Abstract
Objective
The objective of this study was to explore the association of longitudinal CA-125 measurements with overall survival (OS) time by developing a flexible model for patient-specific CA-125 profiles, and to provide a simple and reliable prediction of OS.
Methods
A retrospective study was performed on 275 patients with ovarian cancer who underwent at least one cycle of primary chemotherapy in our institute. Serial measurements of patients' CA-125 levels were performed at different frequencies according to their clinical plans. A statistical model coupling the Cox proportional hazards and the mixed-effects models was applied to determine the association of OS with patient-specific longitudinal CA-125 values. Stage and residual tumor size were additional variables included in the analysis.
Results
A total of 1,601 values of CA-125 were included. Longitudinal CA-125 levels, stage, and the residual tumor size were all significantly associated with OS. A patient-specific survival probability could be calculated. Validation showed that, in average, 85.4% patients were correctly predicted to have a high or low risk of death at a given time point. Comparison with a traditional model using CA-125 half-life and time to reach CA-125 nadir showed that the longitudinal CA-125 model had an improved predicative value.
Conclusion
Longitudinal CA-125 values, measured from the diagnosis of ovarian cancer to the completion of primary chemotherapy, could be used to reliably predict OS after adjusting for the stage and residual tumor disease. This model could be potentially useful in clinical counseling of patients with ovarian cancer.
Keywords: CA-125, Longitudinal analysis, Ovarian cancer, Overall survival, Prediction
INTRODUCTION
Among gynecologic cancers, ovarian cancer is the second leading cause of death worldwide and the leading cause in developed countries [1]. In patients diagnosed with advanced stage III-IV ovarian cancer (International Federation of Gynecology and Obstetrics [FIGO] classification), the 5-year survival rate is ~30%, whereas in those diagnosed at an early stage the 5-year survival rate is ~90% [2]. The overall survival (OS) of patients with ovarian cancer has been extensively researched and many risk factors have been explored, including the serum cancer antigen 125 (CA-125) levels.
CA-125 is the most commonly used biomarker for diagnosis and follow-up of ovarian cancer. It is a high molecular weight glycoprotein with an elevated serum level (>35 U/mL) in 50-90% of patients with ovarian cancer, depending on the cancer stage [3]. Many features of CA-125 have been explored for an association with OS, such as the absolute value of CA-125 at different time points during treatment, and pharmacokinetic parameters, including CA-125 half-life [4]. However, the majority of survival studies have only considered a single CA-125 measurement or a limited number at well-defined time points. However, patients usually have CA-125 measurements taken repeatedly (longitudinally) over time, and the number of measurements, as well as the intervals between measurements, vary between patients for different reasons such as different responses to chemotherapy or unexpected loss to follow-up [5]. Therefore, longitudinal and often misaligned measurements contain information that cannot be represented by single values or characteristics, and it would be beneficial to develop a model that takes into account all longitudinal measurements to give a clear indication of patient-specific CA-125 profiles for the analyses of an association with OS.
Here, a recently published joint model, which couples the Cox proportional hazards and the mixed-effects models, was applied to assess the relationship between longitudinal CA-125 and OS [6]. The aim was to flexibly analyze repeated CA-125 measurements over time, including misaligned observations of CA-125, to provide a simple and reliable OS prediction model in a patient-specific manner. FIGO stage and residual tumor size were included as variables in the model because they are important prognostic factors [2,7].
MATERIALS AND METHODS
1. Data collection
A retrospective chart review was performed in the Kaohsiung Veterans' General Hospital (KSVGH), a public teaching medical center in south Taiwan. Data were collected from 275 patients who were admitted to KSVGH with ovarian cancer between June 1995 and January 2012. A total of 6 cycles of primary chemotherapy with an interval of 3-4 weeks was planned for the patients, with all completing at least one cycle. At each cycle of chemotherapy, as well as before and after surgery, at least one measurement of the CA-125 serum level was taken. A complete list of CA-125 has at least 8 values, spanning 4-6 months. However, patients may often suffer from adverse events after chemotherapy, resulting in treatment interruptions and postponement of the next cycle of chemotherapy. Some patients completed >6 cycles, but their CA-125 values beyond the primary chemotherapy were not included. In addition, some patients may have fewer cycles of chemotherapy due to various reasons such as disease progression or informed personal decision. FIGO stage and residual tumor size were included as covariates. The disease stage was obtained from the pathologic report of each patient, and the residual tumor size was obtained from their surgical notes.
The acquisition of patient data was approved of by the Institutional Review Board (IRB) of KSVGH. All patient information was retrieved from the hospital records, including electronic data and chart data. The extracted data included demographic and prognostic information such as age, blood tests, surgical notes, and pathologic reports.
2. Definitions
The end point of interest was OS, defined as the time from surgery to death or last follow-up. Those whose end point was the date of the last follow-up were defined as censored. Prediction of OS was achieved by calculating the survival probability of a patient at any given time point. A patient was defined as being at a high risk of death at a specific time point if the calculated survival probability was lower than the threshold, and at low risk if the probability was higher than the threshold. The threshold was dynamically estimated with each model fitting.
3. Statistical methods
All statistical analyses were conducted with the statistical computing and graphic drawing language, R [8]. A joint model, that coupled the Cox proportional hazards and the mixed-effects models, was constructed based on a freely available package, JM [9], to assess longitudinal measurements of CA-125 and OS data. The Cox proportional hazards model explores the association between each patient's OS and longitudinal CA-125 profile [10], while the mixed-effects model, often used in longitudinal data analyses, models the time trend of CA-125 profiles [11]. The advantage of using the mixed-effects model is that it can handle patients' misaligned observations of CA-125 across time. The CA-125 profiles of the patients were smoothed using lowess (locally weighted scatterplot smoothing) regression [12], which is a non-parametric regression method that uses locally-weighted polynomials to smooth the data.
To test the accuracy of the prediction, we performed validation based on 3-year survival probabilities. Two-hundred and eighteen patients were used in this part of the study after excluding 57 patients who were censored within 3 years. In order to have a well-fitted model, we unevenly divided these 218 patients by randomly selecting 50 patients as the validation cohort so that a majority (168 patients, or roughly 3/4) were left to train the model and for an estimation of the optimal threshold of high or low risk stratification. Once the model was fitted and an optimal threshold was estimated, we applied it to the 50 patients in the validation cohort to calculate their 3-year survival probabilities. We determined that the prediction was accurate if a patient died within 3 years and was grouped as high risk, and vice versa. The validation course was repeated 10 times, with 50 randomly selected patients each time.
RESULTS
1. CA-125 profiles
A total of 1,601 CA-125 values were collected, representing all the available CA-125 measurements of the 275 patients from the time of diagnosis of ovarian cancer to the completion of the primary chemotherapy. The cut-off date of follow-up was July 31, 2012. At the time, 81 patients had expired and 194 had been censored. Patient and tumor characteristics are summarized in Table 1. CA-125 profiles of the patients were extremely heterogeneous, ranging from 2-14 measurements, spanning over 2-10 months. Some typical examples of the CA-125 profiles are shown in Fig. 1.
Table 1.
Patient and tumor characteristics (n=275)
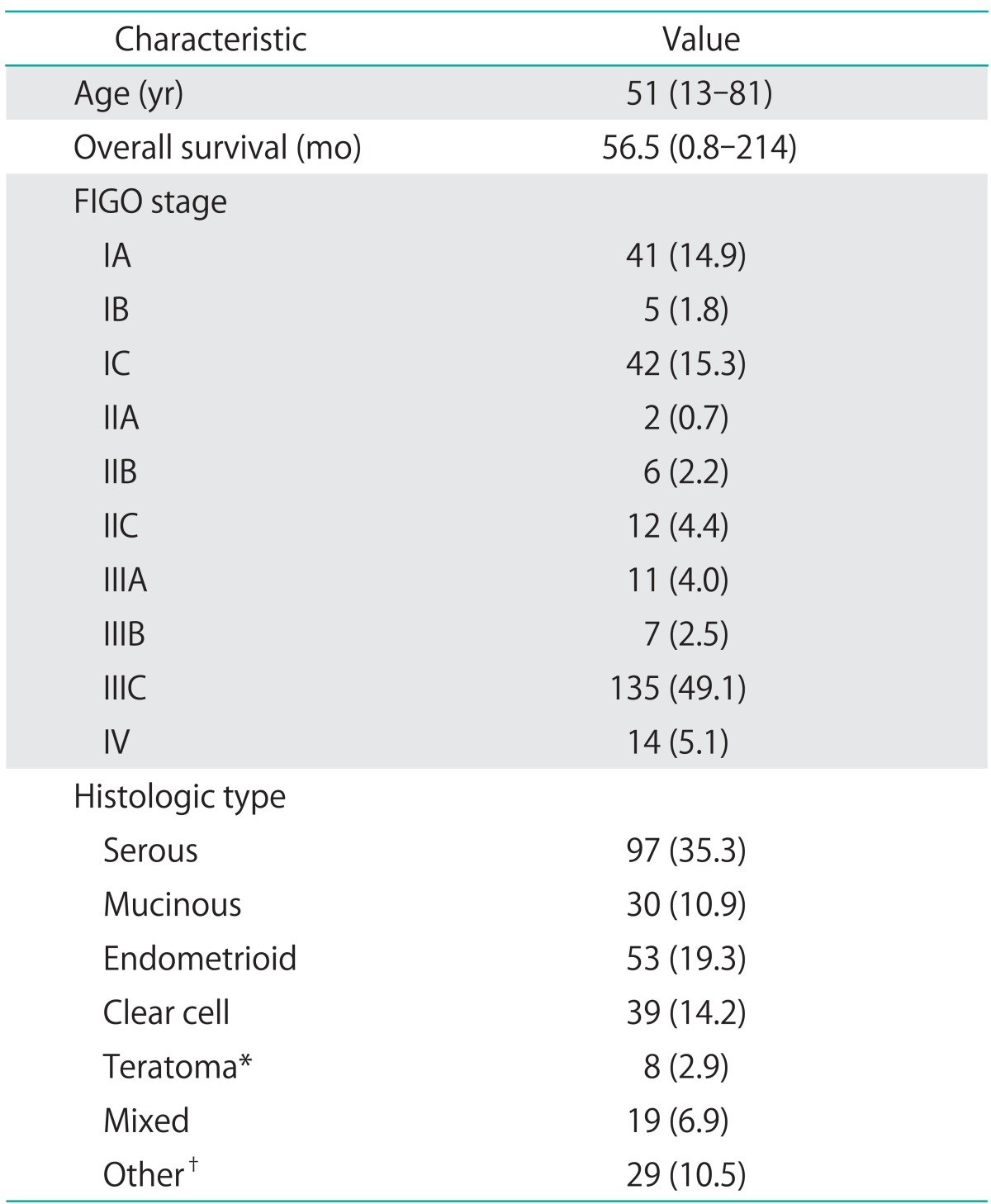
Values are presented as median (range) or number (%).
FIGO, International Federation of Gynecology and Obstetrics.
*Six cases of malignant immature teratoma, 1 squamous cell tumor associated with teratoma, and 1 malignant melanoma arising from teratoma. †Mullerian tumor, granulosa cell tumor, sex cord tumor, transitional cell tumor, dysgerminoma, undifferentiated, and unknown types.
Fig. 1.
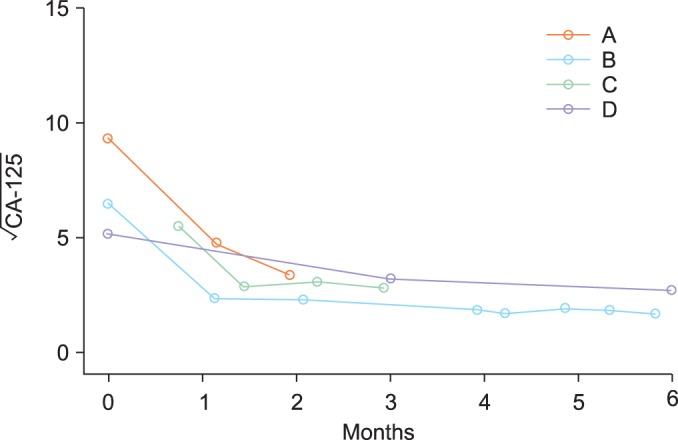
Typical examples of patient-specific CA-125 profiles. Different patients had different numbers of CA-125 measurements taken at different time points spanning different time frames. The profile of patient A contained only 3 measurements in the first two months following admission. Patient B had 8 CA-125 values taken throughout the course from surgery to completion of the primary chemotherapy (approximately 6 months). Patient C had four CA-125 measurements in the first three months and her preoperative CA-125 value was missing. Patient D had three CA-125 observations throughout the course from surgery to completion of the primary chemotherapy (around 6 months).
2. The relationship between longitudinal CA-125 and OS according to FIGO stage and residual tumor size
The joint model was adapted to evaluate the relationship between longitudinal CA-125 and OS according to FIGO stage and residual tumor size as covariates. Residual plots showed that the model fit the data very well (Fig. 2). It could flexibly fit all the various CA-125 profiles, regardless of the number of CA-125 measurements including early-censored patients who only had 2-3 measurements. All the collected CA-125 measurements (n=1,601) were fitted to the model, and the results showed that the longitudinal CA-125 values were significantly associated with OS (p<0.001).
Fig. 2.
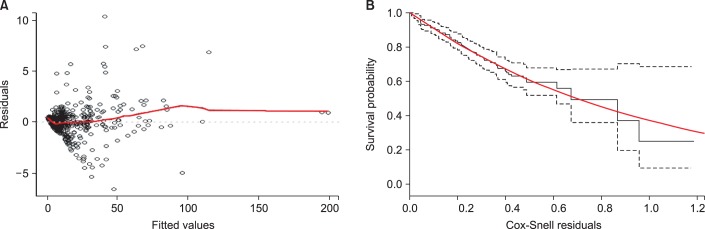
Residual plots of the joint model. (A) The subject specific residual plot for the validity of the mixed-effects model. The red line is the lowess curve of the fitted values. It is very close to the horizontal line from point 0 (the dotted line), suggesting a good fit. (B) The Cox-Snell residual plot for the accuracy of the second part of the joint model. The y axis is the fitted survival time (Kaplan Meier estimates). The solid black line is the Kaplan-Meier curve of the residuals, which corresponds to the ideal curve (the red line) quite well, suggesting a good fit of the model. The two dotted lines give the 95% confidence interval.
To further address the relationship between longitudinal CA-125 and OS, we plotted lowess curves (Fig. 3) for those patients who died within 3 years (the high-risk curve) and those who survived for at least 3 years (the low-risk curve). It is obvious that those patients at high-risk had a higher CA-125 curve. It is also interesting to note that the two curves are farthest apart not at the initial phase, but in the later time period post one month. This is in line with our suggestion that CA-125 changes over time have a significant predictive power.
Fig. 3.
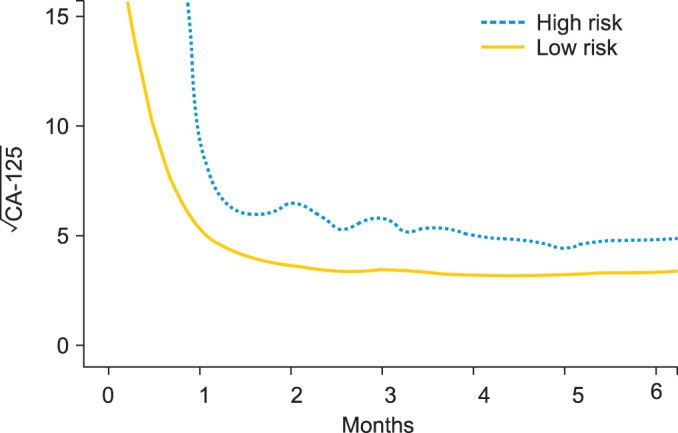
The lowess curves of CA-125 profiles according to high and low risk patients at 3-year survival. Those who died within 3 years after surgery are considered high risk (n=41), and those who survived for at least 3 years are considered low risk (n=177). Patients who were censored within 3 years were excluded from the curves. All the CA-125 values of each entire group were locally smoothened to generate a curve that showed the overall distribution of CA-125 profiles.
Stage and residual disease are both important prognostic factors of OS, and we included them as variables in the model to adjust for the possible confounding effect they may have on estimating OS with longitudinal CA-125. Stage was treated as a binomial variable labeled as early (FIGO I and II) or advanced (FIGO III and IV) stage. Residual tumor size was also treated as a binomial variable with a cut-off value of 1 cm [7]. Our model showed that they were both significantly associated with OS (p=0.004 for stage, and p<0.001 for residual tumor size; data not shown).
3. Prediction of OS and validation
Based on the joint model described above, survival probability could be predicted at any given point of time for a new patient, provided that the CA-125 profile, FIGO stage (early or late), and residual tumor size was available. The patient-specific prediction is flexibly available for new patients even those with only 2-3 measurements of CA-125 over any time frame during the course from diagnosis to the completion of primary chemotherapy, and the survival probability could be calculated with the model at any time point, i.e., 1, 2, 3, 4, 5 years, and so on. The higher the probability, the lower the risk of death is at that time point. (For a program to calculate subject-specific survival probabilities, please go to http://www.math.nsysu.edu.tw/~cchang/predict_survival/.)
The average accuracy of the prediction was 85.4%, ranging from 80.0% to 88.0% (Table 2). These stable results showed that the model could give reliable prediction according to the high and low risk groups.
Table 2.
Validation of the accuracy of predicting overall survival (n=50)*
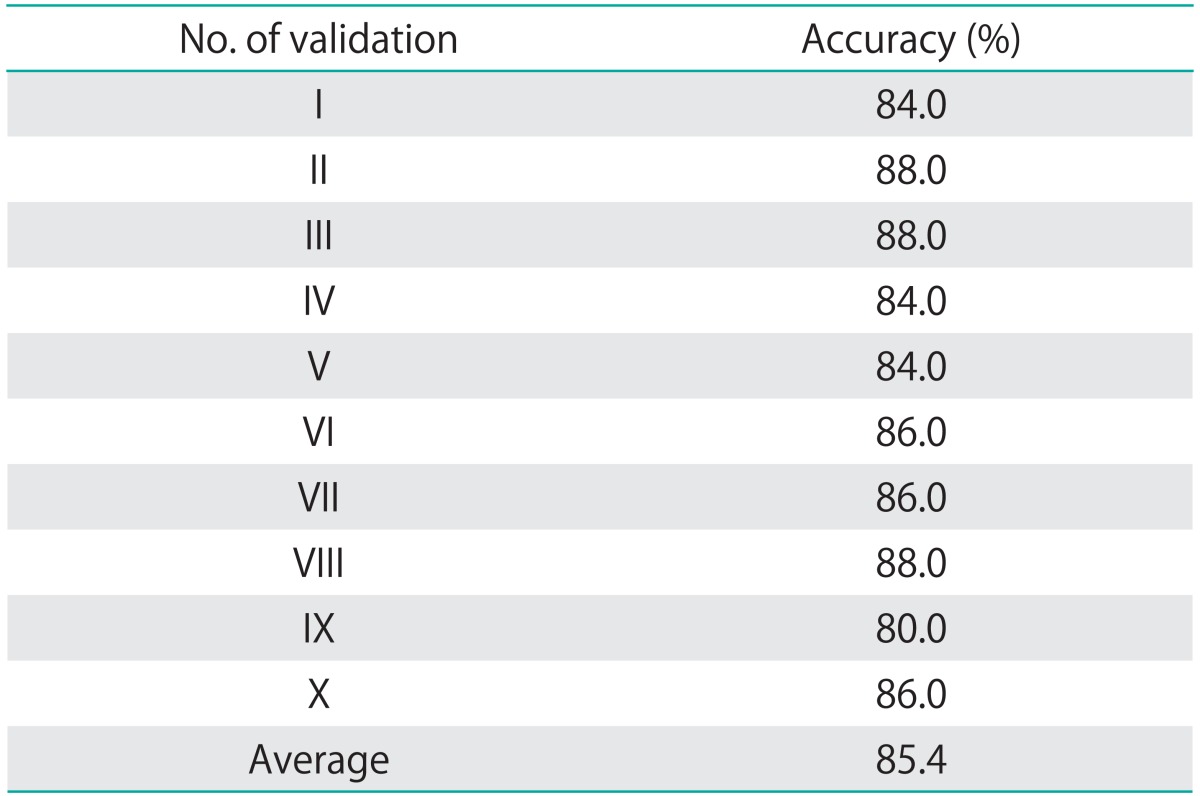
*Validation was done for 10 times. At each time, 50 patients were randomly selected as the validation cohort and the rest of the patients were used to fit the model and for estimation of the optimum threshold of high/low risk. Once the model was fitted, it was applied to the 50 patients to classify them into high or low risk of death at the 3-year time point. From 40 to 44 patients were correctly predicted to be at high or low risk in all the 10 rounds, which gave an average accuracy of prediction of 85.4%.
4. Comparison with CA-125 pharmacokinetic factors
The predicative value of the longitudinal CA-125 was compared with the CA-125 half-life and time-to-nadir predictive values as described previously [13]. Using the Cox proportional hazards model, the survival probability for each patient can also be calculated using CA-125 half-life or time-to-nadir with FIGO stage and residual tumor size as covariates. We ran the same validation course 10 times as described in the above section. The average accuracy of prediction with CA-125 half-life and time-to-nadir were both 82.2%. Compared to the average accuracy of 85.4% of longitudinal CA-125, the longitudinal CA-125 showed a better predictive value than a single kinetic factor (Table 3).
Table 3.
Comparison of the accuracy of predicting overall survival with longitudinal CA-125, CA-125 half-life, and CA-125 nadir (n=10)*

*Validation was performed as described previously and in Table 2. The accuracy presented in this table was the average result of 10 times. Prediction with CA-125 half-life and time-to-nadir was performed with Cox proportional hazards model, while prediction with longitudinal CA-125 was with the joint model. International Federation of Gynecology and Obstetrics (FIGO) stage and residual tumor size were included in each model as covariates.
DISCUSSION
CA-125 is widely used as a tumor marker for the diagnosis and prognosis of ovarian cancer. However, the exact relationship between CA-125 and OS is still unclear [14,15]. Many of the related studies focus on specific values of CA-125. For example, studies on absolute CA-125 values have shown that the CA-125 levels after surgery, after 2, 3, or 6 cycles of chemotherapy are important prediction factors for OS [16-20], but the preoperative CA-125 may not correlate with OS [21]. The results presented here also suggested that those patients survived <3 years had an overall higher CA-125 curve compared to those who survived 3 years or more, even in the first month of treatment, which typically represents the CA-125 changes after the first couple of chemotherapy cycles.
There have also been studies on changes of CA-125 levels as kinetic parameters such as half-life and doubling time of CA-125, time to reach CA-125 nadir, and CA-125 area under the curve (AUC) [13,22-24]. However, focus on single CA-125 values at specific time points may only reveal a limited relationship to OS. A notable feature of CA-125 is that it is measured repeatedly over time. Here, a comparison of the predicative values of using CA-125 half-life and time-to-nadir with longitudinal CA-125 revealed that longitudinal CA-125 performed better in predicting OS. This suggests that longitudinal measurements contain valuable information that cannot be fully represented by examining a single value or a kinetic factor. In addition, studies that considered CA-125 over a period of time treated multiple CA-125 levels individually [20,25,26], and there is so far no report that has taken all the measurements and fitted them concurrently to a survival model.
In contrast, here all available CA-125 measurements of patients regardless of whether a patient has missing data or not were used. Furthermore, the model was constructed based on an assembly of patients regardless of factors such as tumor type, stage, and chemotherapeutic agent, which enabled the model to be applicable to a wide population without recruitment restrictions. This approach would be potentially helpful to gynecologic oncologists to make better informed decisions in patient counseling with all the available information.
No other effort has achieved OS prediction with longitudinal CA-125, which has obvious medical applications. In the management of many diseases, there are important markers that are taken repeatedly over time to monitor prognosis, such as CA 19-9 in pancreatic cancer [27] and CA15-3 in breast cancer [28]. Our model provides a simple alternative to investigating how OS is related to those longitudinally measured markers and is accessible online (http://www.math.nsysu.edu.tw/~cchang/predict_survival/).
We included FIGO stage and residual tumor size as covariates in our model. Patients at an early stage (FIGO I and II) usually have a much better prognosis than those at an advanced stage (FIGO III and IV) [2], and their CA-125 profiles may also be quite different. Similarly, patients who had a small residual tumor size (<1 cm) after surgery had a better prognosis than those with larger residual tumor (≥1 cm) [7]. Inclusion of these covariates could adjust for potential confounding effects, and the validation result confirmed that prediction with CA-125, stage and residual tumor size could reach a high accuracy.
Another prominent feature of our model is that it could give prediction of a future patient's survival probability at any time point with patient-specific CA-125 observations. To test the performance of our prediction, we performed validation based on 3-year survival probability. The accuracy in the multiple validation efforts was very stable, with an average accuracy of 85.4%, ranging from 80.0% to 88.0%. This result confirmed that longitudinal CA-125 could be used as a reliable prediction factor for OS. The fact that multiple validation based on random selections gave stable results suggests that this model could be applied to various patient populations. However, it is necessary to validate the model in a large, heterogeneous external population in the future. It should be noted that since we based the OS prediction on longitudinal CA-125, the survival probability of a patient, who does not show elevated levels of CA-125 throughout the course, might be overestimated in our model. About 20% ovarian cancer patients have low CA-125 concentration [29]. Fitting the model with a low CA-125 patient pool could solve the potential problem, and this relies on acquisition of more patient information.
In conclusion, longitudinal CA-125 measurements from the time of diagnosis to the completion of primary chemotherapy are significantly associated with and can reliably predict OS in patients with ovarian cancer. Future studies should be directed to explore other prognostic factors in their relation to OS, and the model should be tested in a large, external, and heterogeneous population.
ACKNOWLEDGMENTS
The study was supported in part by a grant from KSVGH (VGHKS 101-120) and a grant from the National Science Council of Taiwan (NSC 101-2118-M-110-004).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Sankaranarayanan R, Ferlay J. Worldwide burden of gynaecological cancer: the size of the problem. Best Pract Res Clin Obstet Gynaecol. 2006;20:207–225. doi: 10.1016/j.bpobgyn.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Su Z, Graybill WS, Zhu Y. Detection and monitoring of ovarian cancer. Clin Chim Acta. 2013;415:341–345. doi: 10.1016/j.cca.2012.10.058. [DOI] [PubMed] [Google Scholar]
- 3.Markowska J, Manys G, Kubaszewska M. Value of CA 125 as a marker of ovarian cancer. Eur J Gynaecol Oncol. 1992;13:360–365. [PubMed] [Google Scholar]
- 4.Gupta D, Lis CG. Role of CA125 in predicting ovarian cancer survival: a review of the epidemiological literature. J Ovarian Res. 2009;2:13. doi: 10.1186/1757-2215-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boere IA, van der Burg ME. Review of dose-intense platinum and/or paclitaxel containing chemotherapy in advanced and recurrent epithelial ovarian cancer. Curr Pharm Des. 2012;18:3741–3753. doi: 10.2174/138161212802002634. [DOI] [PubMed] [Google Scholar]
- 6.Rizopoulos D. Dynamic predictions and prospective accuracy in joint models for longitudinal and time-to-event data. Biometrics. 2011;67:819–829. doi: 10.1111/j.1541-0420.2010.01546.x. [DOI] [PubMed] [Google Scholar]
- 7.Polterauer S, Vergote I, Concin N, Braicu I, Chekerov R, Mahner S, et al. Prognostic value of residual tumor size in patients with epithelial ovarian cancer FIGO stages IIA-IV: analysis of the OVCAD data. Int J Gynecol Cancer. 2012;22:380–385. doi: 10.1097/IGC.0b013e31823de6ae. [DOI] [PubMed] [Google Scholar]
- 8.R Development Core Team. R: a language and environment of statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2010. [Google Scholar]
- 9.Rizopoulos D, Verbeke G, Molenberghs G. Multiple-imputation-based residuals and diagnostic plots for joint models of longitudinal and survival outcomes. Biometrics. 2010;66:20–29. doi: 10.1111/j.1541-0420.2009.01273.x. [DOI] [PubMed] [Google Scholar]
- 10.Cox DR. Regression models and life-tables. J R Stat Soc Series B Stat Methodol. 1972;34:187–220. [Google Scholar]
- 11.Ying LS, Xu SH, Su D, Mou HZ, Gu LH, Zhu CH, et al. Study of the metastasis-associated genes and its copy numbers variation in highly metastatic epithelial ovarian cancer. Zhonghua Fu Chan Ke Za Zhi. 2009;44:126–130. [PubMed] [Google Scholar]
- 12.Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc. 1979;74:829–836. [Google Scholar]
- 13.Gadducci A, Cosio S, Fanucchi A, Negri S, Cristofani R, Genazzani AR. The predictive and prognostic value of serum CA 125 half-life during paclitaxel/platinum-based chemotherapy in patients with advanced ovarian carcinoma. Gynecol Oncol. 2004;93:131–136. doi: 10.1016/j.ygyno.2003.12.043. [DOI] [PubMed] [Google Scholar]
- 14.Riedinger JM, Wafflart J, Ricolleau G, Eche N, Larbre H, Basuyau JP, et al. CA 125 half-life and CA 125 nadir during induction chemotherapy are independent predictors of epithelial ovarian cancer outcome: results of a French multicentric study. Ann Oncol. 2006;17:1234–1238. doi: 10.1093/annonc/mdl120. [DOI] [PubMed] [Google Scholar]
- 15.Nolen BM, Lokshin AE. Protein biomarkers of ovarian cancer: the forest and the trees. Future Oncol. 2012;8:55–71. doi: 10.2217/fon.11.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Díaz-Padilla I, Razak AR, Minig L, Bernardini MQ, Maria Del Campo J. Prognostic and predictive value of CA-125 in the primary treatment of epithelial ovarian cancer: potentials and pitfalls. Clin Transl Oncol. 2012;14:15–20. doi: 10.1007/s12094-012-0756-8. [DOI] [PubMed] [Google Scholar]
- 17.Kim HS, Park NH, Chung HH, Kim JW, Song YS, Kang SB. Serum CA-125 level after 6 cycles of primary adjuvant chemotherapy is a useful prognostic factor for complete responders' survival in patients with advanced epithelial ovarian cancer. Onkologie. 2008;31:315–320. doi: 10.1159/000131270. [DOI] [PubMed] [Google Scholar]
- 18.Makar AP, Kristensen GB, Kaern J, Børmer OP, Abeler VM, Trope CG. Prognostic value of pre- and postoperative serum CA 125 levels in ovarian cancer: new aspects and multivariate analysis. Obstet Gynecol. 1992;79:1002–1010. [PubMed] [Google Scholar]
- 19.van Dalen A, Favier J, Burges A, Hasholzner U, de Bruijn HW, Dobler-Girdziunaite D, et al. Prognostic significance of CA 125 and TPS levels after 3 chemotherapy courses in ovarian cancer patients. Gynecol Oncol. 2000;79:444–450. doi: 10.1006/gyno.2000.5982. [DOI] [PubMed] [Google Scholar]
- 20.Ron IG, Inbar M, Gelernter I, Lewysohn O, Ayalon D, Dale J, et al. Use of CA-125 response to predict survival parameters of patients with advanced ovarian carcinoma. Acta Obstet Gynecol Scand. 1994;73:658–662. doi: 10.3109/00016349409013462. [DOI] [PubMed] [Google Scholar]
- 21.Markman M, Federico M, Liu PY, Hannigan E, Alberts D. Significance of early changes in the serum CA-125 antigen level on overall survival in advanced ovarian cancer. Gynecol Oncol. 2006;103:195–198. doi: 10.1016/j.ygyno.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 22.Osman N, O'Leary N, Mulcahy E, Barrett N, Wallis F, Hickey K, et al. Correlation of serum CA125 with stage, grade and survival of patients with epithelial ovarian cancer at a single centre. Ir Med J. 2008;101:245–247. [PubMed] [Google Scholar]
- 23.Bidart JM, Thuillier F, Augereau C, Chalas J, Daver A, Jacob N, et al. Kinetics of serum tumor marker concentrations and usefulness in clinical monitoring. Clin Chem. 1999;45:1695–1707. [PubMed] [Google Scholar]
- 24.Mano A, Falcao A, Godinho I, Santos J, Leitao F, de Oliveira C, et al. CA-125 AUC as a predictor for epithelial ovarian cancer relapse. Cancer Biomark. 2008;4:73–81. doi: 10.3233/cbm-2008-4203. [DOI] [PubMed] [Google Scholar]
- 25.Gupta D, Lammersfeld CA, Vashi PG, Braun DP. Longitudinal monitoring of CA125 levels provides additional information about survival in ovarian cancer. J Ovarian Res. 2010;3:22. doi: 10.1186/1757-2215-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riedinger JM, Bonnetain F, Basuyau JP, Eche N, Larbre H, Dalifard I, et al. Change in CA 125 levels after the first cycle of induction chemotherapy is an independent predictor of epithelial ovarian tumour outcome. Ann Oncol. 2007;18:881–885. doi: 10.1093/annonc/mdl500. [DOI] [PubMed] [Google Scholar]
- 27.Goonetilleke KS, Siriwardena AK. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol. 2007;33:266–270. doi: 10.1016/j.ejso.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Shering SG, Sherry F, McDermott EW, O'Higgins NJ, Duffy MJ. Preoperative CA 15-3 concentrations predict outcome of patients with breast carcinoma. Cancer. 1998;83:2521–2527. [PubMed] [Google Scholar]
- 29.Høgdall EV, Christensen L, Kjaer SK, Blaakaer J, Kjaerbye-Thygesen A, Gayther S, et al. CA125 expression pattern, prognosis and correlation with serum CA125 in ovarian tumor patients. From The Danish "MALOVA" Ovarian Cancer Study. Gynecol Oncol. 2007;104:508–515. doi: 10.1016/j.ygyno.2006.09.028. [DOI] [PubMed] [Google Scholar]


