Abstract
Signal transduction is a process required to conduct information from a receptor to the nucleus. This process is vital for the control of cellular function and fate. The dynamics of signaling activation and inhibition determine processes such as apoptosis, proliferation, and differentiation. Thus, it is important to understand the factors modulating transient and sustained response. To address this question, by applying mathematical approach we have studied the factors which can alter the activation nature of downstream signaling molecules. The factors which we have investigated are loops (feed forward and feedback loops), cross-talk of signal transduction pathways, and the change in the concentration of the signaling molecules. Based on our results we conclude that among these factors feedback loop and the cross-talks which directly inhibit the target protein dominantly controls the transient cellular response.
Cells transmit and receive information through signal transduction process by controling the dynamics of the intracellullar signaling molecules (SMs)1,2,3,4,5. The temporal dynamics of SMs plays critical roles in making cellular decisions5,6,7,8,9. For example, PC-12 cells after NGF treatment causes sustained Erk activation leads to differentiation of the PC-12 cells, whereas transient Erk activation induces proliferation10. From the previous published data11,12,13,14,15, it appears that there are many important diseases which arise due to aberrations in the signal transduction process. The critical point is the cellular response duration (nature) which seems to be directly linked to the cell-fate decision10,16,17,18,19,20. Based on the nature of the cellular response (transient or sustained or partially adapted), the cells undergo apoptosis, proliferation, or differentiation10,18,19. Thus, it is an important step in signal transduction process to understand the interaction of the signaling pathways resulting in transient or sustained cellular response.
In the past, many research groups have focused on the signal transduction pathways and investigated different factors which may play critical roles in controling the cellular response nature and finally the cell-fate (or cell-fate decision)9,21,22,23. The factors which have been investigated so far are the rate of reactions24,25, network topology25, concentration of the SM26,27, feed forward loops (FFLs), feedback loops (FBLs)21,22, or the cross-talk of the signal transduction pathways28,29,30,31,32,33,34,35.
In biological systems, mainly four different types of cross-talks ((i) concomitant signaling, (ii) collaborative signaling, (iii) direct signaling, and (iv) amplification of signaling), have been reported31. Unlike to these previous works, we have started the investigation of a minimal cascade to the complex signaling regulation by adding all the possible interactions in one model.
Some of the FBLs22, FFLs36, and cross-talks28,29,31,37,38,39 have been investigated in biological signaling. In addition to these previously studied possible regulations, we have included more possible FFLs (both positive and negative), FBLs (both positive and negative), the combination of FFLs and FBLs, and increased more cross-talk possibilities (both the cross-interactions between the cascades i.e., inhibition and activation) between the linear cascades in one model and investigated their impact in controling the cellular response nature. From our results, we conclude that FBL and cross-talk plays critical role in determining transient cellular response. This model will help to understand the cellular response nature, to further reveal the new interactions based on the desired output response, and to perturb the output response by targeting the specific SM.
Results
As mentioned in the previous section, some of the FBLs, FFLs, and cross-talks have been investigated in biological signaling. In addition to these previously studied possible regulations, we have included more possible FFLs (both positive and negative), FBLs (both positive and negative), the combination of FFLs and FBLs, and increased more cross-talk possibilities (both the cross-interactions between the cascades i.e., inhibition and activation) between the linear cascades in one model (Figure 1 a, b, c, d, e, f, and g) and investigated their impact in controling the cellular response nature. The major difference between the previous works and our work is the investigation of the combinations of different kinds of FFLs and FBLs and more cross-interactions between the signaling cascades in the presence and absence of FFLs and FBLs than the four positive cross-talks (Figure 1g) reported by Ivaska J and Heino J28,29,30,31,34,40,41,42,43,44,45. In this model, the complex signaling networks have been simplified and represented as receptor level (R), intracellular signaling level (ISM), and target level (TP). So that the effect of different kinds of interactions at different levels on the final cellular response nature can be studied.
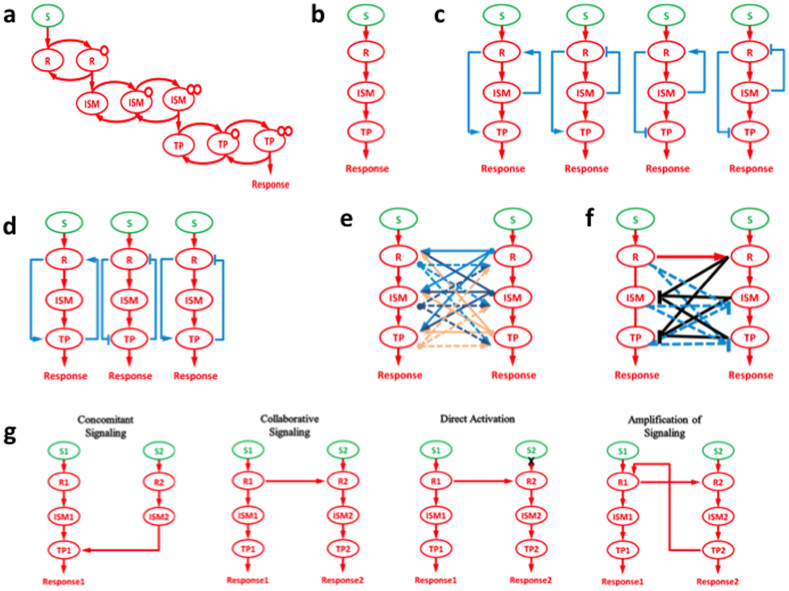
Figure 1. Signaling cascade and its regulations.
S, R, ISM, and TP stand for input signal, receptor, intracellular signaling molecule, and target protein, respectively. (a) A typical linear signaling cascade where R after detecting input signal S becomes active (goes to post-translational modification (e.g., phosphorylated)), active R activates ISM (single or double phosphorylation) and finally active ISM activates TP (single or double phosphorylation), (b) its simplified form, and (c) and (d) represents possible feed forward and feedback regulation (both positive (arrow) and negative regulation (blocked line)). (e) and (f) represent the cross-talks (arrows – activation and lines with blocked end -- inhibition) between signal transduction pathways (cascades). (g) cross-talks known in biological signal transduction31.
A linear cascade always produces sustained cellular response
Here, we have investigated the kinetics of the signaling molecules for linear cascade (a cascade without feed forward loop, feedback loop, and cross-talk between a pair of linear cascades) and linear cascades with feed forward loop and feedback loop (Figure 1 a, b, c, and d).
For this purpose, we have generated linear cascades with different sets of kinetic parameters (kpar). In case of signaling networks, the unit of kpar can be second−1 or minute−1 46. It is known that in general, the signal transduction process is fast and can function on the timescale of seconds to minutes47. Throughout our work, we have written time instead of second or minute. Initially, kpar were randomly generated between 0.001 to 0.1. So, all the cascades have response kinetics close to zero (Figure 2a). Then, we have applied an evolutionary algorithm (EA)24,48 to evolve the cascades. During the evolutionary period, we allowed the change in kpar and the concentration level of SMs. In this period, the signaling cascade adapts the improved kinetic parameters to produce better response.
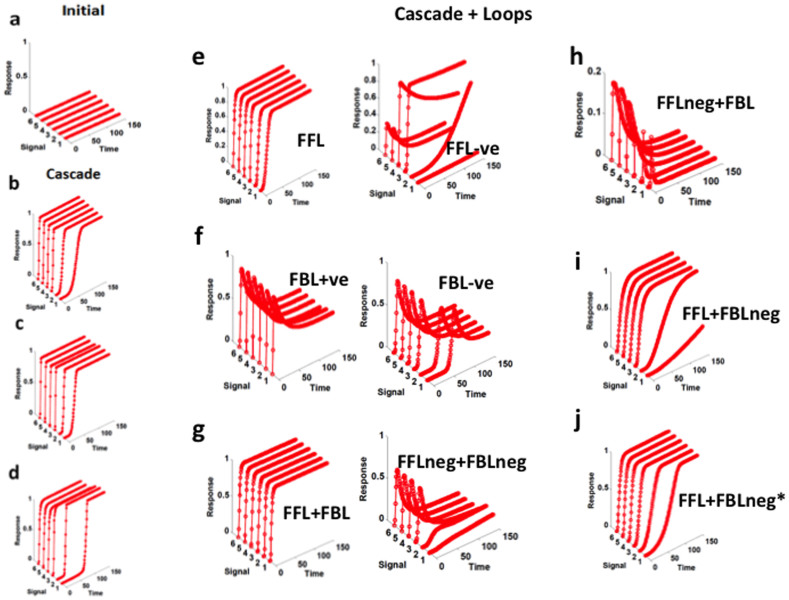
Figure 2. Response kinetics (normalized value) of the signaling cascade.
(a) Initially, kinetics of all the signaling cascade with or without additional regulation (e.g., FFL, FBL, or cross-talks) stays close to zero (kpar are generated randomly between 0.001 and 0.1). (b) kinetics of the fully evolved signaling cascade (the concentration of R, ISM, and TP are fixed and equal i.e., 10 μl, during the evolutionary period the signaling cascades were allowed to adapt new kpar (between 0.1 and 100) values to improve the kinetic response). (c) kinetics of the fully evolved signaling cascade (the concentration of R, ISM, and TP are fixed and unequal i.e., 10 μl, 5 μl, and 1 μl, respectively, during the evolutionary period the signaling cascades were allowed to adapt new kpar (between 0.1 and 100) values to improve the kinetic response). (d) kinetics of the fully evolved signaling cascade (initially the concentration of R, ISM, and TP are fixed and equal i.e., 10 μl, during the evolutionary period the signaling cascades were allowed to adapt new kpar (between 0.1 and 100) values and change in the concentration of R, ISM, and TP to improve the kinetic response). (e) kinetics of the fully evolved signaling cascade in the presence of FFL (the concentration of R, ISM, and TP are fixed and unequal i.e., 10 μl, 5 μl, and 1 μl, respectively, during the evolutionary period the signaling cascades were allowed to adapt new kpar (between 0.1 and 100) values to improve the kinetic response). (f) kinetics of the fully evolved signaling cascade in the presence of FBL, (g) FFL and FBL (both positive and negative), (h) negative FFL and FBL, (i) FFL and negative FBL, and (j) FFL and negative FBL from ISM to R (the concentration of R, ISM, and TP are fixed and unequal i.e., 10 μl, 5 μl, and 1 μl, respectively, during the evolutionary period the signaling cascades were allowed to adapt new kpar (between 0.1 and 100) values to improve the kinetic response).
After analyzing the kinetics of the evolved networks, we observe that in a linear signaling cascade (without any FFL/FBL), the change in the kinetic parameters or the concentration does not produce any transient response (Figure 2b, c, and d). Increase in the concentration (SMs) or the kinetic parameter values leads to improved sustained response (Figure 2b, c, and d).
Addition of a positive FFL in a signaling cascade (Figure 1c) does not change the cascade response and it remains sustained (Figure 2e, left) while the addition of a negative FFL disturbs the output response. The addition of a negative FFL produces mixed response either as transient, or sustained, or complete blocking of the response (Figure 2e, right) which means the activation pattern is not robust. Addition of FBL (positive or negative) leads to transient response (Figure 2f). Presence of one positive FFL and a positive FBL leads to sustained response (Figure 2g, left), presence of one negative FFL and a negative FBL and one negative FFL and a positive FBL leads to transient response (Figure 2 g and h), and presence of one positive FFL and a negative FBL does not change the sustained response to transient nature (Figure 2i). These FFL (positive or negative) and FBL (positive or negative) are from R to TP or TP to R (Figure 1d). When we apply the FBL (positive or negative) and/or FFL (positive or negative) from R to ISM or ISM to R in a cascade, we always observe sustained output response (Figure 2j).
Concomitant inhibition between cascades dominantly produce transient response
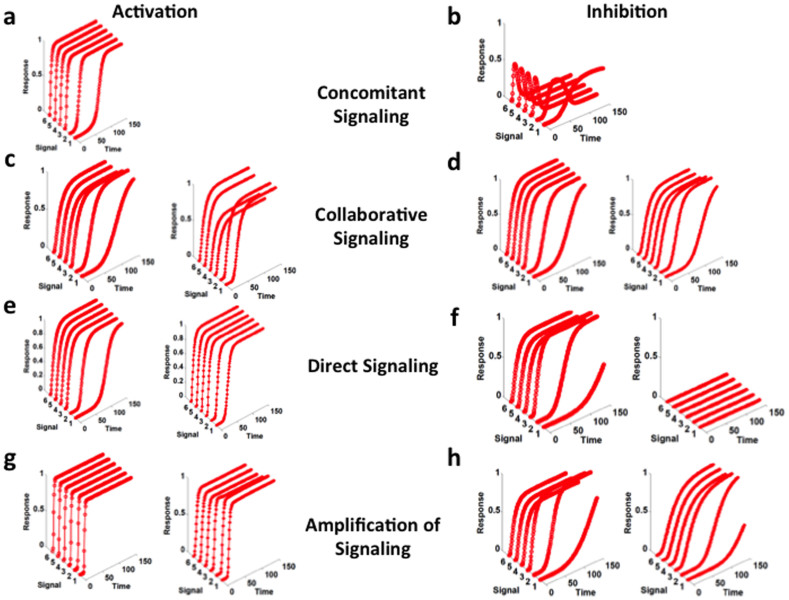
After analyzing the kinetics of signaling cascade response, we investigated the change in the kinetics of the TP of the signaling cascade in the presence of different kinds of cross-talks known from biological system31. We have investigated their inhibitory forms (in biological cross-talks the links between the cascades are activation) also for all the four cross-talks. We found that concomitant signaling (activation link between two cascade) leads to sustained response (Figure 3a) and its inhibitory form produces transient response (Figure 3b). While all the three other kinds of cross-talks (collaborative, direct, and signal amplification) between the cascades help in producing stable sustained response (Figure 3 c, d, e, f, g, and h) irrespective the nature (activation or inhibition) of the links between the cascade. In case of direct signaling, inhibitory interaction between the two cascades leads to only one output response in cascade 1 and complete blockage of the output response of cascade 2 (Figure 3f) because here input signal (S2) is blocked.
Figure 3. Kinetics of output response in case of cross-talk between the signaling cascades.
(a) activation concomitant signaling, (b) inhibitory concomitant signaling, (c) activation type collaborative signaling, (d) inhibition type collaborative signaling, (e) direct signaling – activation, (f) direct signaling – inhibition, (g) amplification of signaling – activation, and (h) amplification of signaling – inhibition. In figure c, d, e, f, g, and h, left side figure represents the kinetics of the output response of cascade 1 and right side figure represents the kinetics of output response of cascade 2.
Increase in the number of inhibitory links leads to transient response or complete blockage of the output response
Finally, we have investigated the effect of all the possible interactions (FFL, FBL, and cross-talks) in a single model. Here, we have two linear cascades in parallel without any cross-interaction. We have generated 200 sets of parallel cascades and evolved them in parallel until 100 generations by allowing the rate of reactions (kpar) to change during evolutionary period to adapt new kpar in order to produce improved kinetic response. After 100 generations, all the new interactions were added one-by-one in a linear signaling cascade (Figure 1a) in each generation. In this work, first we have started addition of negative interactions between two cascades, then FFL and FBL, and finally the positive interactions between cascades.
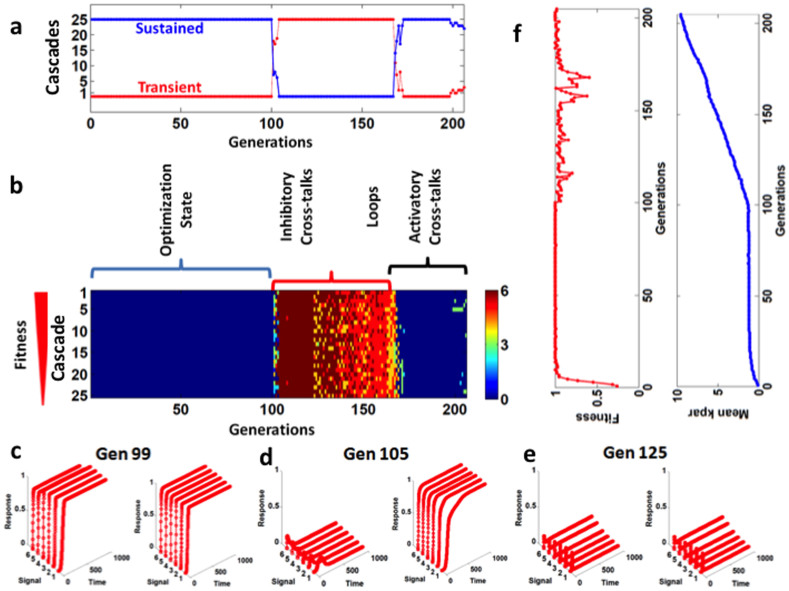
We observe that all the minimal cascades produce sustained output response for all the six different (strength) input signals (Figure 4 a and b). In contrast, addition of new inhibitory interactions between the two cascades, FFL, and FBL leads to transient response which can be seen between generation 100 and 165 in Figure 4 a and b. The response nature has been shown in Figure 4c (for a linear cascade – where both the cascades produce sustained output response before generation 100 (left – pathway 1 and right – pathway 2)). Since, in the beginning we add the interactions through which pathway 2 inhibits pathway 1 so the output response of pathway 1 is transient and pathway 2 remains sustained (Figure 4d). When we add the interactions (inhibitory) between both the pathways then both the pathways produce transient response or completely block the output response of both the pathways (Figure 4e). Addition of positive interactions between the cascades lead to the sustained output response which can be seen in Figure 4 a and b after generation 165 and the kinetics of the output appears similar to Figure 4c. As far as the fitness of the cascades is concerned, as long as the cascades are free from additional interactions, the fitness remain stable and stays at maximum (Figure 4f (left)) because the kinetics of all the cascades for all the input signals easily crosses the threshold level and remain sustained. While addition of new inhibitory interactions between the cascades and the FFL and FBL shows fluctuation in the fitness because the output response becomes either transient or does not crosses the threshold. We further investigated the change in the kpar. In linear cascade which has comparatively less number of reactions so the mean of the kpar is comparatively lower than the cascade with new interactions and the addition of new interactions in each generation leads to the gradual increase in the kpar (Figure 4f (right)). So, we conclude that the irrespective the response nature (sustained or transient) the kpar increases but it does not affect the cellular response nature.
Figure 4. Change in response kinetics of the signaling cascade from simple cascade (without FFL, FBL, and cross-talk) to complex cascade (with FFL, FBL, and cross-talks).
(a) Total number signaling cascades with transient and sustained response among the best 25 signaling cascades. (b) As in our model for each cascade we have six input signals (of different strength) so we have six output response. Here, we show total number of response (transient and/or sustained) in each cascade. (c), (d), and (e) show the kinetics of the output response (cascade 1 (left side figure) and cascade 2 (right side figure)) in generation 99, 105, and 125, respectively. (f) Mean fitness (left side) and the mean of kpar (right side) of cascades (best cascades).
Bistable behavior
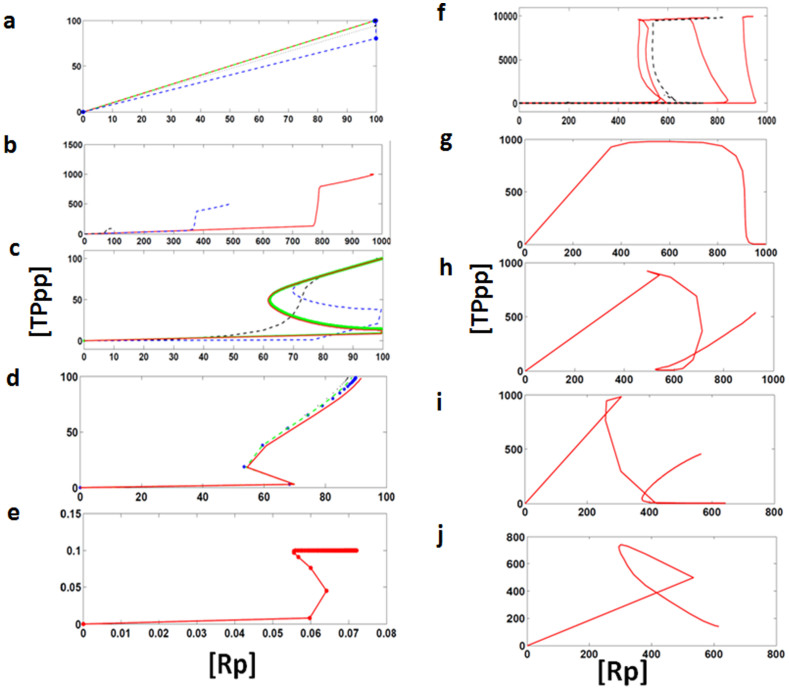
After analyzing the kinetics of the output response, we have also analyzed the bistable behavior49,50,51 for the output response (TPpp) of linear cascade (without FFL, FBL, or cross-talk) and the cascade with FFL, FBL, and cross-talk. We found that in case of a linear cascade and a cascade with positive FFL, and a linear cascade with negative FFL, the response is linear (Figure 5a). We observed bistable behavior for a cascade with FBL (positive/negative) and combination of FFL (positive/negative) and FBL (positive/negative) (Figure 5 b, c, d, e, and f). In case of negative cross-interaction between cascades with few exceptions (Figure 5g) the multistable response behavior appears to be dominant (most of the successful evolved networks show multiple stable states) (Figure 5 h, I, and j).
Figure 5. Dose-response curve.
x-axis represents total concentration of activated receptor ([Rp]) and y-axis represents total concentration of output response ([TPpp]). (a) Red, blue, and black curves are representing dose-response curve for three different sets of kinetic parameters. (b) For one fixed set of kinetic parameters (randomly selected from the evolved cascades), the total output response have been plotted for three different concentration of R and TP. Red, blue, and black curves are representing dose-response curve for three different concentration of R and TP is 1000 μl, 500 μl, and 100 μl, respectively (positive FBL). (c) signal-response curve in the presence of a positive FBL and (d) signal-response curve in the presence of a negative FBL. Black curve represents the dose-response curve plotted for a set of kinetic parameter (one of the evolved cascade randomly selected) then we increase the kinetic parameter values which are involved in positive FBL blue curve (kpar updated with the values between 10 and 100), green curve when positive FBL kpar is between 100 and 500), and red curve where the positive FBL kpar is between 500 and 1000. (e) Signal-response curve in the presence of a FFL and a FBL, (f) signal-response curve in the presence of a negative FFL and a negative FBL. (g), (h), and (i) are the signal-response curve in the presence of inhibitory cross-interactions between the cascades and (j) signal-reponse curve in the presence of activation links between the two cascades.
Discussion
In this study, we have investigated the change in the output response nature (sustained or transient) of the signaling cascade in the presence and absence of the FFL, FBL, and cross-talks between two cascades. The cascade which we have used here, is similar to the MAPK cascade1. Based on our data, we propose that transient signaling responses result from FBL and/or negative cross-interactions between signaling cascades. If the concentration of the TP is lower than the concentration of the R and ISM, and either FBL or negative cross-talks are present then all the cascade produce consistently transient output response. Irrespective of the concentration of the signaling molecules, FFL and all the positive interactions (cross-talks) between the cascades lead to stable and sustained output response.
The evolved networks in a stationary population show stable activation pattern against the change in kinetic parameters for both signaling cascades (until generation 100) and addition of the positive interactions between the signaling cascades (after generation 165 onwards) and the output response as sustained response. This suggests that this type of response is the generic cellular behavior when the presence of a signal is sufficient information for a cell. While in the presence of inhibitory interactions between the signaling cascades and the cascade with FBL and the simultaneous presence of FBL and FFL, the kinetics of the output response is always transient (if the concentration of the TP is less than R, and ISM). The fitness of the cascades fluctuates significantly. This suggests the transient response as the generic solution. If the concentration of R, ISM, and TP is equal then the cascades with inhibitory cross-talks and the cascade with FBL or with combination of FFL and FBL also produces the transient response but not all the cascades (with the exceptions of few cascades having sustained response).
From previous works21,22,23,31,52,53, some interesting facts about the effect of variation in the concentration of SMs, FBL, FFL, and cross-talk of signaling pathways are known. Here, they have investigated the role of change in the concentration of an individual molecule and not investigated in comparison to the other molecules involved in signaling. The FBL, FFL, or cross-talk of pathways have been investigated individually and not in combination of FFL and FBL or cross-talk.
Most of the complex and/or common diseases such as cancer, diabetes, obesity, and asthma are caused by defects in multiple genes and pathways. So, it is not surprising that the current one-target-one-compound approach in drug discovery and development has failed to deliver as many efficacious medicines as expected in the post-genomic era15,37,54. In order to understand such complex diseases and find therapeutic solution, it appears to be promising point to understand the signal transduction process from a simple linear cascade to a complex regulatory mechanism (a linear cascade with different loops and the cross-interactions of the cascade) of signaling network. By applying this approach, we can selectively target the signaling molecules to get the desired output response and will help to target multiple signaling molecules.
The advantage of our model is that it will not only help to understand the effect of the variation in the concentration of the receptor molecules but also help to understand the impact of the concentration of other signaling molecules (such as intermediate and effector molecules) involved in the signal transduction and will give an insight of the different additional regulations such as FFL, FBL, and cross-talk. These models can only be applied to those systems which are known to have such behavior, but often the exact behavior of the STNs is not known. Therefore, the creation of a fitness function that encodes the task that a cell solves under certain experimental conditions, may be more beneficial in determining possible and likely behavior of the underlying signaling cascades.
Conclusion
Based on our data, we conclude that the transient response is controlled by the FBL and the negative cross-interactions between the cascades. If the concentration of the TP is lower than the concentration of the R and ISM, and either FBL or negative cross-talks are present then all the cascade produce consistently transient output response. Irrespective of the concentration of the signaling molecules, FFL and all the positive interactions (cross-talks) between the cascades lead to stable and sustained output response.
Methods
Model
We have set up a signaling cascade which function in the similar way as MAPK signaling cascade works (Figure S1). This signaling cascade is divided into several different levels of signaling such as receptor level (represented as R), intracellular signaling level (as ISM), and the target level represented as TP (the target proteins are those proteins which communicate the information to the nucleus in the form of the output response). Then, we have added different kinds of loops and the cross-interactions (cross-talks) between two signaling cascades at different levels of signaling. In the next step, we have created mass-action kinetic model by using the ordinary differential equations (ODEs) for all the molecules including the complexes formed as result of chemical reaction. In simplified form, the temporal change in the concentration of the signaling components (SMs including the complexes formed) can be represented as:
 |
After the calculation of the kinetics of the all the molecules (1), we have calculated the fitness of all the cascades. For all the calculations, we have used six different input signals (ns = 6). The six different input signals (different in strength) were 0.0001, 0.001, 0.01, 0.1, 1, and 10. For each input signal (n), the kinetics of TP is tested whether it exceeds the threshold level (TL). The threshold ( of the initial concentration of TP) is defined to be the relative fraction of double phosphorylated form of TP. In case of cascade with or without FBL and FFL, If this double phosphorylated form of TP crosses the threshold level at any time point then the cascade is assigned a value 1.0 called fitness factor (Ffactor1) and Ffactor2 = 0. In case of cross-talk of two cascades, in the similar way we check the kinetics of the double phosphorylated form of TP of both the cascades, if the kinetics of cascade 1 crosses the threshold at any time point then we assign fitness factor (Ffactor1) a value of 0.5 and if the kinetics of cascade 2 crosses the threshold at any time point then we assign fitness factor (Ffactor2) a value of 0.5 for cascade 2. After evaluating the kinetics for each cascade for all the six input signals (different in strength), we calculate the fitness (F) by taking the mean of the fitness factors (Ffactor1
and
Ffactor2) which can be represented as:
of the initial concentration of TP) is defined to be the relative fraction of double phosphorylated form of TP. In case of cascade with or without FBL and FFL, If this double phosphorylated form of TP crosses the threshold level at any time point then the cascade is assigned a value 1.0 called fitness factor (Ffactor1) and Ffactor2 = 0. In case of cross-talk of two cascades, in the similar way we check the kinetics of the double phosphorylated form of TP of both the cascades, if the kinetics of cascade 1 crosses the threshold at any time point then we assign fitness factor (Ffactor1) a value of 0.5 and if the kinetics of cascade 2 crosses the threshold at any time point then we assign fitness factor (Ffactor2) a value of 0.5 for cascade 2. After evaluating the kinetics for each cascade for all the six input signals (different in strength), we calculate the fitness (F) by taking the mean of the fitness factors (Ffactor1
and
Ffactor2) which can be represented as:
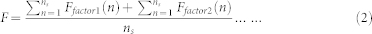 |
Work flow
We have created a set of cascades (total number of cascades 200) with randomly generated kinetic parameters (kpar) between 0.001 and 0.1. EA24,55 has been applied to evolve the signaling cascades. For each cascade, we have F. After calculating F of all the cascades (2), we select the best 50 cascades (successful cascades) based on higher F values. In order to improve the response kinetics, these successful cascades are allowed to adapt new kpar. Four copies of all these 50 cascades with updated kpar are created to keep the total number of cascades equal in each iteration. All these processes are repeated for 200 iterations. Each iteration is called as a generation. Sets of ODEs have been solved with MATLAB 7.9.0.
Additional information
MM and TB were supported in part by grants from the German Ministry of Education and Research (BMBF FORSYS program) [0313922], the State of Sachsen-Anhalt (Dynamic Systems) [XD3639HP/0306], and the European Union (SYBILLA) [HEALTH-F4-2008-201106]. No additional external funding received for this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors declare no potential conflict of interest.
Author Contributions
M.M. carried out research design and performed study, data analysis, and wrote main manuscript text and prepared figures. M.T. helped in designing research. B.I. helped in designing research and contributed in manuscript writing. T.B. and B.S. designed research and contributed to write the manuscript. All authors read and approved the final manuscript.
Supplementary Material
S1: Minimal cascade and its reaction details
References
- Hornberg J. J. et al. Control of MAPK signalling: from complexity to what really matters. Oncogene 24, 5533–5542 (2005). [DOI] [PubMed] [Google Scholar]
- Chaudhri V. K., Kumar D., Misra M., Dua R. & Rao K. V. S. Integration of a phosphatase cascade with the mitogen-activated protein kinase pathway provides for a novel signal processing function. J Biol Chem 285, 1296–1310 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki K., Yamadac M., Kunidac K., Yasudaa S. & Matsudaa M. Processive phosphorylation of ERK MAP kinase in mammalian cells. Proc Natl Acad Sci USA 108, 12675–12680 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell J. E. & Bhatt R. R. Mechanistic studies of the dual phosphorylation of mitogen- activated protein kinase. J Biol Chem 272, 19008–19016 (1997). [DOI] [PubMed] [Google Scholar]
- Acuto O., Bartolo V. D. & Michel F. Tailoring T-cell receptor signals by proximal negative feedback mechanisms. Nat Rev Immunol 8, 699–712 (2008). [DOI] [PubMed] [Google Scholar]
- Purvis J. E. & Lahav G. Encoding and Decoding Cellular Information through Signaling Dynamics. Cell 152, 945–956 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahav G. et al. Dynamics of the p53-Mdm2 feedback loop in individual cells. Nat Genet 36, 147–150 (2004). [DOI] [PubMed] [Google Scholar]
- Ferrell J. E. Jr The biochemical basis of an all-or-none cell fate switch in xenopus oocytes. Science 280, 895–898 (1998). [DOI] [PubMed] [Google Scholar]
- Kubota H. et al. Temporal coding of insulin action through multiplexing of the AKT pathway. Mol Cell 46, 820–832 (2012). [DOI] [PubMed] [Google Scholar]
- Marshall C. J. Specificity of receptor tyrosine kinase review signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell 80, 179–185 (1995). [DOI] [PubMed] [Google Scholar]
- Hanahan D. & Weinberg R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011). [DOI] [PubMed] [Google Scholar]
- Testa J. R. & Tsichlis P. N. AKT signaling in normal and malignant cells. Oncogene 24, 7391–7393 (2005). [DOI] [PubMed] [Google Scholar]
- Altomare D. A. & Testa J. R. Perturbations of the AKT signaling pathway in human cancer. Oncogene 24, 7455–7464 (2005). [DOI] [PubMed] [Google Scholar]
- Gunawardena J. Signals and systems: towards a systems biology of signal transduction. Proc. IEEE 96, 1386–1397 (2008). [Google Scholar]
- Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer 3, 11–22 (2003). [DOI] [PubMed] [Google Scholar]
- Purvis J. E. & Lahav G. Encoding and decoding cellular information through signaling dynamics. Cell 152, 945–956 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D., Srikanth R., Ahlfors H., Lahesmaa R. & Rao K. V. S. Capturing cell-fate decisions from the molecular signatures of a receptor-dependent signaling response. Mol Syst Biol 3, (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstead C. J. & Weaver C. T. Dwelling on T cell fate decisions. Cell 153, 739–741 (2013). [DOI] [PubMed] [Google Scholar]
- Tyson J. J. et al. Dynamic modelling of oestrogen signalling and cell fate in breast cancer cells. Nat Rev Cancer 11, 523–532 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg N. N., Puente L. G., Dawicki W. & Ostergaard H. L. Sustained TCR signaling is required for mitogen-activated protein kinase activation and degranulation by cytotoxic T lymphocytes. J Immunol 161, 2919–2924 (1998). [PubMed] [Google Scholar]
- Loewer A. & Lahav G. Cellular conference call: external feedback affects cell-fate decisions. Cell 124, 1128–1130 (2006). [DOI] [PubMed] [Google Scholar]
- Poltorak M. et al. TCR activation kinetics and feedback regulation in primary human T cells. Cell Commun Signal 11, 4 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer S. L. & Sorger P. K. Measuring and modeling apoptosis in single cells. Cell 144, 926–939 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobashir M., Schraven B. & Beyer T. Simulated evolution of signal transduction networks. PLoS ONE 7, e50905 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francois P. & Hakim V. Design of genetic networks with specified functions by evolution in silico. Proc Natl Acad Sci USA 101, 580–585 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer S. L. & Sorger P. K. Measuring and modeling apoptosis in single cells. Cell 144, 926–939 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer S. L., Gaudet S., Albeck J. G., Burke J. M. & Sorger P. K. Non-genetic origins of cell-to-cell variability in TRAIL-induced apoptosis. Nature 459, 428–432 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClean M. N., Mody A., Broach J. R. & Ramanathan S. Cross-talk and decision making in MAP kinase pathways. Nat Genet 39, 409–414 (2007). [DOI] [PubMed] [Google Scholar]
- Aksamitiene E., Kiyatkin A. & Kholodenko B. N. Cross-talk between mitogenic Ras/MAPK and survival PI3K/Akt pathways: a fine balance. Biochem Soc Trans 40, 139–146 (2012). [DOI] [PubMed] [Google Scholar]
- Negishi H. et al. Cross-interference of RLR and TLR signaling pathways modulates antibacterial T cell responses. Nat Immunol 13, 659–666 (2012). [DOI] [PubMed] [Google Scholar]
- Ivaska J. & Heino J. Cooperation Between Integrins and Growth Factor Receptors in Signaling and Endocytosis. Annu Rev Cell Dev Biol 27, 291–320 (2011). [DOI] [PubMed] [Google Scholar]
- Basak S. & Hoffmann A. Crosstalk via the NF-κB signaling system. Cytokine & Growth Factor Reviews 19, 187–197 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S. et al. Molecular determinants of crosstalk between nuclear receptors and Toll-like Receptors. Cell 122, 707–721 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezbradica J. S. & Medzhitov R. Integration of cytokine and heterologous receptor signaling pathways. Nat Immunol 10, 333–339 (2009). [DOI] [PubMed] [Google Scholar]
- Fraser I. D. C. & Germain R. N. Navigating the network: signaling cross-talk in hematopoietic cells. Nat Immunol 10, 327–331 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doncic A. & Skotheim J. M. Feedforward regulation ensures stability and rapid reversibility of a cellular state. Mol Cell 50, 856–868 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands W. A., Copland M. & Wheadon H. Targeting self-renewal pathways in myeloid malignancies. Cell Commun Signal 11, 1–1 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Noort V. et al. Cross-talk between phosphorylation and lysine acetylation in a genome-reduced bacterium. Mol Syst Biol 8, 1–16 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J. J. Cross-talk between JIP3 and JIP1 during glucose deprivation: SEK1-JNK2 and Akt1 act as mediators. J Biol Chem 280, 26845–26855 (2005). [DOI] [PubMed] [Google Scholar]
- Dumont J. E., Dremier S., Pirson I. & Maenhaut C. Cross signaling, cell specificity, and physiology. AJP: Cell Physiology 283, C2–C28 (2002). [DOI] [PubMed] [Google Scholar]
- van Noort V. et al. Cross-talk between phosphorylation and lysine acetylation in a genome-reduced bacterium. Mol Syst Biol 8, 1–16 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinke D. J., Cheng N. & Chambers E. Quantifying crosstalk among interferon-γ, Iinterleukin-12, and tumor necrosis factor signaling pathways within a TH1 cell model. Science Signaling 5, ra32–ra32 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.-C., Cirit M. & Haugh J. M. PI3K-dependent cross-talk interactions converge with Ras as quantifiable inputs integrated by Erk. Mol Syst Biol 5, 246 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junttila M. R., Li S. P. & Westermarck J. Phosphatase-mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. The FASEB Journal 22, 954–965 (2007). [DOI] [PubMed] [Google Scholar]
- Fraser I. D. C. & Germain R. N. Navigating the network: signaling cross-talk in hematopoietic cells. Nat Immunol 10, 327–331 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofts P. S. et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusible tracer: standardized quantities and symbols. J Magn Reson Imaging 10, 223–232 (1999). [DOI] [PubMed] [Google Scholar]
- Alon U. An Introduction to systems biology: design principles of biological circuits. Chapman & Hall/CRC 1–162 (2007). [Google Scholar]
- Ito Y., Toyota H., Kaneko K. & Yomo T. How selection affects phenotypic fluctuation. Mol Syst Biol 5, 1–7 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legewie S., Blüthgen N. & Herzel H. Mathematical modeling identifies inhibitors of apoptosis as mediators of positive feedback and bistability. PLoS Comput Biol 2, e120 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao L., Nachbar R. B., Kevrekidis I. G. & Shvartsman S. Y. Bistability and oscillations in the Huang-Ferrell model of MAPK signaling. PLoS Comput Biol 3, e184 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla U. S. & Iyengar R. Robustness of the bistable behavior of a biological signaling feedback loop. Chaos 11, 221 (2001). [DOI] [PubMed] [Google Scholar]
- Spencer S. L., Gaudet S., Albeck J. G., Burke J. M. & Sorger P. K. Non-genetic origins of cell-to-cell variability in TRAIL-induced apoptosis. Nature 459, 428–432 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albeck J. G., Burke J. M., Spencer S. L., Lauffenburger D. A. & Sorger P. K. Modeling a snap-action, variable-delay switch controlling extrinsic cell death. Plos Biol 6, e299 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornberg J. J., Bruggeman F. J., Westerhoff H. V. & Lankelma J. Cancer: A systems biology disease. Biosystems 83, 81–90 (2006). [DOI] [PubMed] [Google Scholar]
- Kaneko K. Evolution of Robustness to noise and mutation in gene expression dynamics. PLoS ONE 2, e434 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S1: Minimal cascade and its reaction details