Abstract
Nausea and vomiting are among the most frequently occurring symptoms observed by clinicians. While advances have been made in understanding both the physiological as well as the neurophysiological pathways involved in nausea and vomiting, the final common pathway(s) for emesis have yet to be defined. Regardless of the difficulties in elucidating the precise neurocircuitry involved in nausea and vomiting, it has been accepted for over a century that the locus for these neurocircuits encompasses several structures within the medullary reticular formation of the hindbrain and that the role of vagal neurocircuits in particular are of critical importance. The afferent vagus nerve is responsible for relaying a vast amount of sensory information from thoracic and abdominal organs to the central nervous system. Neurons within the nucleus of the tractus solitarius not only receive these peripheral sensory inputs but have direct or indirect connections with several other hindbrain, midbrain and forebrain structures responsible for the co-ordination of the multiple organ systems. The efferent vagus nerve relays the integrated and co-ordinated output response to several peripheral organs responsible for emesis. The important role of both sensory and motor vagus nerves, and the available nature of peripheral vagal afferent and efferent nerve terminals, provides extensive and readily accessible targets for the development of drugs to combat nausea and vomiting.
Keywords: Vagus, Brainstem, NTS, DVC
1. Introduction
Given the wide range of conditions, diseases and treatments that often result in nausea and vomiting, (medication, infection, toxins, motion sickness, pregnancy, intestinal blockage or slow transit, migraine headaches, hormonal disorders, CNS disorders, kidney failure, radiation therapy, psychiatric disorders, physical or emotional pain, cardiovascular dysfunction, to name a few), it is unsurprising that these are among the most frequently occurring symptoms observed in the clinic. Nausea is often a prodromal symptom of emesis although both nausea and vomiting can occur separately and are considered, at least partially, as separate physiological processes that may engage distinct central nervous system neurocircuitry.
While advances have been made in understanding both the physiological and neurophysiological underpinnings of nausea and vomiting, research has been hampered by lack of suitable animal models that replicate human behavior accurately. Nausea, for example, cannot be studied in non-humans, and surrogate behavioral markers such as excessive salivation, swallowing, conditioned taste aversion and conditioned disgust/gaping have been used to provide some insights into its neural control (Andrews and Horn, 2006; Darmani and Ray, 2009; Parker et al., 2011). Additionally, several commonly used laboratory species (rats, mice, guinea-pigs, rabbits) lack a vomiting reflex requiring investigations into emetic reflexes to either measure alternative outcomes (retching, fictive coughing, conditioned taste aversion and pica, for example), or to study less commonly used laboratory species that have an intact vomiting reflex (cats, dogs, ferrets, shrews); (Andrews and Horn, 2006; Darmani and Ray, 2009; Horn, 2008; Horn et al., 2013). It must be noted, as pointed out in previous review articles (Andrews and Horn, 2006; Horn, 2008) that only a limited number of strains of these laboratory species lacking a vomiting reflex have been tested using a restricted set of stimuli and it is not clear whether all species members lack an emetic reflex.
Regardless of these difficulties in elucidating the precise neurocircuitry involved in nausea and vomiting, it has been accepted for over a century that the locus for these neurocircuits encompasses several structures within the medullary reticular formation of the hindbrain, including the area postrema, nucleus tractus solitarius (NTS), dorsal motor nucleus of the vagus (DMV), the reticular formation and the ventrolateral medulla (Andrews and Horn, 2006; Hornby, 2001; Miller and Ruggiero, 1994). Thumas (1891) described a bilateral structure at the caudal tip of the calamus scriptorius as the site of the vomiting center, as described by Hatcher and Weiss (1923), noting that destruction of the ala cinerea (vagal trigone) prevented vomiting (Hatcher and Weiss, 1923). Further studies noted that destruction of the area postrema, but not the vagal trigone itself, eliminated the emetic response to cardiac glycosides and termed this area the “emetic chemoreceptor trigger zone” (Borison and Wang, 1949; Borison and Brizzee, 1951; Wang and Borison, 1950). The final common pathway for emesis has not been defined, however and the existence of a discrete ‘central pattern generator’ for emesis, rather than a series of localized and integrated nuclei, remains controversial (Miller et al., 1994; Miller and Wilson, 1983).
2. Pathophysiology of vomiting
Understanding the pathophysiological features of vomiting provides an important reminder of the multiple organ systems involved, particularly the multiple autonomic reflexes that must be co-ordinated precisely by the “vomiting center” within the hindbrain, including excessive salivation, inhibition of normal gastric motility, retroperistaltic activity in the duodenum and stomach, relaxation of the lower esophageal sphincter, tachycardia, sweating, breath holding and contraction of abdominal and thoracic muscles (see Fig. 1).
Fig. 1. Schematic diagram illustrating vagal neurocircuits involved in nausea and vomiting.
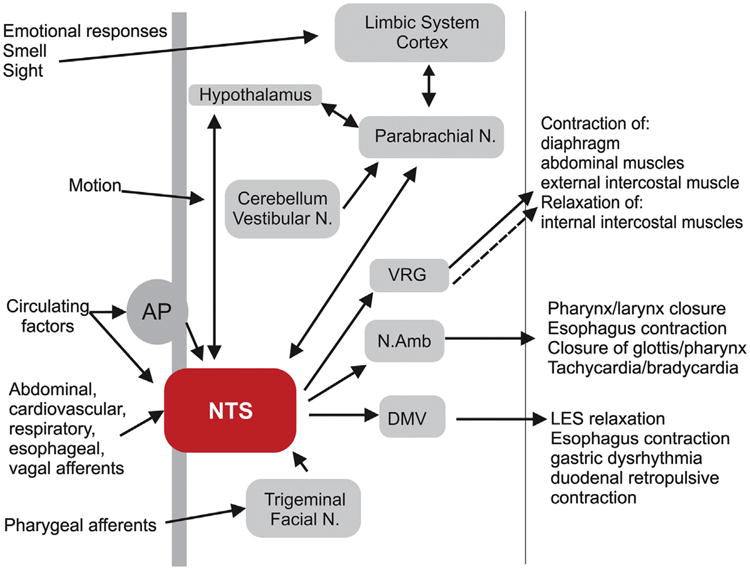
While the exact neural pathways of the central pattern generator responsible for emesis are unknown, the NTS is the recipient of direct or indirect inputs from the abdominal and thoracic vagus, pharyngeal, glossopharyngeal and trigeminal nerves, the spinal tract, the area postrema, the hypothalamus, the cerebellum and vestibular/labyrinthine systems as well as the cerebral cortex and the critical role this nucleus plays in the integration, modulation and regulation of many autonomic reflexes involved in emesis cannot be overstated. Distinct neural outputs from the NTS co-ordinate several of the effector responses of emesis (swallowing, salivation, respiration, cardiovascular, gastrointestinal) in a precisely regulated temporal manner. For simplicity, not all neural pathways and regions are illustrated. AP, area postrema; NTS, nucleus tractus solitarius; Vestibular N., Vestibular Nucleus; Facial N., Facial Nucleus; DMV, dorsal motor nucleus of the vagus; N.Amb., Nucleus ambiguous; VRG, ventral respiratory group; Parabrachial N., Parabrachial nucleus.
3. Role of vagal neurocircuitry in nausea and vomiting
The involvement and importance of vagal neurocircuitry in the generation of nausea and vomiting has been well defined across several species including humans (see reviews by Andrews and Sanger, 2002; Andrews and Horn, 2006; Horn, 2008; Hornby, 2001). The NTS is the recipient of direct neural inputs from the afferent (sensory) vagus as well as direct or indirect inputs from the pharyngeal, glossopharyngeal and trigeminal nerves, the spinal tract, the area postrema, the hypothalamus, the cerebellum and vestibular/labyrinthine systems as well as the cerebral cortex, all of which play important roles in the regulation of medullary reflexes controlling nausea and vomiting, as discussed below (Kalia and Mesulam, 1980a, 1980b; Miller and Ruggiero, 1994; Travagli et al., 2006). Regardless of the means by which it is produced, emesis is accompanied by increased c-fos immunoreactivity in the NTS, suggesting that this region coordinates the emetic response (De Jonghe and Horn, 2009; Ito et al., 2003; Miller and Ruggiero, 1994; Ray et al., 2009; Reynolds et al., 1991). Increased c-fos immunoreactivity was also observed in NTS projection targets which control the motor aspects of vomiting, including the DMV, nucleus ambiguus and phrenic motor nucleus (Ito et al., 2003; Miller and Ruggiero, 1994).
Neurons of the NTS have connections, including reciprocal connections, with several brainstem and higher CNS centers, and the critical role this nucleus plays in the integration, modulation and regulation of many autonomic reflexes involved in nausea and vomiting cannot be overstated. The timing of outputs from the NTS to co-ordinate various output responses of vomiting (swallowing, salivation, respiration, cardiovascular, gastrointestinal etc.) is not random and will be discussed within the context of their inputs and outputs from the NTS.
3.1. Vagal neurocircuits controlling GI functions in nausea and vomiting
Gastrointestinal vagal afferent fibers carry the information about the physiological state of the GI tract to the dorsal vagal complex (DVC; i.e., NTS, DMV, and area postrema) in the caudal brainstem. Vago-vagal reflexes, i.e., reflexes involving vagal sensory as well as vagal motor fibers, are initiated by the activation of low threshold mechano-, osmo- and chemo-receptors in the gut, which convey the information on the movement and composition of nutrients (Blackshaw et al., 2007; Travagli et al., 2006). Gastrointestinal vagal afferent fibers play a critical role in the induction of nausea and the generation of vomiting (Andrews et al., 1990) and the integrity of the abdominal vagus is essential for the generation of emesis. In 1951, for example, Wang and Borison demonstrated that vagotomy prevents the emesis associated with intragastric copper sulfate administration (Wang and Borison, 1951) and electrical stimulation of the abdominal vagus is known to induce an emetic response that includes an increase in blood pressure, licking and retching, increased activity of the abdominal muscles and an increased intragastric pressure (Andrews et al., 1990). Furthermore, in ferrets, stimulation of mucosal chemoreceptors in the stomach or the duodenum results in a long latency but sudden increase in vagal efferent discharge associated with the prodrome of vomiting, suggesting that activation of vagal afferents is involved in the generation of vomiting (Andrews and Wood, 1988).
The cell bodies of vagal afferent fibers are located in the nodose ganglion and transmit afferent signals via glutamatergic synapses to the second-order neurons located in the NTS (Andresen and Yang, 1990; Andresen and Kunze, 1994; Baptista et al., 2005; Smith et al., 1998). The NTS consists of various subnuclei, which are organized in a viscerotopic manner (Altschuler et al., 1989, 1991; Barraco et al., 1992; Travagli et al., 2006). Activation of GI vagal afferents and administration of emetic agents activate neurons in the subnucleus gelatinosus of the NTS, which receives gastric afferent input, the subnucleus centralis, which controls the sensory aspect of swallowing, as well as the medial and ventrolateral NTS, which control cardiovascular and respiratory functions, respectively (Hornby, 2001; Miller and Ruggiero, 1994; Reynolds et al., 1991). Neurons of the NTS involved in GI regulation innervate the adjacent DMV, using predominately glutamate, norepinephrine and especially GABA as neurotransmitters. The DMV contains the preganglionic parasympathetic motoneurons that relay the appropriate integrated neuronal response back to the upper GI tract via the efferent vagus nerve (reviewed: Travagli et al., 2006). Rather than exhibiting a viscerotopic organization, DMV neurons are organized in neuronal “columns” that project to the viscera through each of the five subdiaphragmatic vagal branches (Jarvinen and Powley, 1999; Shapiro and Miselis, 1985; Travagli et al., 2006). DMV neurons within each “column”, however, are not identical but comprise heterogeneous neuronal populations that differ in their neurochemical, morphological and electrophysiological properties (Babic et al., 2011; Browning et al., 1999; Fox and Powley, 1992; Jarvinen and Powley, 1999; Travagli et al., 1991). Regardless of their heterogeneity, as preganglionic parasympathetic neurons, all DMV neurons are a priori cholinergic, and activate nicotinic acetylcholine receptors present on postganglionic neurons within the target organ of interest, in this case the stomach and upper gastrointestinal tract. Postganglionic neurons within the stomach and upper gastrointestinal tract form two distinct pathways; an excitatory cholinergic pathway that induces muscle contraction via activation of muscarinic cholinergic receptors on gastrointestinal smooth muscle, and a non-adrenergic, non-cholinergic inhibitory pathway that induces muscle relaxation via release of nitric oxide and/or vasoactive intestinal polypeptide. The excitatory cholinergic pathway appears to predominate under normal conditions thus gastric relaxation can be produced by either withdrawal/inhibition of the tonically active excitatory cholinergic pathway or activation of the inhibitory non-adrenergic, non-cholinergic pathway (reviewed in Travagli et al., 2006). Under normal conditions, the activity of DMV neurons that innervate the GI tract is controlled by a tonic GABAergic input from the NTS (Sivarao et al., 1998; Travagli et al., 1991, 2006). The activity of synaptic inputs impinging upon DMV neurons, hence the excitability and efferent output of DMV neurons, can be modulated by numerous neurotransmitters and neuromodulators, including those implicated in emetic reflexes such as, for example, opioid peptides (Browning et al., 2004, 2002), serotonin (Browning and Travagli, 1999; Mussa et al., 2008; Travagli and Gillis, 1995), endocannabinoids (Derbenev et al., 2004; Glatzer and Smith, 2005), tachykinins (Ladic and Buchan, 1996; Le et al., 2008; Lewis and Travagli, 2001) and dopamine (Cai et al., 2013; Zheng and Travagli, 2007). Activity within vagal efferent pathways during emetic reflexes results in a large retropulsive wave of intestinal motility accompanied by gastric contraction. Together with temporally co-ordinated relaxation of the antral/pyloric sphincter and the lower esophageal sphincter accompanied by contraction of the abdominal and intercostal muscles, this results in expulsion of gastric contents from the stomach and upper intestine (Lang et al., 1986, 1993; Miller, 1990).
The role of vagal afferent fibers in emesis has been most extensively studied in the context of chemotherapy induced nausea and vomiting (Hesketh, 2004; Andrews and Horn, 2006; Darmani and Ray, 2009). Under normal conditions, ingestion of nutrients, particularly glucose, leads to the release of 5-HT from entero-endocrine cells and subsequent activation of 5-HT3 receptors on vagal afferents (Raybould, 2001, 2002; Raybould et al., 2003; Zhu et al., 2001). This excitatory signal is then relayed to the NTS (Raybould, 1998, 2001; Savastano et al., 2007; Travagli et al., 2006). It has also been shown that many chemotherapy agents, particularly cisplatin and related drugs, also cause the release of 5-HT from entero-endocrine cells and activate 5-HT3 receptors on vagal afferents, while vagotomy decreases vomiting induced by cytotoxic drugs (Andrews et al., 1990; Andrews and Horn, 2006; Darmani and Johnson, 2004; Endo et al., 2000, 1990; Hawthorn et al., 1988). Perhaps the most convincing argument supporting the role of 5-HT in the induction of radio- and chemotherapy-induced nausea and vomiting is the efficacy of 5-HT3 receptor antagonists, particularly in preventing acute-phase chemotherapy induced nausea and vomiting (reviewed in (Andrews and Horn, 2006; Darmani and Ray, 2009). The presumed site of action of these 5-HT3 receptor antagonists is at peripheral vagal afferent terminals (Endo et al., 2000) although the presence of 5-HT3 receptors within the brainstem, including area postrema, NTS and DMV and the ability of centrally applied 5-HT3 receptor antagonists to attenuate chemotherapy induced nausea and vomiting suggests that the actions of 5-HT may not be restricted to peripheral sites (Darmani and Ray, 2009; Leslie et al., 1990; Liu et al., 2003; Reynolds et al., 1989, 1991). It is important to recognize that 5-HT released from enterochromaffin cells may also activate 5-HT receptors present on neurons within the enteric nervous system to modulate gastrointestinal motility (Endo et al., 2000; Glatzle et al., 2002; Tonini, 2005); disruption of normal gastrointestinal motility patterns also contributes to the genesis of nausea and vomiting (see below). A similar mechanism involving the activation of vagal afferents via 5-HT released from enterochromaffin cells has been proposed to be involved in the nausea and vomiting induced by several infectious agents including rotavirus (Hagbom et al., 2011), cholera toxin (Jensen et al., 1997), Salmonella typhimurium (Jensen et al., 1997) and campylobacter (Blakelock and Beasley, 2003). The use of 5-HT3 receptor antagonists in treated gastroenteritis-related vomiting, particularly in infants and children, still requires evaluation, however (Cheng, 2011; Marchetti et al., 2011).
It is of interest to note, though, that tachykinin NK1 receptor antagonists are also efficacious in the treatment of chemotherapy induced nausea and vomiting suggesting that tachykinins may also be involved in this process (Darmani and Ray, 2009; Hesketh, 2004); tachykinins, including substance P, are also found within intestinal enterochromaffin cells and are released by cytotoxic drugs (Ray et al., 2009; Saito et al., 2003). As with serotonergic receptors, neurokinin receptors are also present within central vagal neurocircuits as well as the enteric nervous system hence tachykinins may modulate nausea and vomiting at multiple sites (Endo et al., 2000; Lewis and Travagli, 2001; Saito et al., 2003; Sanger, 2004).
The efficacy of 5-HT3 and NK1 receptor antagonists in the treatment of post-operative nausea, one of the most common post-operative complaints from patients, suggests that 5-HT and/or neurokinins also play a significant role in this form of nausea and vomiting (Diemunsch et al., 2009; Ho and Gan, 2006). Indeed, recent studies have demonstrated that, when administered together, 5-HT3 and NK1 antagonists have synergistic interactions to counteract emesis (Darmani et al., 2011). While the pathophysiology of postoperative nausea and vomiting is incomplete, and the complexity of its multifactorial origins hampers elucidation/definition, it appears that, in addition to the use of inhalational anesthetics, the inherent physical manipulation, particularly in abdominal or gynecological surgical procedures, may cause the release of humoral substances, including 5-HT and neurokinins, which activates vagal afferent signaling to the brainstem and “vomiting center”. Postoperative nausea and vomiting is also particularly common following head and neck surgery; again physical manipulation of the sensory trigeminal nerve, hence activation of inputs terminating within the NTS, appears to be of importance (Becker, 2010).
Glucocorticoids, such as dexamethasone, are used commonly in combination with 5-HT3 and NK-1 receptor antagonists in the treatment of both chemotherapy-induced and postoperative nausea and vomiting (Kris et al., 2011). The antiemetic mechanism of action of dexamethasone is still largely unknown described earlier, some of these effects may involve actions at glucocorticoid receptors within the DVC including the area postrema and NTS (Fukunaka et al., 1998; Ho et al., 2004; Morimoto et al., 1996) although it is important to note that dexamethasone has also been shown to modulate the actions of 5-HT receptors on vagal afferent nerves (Woods and Andrews, 1995).
Disruption of normal gastric motility patterns has also been correlated closely with the development of nausea, most likely via the abnormal activation of mechanosensitive gastrointestinal vagal afferents. The alterations in gastrointestinal motor activity observed during vomiting are regulated by the vagus nerve, since vagotomy and vagal block by anesthetics prevent this activity (Lang et al., 1986, 1993). Information regarding gastrointestinal motility/contractile activity is relayed via the afferent vagus nerve into the brainstem and thence to the paraventricular and supraoptic nuclei of the hypothalamus, the parabrachial nucleus and the limbic and cortical areas (Balaban and Porter, 1998; Karimnamazi et al., 2002; Suzuki et al., 2012; Yuan and Barber, 1991) where it is perceived as either normal or abnormal. Studies into pathophysiology of motion sickness have provided the majority of information regarding the interplay between gastric dysrhythmias and nausea & vomiting in humans. The shift from the normal 3 contractions per minute gastric slow wave rhythm to a faster 4–9 contractions per minute rhythm precedes the development of nausea and the intensity of the tachygastric response/motility pattern appears correlated with the severity of nausea (Xu et al., 1993). In addition to the well-described gastric dysrhythmia associated with motion sickness, gastric dysmotility has also been associated with nausea in patients with diabetic or idiopathic gastroparesis, functional dyspepsia, peptic ulcer disease and pregnancy, for example (Owyang and Hasler, 2002).
Nausea and vomiting are commonly experienced during pregnancy, affecting up to 80% of all pregnant women (review: Lee and Saha, 2011). While the precise etiology of nausea and vomiting during pregnancy is unknown, various metabolic and hormonal factors have been implicated. In particular, the ovarian hormones estrogen and progesterone, are known to alter esophageal, gastric and intestinal motility patterns, resulting in decreased smooth muscle contraction, decreased gastrointestinal motility and decreased gastric emptying (Depue et al., 1987; Koch et al., 1990; Walsh et al., 1996).
Increased activation of mechanosensitive vagal afferents following either gastrointestinal dysrhythmia or dysmotility or following abnormal distention of the stomach, intestine or biliary tract also evokes nausea. While nociception is normally associated with the activation of spinal afferent pathways, some distention-evoked vagal afferents have been shown to encode information into a noxious range (Grundy, 2002; Randich and Gebhart, 1992; Renehan et al., 1995; Traub et al., 1996) hence may also be involved in the transmission of painful stimuli.
3.2. Vagal neurocircuits regulating cardiorespiratory functions in nausea and vomiting
Baro- and chemoreceptor reflexes are critical for blood pressure stabilization and regulation of tissue perfusion. At rest, in most species, there is a tonic level of cardiovascular-related parasympathetic nerve activity (Dergacheva et al., 2010). Activation of baroreceptor afferents by an increase in arterial pressure activates neurons in the NTS, primarily in the medial, commissural and ventral subnuclei. These NTS neurons send excitatory projections to cardiac vagal motoneurons in the nucleus ambiguus (Neff et al., 1998) as well as motoneurons innervating the airways (McAllen and Spyer, 1978). In addition, baroreceptor-responsive NTS neurons also affect sympathetic outflow to the vasculature via projections to the caudal ventrolateral medulla, which, in turn, sends inhibitory projections to the vasomotor center in the rostral ventrolateral medulla (reviewed in Guyenet, 2006; McAllen and Spyer, 1978). Baroreceptor activation, therefore, simultaneously activates the vagal outflow to the heart and inhibits sympathetic activity to the vasculature.
NTS neurons also receive afferent inputs from peripheral chemoreceptors. Although terminal fields of chemoreceptors display a certain degree of overlap with those of baroreceptors, their primary termination sites are in the commissural and medial subnuclei of the NTS. Stimulation of the carotid body chemoreceptors results in a reflex increase in the respiratory rate and volume, glandular secretions and vasoconstriction. These functions are mediated by the projections of NTS neurons to regions that regulate the autonomic functions of the airways as well as motor output to respiratory muscles. NTS neurons send direct projections to the vagal preganglionic neurons in the rostral nucleus ambiguus. These neurons, in turn, project to the intrinsic tracheobronchial ganglia, which provide the innervation to airway smooth muscle, submucosal glands and the vasculature (reviewed in Kc and Martin, 2010). Several tract tracing studies have demonstrated that NTS neurons also send monosynaptic projections to the ventral respiratory group (Alheid et al., 2011; Ellenberger and Feldman, 1990; Rosin et al., 2006). The ventral respiratory group includes several rostrocaudally arranged regions, namely the Bötzinger complex, the pre-Bötzinger complex, the rostral and the caudal VRG. Respiratory neurons in these regions display phases of activity relative to the breathing cycle (reviewed in Smith et al., 2009). A recent study has demonstrated that NTS subnuclei show a dichotomy in their projections to the ventral respiratory group. Specifically, medial and commissural subnuclei of the NTS project predominantly to the retrotrapezoid nucleus, rostral ventrolateral medulla and rostral portions of the ventral respiratory group, with only a small number of neurons projecting to the Bötzinger and pre-Bötzinger complex. In contrast, gelatinosus, dorsolateral and ventrolateral subnuclei were shown to have broader projection targets throughout the ventral respiratory group (Alheid et al., 2011).
Regulation of tissue perfusion is accomplished by adjustments of blood pressure, heart rate and respiration and requires integration of cardiovascular and respiratory functions. Both baro- and chemoreceptors are inhibited during inspiration and facilitated during post-inspiration and expiration. These effects persist following pulmonary denervation, suggesting that the integration of cardiovascular and respiratory functions occurs in the brainstem (Dergacheva et al., 2010). The interaction between these two systems can also be observed in respiratory sinus arrhythmia, a variation in heart rate observed during a respiratory cycle. Heart rate increases during inspiration and decreases during post-inspiration and expiration. Integration of cardiovascular and respiratory inputs has been shown to occur both at the level of the NTS as well as cardiac vagal preganglionic neurons in the nucleus ambiguus.
Changes in cardiovascular and respiratory functions are essential for the generation of vomiting. Vomiting is associated with an increase in blood pressure and a coordinated contraction of respiratory muscles. The co-ordinated activity of respiratory muscles (diaphragm, abdominal, intercostal) is essential to the production of increased thoracic and abdominal pressures required for vomiting. Unlike normal respiration, during emetic-like behavior the diaphragm and abdominal muscles co-contract accompanied by contraction of the external (inspiratory) intercostal muscles and relaxation of the internal (expiratory) intercostal muscles (Miller et al., 1987; Miller and Nonaka, 1990; Miller, 1990). Additionally, the area of the diaphragm surrounding the esophagus relaxes to assist in expulsion of gastric contents. The muscles of the upper airway undergo a temporally connected series of protective movements including raising the soft palate and closure of the glottis (Lang et al., 1993, 2002).
Blocking either baro-or chemo-receptors by internal carotid artery ligation, or carotid body denervation, respectively, suppressed the emetic response (Uchino et al., 2006). In addition, during emesis, there is an activation of both baro- and chemo-receptors and the sensitivity of these reflexes is increased during retching and vomiting. It has been suggested that the predominance of the parasympathetic nervous system activity augments the emetic response, whereas predominance of sympathetic nervous system activity suppresses it. Studies in shrews and ferrets have demonstrated that arterial pressure increases during prodromal phase of retching, whereas heart rate decreases prior to retching, followed by a gradual increase (Andrews et al., 1990; Uchino et al., 2006). Interestingly, studies in mice, a species that does not vomit, have demonstrated that emesis was accompanied by tachycardia in contrast to bradycardia observed in shrews. However, the bradycardia observed in shrews was blocked by vagotomy, suggesting that it was due to baroreceptor activation. In contrast to the shrew, the baroreflex in mice and rats during emesis does not appear to be as sensitive (Uchino et al., 2006). These observations are consistent with findings on human subjects, which have demonstrated that during parabolic flight, subjects that vomited had an increased baroreflex responsiveness, whereas those that did not vomit displayed no changes (Schlegel et al., 2001). Moreover, recruitment of respiratory muscles during retching and vomiting elicit a change in the respiratory pattern (Cohen et al., 1992; Miller, 1990). Retching and vomiting are accompanied by a decrease in arterial oxygen tension and oxygen concentration in the trachea, conditions suggestive of chemoreceptor activation (Fukuda and Koga, 1993; Fukuda and Koga, 1995). The increased sensitivity of baro- and chemo-receptor afferents during emesis, as well as increased c-fos expression, as a marker of neuronal activation, suggest that NTS and area postrema coordinate the cardiovascular and respiratory components of the emetic response (Miller and Ruggiero, 1994; Reynolds et al., 1991).
3.3. Vagal neurocircuits regulating esophageal functions in nausea and vomiting
Neuronal tracing techniques (Broussard and Altschuler, 2000) have demonstrated that vagal motoneurons projecting to the pharynx and larynx are located within the compact as well as the external formation of the nucleus ambiguus. The location of esophageal motoneurons is dependent upon whether the esophagus is composed of smooth or striated muscle which appears to be species dependent (Broussard et al., 1998; Collman et al., 1992; Jean, 2001; Lawn, 1966). Thus, esophageal motoneurons are located within either the nucleus ambiguus (striated muscle; rat) or the dorsal motor nucleus of the vagus (smooth muscle; cat, rabbit, humans).
Regardless of their central location, however, esophageal moto-neurons receive inputs from NTS involved in the central integration of esophageal peristalsis and in the co-ordination of swallowing and airway-protective reflexes. During an emetic reflex, the lower esophageal sphincter relaxes in co-ordination with closure of the epiglottis and closure of the pharynx/larynx to protect the airways (Lang et al., 1993, 2002).
3.4. Area postrema inputs to vagal neurocircuits involved in nausea and vomiting
The area postrema is a chemosensitive organ adjacent to the NTS that was first identified as the emetic chemoreceptor trigger zone in the 1940s and 50s (Borison and Wang, 1949; Borison and Brizzee, 1951; Wang and Borison, 1950). As with other circumventricular organs, the area postrema is extensively vascularized with highly fenestrated capillaries and, by consequence, is not isolated from peripheral circulation by the blood brain barrier (Borison, 1989; Cottrell and Ferguson, 2004; Fry and Ferguson, 2007). The rodent area postrema is composed of three distinct regions, delineated on the basis of the neuronal locations and projections. The mantle zone comprises the dorsal aspect of the area postrema and, similarly to the central zone, is rich in neuronal cell bodies. The ventral zone contains primarily glial cells as well as a monolayer of tanycytes, joined together by tight junctions, which define the boundary with the adjacent NTS (Price et al., 2008). Apical dendrites of area postrema neurons extend toward the basal lamina side of endothelial cells, which allows these dendrites to receive blood-borne information from the vasculature (Price et al., 2008). The area postrema sends dense, excitatory (glutamatergic) projections to the NTS as well as less prominent projections to the nucleus ambiguus, dorsal motor nucleus of the vagus, parabrachial nucleus and the tegmental nuclei (Fry and Ferguson, 2007; Price et al., 2008). Approximately 60% of area postrema neurons exhibit pacemaking activity due to expression of a hyperpolarization-activated cation current (Shinpo et al., 2012); blockade of these channels has been demonstrated to suppress some forms of conditioned taste aversion, most likely by suppressing the area postrema neuronal excitability (Shinpo et al., 2012). The unique positioning of area postrema neurons allows them to receive both neural and humoral signals related to emesis and relay this information to the adjacent NTS, which, in turn, integrates these synaptic inputs and transmits them to motor centers associated with emesis. Area postrema neurons contain receptors for numerous neurotransmitters (dopamine, neurokinins, serotonin) as well as, neuropeptides and hormones involved in the regulation of gastrointestinal (cholecystokinin, glucagon-like peptide-1, ghrelin, peptide YY, amylin, adiponectin, oxynto-modulin), cardiovascular (angiotensin, vasopressin, endothelin, adrenomedullin) and immune (interleukin-1β interleukin-10; tumor necrosis factor-α prostaglandin) functions (review: Darmani and Ray, 2009; Price et al., 2008). Destruction of the area postrema either eliminates or delays the vomiting caused by systemic administration of several substances, including apomorphine, angiotensin II, cisplatin, interleukin-2, copper sulfate, nicotine and epinephrine (Borison, 1989).
From a clinical perspective, it is also important to recognize that the activity of neurons within several nuclei, including the area postrema, hence the ability of their synaptic outputs to influence vagal neurocircuits involved in the regulation of nausea and vomiting, may also be modulated by non-chemosensitive means. Compression of area postrema neurons following disruption of the brainstem blood supply as observed following a subarachnoid hemorrhage (Akpinar et al., 2005; Nozaki et al., 1992), for example, induces nausea and vomiting, as does cerebral artery dysregulation during migraine (Edvinsson and Uddman, 2005). Similarly, an increase in intracranial pressure from trauma, brain tumors or infections of the meninges (bacterial, fungal or viral) also may induce nausea and vomiting via alteration in the activity of area postrema neurons (Goehler et al., 2006; Wuchert et al., 2009). Conversely, modulating the activity of these neurons may present important, and readily available, therapeutic targets to disrupt chemoreceptor-mediated nausea and vomiting. Corti-costeroids such as dexamethasone and methylprednisolone, for example, may exert their anti-emetic effects via actions, at least in part, on glucocorticoid receptors within the dorsal vagal complex, including the area postrema (Morimoto et al., 1996; Sanger, 2004).
3.5. Vagal neurocircuits regulating nociception in nausea and vomiting
NTS neurons have dense reciprocal innervation with the trigeminal complex and facial motor nucleus (Zerari-Mailly et al., 2005); activation of trigeminal and facial nerves during intense headaches/migraine/dental pain activates parasympathetic reflexes that regulate cardiovascular, GI and respiratory functions, including emesis (Caous et al., 2001). As described earlier, NTS neurons can be activated following noxious stimulation of gastrointestinal vagal afferents (Randich and Gebhart, 1992; Renehan et al., 1995; Traub et al., 1996) although nociceptive inputs more often activate NTS neurons via spinal pathways (Boscan et al., 2002; Pickering et al., 2003a, 2003b).
3.6. Cerebellar and labyrinthine inputs to vagal neurocircuits involved in nausea and vomiting
The cerebellum does not appear to be essential for vomiting induced either by electrical stimulation of the vagus nerve or administration of emetic drugs, and while it certainly contributes to vestibular nucleus-induced vomiting associated with motion sickness, it appears to exert a modulatory, rather than principle, influence (Miller et al., 1994). Cerebellar neurons do send projections to the hindbrain vagal neurocircuits responsible for the coordination of emetic reflexes, however, via the vestibular nucleus (cerebellovestibular fibers) as well as via the hypothalamus (cerebellohypothalamic fibers) by way of the parabrachial and Kölliker-Fuse nucleus (see Section 4.7, (Balaban, 2004; Suzuki et al., 2012), hence the cerebellum is able to exert some influence over visceral functions such as nausea and vomiting. Electrical stimulation of the cerebellum can influence gastric and duodenal motility in both a vagally-dependent and vagally–independent manner (Lisander and Martner, 1975; Manchanda et al., 1972).
Activation of neurons within the vestibular system is critically important to the generation of nausea and vomiting associated with motion sickness. Similarly, nausea and vomiting are commonly associated with vestibular system diseases including vertigo, vestibular neuritis, labyrinthitis and benign paroxysmal positional vertigo (Cuomo-Granston and Drummond, 2010). Motion sickness induced by visual sensory inputs, however, does not appear to involve direct stimulation of the vestibular system but instead results from the mis-match of converging information from several sensory systems (vestibular, visual, visceral). Neuronal tracing studies have demonstrated that in species such as the rabbit, rat and cat, neurons of the medial and inferior vestibular system innervate vagal neurocircuits, including neurons within the NTS and reticular formation (Balaban and Beryozkin, 1994; Balaban, 1999; Ruggiero et al., 1996; Takeda et al., 2001) and neurophysiological experiments have demonstrated that some NTS neurons receive convergent inputs from the vestibular nuclei and the gastrointestinal tract (Suzuki et al., 2012). Such vestibulosolitarius and vestibuloreticular inputs may provide the anatomical basis for the integration of abnormal or mis-matched spatial and motion sensory inputs that result in emesis (Balaban and Porter, 1998; Balaban, 1999).
Vestibular neurons have been shown to display functional receptors for several excitatory neurotransmitters, including acet-ylcholine muscarinic receptors, dopamine D2 receptors, serotonin 5-HT2 receptors and histamine H1, and H2 receptors. Theoretically, antagonists of these receptors would decrease the activity of vestibular neurons, hence reduce the activity of their synaptic inputs into the dorsal vagal complex, and potentially be useful in the treatment of motion sickness. In practice, only antagonists of muscarinic cholinergic receptors and antihistamines have proved useful clinically (Horn et al., 2013; Yates et al., 1998).
3.7. Role of other central nervous system inputs to vagal neurocircuits involved in nausea and vomiting
Neurons within central vagal neurocircuits, particularly NTS neurons, have dense reciprocal connections with the hypothalamus which are critically important in the integration and regulation of several autonomic reflexes (Rinaman, 2007; Rinaman, 2010; Spyer et al., 1997; van der Kooy et al., 1984). The hypothalamus itself, however, does not appear to be essential to the genesis of nausea and vomiting (Miller et al., 1994). Hypothalamic subnuclei, particularly those adjacent to the third ventricle, may be activated by circulating neurohormones/neuropeptides resulting in the generation of emetic reflexes (Cottrell and Ferguson, 2004; Rodriguez et al., 2010). Actions of glucagon-like peptide-1, for example, to induce nausea and vomiting may be due, at least in part, to actions within the hypothalamus (Chan et al., 2013).
As described in Section 4.6, the parabrachial and associated Kölliker-Fuse nuclei relay inputs from vestibular nuclei to higher centers within the central nervous system including the hypothalamus, limbic system and forebrain, and are involved in the generation of nausea and vomiting induced by motion sickness. Studies have also demonstrated that parabrachial/Kölliker-Fuse neurons also receive reciprocal inputs from several of these ascending structures, including the cortex, basal forebrain and hypothalamus (Moga et al., 1990) as well as receiving inputs from vagal neurocircuits, particular neurons within the area postrema and NTS (Herbert et al., 1990). Parabrachial/Kölliker-Fuse neurons are activated by a variety of visceral sensations including baroreceptor (Jhamandas et al., 1991; Jhamandas and Harris, 1992), chemoreceptor (Hayward and Felder, 1995; Song et al., 2011), respiratory (Ezure, 2004; Kubin et al., 2006), gastrointestinal (Suzuki et al., 2012) and gustatory/taste (Karimnamazi et al., 2002; Rosen et al., 2011) inputs. Recent studies have also shown that parabrachial/Kölliker-Fuse neurons receive converging inputs from vestibular and visceral vagal afferents (Suzuki et al., 2012), and from orosensory and visceral vagal afferents (Karimnamazi et al., 2002) suggesting that GI irritation may amplify or reduce the sensitivity of parabrachial/Kölliker-Fuse neurons to body motion or taste. While the sensation of nausea is assumed to involve the cerebral cortex, the emetic reflex can be elicited in decerebrate animals demonstrating it can occur independently of forebrain involvement (Miller et al., 1994). Nevertheless, the dorsal vagal complex, particularly the NTS, has reciprocal direct or indirect projections to several higher CNS centers, including the parabrachial nucleus, hypothalamus, limbic system and forebrain (Moga et al., 1990; Terreberry and Neafsey,1987, 1983; van der Kooy et al., 1984) providing a neuroanatomical substrate for the involvement and integration of various sensory, affective and emotional responses to nausea and vomiting.
4. Conclusions
Nausea and vomiting are among the most common symptoms observed in the clinic and can result for a wide variety of conditions (medication, infection, toxins, motion sickness, pregnancy, gastrointestinal motility disorders, migraine headaches, hormonal disorders, CNS disorders, kidney failure, radiation therapy, psychiatric disorders, physical or emotional pain, cardiovascular dysfunction, to name a few). The emetic reflex is arguably the most complicated autonomic reflex involving the precise temporal co-ordination of multiple physiological systems including gastrointestinal, cardiovascular and respiratory. While a final common pathway for emesis has not been defined, and there is clearly much work still to be to describe the “central pattern generator” involved in emesis, the involvement and critical importance of vagal neurocircuitry in the generation of nausea and vomiting has been well defined across several species including humans.
The afferent vagus nerve is responsible for relaying a vast volume of sensory information from the thorax and abdominal viscera to the central nervous system. Neurons within the NTS not only receive these peripheral sensory inputs, but also have direct or indirect inputs with several other hindbrain, midbrain and forebrain nuclei responsible for co-ordinating and regulating the medullary reflexes controlling emesis but also the emotional and affective responses to nausea and vomiting. The efferent vagus nerve is responsible for relaying the integrated and co-ordinated output response to several peripheral organs (including the pharynx, larynx, esophagus, stomach and upper intestine) which results, ultimately, in the expulsion of gastric contents. The important role of both sensory and motor vagus nerves, and the available nature of peripheral vagal afferent and efferent nerve terminals, provides extensive and readily accessible targets for the development of drugs to combat nausea and vomiting.
Acknowledgments
The authors thank Dr, R. Alberto Travagli for comments on previous versions of this manuscript, and W. Nairn Browning for support and encouragement. This manuscript was supported by National Institutes of Health Grants DK078364 (to KNB) and DK55530 (to RAT).
References
- Akpinar G, Acikgoz B, Surucu S, Celik HH, Cagavi F. Ultrastructural changes in the circumventricular organs after experimental subarachnoid hemorrhage. Neurol Res. 2005;27:580–585. doi: 10.1179/016164105X48752. [DOI] [PubMed] [Google Scholar]
- Alheid GF, Jiao W, McCrimmon DR. Caudal nuclei of the rat nucleus of the solitary tract differentially innervate respiratory compartments within the ventrolateral medulla. Neuroscience. 2011;190:207–227. doi: 10.1016/j.neuroscience.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschuler SM, Bao X, Bieger D, Hopkins DA, Miselis RR. Viscerotopic representation of the upper alimentary tract in the rat: sensory ganglia and nuclei of the solitary and spinal trigeminal tracts. J Comp Neurol. 1989;283:248–268. doi: 10.1002/cne.902830207. [DOI] [PubMed] [Google Scholar]
- Altschuler SM, Ferenci DA, Lynn RB, Miselis RR. Representation of the cecum in the lateral dorsal motor nucleus of the vagus nerve and commissural subnucleus of the nucleus tractus solitarii in rat. J Comp Neurol. 1991;304:261–274. doi: 10.1002/cne.903040209. [DOI] [PubMed] [Google Scholar]
- Andresen MC, Kunze DL. Nucleus tractus solitarius–gateway to neural circulatory control. Annu Rev Physiol. 1994;56:93–116. doi: 10.1146/annurev.ph.56.030194.000521. [DOI] [PubMed] [Google Scholar]
- Andresen MC, Yang M. Non-NMDA receptors mediate sensory afferent synaptic transmission in medial nucleus tractus solitarius. Am J Physiol. 1990;259:H1307–H1311. doi: 10.1152/ajpheart.1990.259.4.H1307. [DOI] [PubMed] [Google Scholar]
- Andrews PL, Horn CC. Signals for nausea and emesis: implications for models of upper gastrointestinal diseases. Auton Neurosci. 2006;125:100–115. doi: 10.1016/j.autneu.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews PL, Sanger GJ. Abdominal vagal afferent neurones: an important target for the treatment of gastrointestinal dysfunction. Curr Opin Pharmacol. 2002;2:650–656. doi: 10.1016/s1471-4892(02)00227-8. [DOI] [PubMed] [Google Scholar]
- Andrews PL, Wood KL. Vagally mediated gastric motor and emetic reflexes evoked by stimulation of the antral mucosa in anaesthetized ferrets. J Physiol. 1988;395:1–16. doi: 10.1113/jphysiol.1988.sp016905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews PL, Davis CJ, Bingham S, Davidson HI, Hawthorn J, Maskell L. The abdominal visceral innervation and the emetic reflex: pathways, pharmacology, and plasticity. Can J Physiol Pharmacol. 1990;68:325–345. doi: 10.1139/y90-047. [DOI] [PubMed] [Google Scholar]
- Babic T, Browning KN, Travagli RA. Differential organization of excitatory and inhibitory synapses within the rat dorsal vagal complex. Am J Physiol Gastrointest Liver Physiol. 2011;300:G21–G32. doi: 10.1152/ajpgi.00363.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban CD. Vestibular autonomic regulation (including motion sickness and the mechanism of vomiting) Curr Opin Neurol. 1999;12:29–33. doi: 10.1097/00019052-199902000-00005. [DOI] [PubMed] [Google Scholar]
- Balaban CD. Projections from the parabrachial nucleus to the vestibular nuclei: potential substrates for autonomic and limbic influences on vestibular responses. Brain Res. 2004;996:126–137. doi: 10.1016/j.brainres.2003.10.026. [DOI] [PubMed] [Google Scholar]
- Balaban CD, Beryozkin G. Vestibular nucleus projections to nucleus tractus solitarius and the dorsal motor nucleus of the vagus nerve: potential substrates for vestibulo-autonomic interactions. Exp Brain Res. 1994;98:200–212. doi: 10.1007/BF00228409. [DOI] [PubMed] [Google Scholar]
- Balaban CD, Porter JD. Neuroanatomic substrates for vestibulo-autonomic interactions. J Vestib Res. 1998;8:7–16. [PubMed] [Google Scholar]
- Baptista V, Zheng ZL, Coleman FH, Rogers RC, Travagli RA. Characterization of neurons of the nucleus tractus solitarius pars centralis. Brain Res. 2005;1052:139–146. doi: 10.1016/j.brainres.2005.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraco R, el-Ridi M, Ergene E, Parizon M, Bradley D. An atlas of the rat subpostremal nucleus tractus solitarius. Brain Res Bull. 1992;29:703–765. doi: 10.1016/0361-9230(92)90143-l. [DOI] [PubMed] [Google Scholar]
- Becker DE. Nausea, vomiting, and hiccups: a review of mechanisms and treatment. Anesth Prog. 2010;57:150–156. doi: 10.2344/0003-3006-57.4.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshaw LA, Brookes SJ, Grundy D, Schemann M. Sensory transmission in the gastrointestinal tract. Neurogastroenterol Motil. 2007;19:1–19. doi: 10.1111/j.1365-2982.2006.00871.x. [DOI] [PubMed] [Google Scholar]
- Blakelock RT, Beasley SW. Infection and the gut. Semin Pediatr Surg. 2003;12:265–274. doi: 10.1053/j.sempedsurg.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Borison HL. Area postrema: chemoreceptor circumventricular organ of the medulla oblongata. Prog Neurobiol. 1989;32:351–390. doi: 10.1016/0301-0082(89)90028-2. [DOI] [PubMed] [Google Scholar]
- Borison HL, Brizzee KR. Morphology of emetic chemoreceptor trigger zone in cat medulla oblongata. Proc Soc Exp Biol Med. 1951;77:38–42. doi: 10.3181/00379727-77-18670. [DOI] [PubMed] [Google Scholar]
- Borison HL, Wang SC. Functional localization of central coordinating mechanism for emesis in cat. J Neurophysiol. 1949;12:305–313. doi: 10.1152/jn.1949.12.5.305. [DOI] [PubMed] [Google Scholar]
- Boscan P, Kasparov S, Paton JF. Somatic nociception activates NK1 receptors in the nucleus tractus solitarii to attenuate the baroreceptor cardiac reflex. Eur J Neurosci. 2002;16:907–920. doi: 10.1046/j.1460-9568.2002.02131.x. [DOI] [PubMed] [Google Scholar]
- Broussard DL, Altschuler SM. Brainstem viscerotopic organization of afferents and efferents involved in the control of swallowing. Am J Med. 2000;108(4A):79S–86S. doi: 10.1016/s0002-9343(99)00343-5. [DOI] [PubMed] [Google Scholar]
- Broussard DL, Lynn RB, Wiedner EB, Altschuler SM. Solitarial premotor neuron projections to the rat esophagus and pharynx: implications for control of swallowing. Gastroenterology. 1998;114:1268–1275. doi: 10.1016/s0016-5085(98)70433-0. [DOI] [PubMed] [Google Scholar]
- Browning KN, Travagli RA. Characterisation of the in vitro effects of 5-Hydroxytryptamine (5-HT) on identified neurones of the rat dorsal motor nucleus of the vagus (DMV) Br J Pharmacol. 1999;128:1307–1315. doi: 10.1038/sj.bjp.0702908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning KN, Renehan WE, Travagli RA. Electrophysiological and morphological heterogeneity of rat dorsal vagal neurones which project to specific areas of the gastrointestinal tract. J Physiol. 1999;517:521–532. doi: 10.1111/j.1469-7793.1999.0521t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning KN, Kalyuzhny AE, Travagli RA. Opioid peptides inhibit excitatory but not inhibitory synaptic transmission in the rat dorsal motor nucleus of the vagus. J Neurosci. 2002;22:2998–3004. doi: 10.1523/JNEUROSCI.22-08-02998.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning KN, Kalyuzhny AE, Travagli RA. Mu-opioid receptor trafficking on inhibitory synapses in the rat brainstem. J Neurosci. 2004;24:7344–7352. doi: 10.1523/JNEUROSCI.1676-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai QQ, Zheng LF, Fan RF, Lian H, Zhou L, Song HY, Tang YY, Feng XY, Guo ZK, Wang ZY, Zhu JX. Distribution of dopamine receptors D1-and D2-immunoreactive neurons in the dorsal motor nucleus of vagus in rats. Auton Neurosci. 2013;176:48–53. doi: 10.1016/j.autneu.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Caous CA, de Sousa BH, Lindsey CJ. Neuronal connections of the paratrigeminal nucleus: a topographic analysis of neurons projecting to bulbar, pontine and thalamic nuclei related to cardiovascular, respiratory and sensory functions. Auton Neurosci. 2001;94:14–24. doi: 10.1016/s1566-0702(01)00338-1. [DOI] [PubMed] [Google Scholar]
- Chan SW, Lin G, Yew DT, Yeung CK, Rudd JA. Separation of emetic and anorexic responses of exendin-4, a GLP-1 receptor agonist in Suncus murinus (house musk shrew) Neuropharmacology. 2013;70:141–147. doi: 10.1016/j.neuropharm.2013.01.013. [DOI] [PubMed] [Google Scholar]
- Cheng A. Emergency department use of oral ondansetron for acute gastroenteritis-related vomiting in infants and children. Paediatr Child Health. 2011;16:177–182. doi: 10.1093/pch/16.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MI, Miller AD, Barnhardt R, Shaw CF. Weakness of short-term synchronization among respiratory nerve activities during fictive vomiting. Am J Physiol. 1992;263:R339–R347. doi: 10.1152/ajpregu.1992.263.2.R339. [DOI] [PubMed] [Google Scholar]
- Collman PI, Tremblay L, Diamant NE. The distribution of spinal and vagal sensory neurons that innervate the esophagus of the cat. Gastroenterology. 1992;103:817–822. doi: 10.1016/0016-5085(92)90012-n. [DOI] [PubMed] [Google Scholar]
- Cottrell GT, Ferguson AV. Sensory circumventricular organs: central roles in integrated autonomic regulation. Regul Pept. 2004;117:11–23. doi: 10.1016/j.regpep.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Cuomo-Granston A, Drummond PD. Migraine and motion sickness: what is the link? Prog Neurobiol. 2010;91:300–312. doi: 10.1016/j.pneurobio.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Johnson JC. Central and peripheral mechanisms contribute to the antiemetic actions of delta-9-tetrahydrocannabinol against 5-hydroxytryptophan-induced emesis. Eur J Pharmacol. 2004;488:201–212. doi: 10.1016/j.ejphar.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Ray AP. Evidence for a re-evaluation of the neurochemical and anatomical bases of chemotherapy-induced vomiting. Chem Rev. 2009;109:3158–3199. doi: 10.1021/cr900117p. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Chebolu S, Amos B, Alkam T. Synergistic antiemetic interactions between serotonergic 5-HT3 and tachykininergic NK1-receptor antagonists in the least shrew (Cryptotis parva) Pharmacol Biochem Behav. 2011;99:573–579. doi: 10.1016/j.pbb.2011.05.025. [DOI] [PubMed] [Google Scholar]
- De Jonghe BC, Horn CC. Chemotherapy agent cisplatin induces 48-h Fos expression in the brain of a vomiting species, the house musk shrew (Suncus murinus) Am J Physiol Regul Integr Comp Physiol. 2009;296:R902–R911. doi: 10.1152/ajpregu.90952.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depue RH, Bernstein L, Ross RK, Judd HL, Henderson BE. Hyperemesis gravidarum in relation to estradiol levels, pregnancy outcome, and other maternal factors: a seroepidemiologic study. Am J Obstet Gynecol. 1987;156:1137–1141. doi: 10.1016/0002-9378(87)90126-8. [DOI] [PubMed] [Google Scholar]
- Derbenev AV, Stuart TC, Smith BN. Cannabinoids suppress synaptic input to neurones of the rat dorsal motor nucleus of the vagus nerve. J Physiol. 2004;559:923–938. doi: 10.1113/jphysiol.2004.067470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dergacheva O, Griffioen KJ, Neff RA, Mendelowitz D. Respiratory modulation of premotor cardiac vagal neurons in the brainstem. Respir Physiol Neurobiol. 2010;174:102–110. doi: 10.1016/j.resp.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diemunsch P, Joshi GP, Brichant JF. Neurokinin-1 receptor antagonists in the prevention of postoperative nausea and vomiting. Br J Anaesth. 2009;103:7–13. doi: 10.1093/bja/aep125. [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Uddman R. Neurobiology in primary headaches. Brain Res Brain Res Rev. 2005;48:438–456. doi: 10.1016/j.brainresrev.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Ellenberger HH, Feldman JL. Brainstem connections of the rostral ventral respiratory group of the rat. Brain Res. 1990;513:35–42. doi: 10.1016/0006-8993(90)91086-v. [DOI] [PubMed] [Google Scholar]
- Endo T, Minami M, Monma Y, Saito H, Takeuchi M. Emesis-related biochemical and histopathological changes induced by cisplatin in the ferret. J Toxicol Sci. 1990;15:235–244. doi: 10.2131/jts.15.235. [DOI] [PubMed] [Google Scholar]
- Endo T, Minami M, Hirafuji M, Ogawa T, Akita K, Nemoto M, Saito H, Yoshioka M, Parvez SH. Neurochemistry and neuropharmacology of emesis – the role of serotonin. Toxicology. 2000;153:189–201. doi: 10.1016/s0300-483x(00)00314-0. [DOI] [PubMed] [Google Scholar]
- Ezure K. Respiration-related afferents to parabrachial pontine regions. Respir Physiol Neurobiol. 2004;143:167–175. doi: 10.1016/j.resp.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Fox EA, Powley TL. Morphology of identified preganglionic neurons in the dorsal motor nucleus of the vagus. J Comp Neurol. 1992;322:79–98. doi: 10.1002/cne.903220107. [DOI] [PubMed] [Google Scholar]
- Fry M, Ferguson AV. The sensory circumventricular organs: brain targets for circulating signals controlling ingestive behavior. Physiol Behav. 2007;91:413–423. doi: 10.1016/j.physbeh.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Fukuda H, Koga T. Hypercapnia and hypoxia which develop during retching participate in the transition from retching to expulsion in dogs. Neurosci Res. 1993;17:205–215. doi: 10.1016/0168-0102(93)90048-u. [DOI] [PubMed] [Google Scholar]
- Fukuda H, Koga T. Activation of peripheral and/or central chemoreceptors changes retching activities of Botzinger complex neurons and induces expulsion in decerebrate dogs. Neurosci Res. 1995;23:171–183. doi: 10.1016/0168-0102(95)00938-p. [DOI] [PubMed] [Google Scholar]
- Fukunaka N, Sagae S, Kudo R, Endo T, Hirafuji M, Minami M. Effects of granisetron and its combination with dexamethasone on cisplatin-induced delayed emesis in the ferret. Gen Pharmacol. 1998;31:775–781. doi: 10.1016/s0306-3623(98)00102-5. [DOI] [PubMed] [Google Scholar]
- Glatzer NR, Smith BN. Modulation of synaptic transmission in the rat nucleus of the solitary tract by endomorphin-1. J Neurophysiol. 2005;5:2530–2540. doi: 10.1152/jn.00429.2004. [DOI] [PubMed] [Google Scholar]
- Glatzle J, Sternini C, Robin C, Zittel TT, Wong H, Reeve JR, Jr, Raybould HE. Expression of 5-HT3 receptors in the rat gastrointestinal tract. Gastroenterology. 2002;123:217–226. doi: 10.1053/gast.2002.34245. [DOI] [PubMed] [Google Scholar]
- Goehler LE, Erisir A, Gaykema RP. Neural-immune interface in the rat area postrema. Neuroscience. 2006;140:1415–1434. doi: 10.1016/j.neuroscience.2006.03.048. [DOI] [PubMed] [Google Scholar]
- Grundy D. Neuroanatomy of visceral nociception: vagal and splanchnic afferent. Gut. 2002;51(Suppl 1):I2–I5. doi: 10.1136/gut.51.suppl_1.i2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- Hagbom M, Istrate C, Engblom D, Karlsson T, Rodriguez-Diaz J, Buesa J, Taylor JA, Loitto VM, Magnusson KE, Ahlman H, Lundgren O, Svensson L. Rotavirus stimulates release of serotonin (5-HT) from human entero-chromaffin cells and activates brain structures involved in nausea and vomiting. PLoS Pathog. 2011;7:e1002115. doi: 10.1371/journal.ppat.1002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatcher RA, Weiss S. Studies on vomiting. J Pharmacol Exp Ther. 1923;22:139–193. [Google Scholar]
- Hawthorn J, Ostler KJ, Andrews PL. The role of the abdominal visceral innervation and 5-hydroxytryptamine M-receptors in vomiting induced by the cytotoxic drugs cyclophosphamide and cis-platin in the ferret. Q J Exp Physiol. 1988;73:7–21. doi: 10.1113/expphysiol.1988.sp003124. [DOI] [PubMed] [Google Scholar]
- Hayward LF, Felder RB. Peripheral chemoreceptor inputs to the parabrachial nucleus of the rat. Am J Physiol. 1995;268:R707–R714. doi: 10.1152/ajpregu.1995.268.3.R707. [DOI] [PubMed] [Google Scholar]
- Herbert H, Moga MM, Saper CB. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol. 1990;293:540–580. doi: 10.1002/cne.902930404. [DOI] [PubMed] [Google Scholar]
- Hesketh PJ. Understanding the pathobiology of chemotherapy-induced nausea and vomiting. Providing a basis for therapeutic progress. Oncology (Williston Park) 2004;18:9–14. [PubMed] [Google Scholar]
- Ho CM, Ho ST, Wang JJ, Tsai SK, Chai CY. Dexamethasone has a central antiemetic mechanism in decerebrated cats. Anesth Analg. 2004;99:734–739. doi: 10.1213/01.ANE.0000130003.68288.C7. table. [DOI] [PubMed] [Google Scholar]
- Ho KY, Gan TJ. Pharmacology, pharmacogenetics, and clinical efficacy of 5-hydroxytryptamine type 3 receptor antagonists for postoperative nausea and vomiting. Curr Opin Anaesthesiol. 2006;19:606–611. doi: 10.1097/01.aco.0000247340.61815.38. [DOI] [PubMed] [Google Scholar]
- Horn CC. Why is the neurobiology of nausea and vomiting so important? Appetite. 2008;50:430–434. doi: 10.1016/j.appet.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn CC, Kimball BA, Wang H, Kaus J, Dienel S, Nagy A, Gathright GR, Yates BJ, Andrews PL. Why can't rodents vomit? A comparative behavioral, anatomical, and physiological study. PLoS ONE. 2013;8:e60537. doi: 10.1371/journal.pone.0060537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornby PJ. Central neurocircuitry associated with emesis. Am J Med. 2001;111(Suppl 8A):106S–112S. doi: 10.1016/s0002-9343(01)00849-x. [DOI] [PubMed] [Google Scholar]
- Ito H, Nishibayashi M, Kawabata K, Maeda S, Seki M, Ebukuro S. Induction of Fos protein in neurons in the medulla oblongata after motion-and X-irradiation-induced emesis in musk shrews (Suncus murinus) Auton Neurosci. 2003;107:1–8. doi: 10.1016/S1566-0702(03)00026-2. [DOI] [PubMed] [Google Scholar]
- Jarvinen MK, Powley TL. Dorsal motor nucleus of the vagus neurons: a multivariate taxonomy. J Comp Neurol. 1999;403:359–377. [PubMed] [Google Scholar]
- Jean A. Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev. 2001;81:929–969. doi: 10.1152/physrev.2001.81.2.929. [DOI] [PubMed] [Google Scholar]
- Jensen GM, Grondahl ML, Nielsen CG, Skadhauge E, Olsen JE, Hansen MB. Effect of ondansetron on Salmonella typhimurium-induced net fluid accumulation in the pig jejunum in vivo. Comp Biochem Physiol A Physiol. 1997;118:297–299. doi: 10.1016/s0300-9629(96)00308-8. [DOI] [PubMed] [Google Scholar]
- Jhamandas JH, Harris KH. Influence of nucleus tractus solitarius stimulation and baroreceptor activation on rat parabrachial neurons. Brain Res Bull. 1992;28:565–571. doi: 10.1016/0361-9230(92)90104-6. [DOI] [PubMed] [Google Scholar]
- Jhamandas JH, Aippersbach SE, Harris KH. Cardiovascular influences on rat parabrachial nucleus: an electrophysiological study. Am J Physiol. 1991;260:R225–R231. doi: 10.1152/ajpregu.1991.260.1.R225. [DOI] [PubMed] [Google Scholar]
- Kalia M, Mesulam MM. Brain stem projections of sensory and motor components of the vagus complex in the cat: I. The cervical vagus and nodose ganglion. J Comp Neurol. 1980a;193:435–465. doi: 10.1002/cne.901930210. [DOI] [PubMed] [Google Scholar]
- Kalia M, Mesulam MM. Brain stem projections of sensory and motor components of the vagus complex in the cat: II. Laryngeal, tracheobronchial, pulmonary, cardiac, and gastrointestinal branches. J Comp Neurol. 1980b;193:467–508. doi: 10.1002/cne.901930211. [DOI] [PubMed] [Google Scholar]
- Karimnamazi H, Travers SP, Travers JB. Oral and gastric input to the parabrachial nucleus of the rat. Brain Res. 2002;957:193–206. doi: 10.1016/s0006-8993(02)03438-8. [DOI] [PubMed] [Google Scholar]
- Kc P, Martin RJ. Role of central neurotransmission and chemoreception on airway control. Respir Physiol Neurobiol. 2010;173:213–222. doi: 10.1016/j.resp.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch KL, Stern RM, Vasey M, Botti JJ, Creasy GW, Dwyer A. Gastric dysrhythmias and nausea of pregnancy. Dig Dis Sci. 1990;35:961–968. doi: 10.1007/BF01537244. [DOI] [PubMed] [Google Scholar]
- Kris MG, Tonato M, Bria E, Ballatori E, Espersen B, Herrstedt J, Rittenberg C, Einhorn LH, Grunberg S, Saito M, Morrow G, Hesketh P. Consensus recommendations for the prevention of vomiting and nausea following high-emetic-risk chemotherapy. Support Care Cancer. 2011;19(Suppl 1):S25–S32. doi: 10.1007/s00520-010-0976-9. [DOI] [PubMed] [Google Scholar]
- Kubin L, Alheid GF, Zuperku EJ, McCrimmon DR. Central pathways of pulmonary and lower airway vagal afferents. J Appl Physiol. 2006;101:618–627. doi: 10.1152/japplphysiol.00252.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladic LA, Buchan AM. Association of substance P and its receptor with efferent neurons projecting to the greater curvature of the rat stomach. J Auton Nerv Syst. 1996;58:25–34. doi: 10.1016/0165-1838(96)00114-2. [DOI] [PubMed] [Google Scholar]
- Lang IM, Sarna SK, Condon RE. Gastrointestinal motor correlates of vomiting in the dog: quantification and characterization as an independent phenomenon. Gastroenterology. 1986;90:40–47. doi: 10.1016/0016-5085(86)90072-7. [DOI] [PubMed] [Google Scholar]
- Lang IM, Sarna SK, Dodds WJ. Pharyngeal, esophageal, and proximal gastric responses associated with vomiting. Am J Physiol. 1993;265:G963–G972. doi: 10.1152/ajpgi.1993.265.5.G963. [DOI] [PubMed] [Google Scholar]
- Lang IM, Dana N, Medda BK, Shaker R. Mechanisms of airway protection during retching, vomiting, and swallowing. Am J Physiol Gastrointest Liver Physiol. 2002;283:G529–G536. doi: 10.1152/ajpgi.00062.2002. [DOI] [PubMed] [Google Scholar]
- Lawn AM. The localization, in the nucleus ambiguus of the rabbit, of the cells of origin of motor nerve fibers in the glossopharyngeal nerve and various branches of the vagus nerve by means of retrograde degeneration. J Comp Neurol. 1966;127:293–306. doi: 10.1002/cne.901270210. [DOI] [PubMed] [Google Scholar]
- Le BI, Dufour A, Crest M, Szabo G, Erdelyi F, Baude A. Differential expression of Nk1 and NK3 neurokinin receptors in neurons of the nucleus tractus solitarius and the dorsal vagal motor nucleus of the rat and mouse. Neuroscience. 2008;152:56–64. doi: 10.1016/j.neuroscience.2007.12.024. [DOI] [PubMed] [Google Scholar]
- Lee NM, Saha S. Nausea and vomiting of pregnancy. Gastroenterol Clin North Am. 2011;40:309–334. doi: 10.1016/j.gtc.2011.03.009. vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie RA, Reynolds DJ, Andrews PL, Grahame-Smith DG, Davis CJ, Harvey JM. Evidence for presynaptic 5-hydroxytryptamine3 recognition sites on vagal afferent terminals in the brainstem of the ferret. Neuroscience. 1990;38:667–673. doi: 10.1016/0306-4522(90)90060-h. [DOI] [PubMed] [Google Scholar]
- Lewis MW, Travagli RA. Effects of substance P on identified neurons of the rat dorsal motor nucleus of the vagus. Am J Physiol. 2001;281:G164–G172. doi: 10.1152/ajpgi.2001.281.1.G164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisander B, Martner J. Effects on gastric motility from the cerebellar fastigial nucleus. Acta Physiol Scand. 1975;94:368–377. doi: 10.1111/j.1748-1716.1975.tb05896.x. [DOI] [PubMed] [Google Scholar]
- Liu Y, Hamaue N, Endo T, Hirafuji M, Minami M. 5-hydroxytryptamine (5-HT) concentrations in the hippocampus, the hypothalamus and the medulla oblongata related to cisplatin-induced pica of rats. Res Commun Mol Pathol Pharmacol. 2003:113–114. 97–113. [PubMed] [Google Scholar]
- Manchanda SK, Tandon OP, Aneja IS. Role of the cerebellum in the control of gastro-intestinal motility. J Neural Transm. 1972;33:195–209. doi: 10.1007/BF01245317. [DOI] [PubMed] [Google Scholar]
- Marchetti F, Maestro A, Rovere F, Zanon D, Arrighini A, Bertolani P, Biban P, Da DL, Di PP, Renna S, Guala A, Mannelli F, Pazzaglia A, Messi G, Perri F, Reale A, Urbino AF, Valletta E, Vitale A, Zangardi T, Tondelli MT, Clavenna A, Bonati M, Ronfani L. Oral ondansetron versus domperidone for symptomatic treatment of vomiting during acute gastroenteritis in children: multicentre randomized controlled trial. BMC Pediatr. 2011;11:15. doi: 10.1186/1471-2431-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllen RM, Spyer KM. The baroreceptor input to cardiac vagal moto-neurones. J Physiol. 1978;282:365–374. doi: 10.1113/jphysiol.1978.sp012469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AD. Respiratory muscle control during vomiting. Can J Physiol Pharmacol. 1990;68:237–241. doi: 10.1139/y90-037. [DOI] [PubMed] [Google Scholar]
- Miller AD, Nonaka S. Mechanisms of abdominal muscle activation during vomiting. J Appl Physiol. 1990;69:21–25. doi: 10.1152/jappl.1990.69.1.21. [DOI] [PubMed] [Google Scholar]
- Miller AD, Nonaka S, Jakus J. Brain areas essential or non-essential for emesis. Brain Res. 1994;647:255–264. doi: 10.1016/0006-8993(94)91325-0. [DOI] [PubMed] [Google Scholar]
- Miller AD, Ruggiero DA. Emetic reflex arc revealed by expression of the immediate-early gene c-fos in the cat. J Neurosci. 1994;14:871–888. doi: 10.1523/JNEUROSCI.14-02-00871.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AD, Tan LK, Suzuki I. Control of abdominal and expiratory intercostal muscle activity during vomiting: role of ventral respiratory group expiratory neurons. J Neurophysiol. 1987;57:1854–1866. doi: 10.1152/jn.1987.57.6.1854. [DOI] [PubMed] [Google Scholar]
- Miller AD, Wilson VJ. ‘Vomiting center’ reanalyzed: an electrical stimulation study. Brain Res. 1983;270:154–158. doi: 10.1016/0006-8993(83)90805-3. [DOI] [PubMed] [Google Scholar]
- Moga MM, Herbert H, Hurley KM, Yasui Y, Gray TS, Saper CB. Organization of cortical, basal forebrain, and hypothalamic afferents to the parabrachial nucleus in the rat. J Comp Neurol. 1990;295:624–661. doi: 10.1002/cne.902950408. [DOI] [PubMed] [Google Scholar]
- Morimoto M, Morita N, Ozawa H, Yokoyama K, Kawata M. Distribution of glucocorticoid receptor immunoreactivity and mRNA in the rat brain: an immunohistochemical and in situ hybridization study. Neurosci Res. 1996;26:235–269. doi: 10.1016/s0168-0102(96)01105-4. [DOI] [PubMed] [Google Scholar]
- Mussa BM, Sartor DM, Verberne AJ. Activation of cholecystokinin (CCK 1) and serotonin (5-HT 3) receptors increases the discharge of pancreatic vagal afferents. Eur J Pharmacol. 2008;601:198–206. doi: 10.1016/j.ejphar.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Neff RA, Mihalevich M, Mendelowitz D. Stimulation of NTS activates NMDA and non-NMDA receptors in rat cardiac vagal neurons in the nucleus ambiguus. Brain Res. 1998;792:277–282. doi: 10.1016/s0006-8993(98)00149-8. [DOI] [PubMed] [Google Scholar]
- Nozaki K, Boccalini P, Moskowitz MA. Expression of c-fos-like immunoreactivity in brainstem after meningeal irritation by blood in the subarachnoid space. Neuroscience. 1992;49:669–680. doi: 10.1016/0306-4522(92)90235-t. [DOI] [PubMed] [Google Scholar]
- Owyang C, Hasler WL. Physiology and pathophysiology of the interstitial cells of cajal: from bench to beside VI. Pathogenesis and therapeutic approaches to human gastric dysrhythmias. Am J Physiol Gastrointest Liver Physiol. 2002;283:G8–G15. doi: 10.1152/ajpgi.00095.2002. [DOI] [PubMed] [Google Scholar]
- Parker LA, Rock EM, Limebeer CL. Regulation of nausea and vomiting by cannabinoids. Br J Pharmacol. 2011;163:1411–1422. doi: 10.1111/j.1476-5381.2010.01176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering AE, Boscan P, Paton JF. Nociception attenuates parasympathetic but not sympathetic baroreflex via NK1 receptors in the rat nucleus tractus solitarii. J Physiol. 2003a;551:589–599. doi: 10.1113/jphysiol.2003.046615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering AE, Boscan P, Paton JF. Nociception attenuates parasympathetic but not sympathetic baroreflex via NK1 receptors in the rat nucleus tractus solitarii. J Physiol. 2003b;551:589–599. doi: 10.1113/jphysiol.2003.046615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Hoyda TD, Ferguson AV. The area postrema: a brain monitor and integrator of systemic autonomic state. Neuroscientist. 2008;14:182–194. doi: 10.1177/1073858407311100. [DOI] [PubMed] [Google Scholar]
- Randich A, Gebhart DF. Vagal afferent modulation of nociception. Brain Res Rev. 1992;17:77–99. doi: 10.1016/0165-0173(92)90009-b. [DOI] [PubMed] [Google Scholar]
- Ray AP, Chebolu S, Darmani NA. Receptor-selective agonists induce emesis and Fos expression in the brain and enteric nervous system of the least shrew (Cryptotis parva) Pharmacol Biochem Behav. 2009;94:211–218. doi: 10.1016/j.pbb.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybould HE. Does your gut taste? Sensory transduction in the gastrointestinal tract. News Physiol Sci. 1998;13:275–280. doi: 10.1152/physiologyonline.1998.13.6.275. [DOI] [PubMed] [Google Scholar]
- Raybould HE. Primary afferent response to signals in the intestinal lumen. J Physiol. 2001;530(2):343. doi: 10.1111/j.1469-7793.2001.0343k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybould HE. Visceral perception: sensory transduction in visceral afferents and nutrients. Gut. 2002;51(1):I11–I14. doi: 10.1136/gut.51.suppl_1.i11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybould HE, Glatzle J, Robin C, Meyer JH, Phan T, Wong H, Sternini C. Expression of 5-HT3 receptors by extrinsic duodenal afferents contribute to intestinal inhibition of gastric emptying. Am J Physiol Gastrointest Liver Physiol. 2003;284:G367–G372. doi: 10.1152/ajpgi.00292.2001. [DOI] [PubMed] [Google Scholar]
- Renehan WE, Zhang X, Beierwaltes WH, Fogel R. Neurons in the dorsal motor nucleus of the vagus may integrate vagal and spinal information from the GI tract. Am J Physiol. 1995;268:G780–G790. doi: 10.1152/ajpgi.1995.268.5.G780. [DOI] [PubMed] [Google Scholar]
- Reynolds DJ, Leslie RA, Grahame-Smith DG, Harvey JM. Localization of 5-HT3 receptor binding sites in human dorsal vagal complex. Eur J Pharmacol. 1989;174:127–130. doi: 10.1016/0014-2999(89)90884-4. [DOI] [PubMed] [Google Scholar]
- Reynolds DJ, Barber NA, Grahame-Smith DG, Leslie RA. Cisplatin-evoked induction of c-fos protein in the brainstem of the ferret: the effect of cervical vagotomy and the anti-emetic 5-HT3 receptor antagonist granisetron (BRL 43694) Brain Res. 1991;565:231–236. doi: 10.1016/0006-8993(91)91654-j. [DOI] [PubMed] [Google Scholar]
- Rinaman L. Visceral sensory inputs to the endocrine hypothalamus. Front Neuroendocrinol. 2007;28:50–60. doi: 10.1016/j.yfrne.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman L. Ascending projections from the caudal visceral nucleus of the solitary tract to brain regions involved in food intake and energy expenditure. Brain Res. 2010;1350:18–34. doi: 10.1016/j.brainres.2010.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez EM, Blazquez JL, Guerra M. The design of barriers in the hypothalamus allows the median eminence and the arcuate nucleus to enjoy private milieus: the former opens to the portal blood and the latter to the cerebrospinal fluid. Peptides. 2010;31:757–776. doi: 10.1016/j.peptides.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Rosen AM, Victor JD, Di Lorenzo PM. Temporal coding of taste in the parabrachial nucleus of the pons of the rat. J Neurophysiol. 2011;105:1889–1896. doi: 10.1152/jn.00836.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin DL, Chang DA, Guyenet PG. Afferent and efferent connections of the rat retrotrapezoid nucleus. J Comp Neurol. 2006;499:64–89. doi: 10.1002/cne.21105. [DOI] [PubMed] [Google Scholar]
- Ruggiero DA, Mtui EP, Otake K, Anwar M. Vestibular afferents to the dorsal vagal complex: substrate for vestibular-autonomic interactions in the rat. Brain Res. 1996;743:294–302. doi: 10.1016/s0006-8993(96)01099-2. [DOI] [PubMed] [Google Scholar]
- Saito R, Takano Y, Kamiya HO. Roles of substance P and NK(1) receptor in the brainstem in the development of emesis. J Pharmacol Sci. 2003;91:87–94. doi: 10.1254/jphs.91.87. [DOI] [PubMed] [Google Scholar]
- Sanger GJ. Neurokinin NK1 and NK3 receptors as targets for drugs to treat gastrointestinal motility disorders and pain. Br J Pharmacol. 2004;141:1303–1312. doi: 10.1038/sj.bjp.0705742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savastano DM, Hayes MR, Covasa M. Serotonin-type 3 receptors mediate intestinal lipid-induced satiation and Fos-like immunoreactivity in the dorsal hindbrain. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1063–R1070. doi: 10.1152/ajpregu.00699.2006. [DOI] [PubMed] [Google Scholar]
- Schlegel TT, Brown TE, Wood SJ, Benavides EW, Bondar RL, Stein F, Moradshahi P, Harm DL, Fritsch-Yelle JM, Low PA. Orthostatic intolerance and motion sickness after parabolic flight. J Appl Physiol. 2001;90:67–82. doi: 10.1152/jappl.2001.90.1.67. [DOI] [PubMed] [Google Scholar]
- Shapiro RE, Miselis RR. The central organization of the vagus nerve innervating the stomach of the rat. J Comp Neurol. 1985;238:473–488. doi: 10.1002/cne.902380411. [DOI] [PubMed] [Google Scholar]
- Shinpo K, Hirai Y, Maezawa H, Totsuka Y, Funahashi M. The role of area postrema neurons expressing H-channels in the induction mechanism of nausea and vomiting. Physiol Behav. 2012;107:98–103. doi: 10.1016/j.physbeh.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Sivarao DV, Krowicki ZK, Hornby PJ. Role of GABAA receptors in rat hindbrain nuclei controlling gastric motor function. Neurogastroenterol Motil. 1998;10:305–313. doi: 10.1046/j.1365-2982.1998.00110.x. [DOI] [PubMed] [Google Scholar]
- Smith BN, Dou P, Barber WD, Dudek FE. Vagally evoked synaptic currents in the immature rat nucleus tractus solitarii in an intact in vitro preparation. J Physiol. 1998;512:149–162. doi: 10.1111/j.1469-7793.1998.149bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Abdala AP, Rybak IA, Paton JF. Structural and functional architecture of respiratory networks in the mammalian brainstem. Philos Trans R Soc Lond B Biol Sci. 2009;364:2577–2587. doi: 10.1098/rstb.2009.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song G, Xu H, Wang H, Macdonald SM, Poon CS. Hypoxia-excited neurons in NTS send axonal projections to Kolliker-Fuse/parabrachial complex in dorsolateral pons. Neuroscience. 2011;175:145–153. doi: 10.1016/j.neuroscience.2010.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spyer KM, Lambert JH, Thomas T. Central nervous system control of cardiovascular function: neural mechanisms and novel modulators. Clin Exp Pharmacol Physiol. 1997;24:743–747. doi: 10.1111/j.1440-1681.1997.tb02125.x. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Sugiyama Y, Yates BJ. Integrative responses of neurons in parabrachial nuclei to a nauseogenic gastrointestinal stimulus and vestibular stimulation in vertical planes. Am J Physiol Regul Integr Comp Physiol. 2012;302:R965–R975. doi: 10.1152/ajpregu.00680.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda N, Morita M, Horii A, Nishiike S, Kitahara T, Uno A. Neural mechanisms of motion sickness. J Med Invest. 2001;48:44–59. [PubMed] [Google Scholar]
- Terreberry RR, Neafsey EJ. Rat medial frontal cortex: a visceral motor region with a direct projection to the solitary nucleus. Brain Res. 1983;278:245–249. doi: 10.1016/0006-8993(83)90246-9. [DOI] [PubMed] [Google Scholar]
- Terreberry RR, Neafsey EJ. The rat medial frontal cortex projects directly to autonomic regions of the brainstem. Brain Res Bull. 1987;19:639–649. doi: 10.1016/0361-9230(87)90050-5. [DOI] [PubMed] [Google Scholar]
- Thumas LJ. Über das Brechcentrum and über die Wirkung einiger pharma-koligischer Mittel auf dasselbe [About the vomiting centre and about the effect of a pharmaceutical agent on the same] Arch Patol Anat. 1891;123:44–69. [Google Scholar]
- Tonini M. 5-Hydroxytryptamine effects in the gut: the 3, 4, and 7 receptors. Neurogastroenterol Motil. 2005;17:637–642. doi: 10.1111/j.1365-2982.2005.00716.x. [DOI] [PubMed] [Google Scholar]
- Traub RJ, Sengupta JN, Gebhart GF. Differential c-fos expression in the nucleus of the solitary tract and spinal cord following noxious gastric distention in the rat. Neuroscience. 1996;74:873–884. doi: 10.1016/0306-4522(96)00173-x. [DOI] [PubMed] [Google Scholar]
- Travagli RA, Gillis RA. Effect of 5-HT alone and its interaction with TRH on neurons in rat dorsal motor nucleus of the vagus. Am J Physiol. 1995;263:G292–G299. doi: 10.1152/ajpgi.1995.268.2.G292. [DOI] [PubMed] [Google Scholar]
- Travagli RA, Gillis RA, Rossiter CD, Vicini S. Glutamate and GABA-mediated synaptic currents in neurons of the rat dorsal motor nucleus of the vagus. Am J Physiol. 1991;260:G531–G536. doi: 10.1152/ajpgi.1991.260.3.G531. [DOI] [PubMed] [Google Scholar]
- Travagli RA, Hermann GE, Browning KN, Rogers RC. Brainstem circuits regulating gastric function. Annu Rev Physiol. 2006;68:279–305. doi: 10.1146/annurev.physiol.68.040504.094635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino M, Kuwahara M, Ebukuro S, Tsubone H. Modulation of emetic response by carotid baro- and chemoreceptor activations. Auton Neurosci. 2006;128:25–36. doi: 10.1016/j.autneu.2005.12.006. [DOI] [PubMed] [Google Scholar]
- van der Kooy D, Koda LY, McGinty JF, Gerfen CR, Bloom FE. The organization of projections from the cortex, amygdala, and hypothalamus to the nucleus of the solitary tract in rat. J Comp Neurol. 1984;224:1–24. doi: 10.1002/cne.902240102. [DOI] [PubMed] [Google Scholar]
- Walsh JW, Hasler WL, Nugent CE, Owyang C. Progesterone and estrogen are potential mediators of gastric slow-wave dysrhythmias in nausea of pregnancy. Am J Physiol. 1996;270:G506–G514. doi: 10.1152/ajpgi.1996.270.3.G506. [DOI] [PubMed] [Google Scholar]
- Wang SC, Borison HL. The vomiting center; a critical experimental analysis. Arch Neurol Psychiatry. 1950;63:928–941. doi: 10.1001/archneurpsyc.1950.02310240087005. [DOI] [PubMed] [Google Scholar]
- Wang SC, Borison HL. Copper sulphate emesis; a study of afferent pathways from the gastrointestinal tract. Am J Physiol. 1951;164:520–526. doi: 10.1152/ajplegacy.1951.164.2.520. [DOI] [PubMed] [Google Scholar]
- Woods AJ, Andrews PL. Cisplatin acutely reduces 5-hydroxytryptamine-induced vagal depolarization in the rat: protective action of dexamethasone. Eur J Pharmacol. 1995;278:275–278. doi: 10.1016/0014-2999(95)00174-j. [DOI] [PubMed] [Google Scholar]
- Wuchert F, Ott D, Rafalzik S, Roth J, Gerstberger R. Tumor necrosis factor-alpha, interleukin-1beta and nitric oxide induce calcium transients in distinct populations of cells cultured from the rat area postrema. J Neuroimmunol. 2009;206:44–51. doi: 10.1016/j.jneuroim.2008.10.010. [DOI] [PubMed] [Google Scholar]
- Xu LH, Koch KL, Summy-Long J, Stern RM, Seaton JF, Harrison TS, Demers LM, Bingaman S. Hypothalamic and gastric myoelectrical responses during vection-induced nausea in healthy Chinese subjects. Am J Physiol. 1993;265:E578–E584. doi: 10.1152/ajpendo.1993.265.4.E578. [DOI] [PubMed] [Google Scholar]
- Yates BJ, Miller AD, Lucot JB. Physiological basis and pharmacology of motion sickness: an update. Brain Res Bull. 1998;47:395–406. doi: 10.1016/s0361-9230(98)00092-6. [DOI] [PubMed] [Google Scholar]
- Yuan CS, Barber WD. Parabrachial nucleus: neuronal evoked responses to gastric vagal and greater splanchnic nerve stimulation. Brain Res Bull. 1991;27:797–803. doi: 10.1016/0361-9230(91)90211-2. [DOI] [PubMed] [Google Scholar]
- Zerari-Mailly F, Buisseret P, Buisseret-Delmas C, Nosjean A. Trigemino-solitarii-facial pathway in rats. J Comp Neurol. 2005;487:176–189. doi: 10.1002/cne.20554. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Travagli RA. Dopamine effects on identified rat vagal motoneurons. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1002–G1008. doi: 10.1152/ajpgi.00527.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JX, Wu XY, Owyang C, Li Y. Intestinal serotonin acts as a paracrine substance to mediate vagal signal transmission evoked by luminal factors in the rat. J Physiol. 2001;530:431–442. doi: 10.1111/j.1469-7793.2001.0431k.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]


