL, Pétrilli V, Gross O, Tschopp J, Evans TJ. The Role of Potassium in Inflammasome Activation by Bacteria. Journal of Biological Chemistry. 2010 Mar 26;285(14):10508–10518. doi: 10.1074/jbc.M109.067298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Guarda G, Dostert C, Staehli F, Cabalzar K, Castillo R, Tardivel A, et al. T cells dampen innate immune responses through inhibition of NLRP1 and NLRP3 inflammasomes. Nature. 2009 Sep 7;460(7252):269–273. doi: 10.1038/nature08100. [DOI] [PubMed] [Google Scholar]
- 124.Suzuki T, Nunez G. A role for Nod-like receptors in autophagy induced by Shigella infection. Autophagy. 2008 Jan 1;4(1):73–75. doi: 10.4161/auto.5101. [DOI] [PubMed] [Google Scholar]
- 125.Bortoluci KR, Medzhitov R. Control of infection by pyroptosis and autophagy: role of TLR and NLR. Cell Mol Life Sci. 2010 Mar 13;67(10):1643–1651. doi: 10.1007/s00018-010-0335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Brodsky IE, Palm NW, Sadanand S, Ryndak MB, Sutterwala FS, Flavell RA, et al. A Yersinia Effector Protein Promotes Virulence by Preventing Inflammasome Recognition of the Type III Secretion System. Cell Host Microbe. 2010 May 20;7(5):376–387. doi: 10.1016/j.chom.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ikeda K, Yamaguchi K, Tanaka T, Mizuno Y, Hijikata A, Ohara O, et al. Unique activation status of peripheral blood mononuclear cells at acute phase of Kawasaki disease. Clinical & Experimental Immunology. 2009 Dec 15;160(2):246–255. doi: 10.1111/j.1365-2249.2009.04073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Macaluso F, Nothnagel M, Parwez Q, Petrasch-Parwez E, Bechara FG, Epplen JT, et al. Polymorphisms in NACHT-LRR (NLR) genes in atopic dermatitis. Exp Dermatol. 2007 Aug;16(8):692–698. doi: 10.1111/j.1600-0625.2007.00589.x. [DOI] [PubMed] [Google Scholar]
- 129.Roy N, Mahadevan MS, McLean M, Shutler G, Yaraghi Z, Farahani R, et al. The gene for neuronal apoptosis inhibitory protein is partially deleted in individuals with spinal muscular atrophy. Cell. 1995 Jan 13;80(1):167–178. doi: 10.1016/0092-8674(95)90461-1. [DOI] [PubMed] [Google Scholar]
- 130.Stacey KJ, Ross IL, Hume DA. Electroporation and DNA-dependent cell death in murine macrophages. Immunol Cell Biol. 1993 Apr;71 (Pt 2):75–85. doi: 10.1038/icb.1993.8. [DOI] [PubMed] [Google Scholar]
- 131.Suzuki K, Mori A, Ishii KJ, Saito J, Singer DS, Klinman DM, et al. Activation of target-tissue immune-recognition molecules by double-stranded polynucleotides. Proc Natl Acad Sci USA. 1999 Mar 2;96(5):2285–2290. doi: 10.1073/pnas.96.5.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Muruve DA, Pétrilli V, Zaiss AK, White LR, Clark SA, Ross PJ, et al. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 2008 Feb 20;452(7183):103–107. doi: 10.1038/nature06664. [DOI] [PubMed] [Google Scholar]
- 133.Roberts TL, Idris A, Dunn JA, Kelly GM, Burnton CM, Hodgson S, et al. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science. 2009 Feb 20;323(5917):1057–1060. doi: 10.1126/science.1169841. [DOI] [PubMed] [Google Scholar]
- 134.Bürckstümmer T, Baumann C, Blüml S, Dixit E, Dürnberger G, Jahn H, et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol. 2009 Mar 1;10(3):266–272. doi: 10.1038/ni.1702. [DOI] [PubMed] [Google Scholar]
- 135.Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009 Mar 17;458(7237):514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fernandes-Alnemri T, Yu J, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009 Mar 17;458(7237):509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rathinam VAK, Jiang Z, Waggoner SN, Sharma S, Cole LE, Waggoner L, et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol. 2010 May 1;11(5):395–402. doi: 10.1038/ni.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wu E, Nemerow GR. Virus yoga: the role of flexibility in virus host cell recognition. Trends Microbiol. 2004 Apr;12(4):162–169. doi: 10.1016/j.tim.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 139.Garner JA. Herpes simplex virion entry into and intracellular transport within mammalian cells. Adv Drug Deliv Rev. 2003 Nov 14;55(11):1497–1513. doi: 10.1016/j.addr.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 140.Fernandes-Alnemri T, Yu J, Juliana C, Solorzano L, Kang S, Wu J, et al. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat Immunol. 2010 Mar 28;11(5):385–393. doi: 10.1038/ni.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Clemens DL, Lee B, Horwitz MA. Virulent and avirulent strains of Francisella tularensis prevent acidification and maturation of their phagosomes and escape into the cytoplasm in human macrophages. Infect Immun. 2004 Jun;72(6):3204–3217. doi: 10.1128/IAI.72.6.3204-3217.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mariathasan S. Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. The Journal of experimental medicine. 2005 Oct 10;202(8):1043–1049. doi: 10.1084/jem.20050977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fernandes-Alnemri T, Yu J, Juliana C, Solorzano L, Kang S, Wu J, et al. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat Immunol. 2010 May 1;11(5):385–393. doi: 10.1038/ni.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jones JW, Kayagaki N, Broz P, Henry T, Newton K, O’Rourke K, et al. Absent in melanoma 2 is required for innate immune recognition of Francisella tularensis. Proc Natl Acad Sci USA. 2010 May 25;107(21):9771–9776. doi: 10.1073/pnas.1003738107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ulland TK, Buchan BW, Ketterer MR, Fernandes-Alnemri T, Meyerholz DK, Apicella MA, et al. Cutting Edge: Mutation of Francisella tularensis mviN Leads to Increased Macrophage Absent in Melanoma 2 Inflammasome Activation and a Loss of Virulence. The Journal of Immunology. 2010 Aug 19;185(5):2670–2674. doi: 10.4049/jimmunol.1001610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kim S, Bauernfeind F, Ablasser A, Hartmann G, Fitzgerald KA, Latz E, et al. Listeria monocytogenes is sensed by the NLRP3 and AIM2 inflammasome. Eur J Immunol. 2010 Mar 23;40(6):1545–1551. doi: 10.1002/eji.201040425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sauer J, Witte CE, Zemansky J, Hanson B, Lauer P, Portnoy DA. Listeria monocytogenes Triggers AIM2-Mediated Pyroptosis upon Infrequent Bacteriolysis in the Macrophage Cytosol. Cell Host Microbe. 2010 May 20;7(5):412–419. doi: 10.1016/j.chom.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Warren SE, Armstrong A, Hamilton MK, Mao DP, Leaf IA, Miao EA, et al. Cutting Edge: Cytosolic Bacterial DNA Activates the Inflammasome via Aim2. The Journal of Immunology. 2010 Jul 2;185(2):818–821. doi: 10.4049/jimmunol.1000724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tsuchiya K, Hara H, Kawamura I, Nomura T, Yamamoto T, Daim S, et al. Involvement of Absent in Melanoma 2 in Inflammasome Activation in Macrophages Infected with Listeria monocytogenes. The Journal of Immunology. 2010 Jul 2;185(2):1186–1195. doi: 10.4049/jimmunol.1001058. [DOI] [PubMed] [Google Scholar]
- 150.Wu J, Fernandes-Alnemri T, Alnemri ES. Involvement of the AIM2, NLRC4, and NLRP3 Inflammasomes in Caspase-1 Activation by Listeria monocytogenes. J Clin Immunol. 2010 May 20;30(5):693–702. doi: 10.1007/s10875-010-9425-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Choubey D, Duan X, Dickerson E, Ponomareva L, Panchanathan R, Shen H, et al. Interferon-Inducible p200-Family Proteins as Novel Sensors of Cytoplasmic DNA: Role in Inflammation and Autoimmunity. Journal of Interferon & Cytokine Research. 2010 Jun;30(6):371–380. doi: 10.1089/jir.2009.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Choubey D, Walter S, Geng Y, Xin H. Cytoplasmic localization of the interferon-inducible protein that is encoded by the AIM2 (absent in melanoma) gene from the 200-gene family. FEBS Lett. 2000 May 26;474(1):38–42. doi: 10.1016/s0014-5793(00)01571-4. [DOI] [PubMed] [Google Scholar]
- 153.Yanai H, Ban T, Wang Z, Choi MK, Kawamura T, Negishi H, et al. [Google Scholar]
- 154.DeYoung KL, Ray ME, Su YA, Anzick SL, Johnstone RW, Trapani JA, et al. Cloning a novel member of the human interferon-inducible gene family associated with control of tumorigenicity in a model of human melanoma. Oncogene. 1997 Jul 24;15(4):453–457. doi: 10.1038/sj.onc.1201206. [DOI] [PubMed] [Google Scholar]
- 155.Henry T, Brotcke A, Weiss DS, Thompson LJ, Monack DM. Type I interferon signaling is required for activation of the inflammasome during Francisella infection. The Journal of experimental medicine. 2007 May 7;204(5):987–994. doi: 10.1084/jem.20062665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cresswell KS, Clarke CJP, Jackson JT, Darcy PK, Trapani JA, Johnstone RW. Biochemical and growth regulatory activities of the HIN-200 family member and putative tumor suppressor protein, AIM2. Biochem Biophys Res Commun. 2005 Jan 14;326(2):417–424. doi: 10.1016/j.bbrc.2004.11.048. [DOI] [PubMed] [Google Scholar]
- 157.Choubey D, Panchanathan R. Interferon-inducible Ifi200-family genes in systemic lupus erythematosus. Immunology Letters. 2008 Aug 15;119(1–2):32–41. doi: 10.1016/j.imlet.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci USA. 2003 Mar 4;100(5):2610–2615. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Panchanathan R, Duan X, Shen H, Rathinam VAK, Erickson LD, Fitzgerald KA, et al. Aim2 Deficiency Stimulates the Expression of IFN-Inducible Ifi202, a Lupus Susceptibility Murine Gene within the Nba2 Autoimmune Susceptibility Locus. The Journal of Immunology. 2010 Dec 2;185(12):7385–7393. doi: 10.4049/jimmunol.1002468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Panchanathan R, Shen H, Duan X, Rathinam VAK, Erickson LD, Fitzgerald KA, et al. Aim2 Deficiency in Mice Suppresses the Expression of the Inhibitory Fc Receptor (Fc RIIB) through the Induction of the IFN-Inducible p202, a Lupus Susceptibility Protein. The Journal of Immunology. 2011 May 6; doi: 10.4049/jimmunol.1003638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Dombrowski Y, Peric M, Koglin S, Kammerbauer C, Goss C, Anz D, et al. Cytosolic DNA Triggers Inflammasome Activation in Keratinocytes in Psoriatic Lesions. Science Translational Medicine. 2011 May 11;3(82):82ra38–82ra38. doi: 10.1126/scitranslmed.3002001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Grenier JM, Wang L, Manji GA, Huang WJ, Al-Garawi A, Kelly R, et al. Functional screening of five PYPAF family members identifies PYPAF5 as a novel regulator of NF-kappaB and caspase-1. FEBS Lett. 2002 Oct 23;530(1–3):73–78. doi: 10.1016/s0014-5793(02)03416-6. [DOI] [PubMed] [Google Scholar]
- 163.Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, et al. NLRP6 Inflammasome Regulates Colonic Microbial Ecology and Risk for Colitis. Cell. 2011 May 27;145(5):745–757. doi: 10.1016/j.cell.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chen GY, Liu M, Wang F, Bertin J, Nuñez G. A Functional Role for Nlrp6 in Intestinal Inflammation and Tumorigenesis. The Journal of Immunology. 2011 May 4; doi: 10.4049/jimmunol.1100412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Normand S, Delanoye-Crespin A, Bressenot A, Huot L, Grandjean T, Peyrin-Biroulet L, et al. Nod-like receptor pyrin domain-containing protein 6 (NLRP6) controls epithelial self-renewal and colorectal carcinogenesis upon injury. Proc Natl Acad Sci USA. 2011 May 18; doi: 10.1073/pnas.1100981108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wang L, Manji GA, Grenier JM, Al-Garawi A, Merriam S, Lora JM, et al. PYPAF7, a novel PYRIN-containing Apaf1-like protein that regulates activation of NF-kappa B and caspase-1-dependent cytokine processing. J Biol Chem. 2002 Aug 16;277(33):29874–29880. doi: 10.1074/jbc.M203915200. [DOI] [PubMed] [Google Scholar]
- 167.Lich JD, Williams KL, Moore CB, Arthur JC, Davis BK, Taxman DJ, et al. Monarch-1 suppresses non-canonical NF-kappaB activation and p52-dependent chemokine expression in monocytes. J Immunol. 2007 Feb 1;178(3):1256–1260. doi: 10.4049/jimmunol.178.3.1256. [DOI] [PubMed] [Google Scholar]
- 168.Jeru I, Duquesnoy P, Fernandes-Alnemri T, Cochet E, Yu J, Lackmy-Port-Lis M, et al. Mutations in NALP12 cause hereditary periodic fever syndromes. Proc Natl Acad Sci USA. 2008;105(5):1614–1619. doi: 10.1073/pnas.0708616105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jéru I, Hentgen V, Normand S, Duquesnoy P, Cochet E, Delwail A, et al. Role of IL-1β in NLRP12-associated autoinflammatory disorders and resistance to anti-IL-1 therapy. Arthritis Rheum. 2011 Apr 7; doi: 10.1002/art.30378. [DOI] [PubMed] [Google Scholar]
- 170.Borghini S, Tassi S, Chiesa S, Caroli F, Carta S, Caorsi R, et al. Clinical presentation and pathogenesis of cold-induced autoinflammatory disease in a family with recurrence of an NLRP12 mutation. Arthritis Rheum. 2011 Mar;63(3):830–839. doi: 10.1002/art.30170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Jéru I, Le Borgne G, Cochet E, Hayrapetyan H, Duquesnoy P, Grateau G, et al. Identification and functional consequences of a recurrent NLRP12 missense mutation in periodic fever syndromes. Arthritis Rheum. 2011 May;63(5):1459–1464. doi: 10.1002/art.30241. [DOI] [PubMed] [Google Scholar]