Abstract
Objective:
Stress evokes thoughts about alcohol and enhances alcohol’s rewarding value in drinkers who use alcohol to cope with negative affect. The present study extends prior research by examining whether this effect applies to actual alcohol consumption following a stressor and whether individuals with high and low coping motives for drinking differ in stress reactivity.
Method:
Nondependent drinkers with high scores ( > 1 SD above national norms) on the coping motives subscale on the Drinking Motives Questionnaire (n = 41; 46% women) were enrolled along with age- and gender-matched nondependent drinkers with low coping motives (n = 41). Participants were randomized to receive the Trier Social Stress Test or a no-stress control condition. Following the stress manipulation, participants could consume up to 473 ml of beer in a “taste test,” a covert measure of alcohol consumption. Stress reactivity was measured with both objective and subjective indices, and milliliters of beer consumed was the alcohol-relevant outcome.
Results:
Participants with high coping motives showed a less robust stress response to the Trier Social Stress Test than participants with low coping motives for drinking. However, the stressor did not result in greater consumption of alcohol (i.e., no main effect of stress induction) or differential drinking in the two motive groups (i.e., no Stressor × Coping Motive group interaction).
Conclusions:
Findings suggest that nondependent drinkers with and without coping motives for drinking may experience a stress provocation differently, but exposure to a standardized social stressor does not lead to differential drinking in these groups in a clinical laboratory setting.
Drinking motives, or reasons for using alcohol, are both a powerful and potentially modifiable predictor of future alcohol problems (Cooper, 1994; Cox and Klinger, 1988). Individuals who endorse coping motives for drinking (i.e., drinking to alleviate negative mood) have an increased risk for alcohol-related problems and an increased risk for developing alcohol dependence compared with individuals who drink primarily for enhancement (i.e., drinking to augment positive mood states) or social motives (i.e., drinking to obtain social rewards) (Carey and Correia, 1997; Cooper et al., 1988, 1995; Holahan et al., 2001; Kassel et al., 2000; Merrill and Read, 2010). Evidence also suggests that drinking to cope may mediate the relationship between low response to alcohol and alcohol problems (Schuckit et al., 2012) and the relationship between impulsivity and alcohol problems (Magid et al., 2007).
Coping motives appear to be a risk factor for alcohol dependence even when severity of depression and frequency of negative alcohol consequences are statistically controlled (Carpenter and Hasin, 1999), suggesting that the risk conferred by coping motives is not a result of coping-motivated drinkers having greater affective distress at the outset or of coping-motivated drinkers having greater severity of alcohol problems at the time of initial assessment. Given the substantial evidence that coping-motivated drinkers are truly an at-risk group for future alcohol use disorders, understanding the mechanisms of this effect may help direct interventions to prevent escalation to alcohol dependence.
Regarding potential mechanisms, individuals who endorse coping motives for drinking may experience alcohol as a more powerful reinforcer following feelings of anxiety or distress than individuals without coping motives for drinking, as predicted by social learning theory (Maisto et al., 1999). As a consequence, individuals with coping motives may be more likely to drink and to drink heavily when such internal cues are present. Another possibility is that individuals with strong coping motives for drinking may experience stressors differently than their peers.
Clinical laboratory studies are useful for examining these research issues because they afford the opportunity to apply a stressor and compare groups of interest on both stress reactivity and subsequent alcohol-related behaviors or cognitions. Several such studies have been conducted that compare nondependent drinkers with different drinking motives on their response to a stressor and/or negative affect induction procedure. Field and Quigley (2009) found that a speech stressor increased self-reported anxiety only in participants with high coping motives for drinking. A similar result was found by other investigators using a musical mood induction technique to induce negative affect (Grant et al., 2007), whereby only coping-motivated (and not enhancement-motivated) drinkers reported the expected rise in anxiety. Regarding alcohol-related outcomes, clinical laboratory studies have found that coping motives moderate the impact of stress induction on positive alcohol expectancies (Birch et al., 2004; Grant and Stewart, 2007), attentional bias for alcohol cues (Field and Powell, 2007; Field and Quigley, 2009; Grant et al., 2007), the semantic activation of alcohol concepts in memory (Stewart et al., 2002), and the reinforcing value of alcohol versus money (Rousseau et al., 2011).
In the study most similar to the present one, Rousseau and colleagues (2011) randomized nondependent drinkers to either a negative mood induction condition (in which participants were instructed to think about and make a list of words or phrases that reminded them of their most distressing life event) or to a neutral mood condition. Participants were asked to identify a dollar amount at which they would forego two standard drinks and instead choose money (an increasing amount between $0 and $20.50). The mood condition alone did not influence the relative reinforcing value of alcohol, but there was an interaction between mood condition and coping motives, whereby negative mood significantly predicted choice of alcohol over money among participants who reported higher coping motives for drinking. Rousseau et al.’s study was the first to demonstrate with controlled laboratory procedures that coping motives and negative mood interact to increase the relative reinforcing value of alcohol, an outcome that suggests a similar effect would be observed if participants were given the opportunity to consume alcohol ad libitum.
Thus, the literature suggests that individuals with strong coping motives for drinking may be more sensitive to stress provocation or negative mood induction, and they may be more motivated to drink following induction of negative affect than individuals with low coping motives. It is interesting to note that actual alcohol use has not yet been studied as an outcome, although drinking is ultimately the behavior of interest. In addition, studies examining how different motive groups respond to a stressor or negative mood provocation have used only self-reported distress to capture group differences; it is unknown whether groups differ on objective measures of stress response such as heart rate, blood pressure, or cortisol.
To advance this body of research, the present study was conducted to examine stress reactivity with both subjective and objective indices and to examine actual alcohol consumption as the alcohol-relevant outcome. Using a between-subjects design and the Trier Social Stress Test (TSST) as a psychosocial stressor, we tested the hypotheses that nondependent drinkers with high coping motives for drinking would have a more pronounced response to the TSST and that they would drink more alcohol following the TSST than individuals with low coping motives.
Method
Participants
Because the study was conducted to examine potential mechanisms by which coping-motivated drinkers may be at risk for developing alcohol problems, individuals with current alcohol use disorders were excluded. However, because the study involved ad libitum drinking, it was important to include individuals who consumed alcohol (and specifically, beer) regularly. Thus, nondependent, nonabstinent drinkers were recruited through advertisements in the community and screened by telephone (N = 210). Individuals who passed an initial telephone screening interview (n = 112, 53% of those phone screened) were invited for an in-person interview, at which time they signed an informed consent form approved by the university’s institutional review board.
Inclusion criteria were (a) ages 21–40 years (the lower age limit was selected because alcohol is administered in the protocol; the upper age limit of 40 was selected because the study was proposed to examine mechanisms of risk for developing alcohol problems, and alcohol dependence typically onsets by the mid-40s; see Grant et al., 2004; Vergés et al., 2012), (b) alcohol use on at least 5 days in the prior 30 days (to exclude infrequent drinkers), and (c) a liking for beer (because beer was the alcoholic beverage presented in the challenge).
Participants were excluded if they met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV; American Psychiatric Association), criteria for any of the following: current alcohol abuse; current or lifetime alcohol dependence; current abuse or dependence on other drugs, including nicotine; or other current major Axis I disorders. Participants were also excluded if they reported health conditions (e.g., hypertension, chronic pain, severe obesity) or use of medications (e.g., antihistamines, corticosteroids) that alter hypothalamic-pituitary-adrenal axis functioning. Women who were pregnant or nursing were excluded.
Assessment battery
Individuals were administered an assessment battery that included the Timeline Followback, a calendar-based strategy to assess quantity and frequency of past-month drinking that yields high test-retest reliability (Sobell and Sobell, 1992), and the Social Phobia Inventory (Connor et al., 2000) and Anxiety Sensitivity Index (Peterson and Heilbronner, 1987) to assess individual differences in social anxiety symptoms and sensitivity to the experience of anxiety, respectively. Both the Social Phobia Inventory and the Anxiety Sensitivity Index showed good internal consistency in our sample (Cronbach’s α ≥ .82).
The Drinking Motives Questionnaire (DMQ; Cooper et al., 1992b) was also administered during this visit. The DMQ is a validated 15-item instrument that assesses the relative frequency of alcohol use (on a 4-point scale) for reasons that relate to three different motives—coping, social facilitation, and enhancement. For the present study, we were interested specifically in the coping motives subscale. The DMQ coping subscale showed acceptable internal consistency in our sample (Cronbach’s α = .70). We classified individuals as having high coping motives for drinking if they scored 1.73 or greater on the coping subscale (of 4.0). This value reflects a score that is more than 1 SD above the normative mean as presented in Cooper et al. (1992b) and thus would put an individual above the 80th percentile on the coping motives subscale. With this classification, 41 individuals (19 women) qualified as high coping-motivated drinkers (M = 1.98, SD = 0.22, range: 1.80-2.80). We selected age- (within 3 years) and gender-matched controls who scored less than 1.73 on the coping subscale to populate the group with low coping motives for drinking (n = 41, 19 women) (for this group, M = 1.33, SD = 0.20, range: 1.00-1.60). The grand mean for the entire sample (n = 82) was 1.65 (SD = 0.39).
A date for the challenge session was determined at the end of the in-person visit. In addition, women were asked about their use of birth control pills and the date of their last menstrual cycle, as well as the typical length of their cycle. This information was used to attempt to schedule free-cycling women for testing during the luteal phase (days 21-25 of their menstrual cycle) when cortisol response to the TSST is most similar to that of men (Kirschbaum et al., 1999), thereby reducing variability within groups. Women using oral contraceptives were also included and scheduled for testing during the week before their period. Participants were instructed to eat lunch on the day of the challenge, to complete the meal by 1:00 p.m., and to avoid alcohol on that day.
Laboratory challenge
The laboratory session was typically held within 1 month of the baseline visit (M = 16 days, SD = 13). Participants arrived at 4:30 p.m. and were assessed for compliance with pre-challenge instructions. All participants were compliant. Each participant was administered a breath alcohol test, and women provided a urine sample to test for pregnancy (all were negative). The participant was fitted with a heart rate monitor, and saliva collection procedures (to measure cortisol) were explained. A video camera was mounted unobtrusively in a corner of the room; the participant was informed of its presence during the consent process. The camera was used to collect data for the secondary drinking variables, described below. The participant was instructed to relax and read a travel magazine until 5:00 p.m.
Trier Social Stress Test.
At 5:00 p.m., participants randomized to the no-stress control condition (n = 20 high coping-motivated drinkers; n = 22 low coping-motivated drinkers) continued to read the travel magazine until 5:15 p.m. Participants randomized to receive the stressor were administered the TSST (Kirschbaum et al., 1993), which is considered the gold standard of standardized psychosocial stressors for eliciting a robust stress response (Dickerson and Kemeny, 2004) and is equally effective in men and women (Kudielka et al., 2004). The exact procedure used has been described in detail elsewhere (Thomas et al., 2011b). In brief, each participant prepared for 5 minutes for an impending mock job interview (with a countdown clock in view). Following the preparation/anticipation phase, the participant was instructed to speak about his or her particular qualifications for the position to an audience of three confederates, who remained nonresponsive (5:05-5:10 p.m.). At 5:10 p.m., the participant was instructed to perform a serial subtraction task to the audience until instructed to stop (5 minutes). In total, the TSST lasted 15 minutes.
Taste-test procedure.
The beer taste-test procedure was used in the study because it is an unobtrusive method of measuring alcohol consumption that minimizes demand effects (George et al., 1988; Marlatt et al., 1973) and has been effectively used to index alcohol wanting (Field and Eastwood, 2005; Hobbs et al., 2005). Immediately following the end of the stressor/control exposure (5:15 p.m.) and the subsequent stress response measurement (5:15-5:18 p.m., as described below), the experimenter provided instructions for the taste test to all participants, modeled after the procedure used by Caudill and Marlatt (1975). The participant was informed that two glasses of beer would soon be provided and to taste each beer to determine whether they were identical or different. The participant was told to drink as much as needed to make a decision and that, to receive bonus compensation ($10), his or her judgment must be accurate. The instruction set took approximately 3 minutes, following which the experimenter left the room to prepare the two glasses; the experimenter returned to collect the second post-stress response measurement at 5:25 (as described below).
At 5:30 p.m., the experimenter presented the two glasses of beer, glasses A and B (each containing 8 oz. of cold Budweiser beer for a total of 473 ml)—the equivalent of 1.5 standard drinks. A third glass containing 8 oz. of water was presented to remove the confound of increased thirst that may follow stress induction (de Wit et al., 2003). The experimenter informed the participant that he or she had 10 minutes to complete the task and make a determination about the beers. The experimenter left the room and returned after 10 minutes to collect the glasses and provide the participants with a sham response sheet (on which the subject reported his or her decision). Participants were discharged at 6:30 p.m. unless the breath alcohol reading exceeded .03% (n = 3; all were discharged by 6:45 p.m.). Participants were compensated $100 ($90 plus the $10 bonus, which all participants received). No adverse events occurred in the conduct of the study.
Use of deception.
Deception of participants was used to minimize demand bias. Participants were told that the study was conducted to examine how personality traits influence one’s ability to detect subtle differences in alcoholic flavors. The TSST (which was called a behavioral extraversion test on the informed consent form) was explained as a behavioral test of extraversion and working memory (“to see how outgoing you are, and how well you can solve problems when you are speaking in front of others”). The details of what the participant would be asked to do for this behavioral test were fully presented in the consent form. The taste-test task was used to obscure the true dependent measure collected (amount of alcohol consumed). It is important to note that participants were not deceived about what their participation in the study would entail or about the specific requirements of the TSST.
Outcome measures
Stress reactivity.
Stress response was collected five times for each participant—at baseline (4:55 p.m.), and 15 (5:15 p.m.), 25 (5:25 p.m.), 45 (5:45 p.m.), and 60 minutes (6:00 p.m.) following initiation of the stressor. Stress reactivity was quantified with a self-report measure of subjective distress (the Subjective Units of Distress Scale [SUDS], 0-10 Likert-type scale), and objectively with heart rate (HR, in beats per minute), mean arterial pressure (MAP, mm/Hg), and salivary cortisol (µg/dl).
Saliva samples were collected with cotton salivettes for 2 minutes at each assessment and were stored within 2 hours at -20 °C. Samples were shipped on dry ice to Salimetrics (State College, PA) for enzyme immunoassay. The test has a lower limit of sensitivity of 0.003 µg/dL, standard curve range from 0.012 µg/dL to 3.0 µg/dL, an average intra-assay coefficient of variation of 3.5%, and an average inter-assay coefficient of variation of 5.1%.
Alcohol consumption.
The combined contents of glasses A and B were measured (in milliliters) before and following the taste-test procedure, and a difference score was computed to measure milliliters of beer consumed, which was the primary alcohol outcome variable. Secondary variables were latency to first sip of beer (urgency to drinking following the stress manipulation), average sip size (total ml/number of sips, an index of gulping), and median latency between sips (speed of consumption), which were assessed by scoring the video recording of participants’ taste-test behavior. Two independent raters provided interrater reliabilities of .92 or higher for quantifying the secondary variables.
Data analysis
Before running substantive analyses, demographic variables and potential covariates were compared with analysis of variance (ANOVA) or logistic regression for effects of motive group, TSST group, and their interaction. An equal number of men and women were included in the final sample (and distributed equally across groups) to explore whether gender was a relevant factor in the predicted Stressor × Coping Motive Group interaction, as suggested by Cooper and colleagues (1992a). Analysis of covariance (ANCOVA) on stress reactivity was used as a manipulation check of the effect of the stressor and to test the two-way interaction of stress and motive group and the three-way interaction (Stress × Motive Group × Gender). Models included stress group (two levels: TSST vs. no stress), motive group (two levels: high vs. low coping motives), and gender as between-groups factors. Stress reactivity was analyzed using the first poststress assessment (5:15 p.m.) as the dependent variable with baseline values on the variable of interest covaried. For cortisol, both the first poststress assessment and the second poststress assessment were analyzed because salivary cortisol response to the TSST typically peaks 10 minutes following the end of the stressor (Kirschbaum et al., 1993), which was when the second poststress assessment was collected (5:25 p.m.).
ANCOVA was also used to test the effects of the two-way interaction of stress and motive group and the three-way interaction (Stress × Motive Group × Gender) on alcohol consumption. Drinks per drinking day in the 30 days before the pre-challenge assessment visit was significantly correlated with milliliters of beer consumed in the taste test (r = .32, p = .003); therefore, drinks per drinking day was included as a covariate in all analyses predicting drinking outcomes. There were no main effects or interaction effects for stress group, motive group, or gender on amount of water consumed in the taste task (all ps > .20), nor was milliliters of water consumed correlated with milliliters of beer consumed (r = -.14, p = .22). Therefore, there was no need to control for milliliters of water consumed in the analysis of drinking outcomes. All tests were conducted as two-tailed tests with α = .05.
Results
Descriptives
Table 1 shows demographic and psychological assessment data for participants. Participants were in their mid-20s and most were White, college educated, and never married. Consistent with more liberal use of alcohol during young adulthood (80% of participants were younger than age 30 years), they reported drinking on about 13 days in the 30 days before participating in the study and drank, on average, about five drinks per occasion. Analyses were conducted to determine whether there were any differences between the two motive groups. As designed by the eligibility assessment, drinkers with high coping motives had significantly higher scores on the coping subscale of the DMQ than those with low coping motives. No group differences were present between the stress and no stress groups, nor were there any significant interaction effects between motive group and stress group on these measures (all ps > .10). Table 1 shows groups by coping motive status.
Table 1.
Demographic and descriptive measures
| Variable | High coping motives group (n = 41) | Low coping motives group (n = 41) | Total | p |
| Demographics | ||||
| Age, in years, M (SD) | 27.0(5.16) | 26.5 (4.47) | 26.7 (4.81) | n.s. |
| Women, % | 46% | 46% | 46% | n.s. |
| White, % | 95% | 88% | 92% | n.s. |
| Employed full time, % | 44% | 41% | 43% | n.s. |
| College graduate, % | 71% | 78% | 74% | n.s. |
| Never married, % | 76% | 78% | 77% | n.s. |
| Positive family history | ||||
| alcohol problems, % | 15% | 18% | 16% | n.s. |
| Baseline alcohol use, TLFB, M (SD) | ||||
| No. of drinks per drinking day | 5.3(3.11) | 4.8 (2.64) | 5.1 (2.88) | n.s. |
| No. of drinking days, past 30 | 13.3 (4.92) | 13.3 (4.79) | 13.3 (4.82) | n.s. |
| Drinking motives, DMQ, M (SD) | ||||
| Coping | 2.0 (0.22) | 1.3 (0.20) | 1.7 (0.39) | < .01 |
| Enhancement | 2.2 (0.57) | 2.1 (0.60) | 2.2 (0.58) | n.s. |
| Social facilitation | 2.6 (0.67) | 2.5 (0.55) | 2.5 (0.61) | n.s. |
| Anxiety measures, M (SD) | ||||
| Anxiety sensitivity (ASI) | 14.0 (7.82) | 14.0 (7.00) | 14.0 (7.37) | n.s. |
| Social phobia (SPIN) | 12.1 (8.21) | 11.8(8.66) | 11.9(8.39) | n.s. |
Notes: Group differences for categorical variables were evaluated with logistic regression. Group means (SD) for all other variables were evaluated with analysis of variance. Motive groups differed by coping motive scores (by design). There were no group differences between groups randomized to the TSST nor a significant interaction between Trier Social Stress Test and motive group. TLFB = Timeline Followback; DMQ = Drinking Motives Questionnaire, where subscale scores range from 1 to 4, with higher scores reflecting high endorsement of that motive for drinking; ASI = Anxiety Sensitivity Index, where scores range from 0 to 64, with higher scores reflecting greater propensity to interpret feelings of anxiety as threatening; SPIN = Social Phobia Inventory, where scores range from 0 to 68; scores 31 or greater reflect moderate social anxiety.
Stress response to the Trier Social Stress Test
There was a significant effect of the stress manipulation (TSST > no stress) on subjective distress, heart rate, and mean arterial pressure, F(1, 73) ≥ 21.9, p < .001. For cortisol, the effect reached trend-level significance at the first poststress assessment, F(1, 73) = 2.88, p = .09; when cortisol values at the second poststress assessment (5:25 p.m.) were analyzed, the main effect of the stress manipulation was confirmed, F(1, 73) = 12.22, p = .001. Effect sizes (Cohen’s d) for cortisol were small for the first poststress measure (d = 0.14) and moderate for the second poststress measure (d = 0.61). Effects were large to very large for HR (d = 1.0), MAP (d = 1.3), and SUDS (d = 2.4).
Effects of motive group and interactions with Trier Social Stress Test on stress reactivity
The ANCOVAs showed no main effects of gender or any two- or three-way interactions with gender on any stress-reactivity measure (all F values < 3.5, p values > .05); therefore, gender was excluded from the model for subsequent analyses. There was no main effect of coping motive group on HR, MAP, or SUDS; there was a trend main effect of motive group on cortisol at both the first and second assessment time points, Fs(1, 77) ≥ 3.62, ps ≤ .07, where the low coping motive group was greater than the high coping motive group.
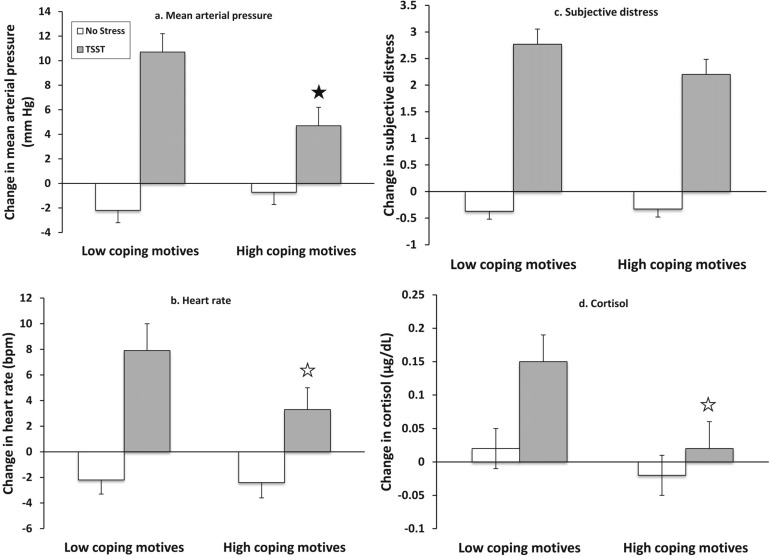
There was a significant or trend-level effect for Motive Group × Stress Group interaction for mean arterial pressure, F(1, 77) = 6.99, p = .01, and heart rate, F(1, 77) = 3.00, p = .09. The interaction failed to reach significance for subjective distress and cortisol (ps > .10). Post hoc ANOVAs conducted only on those who received the TSST showed a significant effect of motive group on mean arterial pressure, F(1, 40) = 5.70, p = .02, and a trend-level effect on cortisol at the second assessment time point (F = 2.92) and heart rate (F = 2.78), ps ≤ .10, reflecting that individuals with high coping motives for drinking generally showed a less robust stress response to the TSST than those with low coping motives. Group means shown in Figures 1a-1d (error bars show SE) show a consistent pattern across all four stress-reactivity indices.
Figure 1.
Four indices of stress reactivity by motive group. Means (error bars are SE) show change from baseline to the first post-stress assessment, collected immediately following the end of the Trier Social Stress Test for (a) mean arterial pressure, (b) heart rate (bpm = beats per minute), and (c) subjective distress. For (d) salivary cortisol, means show change from baseline to the second poststress assessment to capture peak cortisol response. The stress manipulation, the TSST, produced significant effects on all measures (all ps < .01). There were consistent effects for individuals with high coping motives to show a less robust stress response to the TSST compared with individuals with low coping motives. Specifically, post hoc tests were significant for mean arterial pressure (filled star, p = .02) and showed trend level significance for cortisol and heart rate (open star, ps ≤ .10).
Effects of Trier Social Stress Test, motive group, and gender on drinking
The primary hypothesis of the study—that the TSST would induce greater drinking in individuals with high versus low coping motives for drinking—was not supported, Stress Group × Motive Group: F(1, 77) < 1, p = .40, nor was the interaction significant when gender was included in the model, F(1, 73) < 1, p = .54. In a similar manner, when only participants who received the TSST were examined, there was not a main effect of coping motive, F(1, 39) < 1, p = .91.
There were no main effects of either the TSST or motive group on milliliters of beer consumed in the taste test, Fs(1, 77) < 1.1, ps > .20. There was a main effect of gender, whereby men drank more milliliters of beer than women (means adjusted for drinks per drinking day were 290 ml for men vs. 185 ml for women, p < .001). Because men and women were equally distributed across groups, Table 2 shows adjusted group means for milliliters of beer consumed collapsed across gender.
Table 2.
Milliliters of beer consumed in the taste test, adjusted for drinks per drinking day
| Variable | High coping motives group | Low coping motives group |
| Control group | 215.5 (29.2) | 271.6(30.5) |
| TSST group | 246.4 (29.7) | 251.9(28.3) |
Notes: Beer consumed in milliliters by participants in the Trier Social Stress Test (TSST) versus no-stress control group, by drinking motive group. Values show means (SE) adjusted for drinks per drinking day. There were no significant main or interaction effects of TSST or motive group (all ps > .30).
Discussion
The present study examined whether individuals with high coping motives for drinking show heightened response to a psychosocial stress provocation and/or consume more alcohol following a stressor than individuals with low coping motives for drinking. Both hypotheses were based on prior clinical laboratory studies showing that coping-motivated drinkers reported more affective distress to a stressor than individuals without coping motives (Field and Quigley, 2009; Grant et al., 2007), and stress exposure produced greater activation of alcohol cognitions and increased the rewarding value of alcohol in coping-motivated drinkers (Birch et al., 2004; Rousseau et al., 2011; Stewart et al., 2002). Results from our study suggested that drinkers with high versus low coping motives may respond differently to a stress provocation, although in the opposite direction predicted, where coping-motivated drinkers show a less robust stress response. We did not find any evidence for stress-induced drinking or differential drinking by motive groups following the stressor.
Regarding stress reactivity, the TSST was an effective provocation for both motive groups, as evidenced by both objective measures (heart rate, mean arterial pressure, and salivary cortisol) and subjective distress. Effect size estimates for the effect of the TSST were moderate to large, regardless of motive group, confirming the internal validity of the stress manipulation. Confirmation of stress reactivity to the experimental manipulation is a notable strength of the study because stress reactivity is often confirmed only with self-report (Thomas and Bacon, 2013), and in some studies, evidence that the stressor was effective in participants without high coping motives was lacking (Field and Quigley, 2009; Grant et al., 2007). In our study, both motive groups showed elevated responses to the stress provocation.
The measures of stress reactivity suggested that, contrary to expectations, individuals with high coping motives showed a lower stress response than age- and gender-matched drinkers with low coping motives. It is interesting to note that a similar outcome is reported in studies comparing alcohol-dependent and nondependent drinkers (Adinoff et al., 2005; Sinha et al., 2009; Wand and Dobs, 1991; although Munro and colleagues [2005] found no significant differences). Individuals with positive family history of alcoholism also show different stress reactivity than family history-negative individuals, although studies are mixed in the direction of the effect, with some reporting blunted (Lovallo, 2007; Sorocco et al., 2006) and others reporting exaggerated responses (Uhart et al., 2006; Zimmermann et al., 2004) to a stress provocation. In the present study, neither alcohol dependence nor family history of alcohol problems explained the effects observed because alcohol use disorders were exclusionary, and there were no differences between motive groups on percentage with positive family history (15% and 18% of participants with high and low coping motives, respectively, endorsed a family history of alcoholism). This result, if replicated, could lead to a closer inspection of whether and in whom stress reactivity is a useful marker for revealing risk for future alcohol problems.
Despite effectively inducing a stress response, the TSST did not affect drinking differentially in different motive groups (no Stress Group × Motive Group effect), nor did the TSST increase drinking in general (no main effect). In a similar study, Rousseau and colleagues (2011) also found no main effect of their mood manipulation on enhancing the reinforcing value of alcohol, wherein they induced distress by requesting participants to recall their saddest or most distressing life event and generate a list of words that reminded them of the event. In fact, the evidence in the literature for stress-induced drinking is less compelling than is appreciated. Although it is intuitively appealing to assume that aversive affective states induce drinking in nondependent drinkers (and relapse in alcoholics), true experimental studies conducted specifically to examine this issue in both humans and animals show inconsistent evidence of this phenomenon (for reviews, see Thomas and Bacon, 2013; Becker etal., 2011).
There are some limitations to the study that are relevant in interpreting the results. It is possible that the TSST may not have induced drinking because it does not produce the kinds of emotions that evoke the desire to drink in nondependent individuals. Our decision to use the TSST as the stressor in the study was based on several factors. First, the TSST is generally considered the gold standard for inducing a stress response that is verifiable with both objective and subjective measures (Dickerson and Kemeny, 2004), and because we were interested in stress reactivity as a primary outcome (e.g., to examine motive group differences), the TSST was an attractive choice (see Thomas et al., 2012, for review of clinical laboratory stressors used to study alcohol-stress relationships). Second, in a clinical laboratory study in alcohol-dependent participants, the TSST caused more participants to drink all the alcohol available to them compared with participants in the no-stress control condition (Thomas et al., 2011a); therefore, there is a precedent that the TSST can induce drinking.
Another limitation is the way in which drinking was assessed in the laboratory. We used a sham taste test to covertly measure alcohol consumption (to reduce demand effects), which may have diminished ecological validity. On the other hand, others have used a similar taste-test procedure and have shown results that support its value as a method to measure alcohol wanting and urge to drink (Field and Eastwood, 2005; Hobbs et al., 2005). In addition, in the present study, milliliters of beer consumed during the taste test was significantly and positively correlated with participants’ drinks per drinking day (r = .32, p < .01) and drinks per week (r = .32, p < .01) in the 30 days before participating in the study; therefore, participants’ drinking in the laboratory was consistent with their naturalistic drinking practices.
The two coping motive groups were defined based on an a priori grouping approach, in which a participant was classified as a high coping motivated drinker if his or her score was at the 80th percentile or higher on the coping motives subscale (a score of 1.73 or higher) based on normative data based on the report by Cooper et al. (1992b). The highest score in the low coping motive group was 1.60, and the lowest score in the high coping motive group was 1.80. Although this grouping strategy allowed us to observe some differences in stress reactivity between groups, we did not find any evidence for stress-induced drinking or differential drinking by motive groups following the stressor. It is possible that had we recruited more distinct coping motive groups marked by high and low values on the more extreme ends of the potential distribution, we may have found effects in drinking outcomes.
Negative results could be the result of a type II error. We posit that the lack of hypothesized Stressor × Motive Group effects on drinking was not likely attributable to insufficient power because the amount of beer consumed in the taste task following stress exposure was markedly similar across motive groups (Table 2). Ceiling effects were not problematic—only 12 of 82 participants (less than 15%) consumed all the beer available (only 2 of the 12 were high coping-motivated drinkers in the TSST group). Further, our sample size (n = 82) is comparable to or substantially larger than other clinical laboratory studies in which positive effects were observed (Birch et al., 2004; Field and Quigley, 2009; Grant and Stewart, 2007; Rousseau et al., 2011; Stewart et al., 2002).
In summary, studies conducted on drinkers who use alcohol to cope with negative affect support that exposure to a stressor evokes thoughts about alcohol and enhances alcohol’s rewarding value. Although negative affect, distress, and/or anxiety probably do increase drinking in some individuals (and we expected coping-motivated drinkers would be one such group), this assumption lacks empirical support with clinical laboratory studies; in fact, a reliable model of stress-induced drinking among nondependent drinkers remains elusive. Our findings suggest that individuals with high versus low coping motives for drinking may experience a stress provocation differently, but exposure to a stressor does not lead to subsequent drinking, or differential drinking, in these groups.
Acknowledgments
The authors thank the journal’s anonymous reviewers for their suggestions for the manuscript’s improvement.
Footnotes
This research was supported by National Institute on Alcohol Abuse and Alcoholism Grants AA016289 (R21), AA010761 (P50), and AA007474 (T32).
References
- Adinoff B, Krebaum SR, Chandler PA, Ye W, Brown MB, Williams MJ. Dissection of hypothalamic-pituitary-adrenal axis pathology in 1-month-abstinent alcohol-dependent men, part 1: Adrenocortical and pituitary glucocorticoid responsiveness. Alcoholism: Clinical and Experimental Research. 2005;29:517–527. doi: 10.1097/01.ALC.0000158940.05529.0A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. 4th ed. Washington, DC: Author; 1994. Diagnostic and statistical manual of mental disorders. [Google Scholar]
- Becker HC, Lopez MF, Doremus-Fitzwater TL. Effects of stress on alcohol drinking: A review of animal studies. Psychopharma-cology. 2011;218:131–156. doi: 10.1007/s00213-011-2443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch CD, Stewart SH, Wall AM, McKee SA, Eisnor SJ, Theakston JA. Mood-induced increases in alcohol expectancy strength in internally motivated drinkers. Psychology of Addictive Behaviors. 2004;18:231–238. doi: 10.1037/0893-164X.18.3.231. [DOI] [PubMed] [Google Scholar]
- Carey KB, Correia CJ. Drinking motives predict alcohol-related problems in college students. Journal of Studies on Alcohol. 1997;58:100–105. doi: 10.15288/jsa.1997.58.100. [DOI] [PubMed] [Google Scholar]
- Carpenter KM, Hasin DS. Drinking to cope with negative affect and DSM-IV alcohol use disorders: A test of three alternative explanations. Journal of Studies on Alcohol. 1999;60:694–704. doi: 10.15288/jsa.1999.60.694. [DOI] [PubMed] [Google Scholar]
- Caudill BD, Marlatt GA. Modeling influences in social drinking: An experimental analogue. Journal of Consulting and Clinical Psychology. 1975;43:405–415. doi: 10.1037/h0076689. [DOI] [PubMed] [Google Scholar]
- Connor KM, Davidson JR, Churchill LE, Sherwood A, Foa E, Weisler RH. Psychometric properties of the Social Phobia Inventory (SPIN). New self-rating scale. British Journal of Psychiatry. 2000;176:379–386. doi: 10.1192/bjp.176.4.379. [DOI] [PubMed] [Google Scholar]
- Cooper ML. Motivations for alcohol use among adolescents: Development and validation of a four-factor model. Psychological Assessment. 1994;6:117–128. [Google Scholar]
- Cooper ML, Frone MR, Russell M, Mudar P. Drinking to regulate positive and negative emotions: A motivational model of alcohol use. Journal of Personality and Social Psychology. 1995;69:990–1005. doi: 10.1037//0022-3514.69.5.990. [DOI] [PubMed] [Google Scholar]
- Cooper ML, Russell M, George WH. Coping, expectancies, and alcohol abuse: A test of social learning formulations. Journal of Abnormal Psychology. 1988;97:218–230. doi: 10.1037//0021-843x.97.2.218. [DOI] [PubMed] [Google Scholar]
- Cooper ML, Russell M, Skinner JB, Frone MR, Mudar P. Stress and alcohol use: Moderating effects of gender, coping, and alcohol expectancies. Journal of Abnormal Psychology. 1992a;101:139–152. doi: 10.1037//0021-843x.101.1.139. [DOI] [PubMed] [Google Scholar]
- Cooper ML, Russell M, Skinner JB, Windle M. Development and validation of a three-dimensional measure of drinking motives. Psychological Assessment. 1992b;4:123–132. [Google Scholar]
- Cox WM, Klinger E. A motivational model of alcohol use. Journal of Abnormal Psychology. 1988;97:168–180. doi: 10.1037//0021-843x.97.2.168. [DOI] [PubMed] [Google Scholar]
- de Wit H, Söderpalm AHV, Nikolayev L, Young E. Effects of acute social stress on alcohol consumption in healthy subjects. Alcoholism: Clinical and Experimental Research. 2003;27:1270–1277. doi: 10.1097/01.ALC.0000081617.37539.D6. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Field M, Eastwood B. Experimental manipulation of attentional bias increases the motivation to drink alcohol. Psychopharmacology. 2005;183:350–357. doi: 10.1007/s00213-005-0202-5. [DOI] [PubMed] [Google Scholar]
- Field M, Powell H. Stress increases attentional bias for alcohol cues in social drinkers who drink to cope. Alcohol and Alcoholism. 2007;42:560–566. doi: 10.1093/alcalc/agm064. [DOI] [PubMed] [Google Scholar]
- Field M, Quigley M. Mild stress increases attentional bias in social drinkers who drink to cope: A replication and extension. Experimental and Clinical Psychopharmacology. 2009;17:312–319. doi: 10.1037/a0017090. [DOI] [PubMed] [Google Scholar]
- George WH, Phillips SM, Skinner JB. Analogue measurement of alcohol consumption: Effects for task type and correspondence with self-report measurement. Journal of Studies on Alcohol. 1988;49:450–455. doi: 10.15288/jsa.1988.49.450. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991-1992 and 2001-2002. Drug and Alcohol Dependence. 2004;74:223–234. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Grant VV, Stewart SH. Impact of experimentally induced positive and anxious mood on alcohol expectancy strength in internally motivated drinkers. Cognitive Behaviour Therapy. 2007;36:102–111. doi: 10.1080/16506070701223289. [DOI] [PubMed] [Google Scholar]
- Grant VV, Stewart SH, Birch CD. Impact of positive and anxious mood on implicit alcohol-related cognitions in internally motivated undergraduate drinkers. Addictive Behaviors. 2007;32:2226–2237. doi: 10.1016/j.addbeh.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Hobbs M, Remington B, Glautier S. Dissociation of wanting and liking for alcohol in humans: A test of the incentive-sensitisation theory. Psychopharmacology. 2005;178:493–499. doi: 10.1007/s00213-004-2026-0. [DOI] [PubMed] [Google Scholar]
- Holahan CJ, Moos RH, Holahan CK, Cronkite RC, Randall PK. Drinking to cope, emotional distress and alcohol use and abuse: A ten-year model. Journal of Studies on Alcohol. 2001;62:190–198. doi: 10.15288/jsa.2001.62.190. [DOI] [PubMed] [Google Scholar]
- Kassel JD, Jackson SI, Unrod M. Generalized expectancies for negative mood regulation and problem drinking among college students. Journal of Studies on Alcohol. 2000;61:332–340. doi: 10.15288/jsa.2000.61.332. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosomatic Medicine. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’—a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, Kirschbaum C. HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: Impact of age and gender. Psychoneuroendocrinology. 2004;29:83–98. doi: 10.1016/s0306-4530(02)00146-4. [DOI] [PubMed] [Google Scholar]
- Lovallo WR. Individual differences in response to stress and risk for addiction. In: Al’Absi M, editor. Stress and addiction: Biological and psychological mechanisms. San Diego, CA: Elsevier Academic Press; 2007. pp. 227–248. [Google Scholar]
- Magid V, Maclean MG, Colder CR. Differentiating between sensation seeking and impulsivity through their mediated relations with alcohol use and problems. Addictive Behaviors. 2007;32:2046–2061. doi: 10.1016/j.addbeh.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisto SA, Carey KB, Bradizza CM. Social learning theory. In: Leonard KE, Blane HT, editors. Psychological theories of drinking and alcoholism. 2nd ed. New York, NY: Guilford Press; 1999. pp. 106–163. [Google Scholar]
- Marlatt GA, Demming B, Reid JB. Loss of control drinking in alcoholics: An experimental analogue. Journal of Abnormal Psychology. 1973;81:233–241. doi: 10.1037/h0034532. [DOI] [PubMed] [Google Scholar]
- Merrill JE, Read JP. Motivational pathways to unique types of alcohol consequences. Psychology of Addictive Behaviors. 2010;24:705–711. doi: 10.1037/a0020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro CA, Oswald LM, Weerts EM, McCaul ME, Wand GS. Hormone responses to social stress in abstinent alcohol-dependent subjects and social drinkers with no history of alcohol dependence. Alcoholism: Clinical and Experimental Research. 2005;29:1133–1138. doi: 10.1097/01.alc.0000172459.71517.05. [DOI] [PubMed] [Google Scholar]
- Peterson RA, Heilbronner RL. The anxiety sensitivity index: Construct validity and factor analytic structure. Journal of Anxiety Disorders. 1987;1:117–121. [Google Scholar]
- Rousseau GS, Irons JG, Correia CJ. The reinforcing value of alcohol in a drinking to cope paradigm. Drug and Alcohol Dependence. 2011;118:1–4. doi: 10.1016/j.drugalcdep.2011.02.010. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Kalmijn J, Trim RS, Cesario E, Saunders G, Campbell N. Comparison across two generations of prospective models of how the low level of response to alcohol affects alcohol outcomes. Journal of Studies on Alcohol and Drugs. 2012;73:195–204. doi: 10.15288/jsad.2012.73.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, Bergquist K, Bhagwagar Z, Sied-larz KM. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology. 2009;34:1198–1208. doi: 10.1038/npp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring alcohol consumption: Psychosocial and biochemical methods. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Sorocco KH, Lovallo WR, Vincent AS, Collins FL. Blunted hypothalamic-pituitary-adrenocortical axis responsivity to stress in persons with a family history of alcoholism. International Journal of Psychophysiology. 2006;59:210–217. doi: 10.1016/j.ijpsycho.2005.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart SH, Hall E, Wilkie H, Birch C. Affective priming of alcohol schema in coping and enhancement motivated drinkers. Cognitive Behaviour Therapy. 2002;31:68–80. [Google Scholar]
- Thomas S, Bacon AK, Sinha R, Uhart M, Adinoff B. Clinical laboratory stressors uses to study alcohol-stress relationships. Alcohol Research: Current Reviews. 2012;34:459–467. [PMC free article] [PubMed] [Google Scholar]
- Thomas SE, Bacon AK. Stress and affective inductions in addiction research. In: MacKillop J, de Wit H, editors. Handbook of addiction psychopharmacology. New York, NY: Wiley-Blackwell; 2013. pp. 411–434. [Google Scholar]
- Thomas SE, Bacon AK, Randall PK, Brady KT, See RE. An acute psychosocial stressor increases drinking in non-treatment-seeking alcoholics. Psychopharmacology. 2011a;218:19–28. doi: 10.1007/s00213-010-2163-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SE, Randall PK, Brady K, See RE, Drobes DJ. An acute psychosocial stressor does not potentiate alcohol cue reactivity in non-treatment-seeking alcoholics. Alcoholism: Clinical and Experimental Research. 2011b;35:464–473. doi: 10.1111/j.1530-0277.2010.01363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhart M, Oswald L, McCaul ME, Chong R, Wand GS. Hormonal responses to psychological stress and family history of alcoholism. Neuropsychopharmacology. 2006;31:2255–2263. doi: 10.1038/sj.npp.1301063. [DOI] [PubMed] [Google Scholar]
- Vergés A, Jackson KM, Bucholz KK, Grant JD, Trull TJ, Wood PK, Sher KJ. Deconstructing the age-prevalence curve of alcohol dependence: Why “maturing out” is only a small piece of the puzzle. Journal of Abnormal Psychology. 2012;121:511–523. doi: 10.1037/a0026027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wand GS, Dobs AS. Alterations in the hypothalamic-pitu-itary-adrenal axis in actively drinking alcoholics. Journal of Clinical Endocrinology and Metabolism. 1991;72:1290–1295. doi: 10.1210/jcem-72-6-1290. [DOI] [PubMed] [Google Scholar]
- Zimmermann U, Spring K, Kunz-Ebrecht SR, Uhr M, Wittchen HU, Holsboer F. Effect of ethanol on hypothalamic-pituitary-adrenal system response to psychosocial stress in sons of alcohol-dependent fathers. Neuropsychopharmacology. 2004;29:1156–1165. doi: 10.1038/sj.npp.1300395. [DOI] [PubMed] [Google Scholar]