Abstract
Background
Excessive apoptosis of β-cell is closely related to diabetes mellitus. Chronic exposure to high glucose causes β-cell dysfunction and apoptosis in diabetes. Thorn grape (Vitis davidii Foex.) has been used to treat diabetes in Traditional Chinese medicine for many years. In our previous research, thorn grape seeds oil (TGSO) showed promising anti-diabetic effects in animal models. However, it is unknown whether TGSO played an anti-apoptotic role in the anti-diabetic effects and the mechanism regarding signal transduction pathway is unclear either.
Methods
The rattus pancreatic β-cell line RIN-m5F was treated with/without TGSO which was extracted by supercritical carbon dioxide (CO2) fluid extraction and analyzed by Gas Chromatography/Mass Spectrometry (GC/MS). Cell apoptosis was detected by fluorescence activated cell sorting (FACS), insulin secretion was assayed by Enzyme-Linked Immunosorbent Assay (ELISA), and the apoptosis-related genes expressions were evaluated by quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR).
Results
TGSO, containing 87.02% unsaturated fatty acids (UFAs), significantly reduced pancreatic β-cell apoptosis and protected the insulin secretion impaired by high glucose. The expressions of pro-apoptotic genes such as iNOS, Caspase-3, ATF-3, JNK, p38 and Fas were down-regulated while the anti-apoptotic genes Akt and Bcl-2/Bax were up-regulated.
Conclusions
The results indicated that TGSO protected β-cells from high glucose-induced apoptosis and its protective activity may be linked to mitochondrial pathway, endoplasmic reticulum (ER) stress pathway and Fas signal pathway, which implied that TGSO might be an effective complementary or alternative medicine to reduce β-cell apoptosis and dysfunction.
Keywords: Diabetes mellitus, Thorn grape seed oil, Anti-apoptosis, Insulin release
Background
Diabetes mellitus is characterized by hyperglycemia resulting from a relative or absolute insulin deficiency [1]. The prevalence of diabetes has risen dramatically during recent decades, and it is now a serious global health burden [2]. It is reported that more than 371 million people have diabetes (IDF Diabetes Atlas 5th Edition 2012 Update) with the biggest population in China, soaring to 92.3 million, 9.42% of adults (http://www.worlddiabetesday.org/). Insulin deficiency is the consequence of compromised insulin secretion per β-cell and/or reduction in total β-cell mass [3]. Prolonged exposure to high glucose concentrations exerts toxic effects on β-cell, resulting in β-cell dysfunction and ultimately apoptosis [4].
Evidences from both human disease and mouse models have shown that apoptosis is the main form of β-cell death in diabetes mellitus [3]. Beta-cell apoptosis is mainly mediated by three pathways, namely, death receptor signal pathway, mitochondrial pathway and endoplasmic reticulum (ER) stress pathway [5,6]. The death receptor signal pathway is activated upon ligation of the cell surface death receptors, including Fas receptor and tumor necrosis factor receptor-1 (TNFR-1), which in turn activates downstream effector of Caspase family [7]. The assembly of the death inducing signaling complex (DISC), a multi-protein complex consisting of Fas receptor, the adaptor protein Fas-associated death domain, and pro-Caspase-8 (pro-cysteinyl aspartate specific proteinase-8), is the initiating signal for the processing of pro-Caspase-8 to its active form [7]. TNF forms the DISC in the similar way [8]. Depending upon the activity of Caspase-8, the ensuing signal can activate either downstream Caspase cascade or the mitochondrial death pathway or Nuclear Factor-kappa B (NF-kB) for efficient execution [9-11].
Mitochondrial pathway could be activated by the unbalance of the proportion of pro-/anti-apoptotic proteins (such as Bax, Bad, Bcl-2, Bcl-xL), then release several apoptotic factors to cytoplasm, such as cytochrome-c, apoptotic protease-activating factor 1, apoptosis inducing factor, second mitochondria-derived activator of Caspase/direct inhibitor of apoptosis-binding protein with low PI, endonuclease G to induce apoptosis [12-18].
In the processing of ER stress pathway, some pro-apoptotic effectors are activated under ER stress, such as C/EBP homologous protein, c-Jun N-terminal kinase (JNK), activating transcription factor-3 (ATF-3) [19-21]. ER stress could also activate p38 through Ca2+ signal way and induce apoptosis [20,22]. Besides, under ER stress, Akt gene could promote cell survival [23].
Finally, these three pathways converge to activate Caspase-3 to execute cell apoptosis [24-26]. In addition to the three pathway aforementioned, iNOS (inducible nitric oxide synthase) could be induced by environmental exposure leading to NO (Nitric Oxide) formation, which have a direct deleterious effects on β-cells [27,28].
Free fatty acids are important to pancreatic β-cell for glucose-stimulated insulin secretion [29,30]. It is reported that unsaturated fatty acids (UFAs) usually protect β-cell from glucose-induced apoptosis and impaired β-cell proliferation [31,32]. In vivo research showed that UFA could alleviate insulin resistance and promote insulin secretion, while in vitro studies have revealed that omega-6 polyunsaturated fatty acid (PUFA) can prevent chemically induced diabetes and attenuate the oxidant stress that occurs in diabetes mellitus [33,34].
Thorn grape (Vitis davidii Foex.), one of characteristic plants in Hunan province, P. R. China, has been used to treat diabetes in Traditional Chinese medicine for centuries. It is rich in UFA, resveratrol, oligomeric proantho cyanidins, squalene and superoxide dismutase, et al. In thorn grape seed oil (TGSO), UFA is mainly composed of linoleic acid (ω-6 PUFA) and oleic acid (monounsaturated fatty acid, MUFA) [35,36]. In our previous research, TGSO showed remarkable anti-diabetic effects in animal models [37]. However, its anti-diabetic mechanism has not been clarified.
This study aimed to evaluate the effects of TGSO on high glucose-induced rattus pancreatic β-cell apoptosis by detecting the cell apoptotic rate and insulin levels, and reveal its mechanisms regarding signal transduction pathway by measuring related genes expression.
Methods
TGSO extraction and fatty acids analysis
Thorn grapes (Vitis davidii Foex.), authorized by Cultivar Variety Examination and Approving Committee of Hunan Province (No: XPD010-2005), were picked from Mayang county, Huaihua city, Hunan province, P.R. China and deposited in nursery garden of Hunan Agricultural University. The seeds were collected and dried at 50°C for 24 h and then smashed into the powder of 40 meshes. TGSO was extracted by using supercritical CO2 fluid extraction machine (HA231-50-06, Nantong Hua’An Supercritical Extraction Co. Ltd.) at CO2 flow of 20 ~ 30 L/h, extraction pressure of 34.2 Mpa, extraction temperature of 39.3°C, separation pressure of 12 Mpa and separation temperature of 55°C for 95 minutes.
Fatty acid composition of TGSO were analyzed by GC/MS with a QP2010 gas chromatograph-mass spectrometer (Shimadzu, Japan), equipped with flame ionization detector (FID), Rtx-WAX capillary column (30.00 m × 0.25 mm × 0.25 μm). Samples (1 μL) were prepared following the protocols: TGSO (100 mg) were dissolved in 5 mL hexane, 5 mL sodium hydroxide-carbinol solution (0.5 mol/L), then vortex-mixed for 30 s and incubated in a water bath at 80°C for 30 min. After cooled to room temperature, 1 ml hexane and 1 ml deionized water were added, vortex mixed for 30 s and centrifuged for 10 min at 8000 revolutions per minute (RPM). The supernatant were transferred into a GC vial after filtered with 0.45 μm membrane (Shanghai Xingya purification material factory, Shanghai, China) and injected in split mode (1:10) with injector temperature of 250°C for GC/MS analysis. Helium was used as carrier gas at a flow rate of 1.02 mL/min. The oven temperature was 100°C for 3 min, and then increased from 100°C to 200°C at 5°C/min and at 200°C for 17 min. The detector operated in scan mode from m/z 40 to 500. Initial pretreatment of the raw chromatographic data, such as baseline correction, chromatogram alignment, noise reduction, and normalization, were performed by using GC/MS solution software. The peaks were then tentatively identified from their retention characteristics and mass fragmentation patterns by using NIST mass spectrum database.
Cells culture and treatments
Rattus pancreatic β-cell line (Rin-m5F) was purchased from tumor cells bank of Chinese Academy of Medical Sciences. Rin-m5F cells were cultured at 37°C, 5% CO2 atmosphere in RPMI-1640 medium (Hyclone) containing 12% fetal calf serum (Hyclone). On attaining 75-80% confluency, cells (after passages 4) were seeded into 6-well plate at a density of 1 × 106 per well and grown for 24 h. Then the cells were treated as different groups: control group (10 mM glucose), model group (25 mM glucose, 1000 μg/mL BSA (Bovine serum albumin)), 100 μg/mL TGSO group (25 mM glucose, 100 μg/mL TGSO, 1000 μg/mL BSA) and cultured for 48 h. The concentration of glucose, time for glucose exposure of RIN cells and TGSO concentration were obtained from our preliminary experiment. Rin-m5F cell was exposed to the glucose of 5, 15, 25 mmol/L for 12, 24, 36 and 48 hours. The treatment of exposure to 25 mmol/L glucose for 48 h was observed with amount of apoptotic cell and high NO content that it was appropriate for constructing high glucose-induced apoptosis model [38]. TGSO with the concentration of 50, 100, 200 μg/mL were set for cell culture. The results showed that 100 μg/mL TGSO played the best anti-apoptotic role by measuring apoptotic rate and insulin release. All cell cultures were set up in triplicates for following assay.
Detection of cell apoptosis by fluorescence activated cell sorting (FACS)
Flow cytometric analysis was performed to detect the cell apoptosis of each group by using Annexin V-fluorescein isothiocyanate (FITC)/propidiumlodide (PI) kit (Qihaifutai Biotechnology, Shanghai, China) as recommended by manufacturer. After 48 h treatment, culture supernatants which contained floating dying and apoptotic cells were collected, and then merged with the adherent cells which were rinsed with phosphate buffered saline (PBS) and harvested by a brief trypsinization. The mixture was washed with PBS, centrifuged for 5 min at 2000 RPM, and the supernatants were discarded. Cells were re-suspended in 0.5 ml binding buffer, and then double-stained with FITC-conjugated Annexin-V and PI for 15 min at room temperature. Flow cytometric analyses were performed on a FACS Cytomics™ (FC500, Beckman Coulter, USA) within 1 h. The apoptotic cells measured as late or early apoptotic cells were shown respectively in the upper right or lower right quadrant of the FACS histogram.
Insulin secretion assay
Insulin concentration in the supernatant which were collected after 48 h cultivation was determined by ELISA using microplate reader (MB-530, Shenzhen Huisong technology development Co., Ltd, Shenzhen, China). The measurement was conducted according to the instruction of Rat Insulin Elisa kit (R & D Systems, USA).
Isolation of RNA, cDNA synthesis and qRT-PCR
Total RNA was isolated using the TRIzol reagent (CoWin Bioscience, Beijing, China). Its quality was assessed by electrophoresis on 1% agarose gels based on the integrity of 28S, 18S and 5S bands after ethidium bromide staining. The total RNA concentration was quantified by measuring absorbance at 260 nm and 280 nm. cDNA was synthesized from RNA by PrimeScriptTM RT reagent Kit with gDNA Eraser (TaKaRa, Japan) according to the instruction. qRT-PCR was performed on the CFX 96™ Real-Time System (C1000TM Thermal Cycler, BIO-RAD, USA). Primer sequences (Table 1) of GAPDH、Caspase-3、iNOS、Bcl-2、Bax and Fas were referred to our previous study [38] while the others (ATF-3、JNK、p38、Akt) were designed by Primer Premier 5.0 software (Applied Biosystems) with the cDNA sequences obtained from National Center of Biotechnology Information (NCBI) [GenBank: NM_012912.2, NM_053829.1, NM_031020.2, BF563829.1, respectively]. All of these primers were synthesized by Beijing Genome Institute (BGI-Shenzhen, China). For qRT-PCR, each reaction was run in triplicate and contained the following: 10.0 μL Premix Ex TaqTM II (Tli RNaseH Plus, 2 × Conc., TaKaRa, Japan), 0.8 μL Forward primer and Reverse primer (2.5 μM), 0.4 μL ROX Refrence Dye II (50 × Conc., TaKaRa, Japan) and 100 ng cDNA template in a final reaction volume of 20 μL.
Table 1.
Primer sequences of target genes for qRT-PCR
| Target genes | Nucleotide sequences (5′-3′) | Product size | |
|---|---|---|---|
|
GAPDH |
Forward |
AGCCCCCAACACTGAGCAT |
111 bp |
| Reverse |
TGCAGCGAACTTTATTGATGGT |
||
|
Caspase-3 |
Forward |
TTCTCCCTGGACGCCACTT |
111 bp |
| Reverse |
CCTACCCCACTCCCAGTCATT |
||
|
iNOS |
Forward |
GTCTCTCCAAACCCCTCACTGT |
112 bp |
| Reverse |
GGAGCAAAAAAGGGCAACAC |
||
|
Bcl-2 |
Forward |
TGGGATGCCTTTGTGGAACT |
125 bp |
| Reverse |
CAGGTATGCACCCAGAGTGATG |
||
|
Bax |
Forward |
GAGCTGCAGAGGATGATTGCT |
128 bp |
| Reverse |
GCAAAGTAGAAGAGGGCAACCA |
||
|
Fas |
Forward |
GTGTGCAAGGCTCAAGGATGT |
120 bp |
| Reverse |
TGGGATGCCTTTGTGGAACT |
||
|
ATF-3 |
Forward |
TCTAGCCGCTCTCTGGACC |
147 bp |
| Reverse |
TCCTCAAACACCAGTGACCC |
||
|
JNK |
Forward |
TCCAGTTCTCGTACCCGCTA |
135 bp |
| Reverse |
AGCATGGCGTGACACAGTAA |
||
|
p38 |
Forward |
TTTGCTCAGTACCACGACCC |
107 bp |
| Reverse |
TCGTAGGTCAGGCTCTTCCA |
||
|
Akt |
Forward |
GCTGCGGCTCCTCATTCA |
125 bp |
| Reverse | CGCCGAGCTGGGAGTAAA |
After a pre-incubation step at 95°C for 30 s to activate DNA polymerase, PCR amplification was 40 cycles of denaturation at 95°C for 5 s and annealing at 60°C (except 64°C for Akt, Fas, iNOS) for 30 s. After amplification, the melting curves were generated in the range 65–95°C with increments of 0.5°C every 5 seconds. The emission data were quantified using the cycle threshold value. Data were normalized to GAPDH and presented as the mean fold change by comparing with the control group.
Statistical analysis
The qRT-PCR data were analyzed by Livak 2-△△Ct method [39]. Experimental result showed as mean ± standard deviation (SD). Statistical differences between the groups were analyzed by using the one-way analysis of variance (ANOVA). A value of p < 0.05 was considered significant and p < 0.01 represented highly significant.
Results
TGSO contained 87.02% UFA which was 5.7 folds higher than SFA (saturated fatty acid)
For identifying the functional composition in TGSO played the anti-diabetic role, its ingredients were analyzed by GC/MS as shown in Table 2. Twelve kinds of fatty acids were determined. Among them, the contents of UFA reached up to 87.02%, containing 20.16% MUFA and 66.86% PUFA. The rate of UFA and SFA was up to 670%.
Table 2.
Composition and relative contents of fatty acids in TGSO
| No. | Retention time/min | Fatty acids | Relative content (%) |
|---|---|---|---|
| 1 |
17.355 |
Tetradecanoic acid |
0.04 |
| 2 |
21.301 |
Hexadecanoic acid |
7.73 |
| 3 |
21.757 |
11-Hexadecenoic acid |
0.30 |
| 4 |
21.987 |
Ethyl 9-hexadecenoate |
0.06 |
| 5 |
23.146 |
Heptadecanoic acid |
0.08 |
| 6 |
25.233 |
Octadecanoic acid |
4.92 |
| 7 |
25.695 |
9-Octadecenoic acid |
18.91 |
| 8 |
25.845 |
11-Octadecenoic acid |
0.69 |
| 9 |
26.924 |
9,12-Octadecadienoic acid |
66.60 |
| 10 |
28.695 |
9,12,15-Octadecatrienoic acid |
0.26 |
| 11 |
31.296 |
Eicosanoic acid |
0.21 |
| 12 | 32.034 | 11-Eicosenoic acid | 0.20 |
TGSO reduced the percentages of apoptotic cells from 43.80% to 15.44%
To evaluate the effect of TGSO, the apoptotic rate of RINm5F cell exposure to high glucose with/without TGSO were investigated. After exposure to high glucose for 48 h, apoptotic rate of model group was significantly increased to 43.80%, which was 27.8-fold higher than that of the control. However, in the condition of co-culture with 100 μg/mL TGSO, the percentages of apoptotic cells were reduced to 15.44% (Figure 1).
Figure 1.
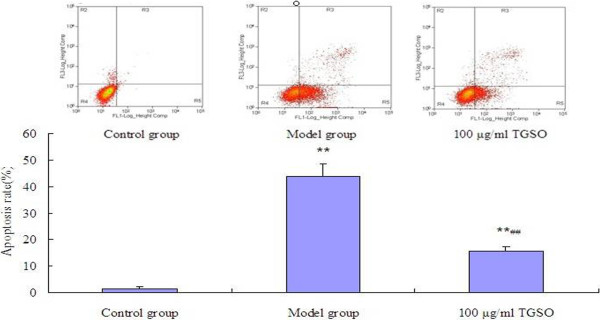
Effects of TGSO on Rin-m5F Cells Apoptosis Induced by High Glucose. Data represent mean ± S.D (n = 3 for each group). The values are statistically significant and presented as follows: **p< 0.01 vs. control group; ##p< 0.01 vs. model group.
TGSO protected β-cells from insulin impairment induced by high glucose
As insulin release is one of the variables to assess high glucose-induced damage and protection afforded by TGSO, insulin concentrations of supernatant were decreased significantly after exposure to high glucose. TGSO recovered insulin secretion damage induced by high glucose to the comparable level of the control in Rin-m5F cells (Figure 2).
Figure 2.
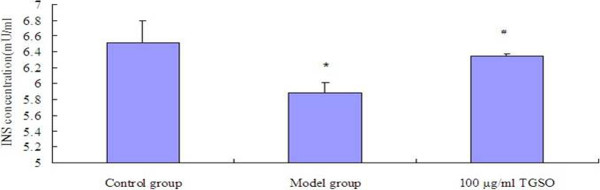
Effects of TGSO on Insulin Secretion in Rin-m5F Cells Induced by High Glucose. Data represent mean ± S.D (n = 3 for each group). The values are statistically significant and presented as follows: *p< 0.05 vs. control group; #p< 0.05 vs. model group.
TGSO down-regulated pro-apoptotic gene expression and up-regulated anti-apoptotic gene expression involved in signal transduction pathways
Related gene expression involved in three pathways was measured for anti-diabetic mechanism identification. After 48 h exposure to high glucose, mRNA expression levels of Caspase-3、iNOS、ATF-3、JNK、p38、Fas genes of model group increased to 298%, 159%, 917%, 264%, 254% and 119%, respectively, while Akt, Bcl-2/Bax genes reduced by 63% and 60% (Figure 3).
Figure 3.
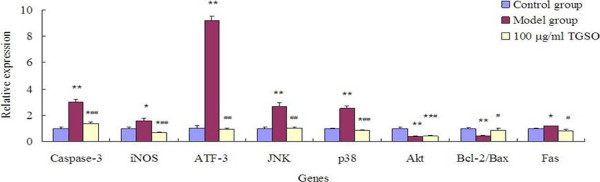
Effects of TGSO on Apoptosis-Related Genes Expression in High Glucose- induced Rin-m5F Cells. Data represent mean ± S.D (n = 3 for each group). The values are statistically significant and presented as follows: *p< 0.05 vs. control group; **p< 0.01 vs. control group; #p< 0.05 vs. model group; ##p< 0.01 vs. model group.
In TGSO group, the expressions of pro-apoptotic genes such as Caspase-3, iNOS, ATF-3, JNK, p38 and Fas gene were down-regulated to 46%, 41%, 11%, 40%, 34% and 68% of the model group. Among them, expressions of iNOS and p38 were even lower than the controls. The anti-apoptotic gene Akt and Bcl-2/Bax gene expressions were up-regulated to 110% and 213% compared with model group (Figure 3).
Discussion
TGSO showed remarkable anti-diabetic effects in animal models, however, functional components in TGSO and its anti-diabetic mechanism have not been identified. In this investigation, the compositions of TGSO were analyzed by GC-MS and its mechanism was illustrated by measuring apoptotic rate, insulin release and related gene expression.
Glucose with supraphysiological level has deleterious effect on pancreatic β-cell function and consequently results in dysfunction and apoptosis [4]. As in our previous study, 25 mM glucose was used in this investigation to construct apoptotic cell models with high apoptotic rate and low insulin secretion [38]. Mechanisms of high glucose induced β-cell apoptosis include increasing iNOS expression together with regulation of apoptosis-related gene expressions involved in three pathways. In consistent with our previous observation, high glucose induced apoptosis via Fas signal pathway and imbalance expression of Bcl-2 versus Bax. The anti-apoptotic gene Bcl-2 expression did not response to high glucose stimulation, whereas pro-apoptotic Bax gene was increased [38], leading to the alteration of the mitochondrial homeostasis, and subsequent activation of apoptosis. In addition, up-regulated expressions of ATF-3, JNK, p38 and down-regulated expressions of Akt revealed the high glucose induced apoptosis is closely related with ER stress pathway too. This is agreed with the review of Bensellam et al. that ER stress pathway plays an important role in β-cell glucotoxicity [4]. Among these tested genes in ER stress pathway, the modification of transcription factor ATF-3 expression in model group is the highest (9.17 folds). This might due to its role as a downstream target of both NF-kB and JNK/SAPK signaling pathways and the induction of NO which is synthesized by iNOS [21].
After co-culture with TGSO, the cells were protected against high glucose-induced apoptosis and insulin secretion was reserved [31]. This may due to the UFA it contained with the ratio of UFA/SFA up to 670%. Though SFA was reported to induce apoptosis by mitochondrial dysfunction and production of reactive oxygen species [40,41], UFA could protect RINm5F cells against SFA-induced cell death, not only induction of apoptosis prevented [32], but also β-cell proliferation promoted [42]. It is reported that the anti-apoptotic UFA displays considerable structural specificity and it appears to regulate a signaling pathway that controls the activity of effector caspase enzymes in the cells [43]. UFA protection from apoptosis mediated through a PI3-kinase-dependent signaling pathway, since PI3-K inhibitor abolished the UFA protection [32]. These were supported by our observation that treatment of β-cell with TGSO causes attenuated expression of caspase-3 and increased expression of Akt, the downstream targets of PI3K.
The regulation of apoptosis-related gene expressions suggested that the protect activity of TGSO was via all of the three pathways, namely ER stress pathway, mitochondrial pathway and Fas signal pathway. In ER stress pathway, ATF3, as a member of the ATF/cAMP response element binding protein (CREB) family of transcription factors, its induction was reported to play an important role in β-cell destruction [44,45]. Li et al.[20] found that ATF-3 down-regulate the expression of insulin receptor substrate 2 (IRS2) while Allen-Jennings et al.[46] demonstrated its inhibition of β-cell proliferation. Hartman et al. speculated that ATF3 may in turn affect the ability of NO to modulate the cell death machinery directly or indirectly [21,47]. Burak Kutlu et al. observed that iNOS inhibitor reduced cytokine-induced ATF-3 expression in INS-1β cell [48]. In this investigation, the extreme high expression of ATF-3 gene induced by high glucose was reduced to the comparable level of control which may be a result of declined NO formation. Besides, the p38 expression might illustrate that the anti-apoptotic action played in ER stress pathway was mainly through the p38 branch rather than JNK. In view of the cytochrome c release block and Bcl-2 expression decrease, mitochondrial pathway was reported to contribute to the protection of MUFA against saturated fatty acid-induced apoptosis in human β-cells [31]. In this research, the expression of Bcl-2/Bax was declined which implied that the protection of TGSO, containing 87.02% UFA, against high glucose was via mitochondrial pathway. In addition to these two pathways, the down-regulation of Fas gene revealed that Fas signal pathway was involved in this anti-apoptotic activity as well.
Conclusions
TGSO protected the Rin-m5F β-cell from high glucose-induced β-cell apoptosis. Its anti-apoptotic action was mediated via ER stress pathway, mitochondrial pathway and Fas signal pathway. ATF-3 might play an important role in the anti-apoptotic activity of TGSO.
Abbreviations
TGSO: Thorn grape seeds oil; CO2: Supercritical carbon dioxide; GC/MS: Gas Chromatography/Mass Spectrometry; ELISA: Enzyme-Linked Immunosorbent Assay; qRT-PCR: Quantitative reverse transcription polymerase chain reaction; SFA: Saturated fatty acids; UFA: Unsaturated fatty acids; PUFA: Polyunsaturated fatty acid; MUFA: Monounsaturated fatty acid; ER: Endoplasmic reticulum; TNFR-1: Tumor necrosis factor receptor-1; DISC: Death inducing signaling complex; NF-kB: Nuclear Factor-kappa B; JNK: c-Jun N-terminal kinase; ATF-3: Activating transcription factor-3; iNOS: Inducible nitric oxide synthase; NO: Nitric oxide; RPM: Revolutions per minute; BSA: Bovine serum albumin; FACS: Fluorescence activated cell sorting; PBS: Phosphate buffered saline.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
DBL conceived this study, participated in its design and coordination, and revised the manuscript. XHL undertook the TGSO extraction, cell culture and statistical analysis, and drafted the manuscript. XCK undertook the qRT-PCR and drafted the manuscript. LMZ, JL participated in the cell culture and TGSO extraction, respectively, while YY designed the primers. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Xihu Lai, Email: laixihu@126.com.
Xincong Kang, Email: kangxincong@163.com.
Luman Zeng, Email: saytoluman@163.com.
Jian Li, Email: lijian1013@aliyun.com.
Yan Yang, Email: hnndyydy@163.com.
Dongbo Liu, Email: chinasaga@163.com.
Acknowledgements
This work was supported by the projects in the National Science & Technology Pillar Program during the Twelfth Five-year Plan Period (2012BAD33B11) and Program of International Science & Technology Cooperation of Ministry of Science and Technology (2013DFG32060).
References
- American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32(Suppl 1):S62–S67. doi: 10.2337/dc09-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahren B. Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nat Rev Drug Discov. 2009;8(5):369–385. doi: 10.1038/nrd2782. [DOI] [PubMed] [Google Scholar]
- Thomas HE, McKenzie MD, Angstetra E, Campbell PD, Kay TW. Beta cell apoptosis in diabetes. Apoptosis. 2009;14(12):1389–1404. doi: 10.1007/s10495-009-0339-5. [DOI] [PubMed] [Google Scholar]
- Bensellam M, Laybutt DR, Jonas JC. The molecular mechanisms of pancreatic beta-cell glucotoxicity: recent findings and future research directions. Mol Cell Endocrinol. 2012;364(1–2):1–27. doi: 10.1016/j.mce.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Donath MY, Storling J, Maedler K, Mandrup-Poulsen T. Inflammatory mediators and islet beta-cell failure: a link between type 1 and type 2 diabetes. J Mol Med. 2003;81(8):455–470. doi: 10.1007/s00109-003-0450-y. [DOI] [PubMed] [Google Scholar]
- Hui H, Dotta F, Di Mario U, Perfetti R. Role of caspases in the regulation of apoptotic pancreatic islet beta-cells death. J Cell Physiol. 2004;200(2):177–200. doi: 10.1002/jcp.20021. [DOI] [PubMed] [Google Scholar]
- Lee SC, Pervaiz S. Apoptosis in the pathophysiology of diabetes mellitus. Int J Biochem Cell Biol. 2007;39(3):497–504. doi: 10.1016/j.biocel.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114(2):181–190. doi: 10.1016/S0092-8674(03)00521-X. [DOI] [PubMed] [Google Scholar]
- Grullich C, Sullards MC, Fuks Z, Merrill AH Jr, Kolesnick R. CD95(Fas/APO-1) signals ceramide generation independent of the effector stage of apoptosis. J Biol Chem. 2000;275(12):8650–8656. doi: 10.1074/jbc.275.12.8650. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281(5381):1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- Eldor R, Yeffet A, Baum K, Doviner V, Amar D, Ben-Neriah Y, Christofori G, Peled A, Carel JC, Boitard C. et al. Conditional and specific NF-kappaB blockade protects pancreatic beta cells from diabetogenic agents. Proc Natl Acad Sci USA. 2006;103(13):5072–5077. doi: 10.1073/pnas.0508166103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr I, Debatin KM. Cellular stress response and apoptosis in cancer therapy. Blood. 2001;98(9):2603–2614. doi: 10.1182/blood.V98.9.2603. [DOI] [PubMed] [Google Scholar]
- Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86(1):147–157. doi: 10.1016/S0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- Zou H, Li Y, Liu X, Wang X. An APAF-1.cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem. 1999;274(17):11549–11556. doi: 10.1074/jbc.274.17.11549. [DOI] [PubMed] [Google Scholar]
- Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102(1):33–42. doi: 10.1016/S0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- Verhagen AM, Ekert PG, Pakusch M, Silke J, Connolly LM, Reid GE, Moritz RL, Simpson RJ, Vaux DL. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell. 2000;102(1):43–53. doi: 10.1016/S0092-8674(00)00009-X. [DOI] [PubMed] [Google Scholar]
- Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M. et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397(6718):441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- Li LY, Luo X, Wang X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature. 2001;412(6842):95–99. doi: 10.1038/35083620. [DOI] [PubMed] [Google Scholar]
- Sundar Rajan S, Srinivasan V, Balasubramanyam M, Tatu U. Endoplasmic reticulum (ER) stress & diabetes. Indian J Med Res. 2007;125(3):411–424. [PubMed] [Google Scholar]
- Li D, Yin X, Zmuda EJ, Wolford CC, Dong X, White MF, Hai T. The repression of IRS2 gene by ATF3, a stress-inducible gene, contributes to pancreatic beta-cell apoptosis. Diabetes. 2008;57(3):635–644. doi: 10.2337/db07-0717. [DOI] [PubMed] [Google Scholar]
- Hartman MG, Lu D, Kim ML, Kociba GJ, Shukri T, Buteau J, Wang X, Frankel WL, Guttridge D, Prentki M. et al. Role for activating transcription factor 3 in stress-induced beta-cell apoptosis. Mol Cell Biol. 2004;24(13):5721–5732. doi: 10.1128/MCB.24.13.5721-5732.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Matsuzawa A, Nishitoh H, Tobiume K, Kishida S, Ninomiya-Tsuji J, Matsumoto K, Ichijo H. Involvement of ASK1 in Ca2 + -induced p38 MAP kinase activation. EMBO Rep. 2004;5(2):161–166. doi: 10.1038/sj.embor.7400072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishigaki S, Fonseca SG, Oslowski CM, Jurczyk A, Shearstone JR, Zhu LJ, Permutt MA, Greiner DL, Bortell R, Urano F. AATF mediates an antiapoptotic effect of the unfolded protein response through transcriptional regulation of AKT1. Cell Death Differ. 2010;17(5):774–786. doi: 10.1038/cdd.2009.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Ichikawa F, Ishiyama-Shigemoto S, Yuan X, Nonaka K. Essential role of caspase-3 in apoptosis of mouse beta-cells transfected with human Fas. Diabetes. 1999;48(3):478–483. doi: 10.2337/diabetes.48.3.478. [DOI] [PubMed] [Google Scholar]
- Salvesen GS, Dixit VM. Caspases: intracellular signaling by proteolysis. Cell. 1997;91(4):443–446. doi: 10.1016/S0092-8674(00)80430-4. [DOI] [PubMed] [Google Scholar]
- Ghavami S, Hashemi M, Ande SR, Yeganeh B, Xiao W, Eshraghi M, Bus CJ, Kadkhoda K, Wiechec E, Halayko AJ. et al. Apoptosis and cancer: mutations within caspase genes. J Med Genet. 2009;46(8):497–510. doi: 10.1136/jmg.2009.066944. [DOI] [PubMed] [Google Scholar]
- Liu D, Darville M, Eizirik DL. Double-stranded ribonucleic acid (RNA) induces beta-cell Fas messenger RNA expression and increases cytokine-induced beta-cell apoptosis. Endocrinology. 2001;142(6):2593–2599. doi: 10.1210/endo.142.6.8188. [DOI] [PubMed] [Google Scholar]
- Eizirik DL, Pavlovic D. Is there a role for nitric oxide in beta-cell dysfunction and damage in IDDM? Diabetes Metab Rev. 1997;13(4):293–307. doi: 10.1002/(SICI)1099-0895(199712)13:4<293::AID-DMR195>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Nolan CJ, Madiraju MS, Delghingaro-Augusto V, Peyot ML, Prentki M. Fatty acid signaling in the beta-cell and insulin secretion. Diabetes. 2006;55(Suppl 2):S16–S23. doi: 10.2337/db06-s003. [DOI] [PubMed] [Google Scholar]
- Zhao YF, Pei J, Chen C. Activation of ATP-sensitive potassium channels in rat pancreatic beta-cells by linoleic acid through both intracellular metabolites and membrane receptor signalling pathway. J Endocrinol. 2008;198(3):533–540. doi: 10.1677/JOE-08-0105. [DOI] [PubMed] [Google Scholar]
- Maedler K, Oberholzer J, Bucher P, Spinas GA, Donath MY. Monounsaturated fatty acids prevent the deleterious effects of palmitate and high glucose on human pancreatic beta-cell turnover and function. Diabetes. 2003;52(3):726–733. doi: 10.2337/diabetes.52.3.726. [DOI] [PubMed] [Google Scholar]
- Beeharry N, Chambers JA, Green IC. Fatty acid protection from palmitic acid-induced apoptosis is lost following PI3-kinase inhibition. Apoptosis. 2004;9(5):599–607. doi: 10.1023/B:APPT.0000038039.82506.0c. [DOI] [PubMed] [Google Scholar]
- Krishna Mohan I, Das UN. Prevention of chemically induced diabetes mellitus in experimental animals by polyunsaturated fatty acids. Nutrition. 2001;17(2):126–151. doi: 10.1016/S0899-9007(00)00468-8. [DOI] [PubMed] [Google Scholar]
- Diakogiannaki E, Welters HJ, Morgan NG. Differential regulation of the endoplasmic reticulum stress response in pancreatic beta-cells exposed to long-chain saturated and monounsaturated fatty acids. J Endocrinol. 2008;197(3):553–563. doi: 10.1677/JOE-08-0041. [DOI] [PubMed] [Google Scholar]
- Cheng Z. Extraction, Purification and Antioxidant Activities of Procyanidin from Seeds of Spine Grape (V. Davidii Foex.) Hunan province, P. R China: Hunan Agricultural University; 2007. [Google Scholar]
- Rencai Wang XX, Fusheng H, Zhitao C, Dongbo L, Jianwen O. Study on functional composition and pharmacological effect of vitis davidii foex. Prog Mod Biomed. 2008;8:1321–1324. [Google Scholar]
- Dongbo L. Study of Active Ingredients Extracted from Horticultural Plants with Anti-Diabetic Function and Hypoglycemic Function of Herbal Formul. Postdoctoral Graduation Thesis. Hunan province, P. R China: Hunan Agricultural University; 2010. [Google Scholar]
- Chen F, Chen Y, Kang X, Zhou Z, Zhang Z, Liu D. Anti-apoptotic function and mechanism of ginseng saponins in Rattus pancreatic beta-cells. Biol Pharm Bull. 2012;35(9):1568–1573. doi: 10.1248/bpb.b12-00461. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C (T) method. Nat Protoc. 2008;3(6):1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Carlsson C, Borg LA, Welsh N. Sodium palmitate induces partial mitochondrial uncoupling and reactive oxygen species in rat pancreatic islets in vitro. Endocrinology. 1999;140(8):3422–3428. doi: 10.1210/endo.140.8.6908. [DOI] [PubMed] [Google Scholar]
- Listenberger LL, Ory DS, Schaffer JE. Palmitate-induced apoptosis can occur through a ceramide-independent pathway. J Biol Chem. 2001;276(18):14890–14895. doi: 10.1074/jbc.M010286200. [DOI] [PubMed] [Google Scholar]
- Maedler K, Spinas GA, Dyntar D, Moritz W, Kaiser N, Donath MY. Distinct effects of saturated and monounsaturated fatty acids on beta-cell turnover and function. Diabetes. 2001;50(1):69–76. doi: 10.2337/diabetes.50.1.69. [DOI] [PubMed] [Google Scholar]
- Morgan NG, Dhayal S, Diakogiannaki E, Welters HJ. The cytoprotective actions of long-chain mono-unsaturated fatty acids in pancreatic beta-cells. Biochem Soc Trans. 2008;36(Pt 5):905–908. doi: 10.1042/BST0360905. [DOI] [PubMed] [Google Scholar]
- Hai T, Hartman MG. The molecular biology and nomenclature of the activating transcription factor/cAMP responsive element binding family of transcription factors: activating transcription factor proteins and homeostasis. Gene. 2001;273(1):1–11. doi: 10.1016/S0378-1119(01)00551-0. [DOI] [PubMed] [Google Scholar]
- Hai T, Wolfgang CD, Marsee DK, Allen AE, Sivaprasad U. ATF3 and stress responses. Gene Expr. 1999;7(4–6):321–335. [PMC free article] [PubMed] [Google Scholar]
- Allen-Jennings AE, Hartman MG, Kociba GJ, Hai T. The roles of ATF3 in glucose homeostasis. A transgenic mouse model with liver dysfunction and defects in endocrine pancreas. J Biol Chem. 2001;276(31):29507–29514. doi: 10.1074/jbc.M100986200. [DOI] [PubMed] [Google Scholar]
- Oyadomari S, Takeda K, Takiguchi M, Gotoh T, Matsumoto M, Wada I, Akira S, Araki E, Mori M. Nitric oxide-induced apoptosis in pancreatic beta cells is mediated by the endoplasmic reticulum stress pathway. Proc Natl Acad Sci USA. 2001;98(19):10845–10850. doi: 10.1073/pnas.191207498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutlu B, Cardozo AK, Darville MI, Kruhoffer M, Magnusson N, Orntoft T, Eizirik DL. Discovery of gene networks regulating cytokine-induced dysfunction and apoptosis in insulin-producing INS-1 cells. Diabetes. 2003;52(11):2701–2719. doi: 10.2337/diabetes.52.11.2701. [DOI] [PubMed] [Google Scholar]


