Abstract
Objectives
This study was designed to assess the relationship between insulin resistance and incident heart failure (HF) in a community-based cohort.
Background
Diabetes mellitus increases the risk for HF, but the association between insulin resistance and HF in individuals without diabetes is unclear.
Methods
We prospectively analyzed 12,606 participants without diabetes mellitus, prevalent HF, or history of myocardial infarction at baseline (1987 to 1989) from the ARIC (Atherosclerosis Risk in Communities) study. We assessed the relationship between insulin resistance and incident HF using the homeostatic model assessment of insulin resistance (HOMA-IR) equation, adjusting for age, sex, race, body mass index, smoking, hypertension, center, and interim myocardial infarction. We tested for interactions by age, sex, obesity, and race.
Results
Participants with insulin resistance, defined as HOMA-IR ≥2.5 (n = 4,810, 39%), were older, more likely female, African American, hypertensive, and had a higher body mass index as compared with those without insulin resistance. There were 1,455 incident HF cases during a median of 20.6 years of follow-up. Insulin resistance defined by this threshold was not significantly associated with an increased risk for incident HF after adjustment (hazard ratio: 1.08, 95% confidence interval: 0.95 to 1.23). However, when analyzed continuously, this relationship was nonlinear, which indicated that risk increased, and was significantly associated with incident HF between HOMA-IR of 1.0 to 2.0, adjusting for baseline covariates; however, values over 2.5 were not associated with additional increased risk in adjusted models.
Conclusions
In a community cohort, insulin resistance, defined by lower levels of HOMA-IR than previously considered, was associated with an increased risk for HF.
Keywords: insulin resistance, heart failure, obesity
Diabetes mellitus has been shown to be associated with a higher risk for incident heart failure (HF) (1,2). Insulin resistance, with or without diabetes, has also been linked to an elevated risk of HF (3), although this association has been inconsistent (4,5), due in part to varying characteristics of study populations, differences in study follow-up time, or dissimilar approaches to modeling the risk for HF and adjusting for covariates. Obesity, known to be a primary determinant of insulin resistance, has also been independently associated with incident HF (6,7). The relationship among obesity, insulin resistance, and HF outcomes is unclear, as is the extent to which the HF risk associated with obesity may be mediated by insulin resistance. Moreover, the association of insulin resistance with HF has not been explored in a middle-aged cohort study with lengthy follow-up. The objectives of this study were to investigate the association of insulin resistance with incident HF and to explore whether factors such as obesity, age, sex, or race modified this association.
Methods
Participants
The ARIC (Atherosclerosis Risk in Communities) study is an ongoing community-based cohort of 15,792 patients, comprised mostly of Caucasian and African-American men and women, age 45 to 64 years at baseline (1987 to 1989), and sampled from 4 U.S. communities: Forsyth County, North Carolina; the northwest suburbs of Minneapolis, Minnesota; Washington County, Maryland; and Jackson, Mississippi (8). Standardized physical examinations and interviewer-administered questionnaires were conducted at baseline and at 3 triennial follow-up examinations (visit 2 [1990 to 1992], visit 3 [1993 to 1995], and visit 4 [1996 to 1998]). Participant follow-up through annual telephone interviews, hospitalization, and vital status is ongoing. Individuals with prevalent HF (n = 752) or who were missing data to determine prevalent HF (n = 287), those with prevalent diabetes (n = 1,870), or those with a history of myocardial infarction (MI) (n = 653) at baseline were excluded. Prevalent HF was defined as follows (7): 1) those answering “yes” to the question, “Were any of the medications you took during the last 2 weeks for HF?” or 2) those with stage 3 HF by applying Gothenburg criteria. Prevalent diabetes was defined as fasting plasma glucose >126 mg/dl, nonfasting glucose >200 mg/dl, self-reported use of diabetes medications, or self-reported physician diagnosis of diabetes. The remaining 12,606 participants were included in this analysis; follow-up time was 20.6 years. The protocol was approved by each site’s institutional review board, and all participants provided written informed consent in accordance with established guidelines for the protection of human subjects.
Exposure definitions and baseline covariates
The homeostatic model assessment of insulin resistance (HOMA-IR) index was calculated from fasting glucose and insulin at visit 1 using the following equation: (fasting glucose [mg/dl] × fasting insulin [uIU/ml])/405 (9). Normal HOMA-IR index has previously been reported as 1.0, although it ranges in healthy individuals from 1.0 to 1.5 and varies by age, sex, and characteristics of the population sampled (9,10). All covariates were collected from the baseline visit. Race was self-reported; smoking status was obtained from interviewer-administered questionnaires.
Ascertainment of events
Incident HF was defined as the first HF hospitalization identified with International Classification of Diseases–Ninth Revision discharge codes of 428.x in any position on the hospital discharge list or a death certificate with death from HF in any position. HF hospitalization and vital status data were available from study baseline through December 31, 2009.
Statistical analysis
Based on the traditional definition of insulin resistance (HOMA-IR ≥2.5) (9), we delineated 2 HOMA-IR categories prior to further analyses: <2.5, and ≥2.5. Baseline demographics between participants in the 2 HOMA-IR categories were compared to identify potential differences. Between-group assessments were performed using unpaired t tests or Wilcoxon rank sum tests for continuous variables and chi-square or Fisher exact tests, as appropriate, for categorical variables.
Logistic regression was used to examine characteristics that were associated with insulin resistance (HOMA-IR ≥2.5) at baseline, adjusting for age, sex, body mass index (BMI) (as a continuous variable), current smoking, hypertension, and center. In order to assess the relationship between baseline HOMA-IR values and incident HF, multiple Cox proportional hazards models were fit in each case, allowing the association with HOMA-IR to be modeled flexibly through the use of restricted cubic splines. Two models were constructed: model 1 adjusted for age and sex, and model 2 also adjusted for race, BMI, smoking, hypertension, and center. As incident MI is a known factor in the pathway for development of HF, we adjusted for incident MI as a time-varying covariate with model 2. As a sensitivity analysis, we assessed the association of HOMA-IR with incident HF while censoring individuals at the time of MI after visit 1. Unadjusted and adjusted models were fit, and for each a baseline HOMA-IR value of 1.0 was used as the reference point. These models were then refit using the common threshold value of HOMA-IR >2.5 to obtain a more interpretable model. The utility and model fit using the standard cutoff value of 2.5 was then compared with other possible cutoff values, ranging from 1.0 to 3.0. Baseline risk for incident HF was modeled using the covariates age, sex, race, BMI, smoking, hypertension, center, and systolic blood pressure. A Cox model was fit, and the resulting estimates provided a baseline risk score for each participant. As an exploratory analysis, we investigated the association between fasting insulin and incident HF. Fasting insulin was log-transformed and was examined in Cox proportional hazards models. The first model adjusted for age and sex, and an expanded model further adjusted for race, BMI, smoking, hypertension, center, and incident MI as a time-varying covariate. To estimate and compare population attributable risks (PARs) between blood pressure measures and HOMA-IR, we created a modified version of our fully-adjusted model, in which the linear systolic and diastolic blood pressure terms were replaced with a single indicator of “elevated blood pressure” (systolic blood pressure ≥140 mm Hg), and our HOMA-IR terms were replaced with a single indicator of “elevated HOMA-IR” (HOMA-IR ≥2.0). Thereafter, the PAR was calculated following elimination of each of these risk factors while keeping all other terms in the model constant. Overall model performance of the HOMA-IR spline models was evaluated with the C-statistic, removing MI as a time-varying covariate. Finally, interactions among baseline HOMA-IR (modeled via cubic splines), baseline covariates (age 55 years or older, sex, and race [African American or Caucasian]), and obesity (defined as BMI ≥30 kg/m2) were assessed, as well as interactions between baseline HOMA-IR and estimated baseline risk of HF. All analyses were conducted using Stata version 11 (StataCorp LP, College Station, Texas).
Results
Demographic characteristics of participants by category of HOMA-IR are shown in Table 1. Individuals with HOMA-IR ≥2.5, as compared with those within the noninsulin resistant category (HOMA-IR <2.5), were more likely African American, had higher BMI, were more likely hypertensive, and exhibited lower HDL and higher triglyceride values. Characteristics that were significantly associated with insulin resistance at baseline in an adjusted model included BMI, hypertension, female sex, older age, and African-American race (Table 2).
Table 1.
Baseline Characteristics by HOMA-IR Category
| Characteristic | HOMA-IR <2.5 (n = 7,566) |
HOMA-IR ≥2.5 (n = 4,810) |
p Value |
|---|---|---|---|
| Age, yrs | 54 ± 5.7 | 54 ± 5.7 | |
| Male | 3,144 (42.0) | 2,309 (48.0) | <0.001 |
| Caucasian | 6,152 (81.3) | 3,288 (68.4) | <0.001 |
| Body mass index (kg/m2) | 25 ± 3.8 | 30 ± 5.3 | <0.001 |
| Current smoker | 2,125 (28) | 1,053 (21.9) | <0.001 |
| Hypertension | 1,616 (21) | 1,978 (41.2) | <0.001 |
| Fasting glucose, mg/dl | 95 ± 8.0 | 103 ± 9.0 | <0.001 |
| Fasting insulin, uIU/ml | 6.4 ± 2.4 | 17.4 ± 8.7 | <0.001 |
| LDL cholesterol, mg/dl | 134 ± 39 | 141 ± 39 | <0.001 |
| HDL cholesterol, mg/dl | 57 ± 17 | 47 ± 14 | <0.001 |
| Triglycerides, mg/dl | 106 ± 56 | 150 ± 95 | <0.001 |
| eGFR, ml/min/1.73 m2 | 93 ± 19 | 92 ± 22 | 0.35 |
Values are mean ± SD or n (%).
eGFR = estimated glomerular filtration rate; HDL = high-density lipoprotein; HOMA-IR = homeostatic model assessment of insulin resistance; LDL = low-density lipoprotein.
Table 2.
Characteristics Associated With Insulin Resistance* at Baseline
| Characteristic | Odds Ratio (95% CI) | Z score | p Value |
|---|---|---|---|
| BMI (per kg/m2) | 1.30 (1.29–1.31) | 48.2 | <0.001 |
| Hypertension | 1.90 (1.79–2.11) | 15.3 | <0.001 |
| Female sex | 0.77 (0.70–0.82) | 7.0 | <0.001 |
| Age (per yr) | 1.02 (1.02–1.03) | 6.98 | <0.001 |
| Black race | 1.37 (1.25–1.51) | 6.72 | <0.001 |
| Current smoking | 0.97 (0.89–1.06) | 0.7 | 0.48 |
Defined as homeostatic model assessment of insulin resistance ≥2.5.
BMI = body mass index; CI = confidence interval.
There were 1,455 cases of incident HF during a median follow-up of 20.6 years. When HOMA-IR was examined in relation to incident HF with a Cox proportional hazards model via flexible cubic spline terms (Fig. 1), crude and adjusted models suggested an increasing hazard associated with increasing HOMA-IR across the observed range beyond approximately 1.0. In models adjusting for baseline covariates and interim MI as a time-varying covariate, we observed a significant nonlinear relationship between HOMA-IR level and the risk for HF (overall spline model C-statistic = 0.76, p = 0.024), with increasing hazard from approximately 1.0 to just above 2.0, after which we observed no additional hazard with increasing HOMA-IR. Table 3 compares the hazard ratio (HR) for incident HF associated with the traditional threshold of HOMA-IR ≥2.5 in both the adjusted and unadjusted models with alternative potential cutoff values, ranging from HOMA-IR ≥1.0 to HOMA-IR ≥3.0. In age- and sex-adjusted models, all HOMA-IR values were associated with incident HF. In fully-adjusted models, only HOMA-IR values 1.5, 2.0, and 2.25 were significantly associated with incident HF, suggesting that the independent association between HOMA-IR and incident HF is maintained only within this range. When the MI time-varying covariate was removed from model 2, findings were similar to those of the model that included MI, such that significant associations between HOMA-IR and incident HF were only observed for HOMA-IR values 1.5, 2.0, and 2.25 (results not shown). Fasting insulin alone was associated with incident HF in the model adjusted for age and sex (HR: 1.45, 95% confidence interval [CI]: 1.34 to 1.58), but not in fully-adjusted models (HR: 1.03, 95% CI: 0.94 to 1.13). The population-attributable risk for HOMA-IR at a threshold of 2.0 was 9% (95% CI: 2% to 16%), which was statistically comparable to the PAR of blood pressure of 7% (95% CI: 4% to 10%), at a threshold of 140/90 mm Hg.
Figure 1. Distribution of HOMA-IR Levels and Relationship Between HOMA-IR and Incident Heart Failure.
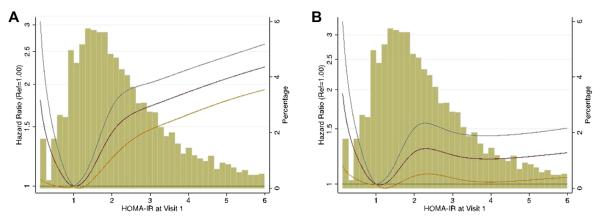
Distribution of homeostatic model assessment of insulin resistance (HOMA-IR) levels (histogram, right axis) and the continuous relationship between HOMA-IR levels and incident heart failure (splines, with 95% confidence intervals, left axis). A shows the relationship adjusted for age and sex (p < 0.001), and B shows the adjusted relationship (p = 0.024).
Table 3.
HOMA-IR Levels and Risk of Incident HF
| HOMA-IR | Model 1* HR (95% CI) |
p Value | Model 2† HR (95% CI) |
p Value |
|---|---|---|---|---|
| 1.00 | 1.34 (1.12–1.61) | 0.001 | 0.95 (0.79–1.14) | 0.564 |
| 1.25 | 1.53 (1.32–1.78) | <0.001 | 1.13 (0.97–1.33) | 0.115 |
| 1.50 | 1.58 (1.39–1.80) | <0.001 | 1.15 (1.00–1.32) | 0.046 |
| 1.75 | 1.55 (1.38–1.74) | <0.001 | 1.11 (0.98–1.26) | 0.094 |
| 2.00 | 1.61 (1.44–1.79) | <0.001 | 1.15 (1.02–1.30) | 0.022 |
| 2.25 | 1.67 (1.50–1.85) | <0.001 | 1.17 (1.04–1.31) | 0.010 |
| 2.50 | 1.58 (1.43–1.76) | <0.001 | 1.10 (0.97–1.23) | 0.127 |
| 2.75 | 1.55 (1.40–1.72) | <0.001 | 1.05 (0.93–1.18) | 0.465 |
| 3.00 | 1.51 (1.35–1.68) | <0.001 | 1.01 (0.89–1.14) | 0.925 |
Model 1 adjusted for age and female sex.
Model 2 adjusted for age, female sex, race, BMI, smoking, hypertension, study center, and myocardial infarction as time-varying covariate.
HF = heart failure; HOMA-IR = homeostatic model assessment of insulin resistance; HR = hazard ratio; other abbreviations as in Table 2.
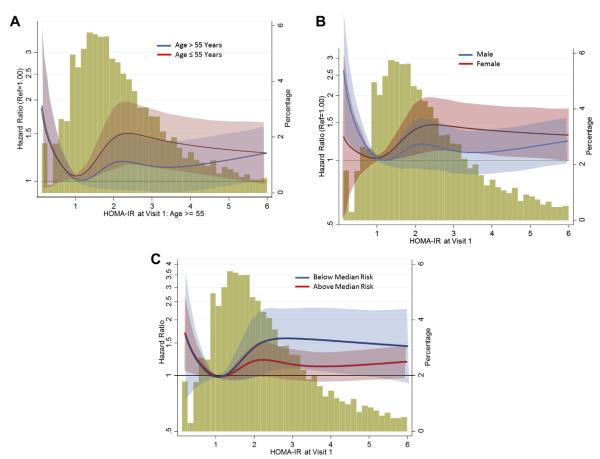
In subgroup analyses, nonsignificant trends were noted for interactions between HOMA-IR and incident HF for age, sex, and estimated baseline risk of HF (p interactions = 0.07, 0.08, and 0.1, respectively), such that the relationship between HOMA-IR and incident HF was stronger for younger individuals, females, and those with lower baseline risk for HF compared with those over age 55 years, males, and individuals with high baseline risk for HF (Fig. 2). Of note, obesity and race did not modify the relationship between insulin resistance and HF, and conversely, insulin resistance did not modify the very significant relationship between obesity and HF incidence.
Figure 2. Subgroup Comparisons Showing Relationship Between Insulin Resistance and Incident Heart Failure.
Subgroup comparisons showing relationship between insulin resistance and incident heart failure with (A) age; (B) sex; and (C) baseline risk for incident heart failure, in adjusted models. HOMA-IR = homeostatic model assessment of insulin resistance.
Discussion
We found in a community-based cohort that insulin resistance, based on HOMA-IR, was associated with an increased risk of incident HF among those without diabetes at baseline. This risk appeared to occur at lower levels than the previously-defined insulin resistance threshold of a HOMA-IR of 2.5 and was not modified by race or BMI, but the relationship between insulin resistance and HF was stronger in younger participants, in females, and in those with lower baseline risk of HF. However, the association between insulin resistance and HF was no longer significant at HOMA-IR levels ≥2.5. Interim MI did not mediate the association between insulin resistance and HF.
The relationship between insulin resistance and incident HF has varied in prior studies. Our findings of a significant association between HOMA-IR, modeled flexibly with the use of cubic splines, and incident HF following adjustment for risk factors for HF corroborate a recent analysis of the Cardiovascular Health Study, which also found significant associations between multiple measures of insulin resistance (fasting insulin, HOMA-IR levels, and oral glucose tolerance testing) and incident HF in an older adult cohort (11). Similar to the Cardiovascular Health Study, we found that insulin levels alone were less predictive of HF than other measures of insulin resistance. It is possible that the combination of hyperglycemia and hyperinsulinemia confers a higher risk, as hyperglycemia develops when insulin levels can no longer reduce glucose levels sufficiently. In contrast, another analysis of a large epidemiologic cohort study did not detect associations of metabolic variables with incident HF (5). In that study, HOMA-IR scores were log-transformed and assessed continuously, and values greater than the 95th percentile were only marginally associated with incident HF. Another analysis of glycemic measures and incident HF in individuals older than age 70 years noted significant associations with fasting glucose, but not with HOMA-IR (4). In a study of older adult Swedish men, HOMA-IR was associated with incident HF in unadjusted models, but not adjusted models (3). However, individuals who exhibited higher oral glucose tolerance test values and lower euglycemic insulin clamp disposal rates had a higher risk, still suggesting that insulin resistance as a whole may be linked to incident HF. Differences in the effect of multivariable adjustment on the relationship between insulin resistance and HF likely reflects differences in patient populations, including differences in baseline risk, different lengths of follow-up time, various ascertainment methods for HF events, and different available covariates.
We found that the relationship between insulin resistance and incident HF was nonlinear, and in adjusted models, this risk began earlier than the traditionally used HOMA-IR index cut-point of 2.5, but not when HOMA-IR was ≥2.5. The established threshold of HOMA-IR 2.5 to define insulin resistance is based on studies validating HOMA-IR against the euglycemic hyperinsulinemic clamp technique and intravenous glucose tolerance tests (9,12). Howe ver, our data suggest that other risk factors attenuate the relationship between insulin resistance and incident HF when HOMAIR rises above approximately the traditional cut-point of 2.5, and the greater contribution of other risk factors, such as increased BMI and hypertension, at higher HOMA-IR levels may dilute the effect of insulin resistance. Moreover, it is possible that because of the large number of participants in the HOMA-IR 1.5 to 2.5 range in the ARIC study, we had greater power to detect an association between lower levels of insulin resistance and incident HF than prior studies. Obesity has previously been associated with incident HF in epidemiologic studies, including the ARIC study(6,7,13–15). The relationship among obesity, insulin resistance, and HF is complex, and it is difficult to separate causative associations with these related risk factors. However, in our analysis, insulin resistance was associated with incident HF independent of BMI, and this relationship was not modified by obesity, suggesting that metabolic derangements beyond obesity may play an important role in target organ damage. Although a recent study has suggested that metabolically-healthy obese people may have a reduced risk for HF (16), in our analysis, obesity was associated with incident HF, and this relationship was also not modified by insulin resistance, suggesting that obesity with or without insulin resistance remained a risk factor for incident HF.
A number of mechanisms may explain the association between insulin resistance and HF. Although insulin resistance and abnormalities of glycemic control have been associated with increased risk for MI, which itself is a key mediator in the development of HF, our results suggest that this relationship was not dependent on interim MI, and suggests a more direct potential mechanism relating insulin resistance and the development of HF. The myocardium utilizes free fatty acids and less glucose in the setting of insulin resistance (17). This metabolic irregularity increases vulnerability to pressure overload or ischemia. Insulin is a recognized growth factor and has been shown to facilitate cardiac remodeling (18–20). Hyperinsulinemia also leads to sodium retention (21), which can potentially cause fluid retention. Moreover, higher insulin levels activate the sympathetic nervous system (22). Insulin resistance has been linked to a more pronounced response to angiotensin II, which may contribute to alterations in cardiac structure (23). However, an elevation in insulin may simply be a marker of metabolic derangement, and may not itself mediate the progression to heart disease.
Study limitations
As with any observational cohort study, residual confounding remains of concern. Identification of HF cases relied on International Classification of Diseases–Ninth Revision codes, which may not accurately reflect HF incidence. Death due to HF was determined from death certificates, which may overestimate or underestimate the number of cases. Additionally, the type of HF was not recorded. However, validation of HF hospitalizations indicated that the positive predictive value of 428.x in the first position was 93% for acute decompensated HF and 97% for chronic HF (24). It is possible that other methods of measuring insulin resistance, such as euglycemic clamping or the intravenous glucose tolerance test, could have yielded different results, although these are impractical in a large cohort study. The HOMA-IR equation has been validated in normoglycemic individuals against insulin sensitivity assessed directly from the euglycemic-hyperinsulinemic clamp technique and has been widely used in epidemiological studies. Because the ARIC study consisted of a middle-age community in the United States, additional studies are needed in younger populations, older adults, or other ethnicities. Finally, although our data suggest that HF risk associated with insulin resistance may begin at a lower level than previously considered, it was not our intention to establish an alternative lower cutoff point for HOMA-IR, as the relationship between lower levels of HOMA-IR and incident HF will need to be assessed in additional cohorts and examined in relation to different outcomes.
Conclusions
In a community cohort, insulin resistance, beginning at a lower HOMA-IR level than previously established but not when HOMA-IR was ≥2.5, was associated with incident HF and was not mediated by an increased risk of MI. Moreover, this relationship was not modified by BMI, suggesting that there is an independent role for insulin resistance on increasing risk for cardiac disease beyond obesity.
Acknowledgments
The ARIC study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN 268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN26820 1100011C, and HHSN268201100012C).
Abbreviations and Acronyms
- BMI
body mass index
- CI
confidence interval
- HF
heart failure
- HOMA-IR
homeostatic model assessment of insulin resistance
- HR
hazard ratio
- MI
myocardial infarction
- PAR
population attributable risk
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
REFERENCES
- 1.Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34:29–34. doi: 10.1016/0002-9149(74)90089-7. [DOI] [PubMed] [Google Scholar]
- 2.Iribarren C, Karter AJ, Go AS, et al. Glycemic control and heart failure among adult patients with diabetes. Circulation. 2001;103:2668–73. doi: 10.1161/01.cir.103.22.2668. [DOI] [PubMed] [Google Scholar]
- 3.Ingelsson E, Sundstrom J, Arnlov J, Zethelius B, Lind L. Insulin resistance and risk of congestive heart failure. JAMA. 2005;294:334–41. doi: 10.1001/jama.294.3.334. [DOI] [PubMed] [Google Scholar]
- 4.Kalogeropoulos A, Georgiopoulou V, Harris TB, et al. Glycemic status and incident heart failure in elderly without history of diabetes mellitus: the health, aging, and body composition study. J Card Fail. 2009;15:593–9. doi: 10.1016/j.cardfail.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bahrami H, Bluemke DA, Kronmal R, et al. Novel metabolic risk factors for incident heart failure and their relationship with obesity: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;51:1775–83. doi: 10.1016/j.jacc.2007.12.048. [DOI] [PubMed] [Google Scholar]
- 6.Kenchaiah S, Evans JC, Levy D. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–13. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 7.Loehr LR, Rosamond WD, Poole C, et al. Association of multiple anthropometrics of overweight and obesity with incident heart failure: the Atherosclerosis Risk in Communities study. Circ Heart Fail. 2009;2:18–24. doi: 10.1161/CIRCHEARTFAILURE.108.813782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The ARIC Investigators The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. AmJ Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 9.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 10.Gayoso-Diz P, Otero-Gonzalez A, Rodriguez-Alvarez MX, et al. Insulin resistance index (HOMA-IR) levels in a general adult population: curves percentile by gender and age. The EPIRCE study. Diabetes Res Clin Pract. 2011;94:146–55. doi: 10.1016/j.diabres.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 11.Banerjee D, Biggs ML, Mercer L, et al. Insulin resistance and risk of incident heart failure: Cardiovascular Health Study. Circ Heart Fail. 2013;6:364–70. doi: 10.1161/CIRCHEARTFAILURE.112.000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ascaso JF, Pardo S, Real JT, Lorente RI, Priego A, Carmena R. Diagnosing insulin resistance by simple quantitative methods in subjects with normal glucose metabolism. Diabetes Care. 2003;26:3320–5. doi: 10.2337/diacare.26.12.3320. [DOI] [PubMed] [Google Scholar]
- 13.He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med. 2001;161:996–1002. doi: 10.1001/archinte.161.7.996. [DOI] [PubMed] [Google Scholar]
- 14.Chen YT, Vaccarino V, Williams CS, Butler J, Berkman LF, Krumholz HM. Risk factors for heart failure in the elderly: a prospective community-based study. Am J Med. 1999;106:605–12. doi: 10.1016/s0002-9343(99)00126-6. [DOI] [PubMed] [Google Scholar]
- 15.Murphy NF, MacIntyre K, Stewart S, Hart CL, Hole D, McMurray JJ. Long-term cardiovascular consequences of obesity: 20-year follow-up of more than 15 000 middle-aged men and women (the Renfrew-Paisley study) Eur Heart J. 2006;27:96–106. doi: 10.1093/eurheartj/ehi506. [DOI] [PubMed] [Google Scholar]
- 16.Voulgari C, Tentolouris N, Dilaveris P, Tousoulis D, Katsilambros N, Stefanadis C. Increased heart failure risk in normal-weight people with metabolic syndrome compared with metabolically healthy obese individuals. J Am Coll Cardiol. 2011;58:1343–50. doi: 10.1016/j.jacc.2011.04.047. [DOI] [PubMed] [Google Scholar]
- 17.Witteles RM, Fowler MB. Insulin-resistant cardiomyopathy clinical evidence, mechanisms, and treatment options. J Am Coll Cardiol. 2008;51:93–102. doi: 10.1016/j.jacc.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 18.Devereux RB, Roman MJ, Paranicas M, et al. Impact of diabetes on cardiac structure and function: the strong heart study. Circulation. 2000;101:2271–6. doi: 10.1161/01.cir.101.19.2271. [DOI] [PubMed] [Google Scholar]
- 19.Rutter MK, Parise H, Benjamin EJ, et al. Impact of glucose intolerance and insulin resistance on cardiac structure and function: sex-related differences in the Framingham Heart Study. Circulation. 2003;107:448–54. doi: 10.1161/01.cir.0000045671.62860.98. [DOI] [PubMed] [Google Scholar]
- 20.Sundstrom J, Lind L, Nystrom N, et al. Left ventricular concentric remodeling rather than left ventricular hypertrophy is related to the insulin resistance syndrome in elderly men. Circulation. 2000;101:2595–600. doi: 10.1161/01.cir.101.22.2595. [DOI] [PubMed] [Google Scholar]
- 21.DeFronzo RA, Cooke CR, Andres R, Faloona GR, Davis PJ. The effect of insulin on renal handling of sodium, potassium, calcium, and phosphate in man. J Clin Invest. 1975;55:845–55. doi: 10.1172/JCI107996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson EA, Hoffman RP, Balon TW, Sinkey CA, Mark AL. Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J Clin Invest. 1991;87:2246–52. doi: 10.1172/JCI115260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sartori M, Ceolotto G, Papparella I, et al. Effects of angiotensin II and insulin on ERK1/2 activation in fibroblasts from hypertensive patients. Am J Hypertens. 2004;17:604–10. doi: 10.1016/j.amjhyper.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 24.Rosamond WD, Chang PP, Baggett C, et al. Classification of heart failure in the atherosclerosis risk in communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail. 2012;5:152–9. doi: 10.1161/CIRCHEARTFAILURE.111.963199. [DOI] [PMC free article] [PubMed] [Google Scholar]