Abstract
The validity of using transcranial Doppler (TCD) measurement of cerebral blood flow velocity (CBFV) to assess cerebral autoregulation (CA) still is a concern. This study measured CBFV in the middle cerebral artery (MCA) using TCD and volumetric cerebral blood flow (CBF) in the internal carotid artery (ICA) using color-coded duplex ultrasonography to assess CA during steady-state changes in mean arterial pressure (MAP). Twenty-one healthy adults participated. MAP was changed stepwise by intravenous infusion of sodium nitroprusside and phenylephrine. Changes in CBFV, CBF, cerebrovascular resistance (CVR = MAP/CBF) or resistance index (CVRi = MAP/CBFV) were measured to assess CA by linear regression analysis. The relationship between changes in ICA diameter and MAP was assessed. All values were normalized as percentage changes from baseline. Drug induced changes in MAP were from −26% to 31%. Changes in CBFV and CVRi in response to MAP were linear and the regresssion slopes were similar between MCA and ICA. However, CBF in ICA remained unchanged despite large changes in MAP. Consistently, a steeper slope of changes in CVR relative to CVRi was observed (0.991 vs. 0.804, P < 0.05). The ICA diameter changed inversely in response to MAP (r = −0.418, P < 0.05). These findings indicate that CA can be assessed with TCD mesurements of CBFV and CVRi in MCA. However, it is likely to be underestimated when compared to the measurements of CBF and CVR in ICA. The inverse relationship between changes in ICA diameter and MAP suggests that large cerebral arteries are involved in CA.
Keywords: cerebral autoregulation, blood pressure, cerebral blood flow, transcranial Doppler, ultrasonography, middle cerebral artery, internal carotid artery
Introduction
Cerebral autoregulation (CA) is essential to maintain a constant cerebral blood flow (CBF) in the context of changes in cerebral perfusion pressure.1 Assessment of CA reflects cerebrovascular function and has been used widely in hypertension studies and other clinical settings.2, 3
Quantitative assessment of CA is challenged by the methods used for CBF measurement. Modern imaging modalities such as SPECT, PET, perfusion CT and MRI are difficult to be applied for CBF measurement in clinical studies of CA because of the cumbersome experimental conditions and/or the limitations of using radioactive isotopes (SPECT and PET) or other imaging contrast agents (CT and MRI) for repeated measurements.4
Recently, transcranial Doppler (TCD) has been used to assess CA due to its bedside availability, non-invasiveness and high temporal resolution in measuring changes in cerebral blood flow velocity (CBFV) in the basal cerebral arteries.5 However, because the diameter of the insonated vessels cannot be measured directly using TCD, the validity of using this technique to assess CA is based on a fundamental assumption that changes in CBFV represent changes in volumetric CBF, i.e., by assuming that the diameter of basal cerebral arteries does not change significantly in the face of changes in blood pressure.5 For its importance, this assumption has been evaluated repeatedly by using a variety of imaging modalities to measure CBF and to compare with TCD measurement of CBFV during changes in arterial pressure.6, 7 However, the findings so far are inconsistent.8–10 One of the major limitations of these studies is that CBF and CBFV often were not measured simultaneously or were measured with different temporal and spatial resolutions thus making it difficult or even impossible for direct comparisons between CBF and CBFV measurements given the nature of spontaneous oscillations in CBF and CBFV.11 In this regard, an earlier study showed that during acute reduction in arterial pressure, changes in CBFV in the middle cerebral artery (MCA) measured with TCD accurately reflected changes in volumetric CBF in the internal carotid artery (ICA) measured simultaneously with electromagnetic flowmetry.7 However, this study was conducted only in seven patients with cerebrovascular diseases under surgical conditions and these observations need to be confirmed.
Color-coded duplex ultrasonography (CDUS) is a non-invasively bedside available technology which has been used to measure volumetric CBF in the ICA.12, 13 Similar to TCD, CDUS also has high temporal resolution.14 In addition, changes in CBF in the ICA most likely reflect those in the MCA and anterior cerebral artery which are the major branches of ICA.
In this study, we simultaneously measured changes in CBFV in the MCA and ICA, and CBF in the ICA to assess CA during stepwise changes in arterial pressure induced by intravenous infusion of sodium nitroprusside and phenylephrine. We tested the hypothesis that CA could be assessed equally well based on the measurements of CBFV in the MCA and volumetric CBF in the ICA. The outcome of this study will provide significant and new information regarding the validity of using TCD to assess CA during steady-state changes in arterial pressure.
Methods
Subjects
Twenty-one healthy adults were recruited from the local community to participate in this study. Subject demographic and clinical characteristics are presented in supplemental Table S1. Subjects were screened to exclude clinical histories of stroke, carotid stenosis, major medical and psychiatric disorders, unstable heart diseases, uncontrolled hypertension and diabetes mellitus. This study conformed to the standards of the Declaration of Helsinki for medical research involving human subjects. All subjects signed the informed consent including the study protocol approved by the Institutional Review Boards of the UT Southwestern Medical Center and Texas Health Presbyterian Hospital of Dallas.
Experimental protocol for changes in arterial pressure
Subjects were asked to refrain from high intensity exercise, alcohol, and caffeinated beverage at least 24 hours before the experiment. Intravenous infusions of sodium nitroprusside (SNP) and phenylephrine were used to induce stepwise changes in arterial pressure.15 Subjects were in the supine position throughout the experiment. After at least 20 minutes of supine rest and baseline data collection, SNP was started with an infusion rate of 0.25 μg/kg/min, and then increased incrementely by 0.25 μg/kg/min until mean arterial presssure (MAP) was reduced by 25% from the baseline or by 20 mmHg which ever came first. The maximum dose used was 1.00 μ/kg/min in the present study. After SNP infusion, a time interval of at least 20 minutes was provided to allow changes in hemodynamics to recover back to baseline. Then, phenylephrine infusion was started with 0.5 μg/kg/min and then increased incrementely by 0.5 μg/kg/min until MAP was increased by 30% from the baseline or by 25 mmHg which ever came first. The maximum dose used was 1.5 μg/kg/min in the present study. At each stage of drug infusion, after changes in pressure were stablized (about 2 minutes for SNP and 5 minutes for phenylephrine), 3 minutes of data were collected. Of note, the numbers of the exact stages of drug infusion were different among individual subjects depending upon their cardiovascular responses to SNP or phenylephrine infusion.
Data collection
TCD was used to measure the time-averaged peak velocity (TAPV) from the spectral envelop of Doppler signal to represent CBFV in the MCA using the standard method (DWL Elektronische Systeme, Germany).16 The TCD probe was placed on the skin over the temporal bone region above the zygomatic arch. A custom made probe holder or a headgear (Spencer Technologies) was used to prevent movement of the TCD probe during the study. Brachial arterial pressure was measured using a sphygmomanometer (Tango+, Suntech). Analog signals of CBFV waveforms, arterial pressure (Finapres, Ohmeda), electrocardiogram (GE Solar 8000M), and respiratory CO2 (Capnogard, Novamatrix) were recorded continuously using a data acquisition system (Acknowledge, BIOPAC Systems). The time-averaged data of CBFV, arterial pressure, heart rate, and breath-by-breath end-tidal CO2 (ETCO2) at the baseline and during each stage of drug infusion were used for statistical analysis.
The CBFV and CBF measurements at the ipsilateral ICA including quantification of the vessel diameter (D) and the cross-sectional area [CSA = π × (D/2)2] of the ICA were performed using the CDUS method (Figure 1).13 For details on these measures, please see the online Data Supplement. Cerebrovascular resistance (CVR) was estimated as MAP divided by CBF and cerebrovascular resistance index (CVRi) was estimated as MAP divided CBFV.13 All of the measurements were obtained from the baseline and when blood pressure was stabilized during each stage of drug infusion.
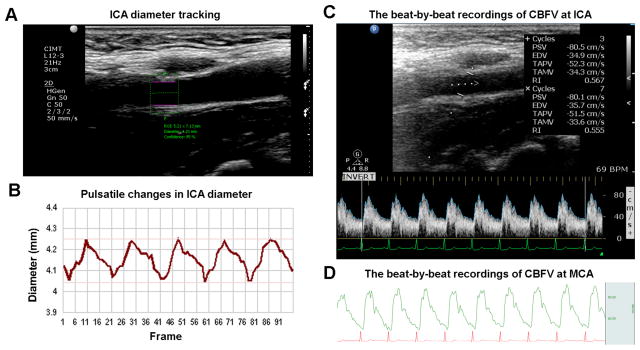
Figure 1.
Representative continuous recordings of pulsatile changes in vessel diameters at the internal carotid artery (ICA) and cerebral blood flow velocity (CBFV) at the ICA and middle cerebral artery (MCA). A. Region of interest is selected for segmental diameter measurements on high-resolution B-mode video using a semiautomatic wall-tacking and edge-detection software (see text); B. Representative ICA diameter pulsatile changes recorded at 21 frames/sec (Hz); C. The time-averaged peak and mean velocities (TAPV and TAMV) were measured at ICA in color Doppler/B mode; D. Continuous recordings of transcranial Doppler derived peak velocity (upper curve in green) at the MCA and electrocardiogram (lower curve in red).
Assessment of cerebral autoregulation
First, CA was assessed by linear regression of percentage changes in CBFV, CBF, CVRi or CVR (Δ%) in responses to percentage changes in MAP (Δ%) based on the pooled data from all subjects. For ΔCBFV% or ΔCBF%, a linear regression slope ≈ 0 has been used to indicate intact autoregulation while a significantly increased slope indicates impaired autoregulation.15 Conversely, a linear regression slope ≈ 0 for ΔCVRi% or ΔCVR% would suggest impaired autoregulation, whereas an slope ≈1.0 indicates intact autoregulation.17 However, it must be acknowledged that the use of these indices to assess CA is likely to be useful only for quantification of relative changes in CA since a clear cut-off threshold for any of these indices to indicate an impaired CA has not been established.17
Furthermore, CA was assessed for each individual subject by linear regression of percentage changes in CVRi or CVR in the MCA and ICA in responses to percentage changes in MAP to account for the individual variability in CA and for group comparisons.
Finally, multiple linear regression analysis was used to account for the influences of percentage changes in ETCO2 (Δ%) on CA: Y = A + B ×ΔMAP% + C × ΔETCO2% + ε, where Y represents ΔCBFV%, ΔCBF%, ΔCVRi% or ΔCVR% (dependent variable), A is a constant, B is the unstandardized linear regression coefficient representing CA after adjustment for the influence from changes in ETCO2, C is the unstandardized linear regression coefficient representing cerebral vasomotor reactivity to changes in CO2 after adjustment of the influence of changes in arterial pressure, and ε represents model random error.18 We used a similar multiple linear regression analysis to assess the influences of percentage changes in ETCO2 and MAP on the ICA diameter or CSA−1 (reciprocal of CSA to reflect the ICA resistance) during steady-state changes in arterial pressure.
Statistical Analysis
We performed linear regression analysis of changes in CBFV, CBF, CVRi or CVR in response to changes in arterial pressure using the general linear model method. For the pooled data from the whole group, we compared CA between the MCA and ICA using the regression slopes of ΔCBFV% or ΔCVRi% to ΔMAP % according to the Steiger method.19 In the ICA, we compared the linear regression slopes of ΔCVRi% and ΔCVR% (i.e., velocity vs. volumetric flow for assessment of changes in cerebrovascular resistance) to ΔMAP% according to the Steiger method19. Slopes are expressed as mean ± standard error. Comparisons of CA based on the individual data were performed using paired t-test. A level of P < 0.05 was considered statistically significant. Data were analyzed using SPSS 19 (IBM SPSS Inc, Chicago, IL).
Results
Baseline systemic and cerebral hemodynamics are presented in Table S1. Drug infusion induced percentage changes in MAP were from −26% to 31% (65~132 mmHg) relative to the baseline. ETCO2 changed concomitantly associated with changes in arterial pressure ranging from −24% to 18% (24~48 mmHg). A positive correlation was found between ΔMAP% and ΔETCO2%(r = 0.396, P < 0.001).
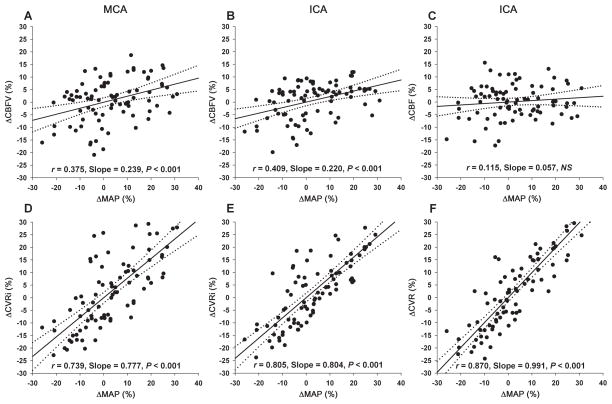
The linear regression slopes of ΔCBFV% and ΔCVRi% to ΔMAP% were positive and similar between the MCA and ICA (ΔCBFV%, 0.239 ± 0.065 vs. 0.220 ± 0.054, P = 0.69; ΔCVRi%: 0.777 ± 0.078 vs. 0.804 ± 0.065, P = 0.09) (Figure 2A vs. Figure 2B; Figure 2D vs. Figure 2E). However, no significant changes in CBF in the ICA were observed despite large changes in MAP (the linear regression slope was small and no statistically different from zero, Figure 2C); Consequently, the slope of ΔCVR% to ΔMAP% was steeper than that of ΔCVRi% to ΔMAP% (0.991 ± 0.062 vs. 0.804 ± 0.065, P < 0.05) (Figure 2F vs. 2E), suggesting that the assessment of CA is likely to be underestimated by the measurement of CVRi instead of CVR. Notably, large individual variabilities of changes in CBFV, CBF, ICA diameter and CSA−1 were observed during changes in arterial pressure (Figure 2 and 4).
Figure 2.
Scatter plots of pooled individual data and simple linear regressions of percentage changes in cerebral blood flow velocity (ΔCBFV%, A and B) and cerebral blood flow (ΔCBF%, C) (top row), and cerebrovascular resistance index (ΔCVRi%, D and E) and cerebrovascular resistance (ΔCVR%, F) (bottom row) relative to the baseline during steady-state changes in mean arterial pressure (ΔMAP%) measured at the middle cerebral artery (MCA, A and D) and internal carotid artery (ICA, B, C, E, and F) without adjustment of changes in end-tidal CO2. The solid lines represent regression slopes and the dotted lines represent 95% confidence intervals.
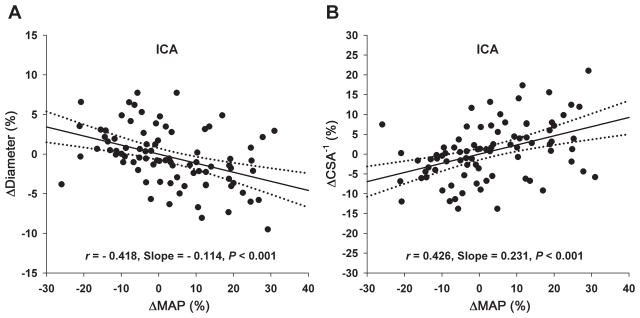
Figure 4.
Scatter plots of pooled individual data and simple linear regression of percentage changes of the internal carotid artery (ICA) diameter and the reciprocal of cross-sectional area (CSA−1) of ICA from the baseline during steady-state changes in mean arterial pressure (ΔMAP%). The solid lines represent regression slopes and the dotted lines represent 95% confidence intervals. These relationships were not influenced by changes in end-tidal CO2 (see text).
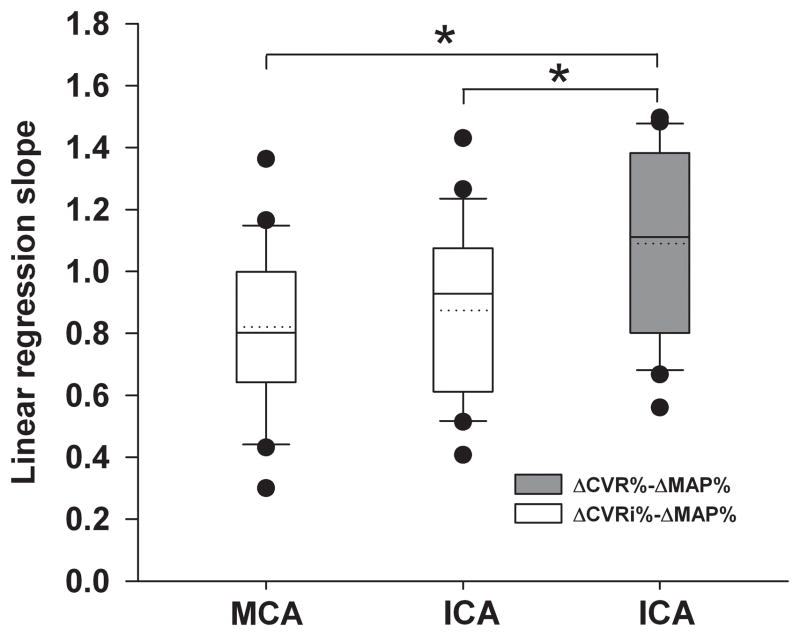
Consistently, for individual data analyses, no group difference in the linear regression slopes of ΔCVRi% to ΔMAP% was found between the MCA and ICA; however these slopes were significantly lower than that of ΔCVR% to ΔMAP% at the ICA (0.820 ± 0.056 vs. 1.090 ± 0.065, P < 0.01; and 0.874 ± 0.059 vs. 1.090 ± 0.065, P < 0.01) (Figure 3).
Figure 3.
Box plots of linear regression slopes between percentage changes in cerebrovascular resistance indices (ΔCVRi%) and mean arterial pressure (ΔMAP%) at the middle cerebral artery (MCA) and internal carotid artery (ICA) (open boxes), and between percentage changes in cerebrovascular resistance (ΔCVR%) and ΔMAP% at the ICA (filled box). The horizontal dotted and solid lines within the box represent the mean and median, respectively. *P < 0.01.
Multiple regression analyses showed that changes in ETCO2 during drug infusion influenced the cerebral hemodynamics (Table S2). After controlling for changes in ETCO2, CBFV and CVRi responses to changes in MAP were similar between the MCA and ICA, consistent with the simple linear regression results (Table S2).
Finally, at ICA, the ΔDiameter% was correlated negatively and the ΔCSA−1% was correlated positively to ΔMAP% (Figure 4). Multiple linear regression analyses showed that these relationships were not influenced by changes in ETCO2 (Table S2).
Discussion
The main findings of this study are twofold. First, we found that changes in CBFV and CVRi in response to changes in arterial pressure measured in the MCA using TCD were similar to those measured in the ICA using CDUS. Notably, the linear regression slopes of changes in CBFV to MAP were positive but relatively small (~ 0.24) and the slopes of changes in CVRi to MAP also were positive but relatively large (~ 0.78), suggesting presence of CA.17 However, volumetric CBF measured in the ICA, as a group average, remained unchanged despite large changes in MAP. Consistently, the linear regression slope of CVR to MAP was steeper than that of CVRi to MAP. Collectively, these findings indicate that CA can be assessed via the mesurement of CBFV and CVRi in the MCA using TCD, but it is likely to be underestimated with these measures. Second, we found an inverse relationship between changes in the ICA diameter and MAP, suggesting that large cerebral arteries participate in CA in human subjects.
Autoregulation of CBFV and CBF
TCD measurement of CBFV in the MCA has been used widely to assess CA. Previous studies in healthy adults found a positive correlation between changes in CBFV and MAP with a linear slope of 0.5~3.0% 3mmHg−1 within a range of MAP from about 60 to 150 mm Hg.15 These observations have been interpreted to indicate that CBF could not be maintained constant in response to change in arterial pressure even with the presence of intact CA.15 Consistent with these prior studies, our study also showed a positive linear slope of 0.24 ΔCBFV (%) ·ΔMAP (%)−1, which is about 0.27% 3mmHg−1 between changes in CBFV and MAP.
The new findings of the present study are that CBFV responses to changes in arterial pressure were similar between the MCA and ICA. As expected, the obtained ΔCVRi% - ΔMAP% slopes were similar between the MCA and ICA (Figure 2D vs. Figure 2E). The slope of linear regressions between ΔCVRi% and ΔMAP% has been used to reflect CA capacity. A slope of 0 has been used to indicate absence of CA, whereas a slope of 1.0 indicates the presence of a perfect CA.17 Thus, assessment of CA based on the CVRi-MAP relationship would suggest that CA is about 70–80% intact during steady-state changes in arterial pressue in the subjects of the present study.
However, as a group average, volumetric CBF in the ICA remained unchanged during changes in arterial pressure. Consistently, the slope of ΔCVR% - ΔMAP% was near to 1.0 which would suggest the presence of a “perfect CA”. Thus, when compared to volumetric CBF or CVR measurements, the assessment of CA could have been underestimated when CBFV or CVRi was used.
There are two important issues related to the data interpretation that need to be discussed. First, consistent with previous studies, we found that there was a positive correlation between changes in ETCO2 and MAP.20 A recent study suggest that baroreflex mediated changes in ventilation may explain changes in ETCO2 associated with transient changes in arterial pressure.21 Whether similar mechanism(s) would apply during steady-state changes in arterial pressure is not known and we did not measure ventilation in this study.
Changes in ETCO2 may reflect changes in arterial CO2 which have profound effects on CBF.22 Previous studies of CA used a correction coefficient of 3–6% changes in CBF or CBFV per mmHg change in ETCO2 to account for the confounding effect of CO2. This method has limitations in that either under or over-corrections may occur because of the existence of large individual variability in cerebral vasomotor reactivity to CO2.23 In this study, multiple linear regression analysis was used to account for the influences of changes in ETCO2 on assessment of CA.24 As expected, the ΔCBFV% - ΔMAP% regression coefficients were reduced and the ΔCVRi% or ΔCVR% - ΔMAP% coefficients were increased after adjustment of ΔETCO2% when compared to simple linear regressions (Figure 2 and Table S2).
Second, large intra- and inter-subject variabilities of CBFV and CBF were present during changes in arterial pressure. In this study, TCD probe was kept in the same position and the insonation angle was maintained constant by using a custom made probe holder or a headgear to reduce artifacts.25 Furthermore, for CBF measurement using the CDUS method, the ICA diameter was measured using a high resolution edge-detection and echo-tracking method to reduce potential measurement variability.13 Thus, the large individual variability of CBFV and CBF observed most likely reflect intrinsic physiological oscillations in CBF under the current experimental conditions and/or the presence of individual variability in CA.11
Autoregulation of large cerebral arteries
Because of technical limitations for measuring cerebral blood vessels directly in human subjects, whether or not large cerebral arteries play a role in CA is not well understood.26 In a previous study, MCA diameter was measured directly during craniotomy. The MCA diameter changed ≤ 4% in response to moderate changes in arterial pressure or arterial CO2.9 Several other studies using MRI or angiography could not detect any changes in the MCA diameter during moderate changes in arterial pressure likely due to the low spatial resolution of these methods.10
Bilateral ICAs are major cerebral arteries which supply about 75% of total CBF in human subjects.12, 13 The extracranial portion of the ICA diameter can be measured accurately and reliably with high temporal and spatial resolutions using the CDUS method.13 In the present study, we found that ICA diameter changed negatively and CSA−1 changed positively with arterial pressure (Figure 4). The range of percentage changes in diameter was from −9.5% to 7.7%, and in CSA−1 was from −13.8% to 21.0% when percentage changes in MAP were from −25.9% to 30.9% relative to the baseline. This observation is consistent with previous studies in animals,26 which demonstrated that the diameter of major intracranial arteries increased during reductions in arterial pressure.27 Resistance of large arteries appears to be greater in the cerebral circulation than other vascular beds and large cerebral arteries are the major determinants of local microvascular pressure.27 Furthermore, diameter changes of large cerebral arteries (MCA, ICA and VA) also have been observed during acute changes in arterial blood gases, e.g., during moderate to severe hypoxia or hypercapnia.28, 29 Taken together, the findings of the present study suggest that large cerebral arteries are involved in CA in human subjects. Notably, the slope of ΔCSA−1% vs. ΔMAP% was far less than 1 (Figure 4B), which indicates that although ICA may involve in CA, its contribution is likely to be disproportionately less than that of intracranial arteries and arterioles.
However, we cannot exclude the possibility that the ICA diameter changes observed in the present study are due to direct pharmacological effects. The α1-adrenergic receptors are located outside the blood–brain barrier (BBB), and the density of α1-adrenergic receptors on extracranial arteries is likely to be much higher than that of intracranial arteries.30 In addition, the arterial endothelial tight junctions are likely to be more intense in the intracranial than extracranial blood vessels. It is generally believed that phenylephrine does not pass BBB and has minimal, if any, direct vasoconstrictive effects on cerebral arteries.31 Thus, phenylephrine may produce greater vasoconstrictions in the ICA than MCA. In this case, the slope of changes in CBFV may be similar to that of volumetric CBF in the MCA, but different from that of ICA volumetric CBF during induced hypertension. SNP is an endothelium-independent nitric oxide (NO) donor, and many studies in animals have shown that NO regulates the vascular tone of large cerebral arteries.32 However, intracarotid infusion of SNP sufficient to reduce arterial pressure does not change CBF in humans.33 Thus, whether or not SNP has direct effects on the ICA diameter needs to be determined. Finally, nine subjects in this study used antihypertensives to control their blood pressure (Table S1). It is not known if the antihypertensives used would have different effects on the MCA CBFV or ICA CBF regulation. However, a subgroup data analysis of these subjects did not differ from those of not using antihypertensives (data not shown). Thus, all the data were pooled together in this study.
Perspectives
TCD has been used widely to study CA in patients with hypertension and other neurovascular diseases. However, one of the major limitations of TCD is that it can only measure CBFV in the basal cerebral arteries, and an assumption has to be made that changes in CBFV in the insonated arteries reflect changes in volumetric CBF. Thus, the validity of using TCD for assessment of CA has been a concern.34 In this study, we found that changes in CBFV in response to steady-state changes in arterial pressure were similar between the MCA and ICA, and that the linear regression slopes between changes in CBFV (or CVRi) and arterial pressure indeed could reflect characteristics of CA. However, assessment of CA using these indices obtained with TCD is likely to be underestimated when compared to the measurements of CBF or CVR in the ICA. Furthermore, we found an inverse relationship between changes in the ICA diameter and arterial pressure which suggests that large cerebral arteries are involved in CA. Given the important role of CA in understanding the control of brain perfusion, these findings have significant clinical implications for evaluation of the methodologies used for CA assessment.
Supplementary Material
Novelty and Significance.
What Is New?
Cerebral autoregulation, a protective vascular mechanism to maintain brain blood flow constant, was assessed by measuring changes in blood flow velocity and volumetric flow simultaneously in the large cerebral arteries during stepwise changes in arterial pressure. This study demonstrates that changes in blood flow velocity in response to changes in arterial pressure are consistent among large cerebral arteries and that the large cerebral arterial arteries, such as the internal carotid artery, are involved in cerebral autoregulation in human subjects.
What Is Relevant?
Study of cerebral autoregulation is fundamentally important for understanding the mechanisms of control of brain perfusion in patients with hypertension and for antihypertensive therapy.
Summary
Transcranial Doppler (TCD) is a widely used method for studying cerebral autoregulation, but has a major limitation in that it only can measure blood flow velocity in the large cerebral arteries. This study showed that changes in TCD measured blood flow velocity in response to changes in arterial pressure indeed reflect the characteristics of cerebral autoregulation. However, assessment of cerebral autoregulation using blood flow velocity is likely to be underestimated when compared to volumetric blood flow. Furthermore, large cerebral arteries are involved in cerebral autoregulation. These novel findings are important for evaluation of the methodologies used for cerebral autoregulation assessments in clinical settings.
Acknowledgments
We thank all our study participants for their willingness, time and effort devoted to this study and all other members of our team for their excellent technical support.
Sources of Funding
This study was supported in part by the NIH grant R01AG033106-01and R01HL102457.
Footnotes
Disclosure
None.
References
- 1.Strandgaard S, Paulson O. Cerebral autoregulation. Stroke. 1984;15:413–416. doi: 10.1161/01.str.15.3.413. [DOI] [PubMed] [Google Scholar]
- 2.Czosnyka M, Smielewski P, Kirkpatrick P, Menon DK, Pickard JD. Monitoring of cerebral autoregulation in head-injured patients. Stroke. 1996;27:1829–1834. doi: 10.1161/01.str.27.10.1829. [DOI] [PubMed] [Google Scholar]
- 3.Zhang R, Witkowski S, Fu Q, Claassen JA, Levine BD. Cerebral hemodynamics after short-and long-term reduction in blood pressure in mild and moderate hypertension. Hypertension. 2007;49:1149–1155. doi: 10.1161/HYPERTENSIONAHA.106.084939. [DOI] [PubMed] [Google Scholar]
- 4.Wintermark M, Sesay M, Barbier E, Borbély K, Dillon WP, Eastwood JD, Glenn TC, Grandin CB, Pedraza S, Soustiel J-F. Comparative overview of brain perfusion imaging techniques. Stroke. 2005;36:2032–2033. doi: 10.1161/01.STR.0000177884.72657.8b. [DOI] [PubMed] [Google Scholar]
- 5.Aaslid R, Lindegaard K-F, Sorteberg W, Nornes H. Cerebral autoregulation dynamics in humans. Stroke. 1989;20:45–52. doi: 10.1161/01.str.20.1.45. [DOI] [PubMed] [Google Scholar]
- 6.Larsen FS, Olsen KS, Hansen BA, Paulson OB, Knudsen GM. Transcranial doppler is valid for determination of the lower limit of cerebral blood flow autoregulation. Stroke. 1994;25:1985–1988. doi: 10.1161/01.str.25.10.1985. [DOI] [PubMed] [Google Scholar]
- 7.Newell DW, Aaslid R, Lam A, Mayberg TS, Winn HR. Comparison of flow and velocity during dynamic autoregulation testing in humans. Stroke. 1994;25:793–797. doi: 10.1161/01.str.25.4.793. [DOI] [PubMed] [Google Scholar]
- 8.Dahl A, Russell D, Nyberg-Hansen R, Rootwelt K. Effect of nitroglycerin on cerebral circulation measured by transcranial doppler and spect. Stroke. 1989;20:1733–1736. doi: 10.1161/01.str.20.12.1733. [DOI] [PubMed] [Google Scholar]
- 9.Giller CA, Bowman G, Dyer H, Mootz L, Krippner W. Cerebral arterial diameters during changes in blood pressure and carbon dioxide during craniotomy. Neurosurgery. 1993;32:737–742. [PubMed] [Google Scholar]
- 10.Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL. Mri measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke. 2000;31:1672–1678. doi: 10.1161/01.str.31.7.1672. [DOI] [PubMed] [Google Scholar]
- 11.Zhang R, Zuckerman JH, Levine BD. Spontaneous fluctuations in cerebral blood flow: Insights from extended-duration recordings in humans. American Journal of Physiology-Heart and Circulatory Physiology. 2000;278:H1848–H1855. doi: 10.1152/ajpheart.2000.278.6.H1848. [DOI] [PubMed] [Google Scholar]
- 12.Scheel P, Ruge C, Petruch UR, Schöning M. Color duplex measurement of cerebral blood flow volume in healthy adults. Stroke. 2000;31:147–150. doi: 10.1161/01.str.31.1.147. [DOI] [PubMed] [Google Scholar]
- 13.Liu J, Zhu Y-S, Khana MA, Brunk E, Martin-Cook K, Weiner MF, Cullum CM, Lu H, Levine BD, Diaz-Arrastia R, Zhang R. Global brain hypoperfusion and oxygenation in amnestic mild cognitive impairment. Alzheimer’s & Dementia. 2013;5260:2420–2425. doi: 10.1016/j.jalz.2013.04.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang R, Zuckerman JH, Giller CA, Levine BD. Transfer function analysis of dynamic cerebral autoregulation in humans. American Journal of Physiology-Heart and Circulatory Physiology. 1998;274:H233–H241. doi: 10.1152/ajpheart.1998.274.1.h233. [DOI] [PubMed] [Google Scholar]
- 15.Lucas SJ, Tzeng YC, Galvin SD, Thomas KN, Ogoh S, Ainslie PN. Influence of changes in blood pressure on cerebral perfusion and oxygenation. Hypertension. 2010;55:698–705. doi: 10.1161/HYPERTENSIONAHA.109.146290. [DOI] [PubMed] [Google Scholar]
- 16.Aaslid R, Markwalder T-M, Nornes H. Noninvasive transcranial doppler ultrasound recording of flow velocity in basal cerebral arteries. Journal of neurosurgery. 1982;57:769–774. doi: 10.3171/jns.1982.57.6.0769. [DOI] [PubMed] [Google Scholar]
- 17.Strebel S, Lam A, Matta B, Mayberg TS, Aaslid R, Newell DW. Dynamic and static cerebral autoregulation during isoflurane, desflurane, and propofol anesthesia. Anesthesiology. 1995;83:66–76. doi: 10.1097/00000542-199507000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Zhu Y-S, Tarumi T, Tseng BY, Palmer DM, Levine BD, Zhang R. Cerebral vasomotor reactivity during hypo-and hypercapnia in sedentary elderly and masters athletes. Journal of Cerebral Blood Flow & Metabolism. 2013;33:1190–1196. doi: 10.1038/jcbfm.2013.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steiger JH. Tests for comparing elements of a correlation matrix. Psychological Bulletin. 1980;87:245–251. [Google Scholar]
- 20.Thomas KN, Cotter JD, Galvin SD, Williams MJ, Willie CK, Ainslie PN. Initial orthostatic hypotension is unrelated to orthostatic tolerance in healthy young subjects. Journal of Applied Physiology. 2009;107:506–517. doi: 10.1152/japplphysiol.91650.2008. [DOI] [PubMed] [Google Scholar]
- 21.Stewart JM, Rivera E, Clarke DA, Baugham IL, Ocon AJ, Taneja I, Terilli C, Medow MS. Ventilatory baroreflex sensitivity in humans is not modulated by chemoreflex activation. American Journal of Physiology-Heart and Circulatory Physiology. 2011;300:H1492–H1500. doi: 10.1152/ajpheart.01217.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peebles K, Celi L, McGrattan K, Murrell C, Thomas K, Ainslie PN. Human cerebrovascular and ventilatory co2 reactivity to end-tidal, arterial and internal jugular vein pco2. The Journal of Physiology. 2007;584:347–357. doi: 10.1113/jphysiol.2007.137075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hetzel A, Braune S, Guschlbauer B, Dohms K. Co2 reactivity testing without blood pressure monitoring? Stroke. 1999;30:398–401. doi: 10.1161/01.str.30.2.398. [DOI] [PubMed] [Google Scholar]
- 24.Menke J, Michel E, Rabe H, Bresser B, Grohs B, Schmitt R, Jorch G. Simultaneous influence of blood pressure, pco2, and po2 on cerebral blood flow velocity in preterm infants of less than 33 weeks’ gestation. Pediatric research. 1993;34:173–177. doi: 10.1203/00006450-199308000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Zhang R, Crandall CG, Levine BD. Cerebral hemodynamics during the valsalva maneuver insights from ganglionic blockade. Stroke. 2004;35:843–847. doi: 10.1161/01.STR.0000120309.84666.AE. [DOI] [PubMed] [Google Scholar]
- 26.Heistad DD, Marcus ML, Abboud FM. Role of large arteries in regulation of cerebral blood flow in dogs. Journal of Clinical Investigation. 1978;62:761–768. doi: 10.1172/JCI109187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faraci FM, Heistad DD. Regulation of large cerebral arteries and cerebral microvascular pressure. Circulation research. 1990;66:8–17. doi: 10.1161/01.res.66.1.8. [DOI] [PubMed] [Google Scholar]
- 28.Wilson MH, Edsell ME, Davagnanam I, Hirani SP, Martin DS, Levett DZ, Thornton JS, Golay X, Strycharczuk L, Newman SP. Cerebral artery dilatation maintains cerebral oxygenation at extreme altitude and in acute hypoxia—an ultrasound and mri study. Journal of Cerebral Blood Flow & Metabolism. 2011;31:2019–2029. doi: 10.1038/jcbfm.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Willie CK, Macleod DB, Shaw AD, Smith KJ, Tzeng YC, Eves ND, Ikeda K, Graham J, Lewis NC, Day TA. Regional brain blood flow in man during acute changes in arterial blood gases. The Journal of Physiology. 2012;590:3261–3275. doi: 10.1113/jphysiol.2012.228551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bevan RD, Dodge J, Nichols P, Penar PL, Walters CL, Wellman T, Bevan JA. Weakness of sympathetic neural control of human pial compared with superficial temporal arteries reflects low innervation density and poor sympathetic responsiveness. Stroke. 1998;29:212–221. doi: 10.1161/01.str.29.1.212. [DOI] [PubMed] [Google Scholar]
- 31.Olesen J. The effect of intracarotid epinephrine, norepinephrine, and angiotensin on the regional cerebral blood flow in man. Neurology. 1972;22:978–978. doi: 10.1212/wnl.22.9.978. [DOI] [PubMed] [Google Scholar]
- 32.Faraci FM, Heistad DD. Regulation of the cerebral circulation: Role of endothelium and potassium channels. Physiological Reviews. 1998;78:53–97. doi: 10.1152/physrev.1998.78.1.53. [DOI] [PubMed] [Google Scholar]
- 33.Joshi S, Young WL, Pile-Spellman J, Fogarty-Mack P, Sciacca RR, Hacein-Bey L, Duong H, Vulliemoz Y, Ostapkovich N, Jackson T. Intra-arterial nitrovasodilators do not increase cerebral blood flow in angiographically normal territories of arteriovenous malformation patients. Stroke. 1997;28:1115–1122. doi: 10.1161/01.str.28.6.1115. [DOI] [PubMed] [Google Scholar]
- 34.Kontos H. Validity of cerebral arterial blood flow calculations from velocity measurements. Stroke. 1989;20:1–3. doi: 10.1161/01.str.20.1.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.